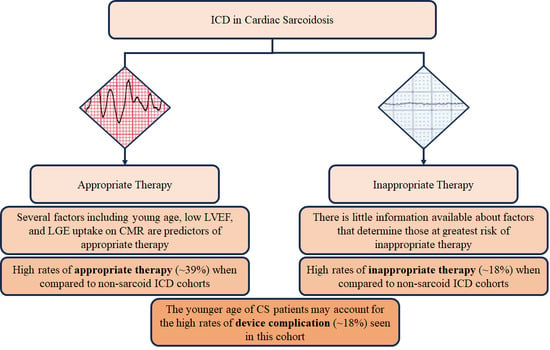
ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications
Abstract
:1. Introduction
2. Factors Associated with Appropriate Therapy
- Patient characteristics;
- Ventricular characteristics;
- Imaging findings.
2.1. Patient Characteristics
2.2. Ventricular Characteristics
2.3. Imaging Findings
2.4. Non-Predictors of Appropriate Therapy
3. Inappropriate Therapy and Device Complications
4. The Association of ICD Device Complications and Risk Factors in Patients with Cardiac Sarcoidosis
5. Strengths and Limitations
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AVB | Atrioventricular Block |
| CHB | Complete Heart Block |
| CI | Confidence Interval |
| CMR | Cardiovascular Magnetic Resonance |
| CS | Cardiac Sarcoidosis |
| CT-PET | Computed Tomography—Positron Emission Tomography |
| ICD | Implantable Cardioverter–Defibrillator |
| LBBB | Left Bundle Branch Block |
| LGE | Late Gadolinium Enhancement |
| LVEF | Left Ventricular Ejection Fraction |
| OR | Odds Ratio |
| RBBB | Right Bundle Branch Block |
| SCD | Sudden Cardiac Death |
| SCD-HeFT | Sudden Cardiac Death In Heart Failure Trial |
| VA | Ventricular Arrhythmia |
| VF | Ventricular Fibrillation |
| VT | Ventricular Tachycardia |
References
- Ellenbogen, K.A.; Levine, J.H.; Berger, R.D.; Daubert, J.P.; Winters, S.L.; Greenstein, E.; Shalaby, A.; Schaechter, A.; Subacius, H.; Kadish, A.; et al. Are Implantable Cardioverter Defibrillator Shocks a Surrogate for Sudden Cardiac Death in Patients with Nonischemic Cardiomyopathy? Circulation 2006, 113, 776–782. [Google Scholar] [CrossRef]
- Xie, J.; Weil, M.H.; Sun, S.; Tang, W.; Sato, Y.; Jin, X.; Bisera, J. High-Energy Defibrillation Increases the Severity of Postresuscitation Myocardial Dysfunction. Circulation 1997, 96, 683–688. [Google Scholar] [CrossRef]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic Importance of Defibrillator Shocks in Patients with Heart Failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Faddis, M.N.; Fetics, B.J.; Zelik, K.E.; Efimov, I.R.; Berger, R.D. Transient Local Injury Current in Right Ventricular Electrogram after ICD Shock Predicts Heart Failure Progression. J. Am. Coll. Cardiol. 2009, 54, 822–828. [Google Scholar] [CrossRef]
- Mactaggart, S.; Ahmed, R. The role of ICDs in patients with sarcoidosis—A comprehensive review. Curr. Probl. Cardiol. 2024, 49, 102483. [Google Scholar] [CrossRef]
- Ahmed, R.; Sawatari, H.; Amanullah, K.; Okafor, J.; Wafa, S.E.I.; Deshpande, S.; Ramphul, K.; Ali, I.; Khanji, M.; Mactaggart, S.; et al. Characteristics and Outcomes of Hospitalised Patients with Heart Failure and Sarcoidosis: A Propenisty-Matched Analysis of the Nationwide Readmissions Database 2010–2019. Am. J. Med. 2024, S0002-9343(24)00206-7. [Google Scholar]
- Ahmed, R.; Sharma, R.; Chahal, C.A.A. Trends and Disparities Around Cardiovascular Mortality in Sarcoidosis: Does Big Data Have the Answers? J. Am. Heart. Assoc. 2024, 13, e034073. [Google Scholar] [CrossRef] [PubMed]
- Schuller, J.L.; Zipse, M.; Crawford, T.; Bogun, F.; Beshai, J.; Patel, A.R.; Sweiss, N.J.; Nguyen, D.T.; Aleong, R.G.; Varosy, P.D.; et al. Implantable Cardioverter Defibrillator Therapy in Patients with Cardiac Sarcoidosis. J. Cardiovasc. Electrophysiol. 2012, 23, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Jimenez, A.; Hood, R.E.; Dickfeld, T.; Saliaris, A.; Shorofsky, S.; Saba, M.M. Cardiac Sarcoidosis: Electrophysiological Outcomes on Long-Term Follow-Up and the Role of the Implantable Cardioverter-Defibrillator. J. Cardiovasc. Electrophysiol. 2014, 25, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.; Stern, E.H.; Gomes, J.A.; Teirstein, A.S.; Eckart, R.E.; Mehta, D. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005, 96, 276–282. [Google Scholar] [CrossRef]
