Abstract
Breast cancer is the most commonly diagnosed cancer in women and is a leading cause of cancer death in women worldwide. Despite the available treatment options, such as surgery, chemotherapy, radiotherapy, endocrine therapy and molecular targeted therapy, breast cancer treatment remains a challenge. The advent of immunotherapy has revolutionized the treatment of breast cancer as it utilizes the host’s immune system to directly target tumor cells. In this literature review, we aim to summarize the recent advancements made in using immunotherapy for treating breast cancer patients. We discuss the different types of existing immunotherapies for breast cancer, including targeted therapy using monoclonal antibodies against breast cancer specific antigens and the use of immune checkpoint inhibitors to elicit an immune response against cancer cells. Finally, we consider the development of breast cancer vaccines that train the immune system to specifically recognize cancer cells and the future perspectives of immunotherapy for breast cancer.
1. Introduction
Immunotherapy refers to harnessing the patient’s own immune system to eradicate cancer. The first immunotherapy attempt was introduced in the late 19th century by two German physicians, Fehleisen and Busch, who noticed significant tumor regression after erysipelas infection [1]. The following years, the role of immunotherapy was extensively studied by William B. Coley, also known as the “Father of Immunotherapy”, who conducted experiments which involved injections of live bacteria S. pyogenes and S. marcescens (referred to as Coley’s toxin) into patients with inoperable bone cancers. In recent years, accumulating data support a key role for the immune system in determining both the response to standard and adjuvant therapy in patients with breast cancer [2].
The immune system consists of monitoring mechanisms that detect and respond to the presence of cancer cells, a process called “immunosurveillance” [3,4]. Specifically, immunosurveillance consists of three phases: elimination, equilibrium and escape [5]. During the elimination phase, the immune system cells recognize tumor-specific antigens and respond by destroying tumor cells. The latter is achieved due to tumor immunogenicity as well as the interaction of tumor-specific antigens with immune system cells. Immunogenicity refers to the ability of a tumor to induce immune responses which inhibit its survival and proliferation. Next, tumor cells that survive the elimination phase, enter a state of equilibrium, which is a transition period from the elimination phase to the onset of the disease [6]. Lastly, tumor cells manage to escape immune surveillance and proliferate after establishing an immune suppressive tumor microenvironment (TME) [7]. The acquired knowledge of immune surveillance and immunogenicity has revolutionized the immunotherapy treatment landscape of several types of cancers, including breast cancer.
Breast cancer (BC) is the most commonly diagnosed cancer among women worldwide with 2.26 million new cases in 2020 and 684,996 deaths [8]. According to the World Health Organization, malignancies account for 107.8 million disability-adjusted life years (DALYs), of which 19.6 million DALYs are attributed to breast cancer [9]. The significant prognostic differences in patient outcomes have led to the classification of the disease based on estrogen receptor (ER) and progesterone receptor (PR) status, as well as the expression of human epidermal growth factor receptor 2 (HER2) into luminal A (defined as ER+ and/or PR+, HER2−), luminal B (defined as ER+ and/or PR+, HER2+), HER2 overexpressing (defined as EGFR+, ER−, PR−) and triple negative breast cancer (TNBC) which is defined by the absence of ER, PR and HER2. TNBCs represent 10–20% of all BCs with 71–91% of them having a basal-like phenotype and approximately 20% expressing ER or HER2 to a certain extent [10,11]. TNBC is diagnosed based on immunohistochemistry (IHC) in clinical practice. However, guidelines recommend additional analysis in IHC samples with ambiguous HER2 status in order to avoid false positive or negative results [12].
The number of risk factors of breast cancer is significant and includes both modifiable factors and non-modifiable factors. Non-modifiable factors include age, sex, ethnicity, genetic mutations, family and reproductive history and previous radiation therapy, while modifiable factors include body mass index (BMI), physical activity, alcohol and processed food intake, smoking, exposure to certain chemicals, vitamin supplementation and chosen drugs. It is estimated that about 80% of patients with BC are white non-Hispanic female individuals aged >50 with mutations in the BRCA1, BRCA2, TP53, CDH1, PTEN and STK11 genes and less often in the ATM, PALB2, BRIP1, CHEK2 and XRCC2 genes. In addition, they present with an increased BMI, long smoking history, insufficient vitamin D supplementation, as well as excessive consumption of alcohol and highly processed meat, which is classified as a Group 1 carcinogen [13].
Currently, the conventional therapeutic approach for BC involves surgery, chemotherapy, radiotherapy, endocrinotherapy and molecular targeted therapy. Due to the potential of immunotherapy, among various chemotherapy agents, it has drawn the attention of researchers. Clinical evidence shows significant variability in the treatment response to programmed cell death-ligand 1 (PD-L1) inhibitors versus standard chemotherapy agents. According to the randomized, phase 3 KEYNOTE-119 study (NCT02555657), there was no significant difference observed in overall survival (OS); however, the results suggested a potential positive association between tumor mutational burden and pembrolizumab versus single-agent chemo per investigator’s choice of capecitabine, eribulin, gemcitabine or vinorelbine [14]. Similarly, positive results were observed in the phase III IMpassion130 trial comparing atezolizumab plus chemotherapy versus chemotherapy plus placebo. In fact, there was an increase in overall survival outcome only in PD-L1+ TNBC patients [15]. The American Society for Radiation Oncology, the Society for Immunotherapy of Cancer, and the US National Cancer Institute have investigated the incorporation of immunotherapy into radiotherapy [16]. This initiative is supported by a single-arm clinical trial of 22 patients with metastatic melanoma, followed by the administration of ipilimumab, which led to a partial reduction in the sizes of non-irradiated metastatic sites in four (18%) patients [17]. Thus, the benefit of immunotherapy versus or combined with conventional treatments is evident and there is ongoing investigation.
This literature review elaborates on various immunotherapies currently being applied for breast cancer treatment. These include 1. monoclonal antibodies; 2. immune checkpoint inhibitors: an approach designed to ‘unleash’ T cell responses; 3. breast cancer vaccines which train the immune system to fight BC; 4. cytokine therapies: an immunomodulatory approach; and 5. adoptive cellular therapies which are based on delivering engineered immune cells into the body to fight cancer. We also discuss preclinical and clinical advancements of various immunotherapies, limitations, and future directions.
2. Monoclonal Antibodies against HER-2 Receptor Protein
According to the National Institute of Health, monoclonal antibodies are artificially made proteins that can recognize specific targets and are widely used as a targeted cancer therapy [18]. Most often, they are categorized into those targeting immune molecules, otherwise known as immune checkpoint inhibitors, or into those targeting oncogenic membrane receptors [19]. Figure 1 demonstrates the two types of monoclonal antibodies that will be discussed in this review.
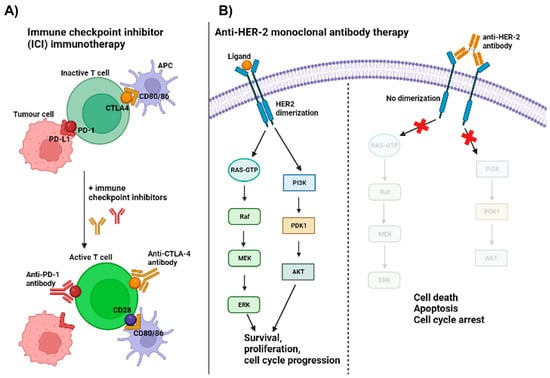
Figure 1.
Monoclonal antibody immunotherapies. (A) Demonstrates the mechanism of action of anti-PD-1 and anti-CTLA-4 immune checkpoint inhibitors. T cells require a total of 3 signals for proper activation. Antigen-specific recognition through T cell receptor (TCR): major histocompatibility complex (MHC) interaction between costimulatory molecules and cytokine signals [20]. Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) are negative checkpoint molecules that drive the inactivation of T cells upon interaction with their corresponding ligands, CD80/86 on antigen-presenting cells (APCs) and PD-L1 on tumor cells. Immune checkpoint inhibitors block that interaction and allow positive checkpoint molecules (e.g., CD28) to interact with their corresponding ligands (i.e., CD80/86) and deliver the 2nd signal for T cell activation. (B) Illustrates the mechanism of action of an anti-HER-2 monoclonal antibody by preventing dimerization of HER subunits. This ultimately leads to the perturbation of downstream signaling events and consequently promotes cell death, apoptosis and cell cycle arrest (created using BioRender.com, URL accessed on 16 May 2023).
Approximately one third of breast cancer patients exhibit upregulation of HER2 receptor which has led to the development of monoclonal antibodies against this particular receptor such as trastuzumab or pertuzumab [21,22]. Trastuzumab, commercially known as Herceptin, was the first humanized monoclonal antibody to be approved by the Food and Drug Administration (FDA) in 1998 for metastatic HER2+ breast cancer. It is known to function through multiple mechanisms, from mediating HER2 receptor internalization and subsequent degradation via recruitment of ubiquitin ligase to dampen HER-2-dependent signaling pathways such as the RAS/MAPK or PI3K/Akt pathways [23]. Patients often develop resistance to trastuzumab despite initial responses which has led to the development of alternative anti-HER2 monoclonal antibodies such as pertuzumab [21,24,25].
Pertuzumab is another humanized monoclonal antibody against a different HER2 extracellular domain (domain II) compared to trastuzumab (domain IV). Pertuzumab prevents the heterodimerization of HER receptors which leads to the inactivation of downstream signaling pathways such as PI3K/Akt [26,27,28,29]. If having to compare the two antibodies, pertuzumab has shown better outcomes in the clinic including a better safety profile and a higher efficacy compared to trastuzumab [21].
There are multiple clinical trials either underway or already completed that look into the effects of a dual HER2 blockade using a trastuzumab plus pertuzumab combination therapy. For patients with previously refractory disease following treatment with trastuzumab, Portera et al., (2008) reported cardiac toxicity in more than 50% of participants following administration of the dual antibody therapy which led to the study being brought to an end [30]. The Baselga study exhibited similar objective response rates (ORRs) to the Portera study (18% versus 24.5%) but reported no cardiac symptoms [30,31]. On the other hand, the CLEOPATRA study is looking into the effects of a dual HER2 blockade in metastatic patients with no prior treatment. So far, evidence suggests that the combination of the two antibodies as a first line treatment in metastatic breast cancer significantly augments the progression-free survival (PFS) rates [32].
