Smoking Cessation Rates among Pregnant Women and Their Relapse Rates in the Postpartum Period in Samsun
Abstract
:1. Introduction
2. Material and Methods
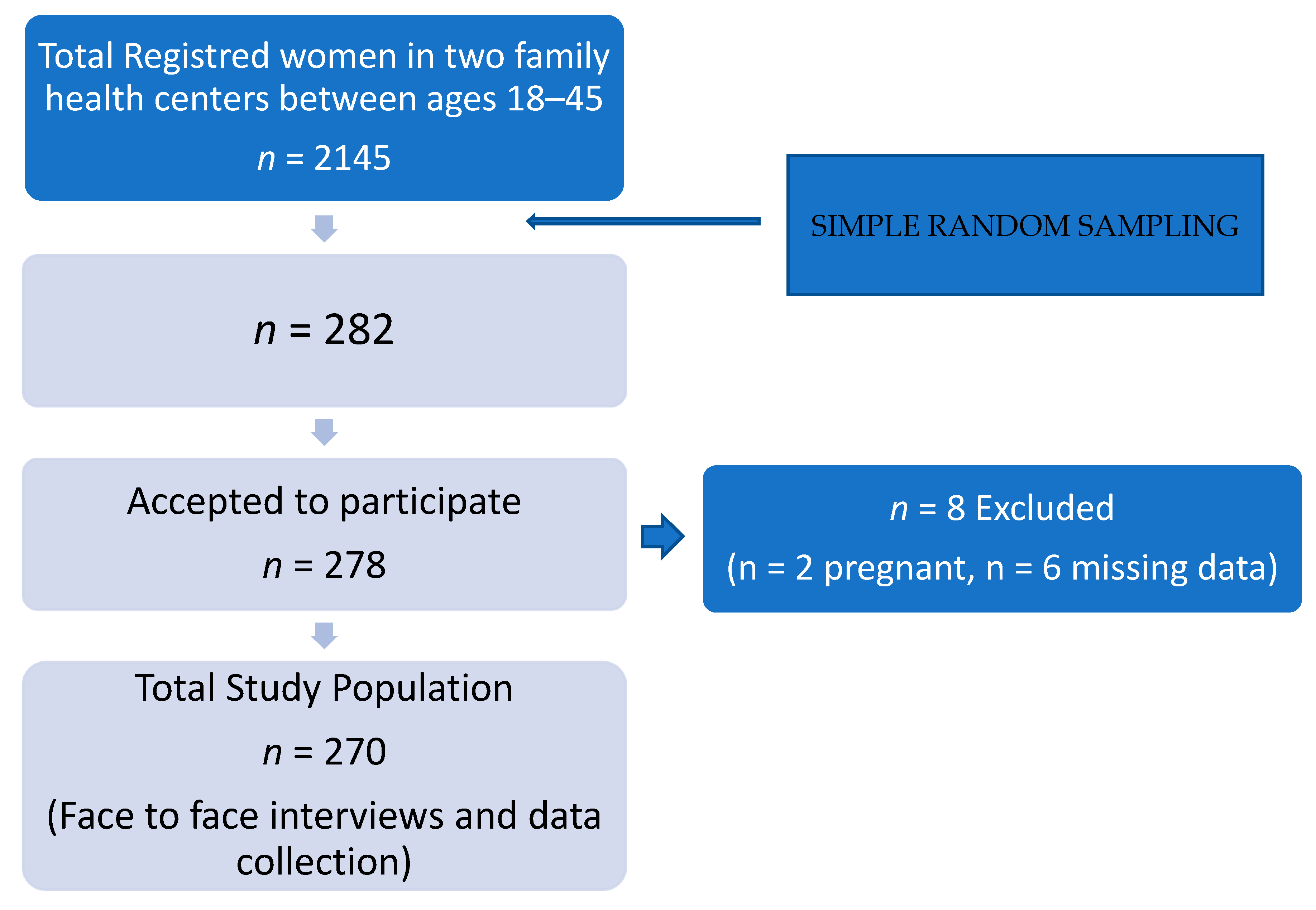
2.1. Type of Study and Selection of Patients
2.2. Data Collection and Statistical Analysis
2.3. Fagerström Nicotine Dependency Test
2.4. Pack Year
2.5. Statistical Analyses
2.6. Ethical Consent
3. Results
3.1. Smoking during Pregnancy
3.2. Knowledge about the Harms of Smoking during Pregnancy
3.3. Weight Change during Pregnancy According to Smoking Status
3.4. Problems in Pregnancy and the Postpartum Period
3.5. Reasons for Relapse
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jafari, A.; Rajabi, A.; Gholian-Aval, M.; Peyman, N.; Mahdizadeh, M.; Tehrani, H. National, regional, and global prevalence of cigarette smoking among women/females in the general population: A systematic review and meta-analysis. Environ. Health Prev. Med. 2021, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, I.B.; Tripp, A.L.; Dean, A.K.; Mbulo, L.; Arrazola, R.A.; Twentyman, E.; King, B.A. Tobacco Smoking Cessation and Quitline Use among Adults Aged ≥ 15 Years in 31 Countries: Findings from the Global Adult Tobacco Survey. Am. J. Prev. Med. 2021, 60 (Suppl. S2), S128–S135. [Google Scholar] [CrossRef]
- Janssen, F.; El Gewily, S.; Bardoutsos, A. Smoking epidemic in Europe in the 21st century. Tob. Control. 2021, 30, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Özer, N.; Tokg, L.; Kay, M.; Bar, C.; Abac, A. Systematic Review, Meta-analysis and Meta-regression of Epidemiological Studies for Cardiovascular Risk Factors. Turk Kardiyol. Dern. Ars. 2018, 46, 602–612. [Google Scholar] [CrossRef]
- Simpson, W.J. A preliminary report on cigarette smoking and the incidence of prematurity. Am. J. Obstet. Gynecol. 1957, 73, 808–815. [Google Scholar] [CrossRef]
- Riaz, M.; Lewis, S.; Naughton, F.; Ussher, M. Predictors of smoking cessation during pregnancy: A systematic review and meta-analysis. Addiction 2017, 113, 610–622. [Google Scholar] [CrossRef]
- Blatt, K.; Moore, E.; Chen, A.; Van Hook, J.; DeFranco, E.A. Association of Reported Trimester-Specific Smoking Cessation with Fetal Growth Restriction. Obstet. Gynecol. 2015, 125, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Hamadneh, S.; Hamadneh, J. Active and Passive Maternal Smoking during Pregnancy and Birth Outcomes: A Study from a Developing Country. Ann. Glob. Health 2021, 87, 122. [Google Scholar] [CrossRef]
- Wallace, J.L.; Aland, K.L.; Blatt, K.; Moore, E.; DeFranco, E.A. Modifying the risk of recurrent preterm birth: Influence of trimester-specific changes in smoking behaviors. Am. J. Obstet. Gynecol. 2017, 216, 310.e1–310.e8. [Google Scholar] [CrossRef]