- Betensky, B.P.; Tschabrunn, C.M.; Zado, E.S.; Goldberg, L.R.; Marchlinski, F.E.; Garcia, F.C.; Cooper, J.M. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012, 9, 884–891. [Google Scholar] [CrossRef]
- Kron, J.; Sauer, W.; Schuller, J.; Bogun, F.; Crawford, T.; Sarsam, S.; Rosenfeld, L.; Mitiku, T.Y.; Cooper, J.M.; Mehta, D.; et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. EP Eur. 2013, 15, 347–354. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Sahoo, D.; Zein, J.; Brunken, R.C.; Tchou, P.J.; Culver, D.A. Outcome of cardiac sarcoidosis after radiofrequency ablation and placement of AICD-A propensity matched analysis. Sarcoidosis. Vasc. Diffuse Lung Dis. Off. J. WASOG 2015, 32, 70–79. [Google Scholar]
- Takaya, Y.; Kusano, K.; Nishii, N.; Nakamura, K.; Ito, H. Early and frequent defibrillator discharge in patients with cardiac sarcoidosis compared with patients with idiopathic dilated cardiomyopathy. Int. J. Cardiol. 2017, 240, 302–306. [Google Scholar] [CrossRef]
- Azoulay, L.D.; Waintraub, X.; Haroche, J.; Amoura, Z.; Cohen Aubart, F. Factors associated with implantable cardioverter defibrillators appropriate therapy in cardiac sarcoidosis: A meta-analysis. Sarcoidosis Vasc. Diffuse Lung Dis. 2020, 37, 17–23. [Google Scholar]
- Kouranos, V.; Khattar, R.S.; Okafor, J.; Ahmed, R.; Azzu, A.; Baksi, J.A.; Wechalekar, K.; Cowie, M.R.; Wells, A.U.; Lüscher, T.F.; et al. Predictors of outcome in a contemporary cardiac sarcoidosis population: Role of brain natriuretic peptide, left ventricular function and myocardial inflammation. Eur. J. Heart Fail. 2023, 25, 2287–2298. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ejhf.3057 (accessed on 13 January 2024). [CrossRef] [PubMed]
- Taha, A.; Assaf, O.; Champsi, A.; Nadarajah, R.; Patel, P.A. Outcomes after transvenous defibrillator implantation in cardiac sarcoidosis: A systematic review. J. Arrhythmia 2022, 38, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A.; Jain, R.; Turagam, M.K.; Kusumoto, F.M.; Woldu, H.G.; Gautam, S. Outcome of implantable cardioverter defibrillator in cardiac sarcoidosis: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2020, 58, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.B.; Marshall, H.; Kennewell, P.; Pham, H.D.; Tully, P.J.; Rattanakosit, T.; Mahadevan, G.; Mahajan, R. Risk and predictors of sudden death in cardiac sarcoidosis: A systematic review and meta-analysis. Int. J. Cardiol. 2021, 328, 130–140. [Google Scholar] [CrossRef]
- Mathijssen, H.; Bakker, A.L.M.; Balt, J.C.; Akdim, F.; van Es, H.W.; Veltkamp, M.; Grutters, J.C.; Post, M.C. Predictors of appropriate implantable cardiac defibrillator therapy in cardiac sarcoidosis. J. Cardiovasc. Electrophysiol. 2022, 33, 1272–1280. [Google Scholar] [CrossRef]
- Patel, N.; Kalra, R.; Doshi, R.; Arora, H.; Bajaj, N.S.; Arora, G.; Arora, P. Hospitalization Rates, Prevalence of Cardiovascular Manifestations, and Outcomes Associated with Sarcoidosis in the United States. J. Am. Heart Assoc. 2018, 7, e007844. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Machado, R.F.; Schraufnagel, D.; Sweiss, N.J.; Baughman, R.P. Racial Difference in Sarcoidosis Mortality in the United States. Chest 2015, 147, 438. [Google Scholar] [CrossRef]