Antibody-drug conjugates (ADCs) are made up of antibodies fused together to cytotoxic agents that allows the efficient delivery of these agents within cancer cells. Antibodies allow for specific recognition of malignant cells followed by ADC internalization and the release of cytotoxic effects mediated by the drug within the cell [33]. Trastuzumab emtansine (T-DM1) is an example of an ADC that is composed of trastuzumab as the monoclonal antibody linked to DM1, a cytotoxic agent that targets microtubule assembly and consequently leading to cell death [34]. In vitro studies have reported remarkable sensitivity to T-DM1 with various different breast cancer cell lines which was also replicated in in vivo animal models [34,35]. There are reports of multiple clinical trials having observed a much better efficacy and safety profile in patients with metastatic breast cancer with T-DM1 compared to trastuzumab or other therapies. Nonetheless, most patients do eventually acquire resistance to T-DM1 [36,37,38].
This has led to the development of alternative ADCs such as Trastuzumab deruxtecan. The latter contains the same antibody as T-DM1 but with a topoisomerase I inhibitor instead and was first approved in the USA in 2019 [39]. Using patients previously treated with T-DM1 and having progressed with it, Trastuzumab deruxtecan showed significant clinical efficacy; however, some noticeable adverse effects related to lung disease were observed in a substantial percentage of those patients [40]. Another study compared the efficacy of the two trastuzumab ADCs in patients previously treated with traszutumab plus a taxane. Cortes et al. (2022) reported a lower risk of death or disease progression in patients treated with trastuzumab deruxtecan than those treated with T-DM1. However, the same pulmonary complications observed by Verma et al. (2020) were also reported here [41].
Worth mentioning is also Sacituzumab govitecan, an ADC directed at trophoblastic cell-surface antigen-2 (Trop-2) expressed in multiple solid tumors including breast cancer. The fact that Trop-2 is highly restricted to tumor cells limits off-target effects and spares healthy tissue and makes it the perfect candidate to be used in cancer therapy [42,43,44]. The drug conjugate used for this ADC is SN-38, another topoisomerase I inhibitor and the ADC as a whole was recently approved in the USA in 2020 for patients with metastatic triple negative breast cancer previously treated and progressed with at least two other therapies [45].
3. Immune Checkpoint Inhibitors (ICIs)
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that specifically target immune checkpoint molecules on the surface of T cells or tumor cells to promote the activation of immune cells and consequently elicit an immune response against the cancer cells [46,47]. Several studies have detected significant levels of the immunosuppressive PD-L1 checkpoint molecule in HER-2+ breast cancer [48,49,50,51] which would suggest that PD-L1 blockade with ICIs would be beneficial.
Several clinical studies are evaluating the use of such antibodies in patients with TNBC—a subtype negative for progesterone, estrogen and HER-2 receptors [52,53]. Atezolizumab is an anti-PD-L1 monoclonal antibody that managed to increase the ORR in patients with metastatic TNBC by approximately 20% compared to non-treated patients [52]. Other PD-L1 checkpoint inhibitors such as avelumab have also been shown to augment the ORR in TNBC patients [53]. In both cases, the efficacy of the antibodies seemed to be dictated by the level of PD-L1 expression in patients. Those with higher expression seemed to benefit more compared to those with lower to no expression [52,53]. Due to the ability of chemotherapy agents to promote antigen presentation and PD-L1 expression, several studies have turned their attention to combining these agents with ICIs [54,55]. The anti-PD-L1 monoclonal antibody, pembrolizumab, was combined with nab-paclitaxel, paclitaxel and gemcitabine/carboplatin chemotherapy in metastatic TNBC patients and led to augmented PFS [54]. This again was restricted to PD-L1-positive patients. Furthermore, the effects of nivolumab combination with different chemotherapy agents again in patients with metastatic TNBC was assessed in the phase 2 TONIC trial. The administration of nivolumab following chemotherapy with doxorubicin rather than cisplatin seemed to be more effective in enhancing the ORRs of these patients—35% versus 23% in doxorubicin versus cisplatin-treated patients, respectively [55].
The KEYNOTE trial was the first clinical trial ever to use an anti-PD-1 agent, pembrolizumab, in patients diagnosed with PD-L1+ metastatic TNBC. Overall, the different trial phases suggest that pembrolizumab exhibits a low toxicity profile; however, response rates including ORR and OS remained relatively low. Furthermore, the study failed to show that pembrolizumab was superior to chemotherapy as a monotherapy treatment for metastatic TNBC patients [56,57,58]. The PANACEA trial enrolled patients diagnosed with HER2+ breast cancer previously progressed on trastuzumab monotherapy to assess the effect of pembrolizumab plus trastuzumab combination therapy. Overall, 15% of PD-L1+ patients had an objective response as opposed to none of the PD-L1- patients in the study. Moreover, no dose-limiting toxicities were reported, and fatigue was the most common treatment-related side effect observed in patients [59].
There are several other clinical trials either underway or already completed that assess the efficacy of different checkpoint inhibitors. Table 1 lists some of those trials.

Table 1.
List of clinical trials for immune checkpoint inhibitors.
Table 1 lists some of the clinical trials either underway or completed that have evaluated efficacy of different immune checkpoint inhibitor monotherapies or combined with other modalities such as chemotherapy or radiotherapy. The trial phase, subtype of breast cancer and sample size are also provided for each trial.
4. Breast Cancer Vaccines
Cancer vaccines is another type of immunotherapy that uses tumorigenic antigens as a way to train the immune system to recognize those antigens and initiate an immune response against them [66]. There are several types of cancer vaccines including those who utilize a patient’s own cancer antigens in order for the vaccine to be specific for the patient’s tumor or those who make use of a patient’s own immune or tumor cells [67]. Protein-based vaccines are considered to be the conventional method of vaccination and Figure 2 briefly summarizes how they function.
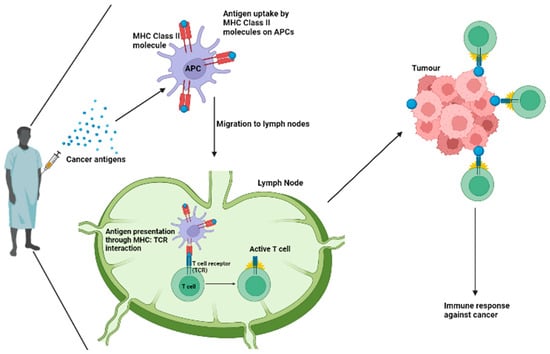
Figure 2.
Mechanism of action of protein-based cancer vaccines. Protein-based cancer vaccines are used to train T cells to recognize specific tumorigenic antigens. Cancer antigens are taken up by antigen presenting cells (APCs) such as dendritic cells through MHC Class II molecules. Active APCs migrate to lymph nodes and present antigens to T cells through MHC: TCR interactions which lead to T cell activation. Active T cells are then able to recognize those same antigens on the surface of cancer cells and initiate an immune response against those tumor cells (created using BioRender.com, URL accessed on 16 May 2023).
As of 2021, there are several clinical trials underway that evaluate the effectiveness of cancer vaccines in a breast cancer setting. The majority of those trials make use of peptide-based vaccines; however, some of them assess cell-based vaccines as well [68,69].
HER-2 positive breast cancer has been reported as a subtype with a strong association between its tumor immune landscape and disease progression [70]. More specifically, the NeoALTTO trial has observed a reduction in disease progression in patients with this particular breast cancer subtype following tumorigenic T cell infiltration [71]. Moreover, studies have also provided evidence that immune responses against HER-2 positive tumors are initiated by both CD8+ and CD4+ T lymphocytes [72]. The evidence for the link between disease recurrence and immune cell infiltration for this cancer subtype is overwhelming; thus, several clinical trials have been targeting this receptor using either protein-based or cell-based vaccination. For example, some of these make use of anti-HER-2/3 vaccines, dendritic cells, autologous or allogenic tumor cells and anti-Arg1 vaccines [69].
The field of DNA vaccine development is rapidly evolving, especially having gone through the COVID-19 pandemic. The AST-301 (pNGVL3-hICD) is a DNA-based vaccine that contains the pNGVL3-hICD plasmid which encodes the intracellular domain of HER-2 receptor protein [73]. A clinical trial evaluating the clinical efficacy of the following vaccine plus GM-CSF therapy is still underway. Results so far have shown that immunization with the following vaccine promotes the activation of anti-HER-2 T lymphocytes [74,75]. In vivo studies with HER-2+ gastric cancer models have shown a favorable safety profile and significant activation of anti-tumor immune responses and consequently tumor growth regression in mice treated with the vaccine [73].
Other than HER-2 specific vaccines, other tumorigenic antigens are also being explored as candidates for vaccine development. For example, Globo H is a carbohydrate antigen found on tumor cells including breast cancer [76]. Adagloxad simolenin (OBI-822) is a protein-based vaccine that encompasses the Globo H antigen covalently linked to an immunogenic carrier protein known as keyhole limpet hemocyanin (KLH). The latter allows for the vaccine to be delivered in close proximity to T cells situated specifically near the targeted protein—in this case Globo H. A phase II trial made use of the following vaccine in patients diagnosed with metastatic breast cancer and has demonstrated enhanced immune responses against Globo H-positive cells and an overall promising tolerability profile [77].
5. Cytokine Therapies
Cytokines consist of small proteins that are released upon specific stimuli to promote numerous cellular processes. Cytokines can have both anti- and pro-inflammatory effects and can promote cell growth or death [78]. For example, interleukin-1β (IL-1β) is a cytokine often found within the TME and can have both anti- and pro-tumorigenic effects. In vivo studies using breast cancer models reported that IL-1β deficiency results in tumor regression due to the intratumoral recruitment and differentiation of inflammatory monocytes. IL-1β deficiency caused low levels of macrophages and recruitment of CD11b+ dendritic cells which consequently led to an interleukin-12 (IL-12) upregulation and interleukin-10 (IL-10) downregulation. This promoted anti-tumor immunity and CD8+ lymphocyte activation. For that reason, the rationale of combining anti-IL-1β and anti-PD-1 ICI treatments was suggested which led to tumor regression. Blocking IL-1β not only reduced tumor progression but also facilitated checkpoint inhibition [79].