- Kamai, E.M.; McElrath, T.F.; Ferguson, K.K. Fetal growth in environmental epidemiology: Mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ. Health 2019, 18, 43. [Google Scholar] [CrossRef] [Green Version]
- Dehmel, S.; Nathan, P.; Bartel, S.; El-Merhie, N.; Scherb, H.; Milger, K.; John-Schuster, G.; Yildirim, A.O.; Hylkema, M.; Irmler, M.; et al. Intrauterine smoke exposure deregulates lung function, pulmonary transcriptomes, and in particular insulin-like growth factor (IGF)-1 in a sex-specific manner. Sci. Rep. 2018, 8, 7547. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Bauld, L. Maternal smoking and preterm birth: An unresolved health challenge. PLOS Med. 2020, 17, e1003386. [Google Scholar] [CrossRef]
- Liu, B.; Xu, G.; Sun, Y.; Qiu, X.; Ryckman, K.K.; Yu, Y.; Snetselaar, L.G.; Bao, W. Maternal cigarette smoking before and during pregnancy and the risk of preterm birth: A dose–response analysis of 25 million mother–infant pairs. PLOS Med. 2020, 17, e1003158. [Google Scholar] [CrossRef]
- Ananth, C.V.; Savitz, D.A.; Luther, E.R. Maternal Cigarette Smoking as a Risk Factor for Placental Abruption, Placenta Previa, and Uterine Bleeding in Pregnancy. Am. J. Epidemiol. 1996, 144, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Isayama, T.; Ye, X.Y.; Dunn, M.; Da Silva, O.; Alvaro, R.; Lee, S.K.; Shah, P.S. Adverse Impact of Maternal Cigarette Smoking on Preterm Infants: A Population-Based Cohort Study. Am. J. Perinatol. 2015, 32, 1105–1111. [Google Scholar] [CrossRef]
- Nakamura, A.; François, O.; Lepeule, J. Epigenetic Alterations of Maternal Tobacco Smoking during Pregnancy: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5083. [Google Scholar] [CrossRef]
- Glover, M.; Kira, A.; Smith, C. Enlisting “Aunties” to Support Indigenous Pregnant Women to Stop Smoking: Feasibility Study Results. Nicotine Tob. Res. 2015, 18, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Osei-Poku, G.K.; Thomas, S.; Mwananyanda, L.; Lapidot, R.; Elliott, P.A.; Macleod, W.B.; Somwe, S.W.; Gill, C.J. A systematic review of the burden and risk factors of sudden infant death syndrome (SIDS) in Africa. J. Glob. Health 2021, 11, 04075. [Google Scholar] [CrossRef]
- Galéra, C.; Salla, J.; Montagni, I.; Hanne-Poujade, S.; Salamon, R.; Grondin, O.; Guichard, E.; Bouvard, M.; Tzourio, C.; Michel, G. Stress, attention deficit hyperactivity disorder (ADHD) symptoms and tobacco smoking: The i-Share study. Eur. Psychiatry 2017, 45, 221–226. [Google Scholar] [CrossRef]
- Tong, V.T.; Dietz, P.M.; Morrow, B.; D’Angelo, D.V.; Farr, S.L.; Rockhill, K.M.; England, L.J. Trends in Smoking before, during, and after Pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6206a1.htm?s_cid=ss6206a1_e (accessed on 8 November 2013).