- Ahmed, R.; Shahbaz, H.; Ramphul, K.; Mactaggart, S.; Dullay, M.S.; Okafor, J.; Azzu, A.; Khattar, R.; Wells, A.U.; Wechalekar, K.; et al. Racial Disparities Among Patients with Cardiac Sarcoidosis and Arrhythmias in the United States: A propensity matched-analysis from the National Inpatient Sample Database 2016–2020. Curr. Probl. Cardiol. 2024, 102450. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Nielsen, J.C.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Heart Rhythm. 2018, 15, e73–e189. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Nordenswan, H.K.; Lehtonen, J.; Ekström, K.; Kandolin, R.; Simonen, P.; Mäyränpää, M.; Vihinen, T.; Miettinen, H.; Kaikkonen, K.; Haataja, P.; et al. Outcome of Cardiac Sarcoidosis Presenting with High-Grade Atrioventricular Block. Circ. Arrhythm. Electrophysiol. 2018, 11, e006145. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Graner, M.; Schildt, J.; Salmenkivi, K.; Kivistö, S.M.; Kupari, M. Diagnosing isolated cardiac sarcoidosis. J. Intern. Med. 2011, 270, 461–468. [Google Scholar] [CrossRef]
- Kusano, K.; Ishibashi, K.; Noda, T.; Nakajima, K.; Nakasuka, K.; Terasaki, S.; Hattori, Y.; Nagayama, T.; Mori, K.; Takaya, Y.; et al. Prognosis and Outcomes of Clinically Diagnosed Cardiac Sarcoidosis Without Positive Endomyocardial Biopsy Findings. JACC: Asia 2021, 1, 385–395. [Google Scholar] [CrossRef]
- Kitai, T.; Nabeta, T.; Naruse, Y.; Taniguchi, T.; Yoshioka, K.; Miyakoshi, C.; Kurashima, S.; Miyoshi, Y.; Tanaka, H.; Okumura, T.; et al. Comparisons between biopsy-proven versus clinically diagnosed cardiac sarcoidosis. Heart 2022, 108, 1887–1894. [Google Scholar] [CrossRef]
- Crawford, T.; Mueller, G.; Sarsam, S.; Prasitdumrong, H.; Chaiyen, N.; Gu, X.; Schuller, J.; Kron, J.; Nour, K.A.; Cheng, A.; et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ. Arrhythm. Electrophysiol. 2014, 7, 1109–1115. [Google Scholar] [CrossRef]
- Velangi, P.S.; Chen, K.H.A.; Kazmirczak, F.; Okasha, O.; von Wald, L.; Roukoz, H.; Farzaneh-Far, A.; Markowitz, J.; Nijjar, P.S.; Bhargava, M.; et al. Right Ventricular Abnormalities on Cardiovascular Magnetic Resonance Imaging in Patients with Sarcoidosis. JACC Cardiovasc. Imaging 2020, 13, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Smedema, J.P.; van Geuns, R.J.; Ainslie, G.; Ector, J.; Heidbuchel, H.; Crijns, H.J.G.M. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Fail. 2017, 4, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ekström, K.; Lehtonen, J.; Nordenswan, H.K.; Mäyränpää, M.I.; Räisänen-Sokolowski, A.; Kandolin, R.; Simonen, P.; Pietilä-Effati, P.; Alatalo, A.; Utriainen, S.; et al. Sudden death in cardiac sarcoidosis: An analysis of nationwide clinical and cause-of-death registries. Eur. Heart J. 2019, 40, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jørgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Brady, P.A.; Meverden, R.A.; Hodge, D.O.; Uslan, D.Z.; Hayes, D.L. Age and Gender Trends in Implantable Cardioverter Defibrillator Utilization. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2008, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Nonin, E.; Pinot, J.; Morel, E.; Flys, C.; Scridon, A.; Chevalier, P. Risk of Sprint Fidelis defibrillator lead failure is highly dependent on age. Arch. Cardiovasc. Dis. 2011, 104, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Suzuki, M.; Shimizu, M.; Shimada, H.; Tsunoda, T.; Miyazaki, H.; Misu, Y.; Yamakami, Y.; Yamaguchi, M.; Kato, N.; et al. Risk prediction of inappropriate implantable cardioverter-defibrillator therapy using machine learning. Sci. Rep. 2023, 13, 19586. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
| Authors | Publication Year | Location | Study Design | Cohort Size | Findings Relevant to This Section |
|---|---|---|---|---|---|