Interleukin-2 (IL-2) is a cytokine often used in cancer immunotherapy mostly due to its ability to activate and expand immune cells, including T lymphocytes and natural killer (NKs) cells. For that reason, studies are looking into IL-2 intervention as a treatment course for cancer in general. For example, a recently commenced clinical trial is aiming to recruit TNBC patients to assess the efficacy of IL-2 administration [80]. In addition, the combination of IL-2 with chemotherapy has also been proven to improve the response rate and survival of patients with advanced breast cancer [78]. TNBC patients are often reported to be positive for epidermal growth factor receptor (EGFR) which has given enough reason for anti-EGFR antibodies such as cetuximab to be considered as a treatment option [81]. Roberti et al. (2011) have demonstrated that administration of either IL-1 or IL-15 can enhance the cetuximab-mediated antibody dependent cell cytotoxicity (ADCC) by NKs in different EGFR-positive TNBC cell lines. Although both cytokines doubled the lytic activity of NKs, IL-15 showed slightly superior results compared to IL-2 [81].
Interleukin-12 (IL-12) is a cytokine that plays a crucial role in the immune response against cancer. Similar to IL-2, it has been shown to activate T cells, NKs and dendritic cells which together can lead to tumor regression. For that reason, several studies have explored the approach of using this cytokine as another immunotherapy. For example, an in vitro study using SCK murine mammary carcinoma cells, described that IL-12 and IL-18 synergistically inhibit tumor angiogenesis and tumor development [82]. Furthermore, clinical studies have also demonstrated a favorable safety profile and high efficacy of IL-12 therapy in patients diagnosed with metastatic breast cancer [83]. In addition, the study conducted by Telli et al. (2021) investigated the effects of intratumoral injection of plasmid IL-12 on CD8+ T cells and C-X-C motif chemokine receptor 3 (CXCR3) gene expression in triple-negative breast tumors. The treatment induced a CXCR3 gene signature in the tumors associated with an upregulation of checkpoint molecules such as PD-1/PD-L1 and CD8+ T cell recruitment. Consequently, this sensitized patients to anti-PD-1 therapy, resulting in improved clinical outcomes. This study highlights the potential of IL-12 administration as a therapeutic adjuvant option together with checkpoint inhibition for triple-negative breast tumors as well as the importance of the CXCR3 gene signature as a predictive biomarker for anti-PD-1 therapy [84]. Cytokine therapy has also been shown to be a promising adjuvant for other conventional treatments such as irradiation. For example, Formenti et al. (2018) investigated the effects of focal irradiation and systemic transforming growth factor-β (TGF-β) blockade in metastatic breast cancer. The latter combination resulted in a decrease in the number of circulating tumor cells and an increase in T cell-mediated tumor cell killing. Furthermore, the combination therapy was well-tolerated by patients and resulted in improved overall survival. The study highlights the potential of combining focal irradiation with systemic TGF-β blockade as a promising therapeutic strategy for metastatic breast cancer [85].
Studies have also evaluated the potential of several other interleukins as immunotherapies in breast cancer. For instance, interleukin-15 (IL-15) is a cytokine that shares many similarities with IL-2 and has been shown to have potent anti-tumor effects. A preclinical study using a mouse model of breast cancer found that IL-15 therapy increased the number and activity of NKs and T cells, resulting in the inhibition of tumor growth and metastasis [86]. A phase I clinical trial has also demonstrated the safety and potential efficacy of IL-15 therapy in patients with metastatic breast cancer [87]. Moreover, interleukin-21 (IL-21) is similar to IL-12 in terms of immune cell activation. Preclinical studies using breast cancer models have found that IL-21 therapy inhibits tumor growth and metastasis by increasing the activity of NKs and T cells [88]. A phase I clinical trial also demonstrated the safety and potential efficacy of IL-21 therapy in patients with metastatic breast cancer [89].
Tumor necrosis factor-alpha (TNF-α) is another type of cytokine and there is a lot of debate in terms of its potential in cancer therapeutics mostly because it has been shown to exert both pro- and anti-tumorigenic effects. For example, studies have reported TNFα to recruit immunosuppressive immune cells to the tumor microenvironment whilst other have shown that similar to other ILs, TNFα can activate the immune system as well [90]. However, its use in breast cancer treatment has been limited by its toxic side effects. For that reason, a modified form of TNF-α called PEGylated TNF-α (PEG-TNF-α) has been developed that has a longer half-life and reduced toxicity. Clinical trials have shown that PEG-TNF-α can have antitumor effects in breast cancer, but further studies are needed to determine its optimal use [91]. In vitro studies of TNF-α in TNBC models have illustrated that the expression of TNF-α promotes cellular proliferation while inhibiting apoptotic pathways. When TNF-α was knocked down, cell proliferation was halted, and apoptosis was induced [92]. In vivo models of TNBC have reported that the administration of TNF-α prior to doxorubicin treatment can enhance tumor accumulation of doxorubicin which generates the rationale of testing this combination in patients [93].
Chemokines are small cytokines often found to be involved in cancer progression, Particularly, the C-C chemokine motif ligand 2/C-C chemokine receptor 2 (CCL2/CCR2) pathway has been shown to drive a pro-inflammatory microenvironment which favors tumor progression [94]. The following study evaluated the safety profile and potential efficacy of a CCL2 inhibitor, propagermanium (PG), as an antimetastatic drug in perioperative patients with primary breast cancer. Overall, a low-grade adverse event profile was reported. IL-6 levels were reduced in a dose-dependent manner which, given the fact that IL-6 is implicated in the metastatic dissemination of breast cancer [95], suggests that PG may have the ability to hinder metastasis in human BC. Furthermore, serum CCL2 levels increased and remained high for at least 2 months post-surgery, possibly promoting cancer metastasis. PG might be preferable for CCL2 inhibition compared to anti-CCL2 neutralizing antibodies in human BC. Finally, the study provided a rationale for the efficacy of PG for the inhibition of metastasis in BC patients based on the FBXW7/CCL2 axis, which is important for BC progression. However, further studies will be required to clarify the mechanism of the downregulation of FBXW7 mRNA levels in some BC patients [96].
Cytokine therapies have shown promising results in the treatment of breast cancer. Multiple ILs have been shown to mediate potent anti-tumor effects through immune cell activation and inhibition of tumor growth and metastasis [96,97]. Further clinical trials are needed to determine the safety and efficacy of these cytokine therapies in the treatment of breast cancer.
6. Adoptive Cell Therapies (ACTs)
Despite the vast availability of cancer immunotherapies, there is still a substantial proportion of patients who become resistant to those therapies and exhibit disease progression with time. For that reason, adoptive cellular therapies (ACT) which involve manipulation of either intratumoral or peripheral blood immune cells can serve as another option for these patients [98,99]. There are three types of ACTs including tumor-infiltrating lymphocytes (TILs), TCR—modified lymphocytes and chimeric antigen receptor (CAR) T cells [100,101].
6.1. Tumor-Infiltrating Lymphocyte (TIL) Therapy
TIL therapy often involves the ex vivo expansion of either CD8+ or CD4+ T cells and their infusion back into the patient [100]. Both allogeneic and autologous transplants have been attempted for breast cancer patients in the past with each approach having its own harms and benefits. For example, allogeneic transplants exhibited significant toxicity whereas autologous transplants were less efficacious compared to allogeneic [102,103,104,105]. TIL therapy has been found to be most effective in patients with a high somatic mutational burden as it allows for the expansion of TILs specifically against tumor-specific neoantigens. For example, a case study for a 49-year-old patient diagnosed with ER-positive metastatic breast cancer reported disease regression following a combination of TIL therapy, specifically against proteins mutated in that patient, IL-2 cytokine therapy and PD-1 immune checkpoint inhibition [106]. A similar study identified 72 mutations in a patient with metastatic TNBC generated TILs against that patient’s own mutated genes; however, results of this study were not published [107,108]. The presence of TILs versus immunosuppressive cells such as tumor-associated macrophages (TAMs) dictate the prognosis of TNBC patients [109,110,111]. Those with higher TAM content are more likely to progress and for their tumors to recur compared to those with greater TIL accumulation [109]. Furthermore, those with greater TIL accumulation are also shown to be more sensitive to chemotherapy treatment [110]; hence, one could perhaps suggest the potential combination of TIL therapy and chemotherapy. There are several clinical trials either underway or completed that make use of TIL therapy either alone or in conjunction with another immunotherapy such as ICIs or cytokine therapy that study the effectiveness of given therapies in patients diagnosed with various breast cancer subtypes [112].
6.2. Engineered TCR Therapy
Engineered TCR therapy describes the genetic engineering of the TCR of a patient’s own T cells to specifically recognize tumor antigens [113]. Most αβTCR-engineered cells can only be used against antigens presented by MHC molecules expressed on immune cells such as dendritic cells [99]. For instance, Qiongshu et al. (2018) successfully engineered CD8+ T cells to specifically recognize PLAC1 in different breast cancer cell lines. PLAC1 is an example of an antigen known to be often expressed in breast cancer [114]. The study reported robust activation of these cells, evident by the enhanced release of interferon-γ (IFNγ) and TNF-α cytokines. Moreover, an increased number of lysed cells were observed as a result of CD8+ T cell activation in PLAC1-positive cell lines as opposed to either PLAC1-negative or PLAC1-silenced cells. Most importantly, the study reported a significantly decelerated tumor growth in vivo following administration of these CD8+ T cells [115].
γδT engineered cells are more potent compared to the conventional αβTCR-engineered cells. This is mainly because they express both γδTCRs and killer cell immunoglobulin-like receptors (KIRs) [116,117]. Several studies have reported the potential of these types of therapy in breast cancer as either a monotherapy [118] or combined with trastuzumab [119]. Regardless, this type of therapy has also been shown to convey immunosuppressive effects on several other immune cells in breast cancer, suggesting that the inhibition of these cells instead could confer anti-tumorigenic effects [120]. For that reason, additional research is warranted as to their potential use in immunotherapeutics. Overall, numerous clinical trials are underway to study the effectiveness of different engineered-TCR therapies in breast cancer against already established cancer targets such as NYESO-1 or MAGE-A3 or other neoantigens [112].
6.3. Chimeric Antigen Receptor (CAR) T Cell Therapy
Chimeric antigen receptor (CAR) therapy includes the genetic modification of a patient’s own T cells that involves the addition of a CAR gene. The latter encodes for a receptor that specifically recognizes certain cancer antigens. This allows for a robust and potent anti-tumor response [107,120]. For example, the success of the anti-CD19 CAR T cell therapy against B cell lymphoma leading to its FDA approval is well known [121,122,123,124]. Initial testing on the first-generation CAR cells showed low efficacy and failure in immune cell activation [125,126,127]. For that reason, second- and third-generation CAR cells were engineered that include co-stimulatory signals that allow complete activation and expansion of T cells [128,129,130,131,132].