- Vivilaki, V.G.; Diamanti, A.; Tzeli, M.; Patelarou, E.; Bick, D.; Papadakis, S.; Lykeridou, K.; Katsaounou, P. Exposure to active and passive smoking among Greek pregnant women. Tob. Induc. Dis. 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Recommendations for the Prevention and Management of Tobacco Use and Second-Hand Smoke Exposure in Pregnancy. Published 2013. Available online: https://www.who.int/tobacco/publications/pregnancy/guidelinestobaccosmokeexposure/en/ (accessed on 9 January 2019).
- World Health Organization. Gender, Women, and the Tobacco Epidemic. World Health Organization; 2010: i-xii, 1–253. Available online: https://www.who.int/tobacco/publications/gender/women_tob_epidemic/en/ (accessed on 9 January 2019).
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Uysal, M.A.; Kadakal, F.; Karşidağ, C.; Bayram, N.G.; Uysal, O.; Yilmaz, V. Fagerstrom test for nicotine dependence: Reliability in a Turkish sample and factor analysis. Tuberk. Ve Toraks 2004, 52, 115–121. [Google Scholar]
- Marakoglu, K.; Sezer, R.E. Smoking during Pregnancy in Sivas. Cumhur. Üniversitesi Tıp Fakültesi Derg. 2003, 25, 157–164. [Google Scholar]
- Davas, I.; Varolan, A.; Yazgan, A.; Yılmaz, Ö.; Yardım, Ç.; Akyol, A.; Baksu, B. The effect of smoking on maternal ve fetal complications. Sisli Etfal Tıp Bülteni 2022, 41, 19–23. [Google Scholar]
- Cnattingius, S. Maternal Age Modifies the Effect of Maternal Smoking on Intrauterine Growth Retardation but Not on Late Fetal Death and Placental Abruption. Am. J. Epidemiol. 1997, 145, 319–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.Q.; Windsor, R.A.; Perkins, L.; Goldenberg, R.L.; Lowe, J.B. The impact on infant birth weight and gestational age of co-tinine-validated smoking reduction during pregnancy. JAMA 1993, 269, 1519–1524. [Google Scholar] [CrossRef]
- Moore, E.; Blatt, K.; Chen, A.; Van Hook, J.; DeFranco, E.A. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 109.e1–109.e6. [Google Scholar] [CrossRef] [Green Version]
- Law, K.L.; Stroud, L.R.; LaGasse, L.L.; Niaura, R.; Liu, J.; Lester, B.M. Smoking during Pregnancy and Newborn Neurobehavior. Pediatrics 2003, 111 Pt 1, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Committee Opinion No. 721: Smoking Cessation During Pregnancy . Obs. Gynecol. 2017, 130, e200–e204. [CrossRef]
- Riaz, M.; Lewis, S.; Coleman, T.; Aveyard, P.; West, R.; Naughton, F.; Ussher, M. Which measures of cigarette dependence are predictors of smoking cessation during pregnancy? Analysis of data from a randomized controlled trial. Addiction 2016, 111, 1656–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, G.; Ellison, B.B.; Meade, C.; Roach, C.N.; Lopez, E.; Albrecht, T.; Brandon, T.H. Adapting Smoking Relapse–Prevention Materials for Pregnant and Postpartum Women: Formative Research. Matern. Child Health J. 2006, 10, 235–245. [Google Scholar] [CrossRef]