| Ellenbogen et al. [1] | 2006 | North America | Prospective | 458 | “Appropriate therapy” is an effective proxy of SCD in non-ischaemic cardiomyopathies. |
| Azoulay et al. [15] | 2020 | Global | Retrospective | 464 | Statistically significant factors in predicting appropriate therapy are as follows: young age, male sex, low LVEF, ventricular pacing, and complete heart block. Statistically non-significant factors in predicting appropriate therapy are as follows: LBBB/RBBB, positive CMR, and syncope. |
| Taha et al. [17] | 2022 | Global | Retrospective | 530 | (Includes several non-predictors of appropriate therapy; please see main text.) |
| Schuller et al. [8] | 2012 | North America | Retrospective | 112 | Higher rates of appropriate therapy in those with RV dysfunction. |
| Halawa et al. [18] | 2020 | Global | Mixed prospective and retrospective | 585 | Rates of appropriate therapy higher in those with AVB. |
| Franke et al. [19] | 2020 | Global | Mixed prospective and retrospective | 1247 | Higher rates of appropriate therapy in those in whom an ICD was implanted for secondary prevention. |
| Mathijssen et al. [20] | 2022 | Netherlands | Retrospective | 105 | Higher rates of appropriate ICD therapy in male sex, 2nd/3rd degree AVB, prior VA, and presence of LGE on CMR—most strongly with LGE in the area of RV. |
| Kron et al. [12] | 2013 | North America | Retrospective | 33 | Young age and reduced LVEF predict appropriate ICD therapy. |
| Authors | Publication Year | Location | Study Design | Cohort Size | Findings Relevant to This Section |
|---|---|---|---|---|---|
| Franke et al. [19] | 2020 | Global | Mixed prospective and retrospective | 1247 | Rates of inappropriate therapy were ~18%. |
| Mathijssen et al. [20] | 2022 | Netherlands | Retrospective | 105 | Low rates of inappropriate ICD therapy in those with CS. Device complications present in ~18% cases. |
| Betensky et al. [11] | 2013 | North America, Canada & Japan | Retrospective | 235 | Those with CS experience high rates of inappropriate therapy, most commonly caused by supraventricular tachyarrhythmias. |
| Kron et al. [12] | 2012 | Global | Retrospective | 235 | Rates of inappropriate therapy were ~25%. Adverse events were present in ~17% cases. |
| Class | 2014 HRS Consensus | 2017 AHA/ACC/HRS Guideline | 2022 ESC Guidelines |
|---|---|---|---|
| I | Sustained VT Survivors of SCA LVEF < 35% (a)(b) | ||
| IIa | LVEF > 35% with syncope (a)(b) | ||
| LVEF > 35% with evidence of myocardial scar on CMR or PET | LVEF > 35% with significant myocardial LGE on CMR after resolution of acute inflammation | ||
| Inducible sustained VA on EP study | Inducible, sustained monomorphic VA on EP study in those with LVEF 35–50% and minor LGE on CMR | ||
| Those with an indication for permanent pacing and LVEF > 35% | |||
| IIb | LVEF between 35 and 50% or RVEF < 40% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mactaggart, S.; Ahmed, R. ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications. J. Respir. 2024, 4, 102-111. https://doi.org/10.3390/jor4020009
Mactaggart S, Ahmed R. ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications. Journal of Respiration. 2024; 4(2):102-111. https://doi.org/10.3390/jor4020009
Chicago/Turabian StyleMactaggart, Sebastian, and Raheel Ahmed. 2024. "ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications" Journal of Respiration 4, no. 2: 102-111. https://doi.org/10.3390/jor4020009
APA StyleMactaggart, S., & Ahmed, R. (2024). ICD in Cardiac Sarcoidosis: Variables Associated with Appropriate Therapy, Inappropriate Therapy, and Device Complications. Journal of Respiration, 4(2), 102-111. https://doi.org/10.3390/jor4020009