On the contrary, CAR T cell therapy has not been as successful in solid malignancies such as breast cancer mainly because of the presence of an immunosuppressive tumor microenvironment as opposed to haematological malignancies where there is none. An important factor to consider is choosing an appropriate target antigen that is highly expressed in tumor cells but not so much in healthy tissue [133]. For that reason, there are several studies that have investigated the impact of many different targets for TNBC which is one of the most aggressive types of breast cancer. For example, in vitro studies have shown that anti-AXL CAR T cells exhibit potent anti-tumor responses in TNBC cells [123,134]. Most importantly, the same anti-tumor response was also confirmed using mouse models [111,135] and Wei et al. (2018) have even reported that having a constitutively active IL-7R enhances the activity of the anti-AXL CAR T cell receptor [136]. Moreover, anti-EGFR CAR T cell receptors were reported to induce tumor lysis in vitro and perturb tumor growth in vivo [137]. There are several CAR T cells already developed and in clinical trials for TNBC treatment such as ROR1+, MUC1 and NKG2D CAR cells [138,139,140]. Moreover, TEM8/ANTXR1-specific CAR T cells have shown remarkable efficacy in TNBC models [141].
Overall, several other antigens have been identified so far that appear to be promising targets for the generation of such CAR T cells such as [142,143,144]. For example, receptor tyrosine kinases (RTKs) drive the activation of several signaling pathways such as the PI3K, MAPK or JAK/STAT pathways, leading to numerous cellular processes such as proliferation, differentiation and apoptosis [145,146]. RTKs identified in breast cancer as promising candidates for CAR cells include HER-2, EGFR and ROR1 [144,147,148,149,150,151,152,153,154]. Cell surface antigens present on tumor cells are another possible candidate for CAR cells. For instance, MUC1 upregulation was detected in the majority of breast cancer subtypes and promotes the metastatic dissemination of tumor cells through the activation of ERK1/2 and NF-kB pathways [143,144,145]. Furthermore, mesothelin overexpression has also been identified in aggressive breast cancer subtypes and is often associated with poor prognosis and resistance to chemotherapy treatment [143,146,147]. Currently, there are several clinical trials undergoing that investigate different targets for CAR T cells in breast cancer (Table 2—from clinicaltrials.gov). Figure 3 demonstrates the different types of ACT therapy.

Table 2.
Clinical trials for CAR T cell therapy in breast cancer.
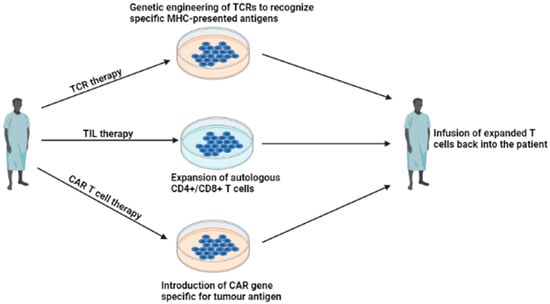
Figure 3.
Adoptive Cellular Therapy. Illustrates the three types of ACT that exist. TIL therapy involves the ex vivo expansion of T lymphocytes. TCR therapy is the genetic modification of TCRs to specifically recognize cancer antigens. CAR T cell therapy involves the introduction of a CAR gene specific for an antigen present in a patient’s tumor (created using Biorender.com, URL accessed on 16 May 2023).
Table 2 lists some of the clinical studies currently underway that investigate the use of CAR T cells in breast cancer subtypes. The different targets, trial phase and number of participants for each study are also displayed.
7. Limitations
7.1. Immune Checkpoint Inhibitors (ICIs)
Despite the worldwide implementation of ICIs in clinical practice, there are multiple challenges which must be taken into consideration. Firstly, predictors of response are considered the most important challenge among physicians, especially in the case of non-small-cell lung cancer (NSCLC), since only 30–40% of patients seem to benefit from ICIs [148,149]. Up to date, there are gaps in clinical evidence for concurrent administration of steroids and ICIs. Retrospective studies showed that patients taking steroids had a lower progression-free survival or overall survival [150,151,152,153,154] but were not negatively affected while taking steroids for managing side effects [155]. It remains unclear if steroids reduce the efficacy of ICIs or if there is an eventual difference driven by a more aggressive form of disease. ICI-induced adverse effects, also known as immune-related adverse effects (irAEs), are more prevalent in organs such as the skin, lungs, liver, kidneys, gastrointestinal and nervous system. ICIs have been associated with gastrointestinal and hepatic adverse events, with colitis being the most common side effect. According to comprehensive systematic review by Wang et al., the majority of deaths were attributed to colitis (70%) with anti-CTLA-4 therapy and pneumonitis and hepatitis (22%) with anti-PD-1/PD-L1-therapy. With combination PD-1/CTLA-4 therapy, deaths were frequently due to colitis (37%) and myocarditis (25%) [156]. The median time of onset of neurological adverse events (nAEs) was 6 weeks after ICIs treatment initiation. The incidence of severe nAEs (grade 3 and 4) with anti-CTLA4 therapy was slightly higher (0.7%) than with anti-PD1 therapy (0.4%) [157]. The incidence of ICI-induced liver dysfunction is much lower compared to diarrhoea and is reported in about 1–6% of patients, mostly at grades 1 and 2 [158,159]. The greater incidence and severity of irAEs with anti-CTLA-4 therapy compared to ani-PD-1/PD-L1 therapies reflects the potentially more dominant role of CTLA-4 compared to PD-1 as a negative T-cell regulator. It is worth mentioning that irAEs of combination ICIs are more severe than those of monotherapy [160]. Combination ICIs have been associated with endocrinopathies such as thyroid disorders and hypophysitis, which can be life-threatening if not recognized early [161].
To tackle the aforementioned challenges, microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) could be implemented, as it correlates with stronger responses with ICIs. In fact, it has already found application in as many as 24 cancer types with the highest percentage of MSI-H in endometrial cancer (17%), gastric adenocarcinoma (9%), small intestinal malignancies (8%), colorectal adenocarcinoma (6%) and lower for breast carcinoma (0.6%) [162]. The management of grade 1–2 hepatic dysfunction generally involves close monitoring with liver tests to avoid grade 3–4 liver toxicity. The management of grade 3–4 hepatic dysfunction requires high dose intravenous glucocorticoids for 24–48 h, followed by an oral steroid over at least a period of 30 days [159]. Regarding neurological AEs, no standard treatment has yet been defined. Clinical improvements have only been reported after the ICI treatment discontinuation [160]. On the contrary, endocrinopathies are reversible and can have a small impact on the patient’s quality of life when managed properly. Thus, treatment discontinuation is not necessary on the onset of hypophysitis and can be managed through the replacement of pituitary hormones, which is safe and common in clinical practice [161].
7.2. Breast Cancer Vaccines
BC vaccines present with good tolerance but without significant clinical benefit. Particularly, the Theratope® (STn) vaccine for metastatic BC and the NeuVax™ [Nelipepimut-S (NPS), or E75] vaccine for adjuvant BC both failed to display clinical benefit during phase 3 studies [163,164]. According to the E75 Phase 2 trial, the effect of BC vaccines seems to fade over time, leading to increased recurrences. The short-lasting effect may be attributed to the following factors: poor vaccine formulations, immune tolerance to specific antigens and immune suppressive microenvironment [165].
Allogeneic vaccines offer a promising solution when the immunosuppressive microenvironment becomes an insurmountable barrier for the immune system to overcome. Taking into account the up-to-date limited clinical benefit of BC vaccines, allogeneic vaccines could be implemented in clinical practices as adjuvant treatment [166]. It should be noted though that the extensive intratumoral heterogeneity poses a challenge for the development of allogeneic vaccines [167]. The clinical benefit may also be amplified through the co-administration of multiple antigens alongside BC vaccines. Through the years, there has been progress made with respect to delivery methods, with the development of lipid nanoparticles to genetically modified viruses; however, the extent to which the patient’s immune systems can respond remains unknown [168].
7.3. Cytokine Therapies
Just like with every treatment, cytokine therapy has its own limitation—not particularly specific for breast cancer. The major challenges presented here originate from the core principles of cytokines. First of all, being pleiotropic makes their targeting not specific to the TME; thus, off-target effects often occur. Additionally, due to the fact that different cytokines can regulate the same pathways, blocking one of them may not be enough as the others can compensate for that loss. Finally, due to their ability to regulate the immune system of the host, a blockade of any cytokines can lead to impairment of the immune system and can lead to autoimmunity or tissue damage [169]. The introduction of point mutations or fusion with their receptor counterparts is a way of overcoming the broad specificity of cytokines [170]. For example, IL-2 can activate both T cells and NKs but regulatory T cells (Tregs) as well. The latter lead to immunosuppression whereas the former to activation of the immune system [171]. Several studies have used site-directed mutagenesis to reduce the affinity of IL-2 for the receptor on Tregs. Overall, these mutants have shown comparable activity to their wild-type forms and enhanced efficacy in in vivo settings [172,173,174].
To reduce the off-target effects and adverse reactions reported with cytokine therapy, studies have looked into the fusion of these cytokines with targeting antibody that directs the cytokine to the TME [170]. In cancer, these antibodies often target oncogenic antigens which are usually overexpressed such as HER-2 in HER-2+ breast cancer [175]. For example, F16-IL2 consists of IL-2 fused with an antibody against the large isoform of tenascin c which is undetectable in healthy tissues but is overexpressed within the TME [176]. In vivo models of breast cancer have shown that F16-IL2 had a superior efficacy to IL-2 alone. Furthermore, when combined with either doxorubicin or paclitaxel, survival rates and overall response drastically increased, respectively [177]. Di Trani et al. (2022) mention some of the clinical trials underway that investigate the use of these antibody-cytokine complexes [170].
Cytokines often are presented with a short half-life which limits their activity and are therefore given in multiple injections. Consequently, this leads to systemic toxicity [162]. Protein PEGylation is widely used to extend a protein’s half-life as it shields the protein from immune cells and prevents proteolysis [178]. Several PEGylated cytokines (e.g., IL-2 or IL-10) are currently under investigation in clinical trials for patients with breast cancer amongst other solid malignancies (NCT02983045, NCT02009449). Some of these PEGylated cytokines have even been combined with ICIs and have demonstrated higher efficacy and tumor control compared to their monotherapy counterparts [179].