- Cooper, S.; Orton, S.; Leonardi-Bee, J.; Brotherton, E.; Vanderbloemen, L.; Bowker, K.; Naughton, F.; Ussher, M.; Pickett, K.E.; Sutton, S.; et al. Smoking and quit attempts during pregnancy and postpartum: A longitudinal UK cohort. BMJ Open 2017, 7, e018746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Rosenberg, K.D.; Sandoval, A.P. Breastfeeding Duration and Perinatal Cigarette Smoking in a Population-Based Cohort. Am. J. Public Health 2006, 96, 309–314. [Google Scholar] [CrossRef]
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update: A U.S. Public Health Service Report. Am. J. Prev. Med. 2008, 35, 158–176. [Google Scholar] [CrossRef] [Green Version]
- Siu, A.L. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2015, 163, 622–634. [Google Scholar] [CrossRef] [Green Version]
- Ashford, K.B.; Hahn, E.; Hall, L.; Rayens, M.K.; Noland, M. Postpartum Smoking Relapse and Secondhand Smoke. Public Health Rep. 2009, 124, 515–526. [Google Scholar] [CrossRef]
- Orleans, C.T.; Barker, D.C.; Kaufman, N.J.; Marx, J.F. Helping pregnant smokers quit: Meeting the challenge in the next decade. Tob. Control. 2000, 9 (Suppl. S3), iii6–iii11. [Google Scholar] [CrossRef] [Green Version]
- Patnode, C.D.; Henderson, J.T.; Thompson, J.H.; Senger, C.A.; Fortmann, S.P.; Whitlock, E.P. Behavioral Counseling and Pharma-cotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2015, 163, 608–621. [Google Scholar] [CrossRef] [Green Version]
- Flemming, K.; Graham, H.; McCaughan, D.; Angus, K.; Bauld, L. The barriers and facilitators to smoking cessation experienced by women’s partners during pregnancy and the post-partum period: A systematic review of qualitative research. BMC Public Health 2015, 15, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenaker, D.A.J.M.; Ploubidis, G.B.; Goodman, A.; Mishra, G.D. Factors across the life course predict women’s change in smoking behaviour during pregnancy and in midlife: Results from the National Child Development Study. J. Epidemiol. Community Health 2017, 71, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
| Variables | Results |
|---|---|
| Mean Age (years) | 35.75 ± 6.9 (Min = 18, Max= 45) |
| Marital status | |
| Married | n = 256 (94.8%) |
| Single/divorced/widowed | n = 14 (5.2%) |
| Educational status | |
| <High school | n = 134 (49.6%) |
| High school | n = 50 (18.5%) |
| University | n = 86 (31.9%) |
| Occupation | |
| Housewife | n = 188 (69.6%) |
| Civil servant | n = 105 (19.3%) |
| Other | n = 60 (11.1%) |
| Income | |
| <Minimum wage | n = 130 (48.1%) |
| Minimum wage | n = 74 (27.4%) |
| >Minimum wage | n = 66 (24.4%) |
| Total weight before pregnancy (kg) | 64.78 ± 11.93 |
| Total weight in the postnatal period (kg) | 68.91 ± 12.01 |
| Current weight (kg) | 67.85 ± 10.94 |
| Number of term deliveries | 2.14 ± 0.09 (Min = 1, Max= 6) |