7.4. Adoptive Cellular Therapies (ACTs)
Each type of ACT from TIL to CAR T cell therapy has its own disadvantages as well. For example, TIL therapy is limited by the fact that we use the patient’s own T cells. Some patients have non-functional T cells or even no T cells at all as they have what is known as a “cold” tumor (i.e., not immunogenic) [180,181] and therefore must be further modified to be applied in those patients. For example, they must either be co-cultured with IL-2 to further expand and activate the cells or select T cells that are tumor-specific [182]. Furthermore, there are cases where these TILs do not exhibit potent anti-tumor responses and must therefore be combined with additional therapies as well. For example, several clinical trials are combining TILs with ICIs in tumors other than breast cancer to suppress the negative co-stimulation of T cells and promote their activation instead (NCT02621021; NCT03645928; NCT03215810).
We often tend to compare CAR cells with TCR-modified cells as one can be the solution for a challenge posed by the other. For example, TCR-modified T cells target specific antigens; however, the fact that they rely on MHC-presented antigens limits their application. This can be overcome by CAR cells which target a broader range of cancer-associated antigens independent of MHC presentation [183]. On the other hand, antigen recognition by CAR cells is limited to surface antigens whereas TCR-modified cells can recognize both surface and intracellular proteins given that they are presented by HLA molecules [183,184,185]. One major challenge posed by solid malignancies such as breast cancer compared to haematological cancers is the presence of an immunosuppressive TME which can dampen the activity of these therapies [186,187,188]. This is one of the main reasons why ACT has reported remarkable success in blood rather than solid cancers and why it is often combined with ICIs in solid malignancies as aforementioned. Finally, CAR T cell therapy in particular has often be associated with a severe side-effect profile due to inappropriate activation of T cells leading to excessive cytokine release, also known as cytokine release syndrome (CRS) [189,190,191]. Moreover, a wide range of neurotoxic side effects are also observed with CAR cells which may be associated with cytokines surpassing the blood-brain barrier and gaining entry to the central nervous system (CNS) [192,193].
8. Conclusions
Immunotherapy has revolutionized the treatment of breast cancer, significantly improving the curative effect when added to standard therapies. Despite challenges, immunotherapy remains a promising therapeutic strategy and ongoing trials will confirm its clinical benefit in combination with conventional therapies. Further knowledge about the immunosuppressive tumor microenvironment could aid the selection of patients who are most likely to respond to the aforementioned regimens.
Author Contributions
Conceptualization, M.V. and S.P.; methodology, M.V. and S.P.; resources, M.V. and S.P.; writing—original draft preparation, M.V. and S.P.; writing—review and editing, M.V., S.P. and N.P.N.; visualization, M.V., S.P. and N.P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figure 1, Figure 2 and Figure 3 were created using https://biorender.com. URL accessed on 16 May 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mishra, A.K.; Ali, A.; Dutta, S.; Banday, S.; Malonia, S.K. Emerging Trends in Immunotherapy for Cancer. Diseases 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat. Rev. Clin. Oncol. 2015, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer Immunosurveillance and Immunoediting: The Roles of Immunity in Suppressing Tumor Development and Shaping Tumor Immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Zitvogel, L.; Tesniere, A.; Kroemer, G. Cancer despite Immunosurveillance: Immunoselection and Immunosubversion. Nat. Rev. Immunol. 2006, 6, 715–727. [Google Scholar] [CrossRef]
- Bhatia, A.; Kumar, Y. Cellular and Molecular Mechanisms in Cancer Immune Escape: A Comprehensive Review. Expert Rev. Clin. Immunol. 2013, 10, 41–62. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Obser-Vatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 10 April 2023).
- World Health Organization. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.who.int/healthinfo/global_burden_disease/esti-mates/en/index1.html (accessed on 10 April 2023).
- Penault-Llorca, F.; Viale, G. Pathological and Molecular Diagnosis of Triple-Negative Breast Cancer: A Clinical Perspective. Ann. Oncol. 2012, 23, vi19–vi22. [Google Scholar] [CrossRef]
- Pal, S.K.; Childs, B.H.; Pegram, M. Triple Negative Breast Cancer: Unmet Medical Needs. Breast Cancer Res. Treat. 2010, 125, 627–636. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Testa, L.; Witzel, I.; Ohtani, S.; et al. Association of Tumor Mutational Burden (TMB) and Clinical Outcomes with Pembrolizumab (Pembro) versus Chemotherapy (Chemo) in Patients with Metastatic Triple-Negative Breast Cancer (MTNBC) from KEYNOTE-119. J. Clin. Oncol. 2020, 38 (Suppl. 15), 1013. [Google Scholar] [CrossRef]
- Marra, A.; Viale, G.; Curigliano, G. Recent Advances in Triple Negative Breast Cancer: The Immunotherapy Era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Walker, J.M.; McGee, H.; Kim, M.M.; Kunos, C.A.; Monjazeb, A.M.; Shiao, S.L.; Tran, P.T.; Ahmed, M.M. Incorporating Radiation Oncology into Immunotherapy: Proceedings from the ASTRO-SITC-NCI immunotherapy workshop. J. Immunother. Cancer 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and Dual Checkpoint Blockade Activate Non-Redundant Immune Mechanisms in Cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Monoclonal Antibodies—NCI 2019. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/monoclonal-antibodies (accessed on 3 April 2023).
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast Cancer Immunotherapy: An Update. Breast Cancer Basic Clin. Res. 2018, 12, 117822341877480. [Google Scholar] [CrossRef]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Capelan, M.; Pugliano, L.; De Azambuja, E.; Bozovic, I.; Saini, K.S.; Sotiriou, C.; Loi, S.; Piccart-Gebhart, M.J. Pertuzumab: New Hope for Patients with HER2-Positive Breast Cancer. Ann. Oncol. 2012, 24, 273–282. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.-S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Vu, T.; Claret, F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef]
- Nagata, Y.; Lan, K.-H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN Activation Contributes to Tumor Inhibition by Trastuzumab, and Loss of PTEN Predicts Trastuzumab Resistance in Patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Sebzari, A.; Kordi-Tamandani, D.M.; Dastjerdi, K. Involvement of the Dysregulation of MiR-23b-3p, MiR-195-5p, MiR-656-5p, and MiR-340-5p in Trastuzumab Resistance of HER2-Positive Breast Cancer Cells and System Biology Approach to Predict Their Targets Involved in Resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.W.; Allison, D.E.; Flagella, K.; Presta, L.; Clarke, J.; Dybdal, N.; McKeever, K.; Sliwkowski, M.X. Humanization of a Recombinant Monoclonal Antibody to Produce a Therapeutic HER Dimerization Inhibitor, Pertuzumab. Cancer Immunol. Immunother. 2005, 55, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J. Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Fendly, B.M.; Winget, M.; Hudziak, R.M.; Lipari, M.T.; Napier, M.A.; Ullrich, A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990, 50, 1550–1558. [Google Scholar]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB Signaling from the Structure of the ErbB2-Pertuzumab Complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Portera, C.C.; Walshe, J.M.; Rosing, D.R.; Denduluri, N.; Berman, A.W.; Vatas, U.; Velarde, M.; Chow, C.K.; Steinberg, S.M.; Nguyen, D.; et al. Cardiac Toxicity and Efficacy of Trastuzumab Combined with Pertuzumab in Patients with Trastuzumab-Insensitive Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer. Clin. Cancer Res. 2008, 14, 2710–2716. [Google Scholar] [CrossRef]
- Baselga, J.; Gelmon, K.A.; Verma, S.; Wardley, A.; Conte, P.; Miles, D.; Bianchi, G.; Cortes, J.; McNally, V.A.; Ross, G.A.; et al. Phase II Trial of Pertuzumab and Trastuzumab in Patients with Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer That Progressed during Prior Trastuzumab Therapy. J. Clin. Oncol. 2010, 28, 1138–1144. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab Emtansine: Mechanisms of Action and Drug Resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Tanner, M.; Köninki, K.; Isola, J. Trastuzumab-DM1 Causes Tumour Growth Inhibition by Mitotic Catastrophe in Trastuzumab-Resistant Breast Cancer Cells in Vivo. Breast Cancer Res. 2011, 13, R46. [Google Scholar] [CrossRef]
- Wildiers, H.; Kim, S.B.; Gonzalez-Martin, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.; Krop, I.E. T-DM1 for HER2-positive metastatic breast cancer (MBC): Primary results from TH3RESA, a phase 3 study of T-DM1 vs. treatment of physician’s choice. Eur. J. Cancer 2013, 49, S7–S8. [Google Scholar]
- Hurvitz, S.A.; Dirix, L.; Kocsis, J.; Bianchi, G.V.; Lu, J.; Vinholes, J.; Guardino, E.; Song, C.; Tong, B.; Ng, V.; et al. Phase II Randomized Study of Trastuzumab Emtansine versus Trastuzumab plus Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer. J. Clin. Oncol. 2013, 31, 1157–1163. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Trastuzumab Deruxtecan: First Approval. Drugs 2020, 80, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Arrojo, R.; Liu, D.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody–Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjugate Chem. 2015, 26, 919–931. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496. [Google Scholar] [CrossRef]
- Pondé, N.; Aftimos, P.; Piccart, M. Antibody-drug conjugates in breast cancer: A comprehensive review. Curr. Treat. Options Oncol. 2019, 20, 37. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor Res. 2015, 42, 55–66. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Kim, A.; Lee, S.J.; Kim, Y.K.; Park, W.Y.; Park, D.Y.; Kim, J.Y.; Lee, C.H.; Gong, G.; Huh, G.Y.; Choi, K.U. Programmed Death-Ligand 1 (PD-L1) Expression in Tumour Cell and Tumour Infiltrating Lymphocytes of HER2-Positive Breast Cancer and Its Prognostic Value. Sci. Rep. 2017, 7, 11671. [Google Scholar] [CrossRef]
- Polónia, A.; Pinto, R.; Cameselle-Teijeiro, J.F.; Schmitt, F.C.; Paredes, J. Prognostic Value of Stromal Tumour Infiltrating Lymphocytes and Programmed Cell Death-Ligand 1 Expression in Breast Cancer. J. Clin. Pathol. 2017, 70, 860–867. [Google Scholar] [CrossRef]
- Li, Y.; Opyrchal, M.; Yao, S.; Peng, X.; Yan, L.; Jabbour, H.; Khoury, T. The Role of Programmed Death Ligand-1 and Tumor-Infiltrating Lymphocytes in Breast Cancer Overexpressing HER2 Gene. Breast Cancer Res. Treat. 2018, 170, 293–302. [Google Scholar] [CrossRef]
- Bae, S.B.; Cho, H.D.; Oh, M.-H.; Lee, J.-H.; Jang, S.-H.; Hong, S.A.; Cho, J.; Kim, S.Y.; Han, S.W.; Lee, J.E.; et al. Expression of Programmed Death Receptor Ligand 1 with High Tumor-Infiltrating Lymphocytes Is Associated with Better Prognosis in Breast Cancer. J. Breast Cancer 2016, 19, 242. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P.; et al. Long-Term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 74. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an Anti-PD-L1 Antibody, in Patients with Locally Advanced or Metastatic Breast Cancer: A Phase 1b JAVELIN Solid Tumor Study. Breast Cancer Res. Treat. 2017, 167, 671–686. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. KEYNOTE-355: Randomized, Double-Blind, Phase III Study of Pembrolizumab + Chemotherapy versus Placebo + Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2020, 38 (Suppl. 15), 1000. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune Induction Strategies in Metastatic Triple-Negative Breast Cancer to Enhance the Sensitivity to PD-1 Blockade: The TONIC Trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort a of the Phase II KEYNOTE-086 Study. Ann. Oncol. 2018, 30, 397–404. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Du, C.; Yuan, M.; Fu, P.; He, Q.; Yang, B.; Cao, J. PD-1/PD-L1 Counterattack Alliance: Multiple Strategies for Treating Triple-Negative Breast Cancer. Drug Discov. Today 2020, 25, 1762–1771. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet. Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, H.; Wang, T.; Zhang, S.; Song, H.; Xu, M.; Yao, S.; Jiang, Z. JS001, an Anti-PD-1 MAb for Advanced Triple Negative Breast Cancer Patients after Multi-Line Systemic Therapy in a Phase I Trial. Ann. Transl. Med. 2019, 7, 435. [Google Scholar] [CrossRef]
- Takada, M.; Yoshimura, M.; Kotake, T.; Kawaguchi, K.; Uozumi, R.; Kataoka, M.; Kato, H.; Yoshibayashi, H.; Suwa, H.; Tsuji, W.; et al. Phase Ib/II Study of Nivolumab Combined with Palliative Radiation Therapy for Bone Metastasis in Patients with HER2-Negative Metastatic Breast Cancer. Sci. Rep. 2022, 12, 22397. [Google Scholar] [CrossRef]
- Waks, A.G.; Keenan, T.E.; Li, T.; Tayob, N.; Wulf, G.M.; Richardson, E.T.; Attaya, V.; Anderson, L.; Mittendorf, E.A.; Overmoyer, B.; et al. Phase Ib Study of Pembrolizumab in Combination with Trastuzumab Emtansine for Metastatic HER2-Positive Breast Cancer. J. ImmunoTherapy Cancer 2022, 10, e005119. [Google Scholar] [CrossRef]
- Adams, S.; Othus, M.; Patel, S.P.; Miller, K.D.; Chugh, R.; Schuetze, S.M.; Chamberlin, M.D.; Haley, B.J.; Storniolo, A.M.V.; Reddy, M.P.; et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART, SWOG S1609). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 28, 271–278. [Google Scholar] [CrossRef]
- De la Cruz-Merino, L.; Gion, M.; Cruz, J.; Alonso-Romero, J.L.; Quiroga, V.; Moreno, F.; Andrés, R.; Santisteban, M.; Ramos, M.; Holgado, E.; et al. Pembrolizumab in Combination with Gemcitabine for Patients with HER2-Negative Advanced Breast Cancer: GEICAM/2015–04 (PANGEA-Breast) Study. BMC Cancer 2022, 22, 1258. [Google Scholar] [CrossRef]
- Ozaki, Y.; Tsurutani, J.; Mukohara, T.; Iwasa, T.; Takahashi, M.; Tanabe, Y.; Kawabata, H.; Masuda, N.; Futamura, M.; Minami, H.; et al. Safety and Efficacy of Nivolumab plus Bevacizumab, Paclitaxel for HER2-Negative Metastatic Breast Cancer: Primary Results and Biomarker Data from a Phase 2 Trial (WJOG9917B). Eur. J. Cancer 2022, 171, 193–202. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Treatment Vaccines—Immunotherapy; NCI: Bethesda, MD, USA, 2019. [Google Scholar]
- Pallerla, S.; Abdul, A.u.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Corti, C.; Giachetti, P.P.M.B.; Eggermont, A.M.M.; Delaloge, S.; Curigliano, G. Therapeutic Vaccines for Breast Cancer: Has the Time Finally Come? Eur. J. Cancer 2022, 160, 150–174. [Google Scholar] [CrossRef]
- Search of: Vaccine|Breast Cancer—List Results. 2023. Available online: ClinicalTrials.gov (accessed on 3 April 2023).
- Mittendorf, E. High Expression of Lymphocyte-Associated Genes in Node-Negative HER2+ Breast Cancers Correlates with Lower Recurrence Rates. Breast Dis. Year Book Q. 2008, 3, 223–224. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations with Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated with Lapatinib and Trastuzumab. JAMA Oncol. 2015, 1, 448. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Czerniecki, B.J. Clinical Development of Immunotherapies for HER2+ Breast Cancer: A Review of HER2-Directed Monoclonal Antibodies and Beyond. NPJ Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.S.; Joung, E.; Shin, J.H.; Lee, H.L.; Lim, J.; Kim, Y.; Park, H.-H.; Shin, H.; Jung, H. 776 the Anti-Tumor Activity of HER-2/Neu ICD Therapeutic Cancer Vaccine (AST-301, PNGVL3-HICD) in Her2-Expressed Gastric Cancer Xenograft Model. J. Immunother. Cancer 2021, 9 (Suppl. 2), A811. [Google Scholar] [CrossRef]
- Vaccine Therapy with Sargramostim (GM-CSF) in Treating Patients with Her-2 Positive Stage III–IV Breast Cancer or Ovarian Cancer—Full Text View. 2023. Available online: ClinicalTrials.gov (accessed on 3 April 2023).
- Disis, M.L.; Guthrie, K.A.; Liu, Y.; Coveler, A.L.; Higgins, D.M.; Childs, J.S.; Dang, Y.; Salazar, L.G. Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients with Advanced-Stage ERBB2-Positive Breast Cancer: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2022, 9, 71. [Google Scholar] [CrossRef]
- Chang, W.-W.; Lee, C.H.; Lee, P.; Lin, J.; Hsu, C.-W.; Hung, J.-T.; Lin, J.-J.; Yu, J.-C.; Shao, L.; Yu, J.; et al. Expression of Globo H and SSEA3 in Breast Cancer Stem Cells and the Involvement of Fucosyl Transferases 1 and 2 in Globo H Synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 11667–11672. [Google Scholar] [CrossRef]
- Huang, C.-S.; Yu, A.L.; Tseng, L.-M.; Chow, L.W.C.; Hou, M.-F.; Hurvitz, S.A.; Schwab, R.B.; Murray, J.L.; Chang, H.-K.; Chang, H.-T.; et al. Globo H-KLH Vaccine Adagloxad Simolenin (OBI-822)/OBI-821 in Patients with Metastatic Breast Cancer: Phase II Randomized, Placebo-Controlled Study. J. ImmunoTherapy Cancer 2020, 8, e000342. [Google Scholar] [CrossRef]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef]
- Efficacy of Intralesional IL-2 for Resectable Triple Negative Breast Cancer—Full Text View. 2023. Available online: ClinicalTrials.gov (accessed on 25 May 2023).
- Roberti, M.P.; Rocca, Y.S.; Amat, M.; Pampena, M.B.; Loza, J.; Coló, F.; Fabiano, V.; Loza, C.M.; Arriaga, J.M.; Bianchini, M.; et al. IL-2-or IL-15-activated NK cells enhance Cetuximab-mediated activity against triple-negative breast cancer in xenografts and in breast cancer patients. Breast Cancer Res. Treat. 2012, 136, 659–671. [Google Scholar] [CrossRef]
- Coughlin, C.M.; Salhany, K.E.; Wysocka, M.; Aruga, E.; Kurzawa, H.L.; Chang, A.E.; Hunter, C.A.; Fox, J.A.; Trinchieri, G.; Lee, W.M. Interleukin-12 and Interleukin-18 Synergistically Induce Murine Tumor Regression Which Involves Inhibition of Angiogenesis. J. Clin. Investig. 1998, 101, 1441–1452. [Google Scholar] [CrossRef]
- Parihar, R.; Nadella, P.; Lewis, A.S.; Jensen, R.; De Hoff, C.; Dierksheide, J.; VanBuskirk, A.M.; Magro, C.M.; Young, D.C.; Shapiro, C.L.; et al. A Phase I Study of Interleukin 12 with Trastuzumab in Patients with Human Epidermal Growth Factor Receptor-2-Overexpressing Malignancies. Clin. Cancer Res. 2004, 10, 5027–5037. [Google Scholar] [CrossRef]
- Telli, M.L.; Nagata, H.; Wapnir, I.; Acharya, C.R.; Zablotsky, K.; Fox, B.A.; Bifulco, C.B.; Jensen, S.M.; Ballesteros-Merino, C.; Le, M.H.; et al. Intratumoral Plasmid IL12 Expands CD8+ T Cells and Induces a CXCR3 Gene Signature in Triple-Negative Breast Tumors That Sensitizes Patients to Anti–PD-1 Therapy. Clin. Cancer Res. 2021, 27, 2481–2493. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic Transforming Growth Factor β Blockade in Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef]