| Number of living children | 2.02 ± 0.07 (Min = 1, Max = 6) |
| Mean duration of pregnancy (weeks) | 38.42 ± 1.9 (Min = 27, Max = 42) |
| Mean birth weight (g) | 3253 ± 581.17 (Min = 1000, Max = 4500) |
| Age at first pregnancy (years) | 24.85 ± 7.2 years (Min = 15, Max = 42) |
| History of abortus | |
| Yes | n = 64 (23.7%) |
| No | n = 206 (76.3%) |
| Mean number of abortus (n = 32) | 1.44 ± 0.759 (Min = 1, Max = 4). |
| Smoking status | |
| Smoker | n = 76 (28.1%) |
| Non-smoker | n = 170 (63%) |
| Ex-smoker | n = 24 (8.9%) |
| Age at starting smoking (years) | 20.96 ± 5.3, (Min = 14, Max = 40) |
| Fagerström score | 1.87 ± 0.338, (Min = 0, Max = 9) |
| Package/year | 20.1 ± 1.4, (Min = 4, Max = 34) |
| Smoking status of spouse/partner | |
| Smoker | n = 98 (38.2%) |
| Non-smoker | n = 92 (35.9%) |
| Ex-smoker | n = 46 (17.9%) |
| Variable | Smoker | Non-Smoker | p | |
|---|---|---|---|---|
| Miscarriage | Yes | 18 (40.9%) | 26 (59%) | 0.521 |
| No | 66 (29.2%) | 160 (70.7%) | ||
| Preterm delivery | Yes | 8 (14.8%) | 46 (85.2%) | 0.546 |
| No | 32 (7.4%) | 402 (92.6%) | ||
| Risk of abortion | Yes | 14 (20%) | 56 (80%) | 0.256 |
| No | 50 (11.7%) | 374 (88.2%) | ||
| Problems in the first year of childhood * | Yes | 30 (42.8%) | 40 (57.1%) | 0.06 |
| No | 76 (18.2%) | 340 (81.7%) | ||
| Fetus birth weight (g) | 3268.4 ± 595.7 (Min = 2598, Max = 4500) | 3125 ± 466.1 (Min = 1000, Max = 4500) | 0.025 | |
| Health problems during pregnancy # | Yes | 12 (22.2%) | 42 (77.8%) | 0.841 |
| No | 330 (76.2%) | 104 (23.8%) | ||
| 95% C.I. for EXP(B) | |||||||
|---|---|---|---|---|---|---|---|
| Variables | B | S.E. | Wald | p | Exp(B) | Lower | Upper |
| Smoking spouse | −0.874 | 0.416 | 4.422 | 0.035 | 0.420 | 0.043 | 0.099 |
| GWG * (kg) | 0.015 | 0.006 | 6.687 | 0.010 | 0.985 | 0.561 | 1.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin, B.M.; Kertmen, T.; Ustaoglu, M. Smoking Cessation Rates among Pregnant Women and Their Relapse Rates in the Postpartum Period in Samsun. J. Respir. 2023, 3, 118-129. https://doi.org/10.3390/jor3030012
Yalcin BM, Kertmen T, Ustaoglu M. Smoking Cessation Rates among Pregnant Women and Their Relapse Rates in the Postpartum Period in Samsun. Journal of Respiration. 2023; 3(3):118-129. https://doi.org/10.3390/jor3030012
Chicago/Turabian StyleYalcin, Bektas Murat, Tugba Kertmen, and Muge Ustaoglu. 2023. "Smoking Cessation Rates among Pregnant Women and Their Relapse Rates in the Postpartum Period in Samsun" Journal of Respiration 3, no. 3: 118-129. https://doi.org/10.3390/jor3030012
APA StyleYalcin, B. M., Kertmen, T., & Ustaoglu, M. (2023). Smoking Cessation Rates among Pregnant Women and Their Relapse Rates in the Postpartum Period in Samsun. Journal of Respiration, 3(3), 118-129. https://doi.org/10.3390/jor3030012