- Isvoranu, G.; Surcel, M.; Munteanu, A.N.; Bratu, O.G.; Ionita-Radu, F.; Neagu, M.T.; Chiritoiu-Butnaru, M. Therapeutic potential of interleukin-15 in cancer (Review). Exp. Ther. Med. 2021, 22, 675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Husman, T.; Cen, X.; Tsao, T.; Brown, J.; Bajpai, A.; Li, M.; Zhou, K.; Yang, L. Interleukin 15 in Cell-Based Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 7311. [Google Scholar] [CrossRef]
- Lewis, K.E.; Selby, M.J.; Masters, G.; Valle, J.; Dito, G.; Curtis, W.R.; Garcia, R.; Mink, K.A.; Waggie, K.S.; Holdren, M.S.; et al. Interleukin-21 Combined with PD-1 or CTLA-4 Blockade Enhances Antitumor Immunity in Mouse Tumor Models. OncoImmunology 2017, 7, e1377873. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Sun, Z.; Qiao, J.; Liang, Y.; Liu, L.; Dong, C.; Shen, A.; Wang, Y.; Tang, H.; Fu, Y.-X.; et al. Targeting Tumors with IL-21 Reshapes the Tumor Microenvironment by Proliferating PD-1intTim-3-CD8+ T Cells. JCI Insight 2020, 5, e132000. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Gamm, H.; Lindemann, A.; Mertelsmann, R.; Herrmann, F. Phase I Trial of Recombinant Human Tumour Necrosis Factor α in Patients with Advanced Malignancy. Eur. J. Cancer 1991, 27, 856–863. [Google Scholar] [CrossRef]
- Pileczki, V.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. TNF-α gene knockout in triple negative breast cancer cell line induces apoptosis. Int. J. Mol. Sci. 2012, 14, 411–420. [Google Scholar] [CrossRef]
- Greish, K.; Taurin, S.; Morsy, M.A. The effect of adjuvant therapy with TNF-α on animal model of triple-negative breast cancer. Ther. Deliv. 2018, 9, 333–342. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef]
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931. [Google Scholar] [CrossRef]
- Goldberg, E.; Jodi, L.; Schwertfeger, K. Proinflammatory Cytokines in Breast Cancer: Mechanisms of Action and Potential Targets for Therapeutics. Curr. Drug Targets 2010, 11, 1133–1146. [Google Scholar] [CrossRef]
- Klampatsa, A.; Dimou, V.; Albelda, S.M. Mesothelin-Targeted CAR-T Cell Therapy for Solid Tumors. Expert Opin. Biol. Ther. 2020, 21, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. BioMed Res. Int. 2020, 2020, 4795171. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qian, M.; Ho, M. The role of mesothelin in tumor progression and targeted therapy. Curr. Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Riddell, S.R.; Schumacher, T.N. Adoptive Cellular Therapy: A Race to the Finish Line. Sci. Transl. Med. 2015, 7, 280ps7. [Google Scholar] [CrossRef]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A Phase I Study on Adoptive Immunotherapy Using Gene-Modified T Cells for Ovarian Cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.-F. Tumor-Infiltrating γδ T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Assadipour, Y.; Zacharakis, N.; Crystal, J.S.; Prickett, T.D.; Gartner, J.J.; Somerville, R.P.T.; Xu, H.; Black, M.A.; Jia, L.; Chinnasamy, H.; et al. Characterization of an Immunogenic Mutation in a Patient with Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2017, 23, 4347–4353. [Google Scholar] [CrossRef]
- Bottai, G.; Raschioni, C.; Losurdo, A.; Di Tommaso, L.; Roncalli, M.; Santarpia, L. Abstract P4-04-06: Immune balance between tumor-infiltrating lymphocytes and tumor-associated macrophages impacts the outcome of triple negative breast cancer patients. Cancer Res. 2016, 76, P4-04-06. [Google Scholar] [CrossRef]
- Matsumoto, H.; Koo, S.-L.; Dent, R.; Tan, P.H.; Iqbal, J. Role of inflammatory infiltrates in triple negative breast cancer: Table 1. J. Clin. Pathol. 2015, 68, 506–510. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, Y.; Lu, W.; Jiang, Y.; Wang, J. Immunotherapeutic interventions of Triple Negative Breast Cancer. J. Transl. Med. 2018, 16, 147. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.-C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune Recognition of Somatic Mutations Leading to Complete Durable Regression in Metastatic Breast Cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Wu, M.; Zhou, X.; Wang, S.; Hu, Y.; Wang, Y.; He, Y.; Zeng, X.; Chen, J.; et al. PLAC1-Specific TCR-Engineered T Cells Mediate Antigen-Specific Antitumor Effects in Breast Cancer. Oncol. Lett. 2018, 15, 5924–5932. [Google Scholar] [CrossRef]
- Janssen, A.; Villacorta Hidalgo, J.; Beringer, D.X.; van Dooremalen, S.; Fernando, F.; van Diest, E.; Terrizi, A.R.; Bronsert, P.; Kock, S.; Schmitt-Gräff, A.; et al. γδ T-Cell Receptors Derived from Breast Cancer–Infiltrating T Lymphocytes Mediate Antitumor Reactivity. Cancer Immunol. Res. 2020, 8, 530–543. [Google Scholar] [CrossRef]
- Borg, Å.; Tandon, A.K.; Sigurdsson, H.; Clark, G.M.; Fernö, M.; Fuqua, S.A.W.; Killander, D.; McGuire, W.L. HER-2/Neu Amplification Predicts Poor Survival in Node-Positive Breast Cancer1. Cancer Res. 1990, 50, 4332–4337. [Google Scholar]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Sparano, J.A.; Fisher, R.I.; Weiss, G.R.; Margolin, K.; Aronson, F.R.; Hawkins, M.J.; Atkins, M.B.; Dutcher, J.P.; Gaynor, E.R.; Boldt, D.H.; et al. Phase II Trials of High-Dose Interleukin-2 and Lymphokine-Activated Killer Cells in Advanced Breast Carcinoma and Carcinoma of the Lung, Ovary, and Pancreas and Other Tumors. J. Immunother. 1994, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, H.; Zhang, A.; Wu, X.; Li, Y.; Liu, J.; Duan, Y.; Xiao, F.; Wang, H.; Lv, M.; et al. A Novel AXL Chimeric Antigen Receptor Endows T Cells with Anti-Tumor Effects against Triple Negative Breast Cancers. Cell. Immunol. 2018, 331, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wallstabe, L.; Göttlich, C.; Nelke, L.C.; Kühnemundt, J.; Schwarz, T.; Nerreter, T.; Einsele, H.; Walles, H.; Dandekar, G.; Nietzer, S.L.; et al. ROR1-CAR T Cells Are Effective against Lung and Breast Cancer in Advanced Microphysiologic 3D Tumor Models. JCI Insight 2019, 4, e126345. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing Neoantigen-Targeted T Cell–Based Treatments for Solid Tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Capietto, A.-H.; Martinet, L.; Fournié, J.-J. Stimulated γδ T Cells Increase the in Vivo Efficacy of Trastuzumab in HER-2+Breast Cancer. J. Immunol. 2011, 187, 1031–1038. [Google Scholar] [CrossRef]
- Wu, Y.; Kyle-Cezar, F.; Woolf, R.T.; Naceur-Lombardelli, C.; Owen, J.; Biswas, D.; Lorenc, A.; Vantourout, P.; Gazinska, P.; Grigoriadis, A.; et al. An Innate-like Vδ1 + γδ T Cell Compartment in the Human Breast Is Associated with Remission in Triple-Negative Breast Cancer. Sci. Transl. Med. 2019, 11, eaax9364. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J.B.A.G. Adoptive cellular therapies: The current landscape. Virchows Arch. 2018, 474, 449–461. [Google Scholar] [CrossRef]
- Hsu, J.L.; Hung, M.-C. The Role of HER2, EGFR, and Other Receptor Tyrosine Kinases in Breast Cancer. Cancer Metastasis Rev. 2016, 35, 575–588. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Yang, Y.; Vedvyas, Y.; McCloskey, J.E.; Min, I.M.; Jin, M.M. Abstract 2322: ICAM-1 Targeting CAR T Cell Therapy for Triple Negative Breast Cancer. Cancer Res. 2019, 79 (Suppl. 13), 2322. [Google Scholar] [CrossRef]
- Lamers, C.H.J.; Sleijfer, S.; Vulto, A.G.; Kruit, W.H.J.; Kliffen, M.; Debets, R.; Gratama, J.W.; Stoter, G.; Oosterwijk, E. Treatment of Metastatic Renal Cell Carcinoma with Autologous T-Lymphocytes Genetically Retargeted against Carbonic Anhydrase IX: First Clinical Experience. J. Clin. Oncol. 2006, 24, e20–e22. [Google Scholar] [CrossRef]
- Pegram, M.; Slamon, D. Biological Rationale for HER2/Neu (C-ErbB2) as a Target for Monoclonal Antibody Therapy. Semin. Oncol. 2000, 27 (Suppl. 9), 13–19. [Google Scholar]
- Park, J.R.; DiGiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.-C.; Ostberg, J.R.; et al. Adoptive Transfer of Chimeric Antigen Receptor Re-Directed Cytolytic T Lymphocyte Clones in Patients with Neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef]
- Byrd, T.T.; Fousek, K.; Pignata, A.; Szot, C.; Samaha, H.; Seaman, S.; Dobrolecki, L.; Salsman, V.S.; Oo, H.Z.; Bielamowicz, K.; et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Cancer Res. 2018, 78, 489–500. [Google Scholar] [CrossRef]
- Song, D.-G.; Ye, Q.; Poussin, M.; Chacon, J.A.; Figini, M.; Powell, D.J. Effective Adoptive Immunotherapy of Triple-Negative Breast Cancer by Folate Receptor-Alpha Redirected CAR T Cells Is Influenced by Surface Antigen Expression Level. J. Hematol. Oncol. 2016, 9, 56. [Google Scholar] [CrossRef]
- D’Aloia, M.M.; Zizzari, I.G.; Sacchetti, B.; Pierelli, L.; Alimandi, M. CAR-T Cells: The Long and Winding Road to Solid Tumors. Cell Death Dis. 2018, 9, 282. [Google Scholar] [CrossRef]
- Fuentes-Antrás, J.; Guevara-Hoyer, K.; Baliu-Piqué, M.; García-Sáenz, J.A.; Pérez-Segura, P.; Pandiella, A.; Ocaña, A. Adoptive Cell Therapy in Breast Cancer: A Current Perspective of Next-Generation Medicine. Front. Oncol. 2020, 10, 7. [Google Scholar] [CrossRef]
- Ueno, N.T.; Rizzo, J.D.; Demirer, T.; Cheng, Y.C.; Hegenbart, U.; Zhang, M.-J.; Bregni, M.; Carella, A.; Blaise, D.; Bashey, A.; et al. Allogeneic Hematopoietic Cell Transplantation for Metastatic Breast Cancer. Bone Marrow Transplant. 2008, 41, 537–545. [Google Scholar] [CrossRef]
- Fultang, N.; Illendula, A.; Lin, J.; Pandey, M.K.; Klase, Z.; Peethambaran, B. ROR1 Regulates Chemoresistance in Breast Cancer via Modulation of Drug Efflux Pump ABCB1. Sci. Rep. 2020, 10, 1821. [Google Scholar] [CrossRef]
- Wright, S.E. Immunotherapy of Breast Cancer. Expert Opin. Biol. Ther. 2012, 12, 479–490. [Google Scholar] [CrossRef]
- Kachala, S.S.; Bograd, A.J.; Villena-Vargas, J.; Suzuki, K.; Servais, E.L.; Kadota, K.; Chou, J.; Sima, C.S.; Vertes, E.; Rusch, V.W.; et al. Mesothelin Overexpression Is a Marker of Tumor Aggressiveness and Is Associated with Reduced Recurrence-Free and Overall Survival in Early-Stage Lung Adenocarcinoma. Clin. Cancer Res. 2014, 20, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive Cellular Therapy in Solid Tumor Malignancies: Review of the Literature and Challenges Ahead. J. Immunother. Cancer 2021, 9, e002723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdanifar, M.; Roy, L.D.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, H.; Neudorfer, J.; Gebhard, K.; Conrad, H.; Hermann, C.; Nährig, J.; Fend, F.; Weber, W.; Busch, D.H.; Peschel, C. Adoptive Transfer of Autologous, HER2-Specific, Cytotoxic T Lymphocytes for the Treatment of HER2-Overexpressing Breast Cancer. Cancer Immunol. Immunother. 2007, 57, 271–280. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Huang, K.-H.; Li, Y.; Fang, X.; An, L.; Wang, F.; Chen, Q.; Zhang, Y.; Shi, A.; et al. EGFR-Specific CAR-T Cells Trigger Cell Lysis in EGFR-Positive TNBC. Aging 2019, 11, 11054–11072. [Google Scholar] [CrossRef]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.L.; et al. MUC1 Enhances Invasiveness of Pancreatic Cancer Cells by Inducing Epithelial to Mesenchymal Transition. Oncogene 2010, 30, 1449–1459. [Google Scholar] [CrossRef]
- Tebbutt, N.; Pedersen, M.W.; Johns, T.G. Targeting the ERBB Family in Cancer: Couples Therapy. Nat. Rev. Cancer 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Tozbikian, G.; Brogi, E.; Kadota, K.; Catalano, J.; Akram, M.; Patil, S.; Ho, A.Y.; Reis-Filho, J.S.; Weigelt, B.; Norton, L.; et al. Mesothelin Expression in Triple Negative Breast Carcinomas Correlates Significantly with Basal-like Phenotype, Distant Metastases and Decreased Survival. PLoS ONE 2014, 9, e114900. [Google Scholar] [CrossRef]
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, 1305. [Google Scholar] [CrossRef]
- Suwa, T.; Hinoda, Y.; Makiguchi, Y.; Takahashi, T.; Itoh, F.; Adachi, M.; Hareyama, M.; Imai, K. Increased invasiveness of MUCI1 cDNA-transfected human gastric cancer MKN74 cells. Int. J. Cancer 1998, 76, 377–382. [Google Scholar] [CrossRef]
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non–Small-Cell Lung Cancer. Eur. J. Cancer 2019, 106, 144–159. [Google Scholar] [CrossRef]
- Addeo, A.; Banna, G.L.; Metro, G.; Di Maio, M. Chemotherapy in Combination with Immune Checkpoint Inhibitors for the First-Line Treatment of Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front. Oncol. 2019, 9, 8–10. [Google Scholar] [CrossRef]
- Fucà, G.; Poggi, M.; Galli, G.; Imbimbo, M.; Lo Russo, G.; Signorelli, D.; Vitali, M.; Ganzinelli, M.; Zilembo, N.; de Braud, F.; et al. Impact of Early Steroids Use on Clinical Outcomes of Patients with Advanced NSCLC Treated with Immune Checkpoint Inhibitors. Ann. Oncol. 2018, 29, viii500. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated Analysis of Concomitant Medications and Oncological Outcomes from PD-1/PD-L1 Checkpoint Inhibitors in Clinical Practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef]
- Fucà, G.; Galli, G.; Poggi, M.; Lo Russo, G.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of Peripheral Blood Immune Cells by Early Use of Steroids and Its Association with Clinical Outcomes in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. ESMO Open 2019, 4, E000457. [Google Scholar] [CrossRef]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological Adverse Events Associated with Immune Checkpoint Inhibitors: Review of the Literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T.; et al. Ipilimumab Monotherapy in Patients with Pretreated Advanced Melanoma: A Randomised, Double-Blind, Multicentre, Phase 2, Dose-Ranging Study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T.K.; Hassel, J.C.; Berking, C.; Aberle, J.; Bachmann, O.; Grünwald, V.; Kähler, K.C.; Loquai, C.; Reinmuth, N.; Steins, M.; et al. Diagnosis, Monitoring and Management of Immune-Related Adverse Drug Reactions of Anti-PD-1 Antibody Therapy. Cancer Treat. Rev. 2016, 45, 7–18. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite Instability Status Determined by Next-Generation Sequencing and Compared with PD-L1 and Tumor Mutational Burden in 11,348 Patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef]
- Miles, D.; Roché, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A.; et al. Phase III Multicenter Clinical Trial of the Sialyl-TN (STn)-Keyhole Limpet Hemocyanin (KLH) Vaccine for Metastatic Breast Cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final Report of the Phase I/II Clinical Trial of the E75 (Nelipepimut-S) Vaccine with Booster Inoculations to Prevent Disease Recurrence in High-Risk Breast Cancer Patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef]
- Emens, L.A.; Machiels, J.P.; Reilly, R.T.; Jaffee, E.M. Chemotherapy: Friend or foe to cancer vaccines? Curr. Opin. Mol. Ther. 2001, 3, 77–84. [Google Scholar]
- Glöckner, S.; Buurman, H.; Kleeberger, W.; Lehmann, U.; Kreipe, H. Marked intratumoral heterogeneity of c-myc and cyclinD1 but not of c-erbB2 amplification in breast cancer. Lab. Investig. 2002, 82, 1419–1426. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations. Int. J. Cell Biol. 2016, 2016, 9259646. [Google Scholar] [CrossRef]
- Di Trani, C.A.; Cirella, A.; Arrizabalaga, L.; Fernandez-Sendin, M.; Bella, A.; Aranda, F.; Melero, I.; Berraondo, P. Overcoming the Limitations of Cytokines to Improve Cancer Therapy. Int. Rev. Cell Mol. Biol. 2022, 369, 107–141. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the Crossroads of Effector Responses, Tolerance, and Immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Carmenate, T.; Pacios, A.; Enamorado, M.; Moreno, E.; Garcia-Martínez, K.; Fuente, D.; León, K. Human IL-2 Mutein with Higher Antitumor Efficacy than Wild Type IL-2. J. Immunol. 2013, 190, 6230–6238. [Google Scholar] [CrossRef]
- Chen, X.; Ai, X.; Wu, C.; Wang, H.; Zeng, G.; Yang, P.; Liu, G. A Novel Human IL-2 Mutein with Minimal Systemic Toxicity Exerts Greater Antitumor Efficacy than Wild-Type IL-2. Cell Death Dis. 2018, 9, 989. [Google Scholar] [CrossRef]
- Vazquez-Lombardi, R.; Loetsch, C.; Zinkl, D.; Jackson, J.; Schofield, P.; Deenick, E.K.; King, C.; Phan, T.G.; Webster, K.E.; Sprent, J.; et al. Potent Antitumour Activity of Interleukin-2-Fc Fusion Proteins Requires Fc-Mediated Depletion of Regulatory T-Cells. Nat. Commun. 2017, 8, 15373. [Google Scholar] [CrossRef]
- Dela Cruz, J.S.; Trinh, K.R.; Morrison, S.L.; Penichet, M.L. Recombinant Anti-Human HER2/Neu IgG3-(GM-CSF) Fusion Protein Retains Antigen Specificity and Cytokine Function and Demonstrates Antitumor Activity. J. Immunol. 2000, 165, 5112–5121. [Google Scholar] [CrossRef]
- Borsi, L.; Carnemolla, B.; Nicolò, G.; Spina, B.; Tanara, G.; Zardi, L. Expression of Different Tenascin Isoforms in Normal, Hyperplastic and Neoplastic Human Breast Tissues. Int. J. Cancer 1992, 52, 688–692. [Google Scholar] [CrossRef]
- Mårlind, J.; Kaspar, M.; Trachsel, E.; Sommavilla, R.; Hindle, S.; Bacci, C.; Giovannoni, L.; Neri, D. Antibody-Mediated Delivery of Interleukin-2 to the Stroma of Breast Cancer Strongly Enhances the Potency of Chemotherapy. Clin. Cancer Res. 2008, 14, 6515–6524. [Google Scholar] [CrossRef]
- Nischan, N.; Hackenberger, C.P.R. Site-Specific PEGylation of Proteins: Recent Developments. J. Org. Chem. 2014, 79, 10727–10733. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A.; et al. Pegilodecakin Combined with Pembrolizumab or Nivolumab for Patients with Advanced Solid Tumours (IVY): A Multicentre, Multicohort, Open-Label, Phase 1b Trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, L.L.; Gorris, M.A.J.; Halilovic, A.; Figdor, C.G.; de Vries, I.J.M. Migrating into the Tumor: A Roadmap for T Cells. Trends Cancer 2017, 3, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T Cell Dysfunction and Exclusion Predict Cancer Immunotherapy Response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Ruella, M.; June, C.H. Emerging Cellular Therapies for Cancer. Annu. Rev. Immunol. 2019, 37, 145–171. [Google Scholar] [CrossRef]
- He, Q.; Jiang, X.; Zhou, X.; Weng, J. Targeting Cancers through TCR-Peptide/MHC Interactions. J. Hematol. Oncol. 2019, 12, 139. [Google Scholar] [CrossRef]
- Newick, K.; Moon, E.; Albelda, S.M. Chimeric Antigen Receptor T-Cell Therapy for Solid Tumors. Mol. Ther.-Oncolytics 2016, 3, 16006. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef]
- Yeku, O.; Li, X.; Brentjens, R.J. Adoptive T-Cell Therapy for Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 193–204. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuramitsu, S.; Posey, A.D.; June, C.H. Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Front. Immunol. 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing Cytokine Release Syndrome Associated with Novel T Cell-Engaging Therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and Activity of Blinatumomab for Adult Patients with Relapsed or Refractory B-Precursor Acute Lymphoblastic Leukaemia: A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Holtzman, N.G.; Xie, H.; Bentzen, S.; Kesari, V.; Bukhari, A.; El Chaer, F.; Lutfi, F.; Siglin, J.; Hutnick, E.; Gahres, N.; et al. Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T-Cell Therapy for Lymphoma: Predictive Biomarkers and Clinical Outcomes. Neuro-Oncology 2021, 23, 112–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).