Abstract
The metabolism of T-lymphocytes has recently emerged as a pivotal area of investigation, offering insights into the supra-genic modulations that can influence the genetic mechanisms underlying lymphocyte clustering processes. Furthermore, it has become a crucial aspect in understanding lymphocyte plasticity within the immune microenvironment, both in physiological and pathological contexts. T-lymphocyte metabolism has recently emerged as a pivotal factor in both targeted therapy and the genetic signature of the T-lymphocyte, as a result of its influence on gatekeeper processes. From this perspective, the interconnections between the metabolic processes traditionally associated with energy production and the capacity to influence the genetic fate of the T lymphocyte have identified purine metabolism and nuclear/cytoplasmic signaling as pivotal elements in comprehending the intricacies of these molecular phenomena. The two aspects of purine metabolism and metabolic/molecular control of the nuclear envelope have been the subject of a number of significant studies published in recent years. However, from a certain perspective, the existing evidence remains sparse and inconclusive, hindering a comprehensive understanding of the subject matter. In this review, we endeavor to establish a connection between these aspects for the first time and to present a review of the molecular, immunological and genetic events that determine how these aspects, which have hitherto received insufficient attention, may represent a new avenue for lymphocyte reprogramming in the therapeutic field. This will be achieved by understanding the connections between nuclear control and purine flux within and outside the cell.
Keywords:
purine metabolism; GDP; ATP; lymphocytes T; nuclear envelope; nuclear transport; genetic modulation 1. Introduction
Purines and purine derivatives serve as key regulators of intracellular energy balance and nucleotide production. As first hypothesized by Albert Szent-Györgyi and Alan Drury in 1929, they also act as chemical messengers. Binding of extracellular purines to purinergic receptors initiates signal transduction [,]. Despite being predominantly intracellular, nucleotides such as adenosine 5′-triphosphate (ATP) and uridine 5′-triphosphate (UTP) can be released by cells into extracellular fluids following various stimuli, including stress and injury []. In the extracellular milieu, nucleotides are rapidly degraded by ectonucleotidases, such as ENTPDases, including CD39, which catalyzes the conversion of ATP to adenosine 5′-diphosphate (ADP) and ADP to adenosine monophosphate (AMP), and CD73/5′-nucleotidase, a key enzyme in purine metabolism, which converts AMP to adenosine []. The purinergic receptors are traditionally classified into two principal categories based on their agonist selectivity: the adenosine P1 receptors and the ATP P2 receptors []. These, in turn, are further subdivided into different subtypes that are widely expressed in different tissues. When activated by different purine derivatives, they exert specific physiological functions in various processes, such as neurotransmission and blood coagulation [,]. It is well established that purines regulate a multitude of cellular processes, including proliferation, differentiation, and cell death, through the mechanism of purinergic signaling []. This occurs through the complex modulation of other molecular signals in an intricate signalling network, which in turn affects the dynamics of nuclear transport by influencing the expression of nuclear envelope regulatory proteins []. Importantly they can act on almost all subsets of immune cells [,,]. Equally important, the nuclear envelope (NE) regulates cellular function in the innate and adaptive immune systems [,,]. Traditionally viewed as a simple physical barrier separating the nucleoplasm from the cytoplasm, it is now recognized as a complex regulator of cellular signalling and regulatory pathways. []. For example, activation of T lymphocytes leads to chromatin decondensation and disruption of the nuclear envelope, releasing DNA into the cytoplasm. This process is also influenced by cellular metabolism. Indeed, components of the NE, such as the nuclear lamina and nuclear pore complex, have been demonstrated to regulate the proliferation and function of immune cells, particularly T lymphocytes []. The nuclear transport complex, which modulates the selective activities of nuclear pores and participates in the metabolic and functional balance of the nucleus, is controlled by a delicate GTP/GDP purine gradient []. This gradient allows for the organization of specific nuclear transport co-receptors, enabling structural and regulatory micro-RNAs, proteins, and mRNAs to facilitate a dense and continuous communicative network between the nucleus and the cytoplasm [,,]. In this process, RAN (Ras-related Nuclear Protein), which is involved in nuclear/cytoplasmic transport and mitotic stability, plays a crucial role []. Moreover, the overall purine arrangement around the nuclear envelope is capable of influencing the metabolic and energy levels between the nucleus and the mitochondrion. This process is achieved, at least in part, through the mediation of GTPases such as RANBP1 (Ran-binding protein 1) in nuclear-cytoplasmic transport [,]. The balance between ATP and adenosine, between GTP and guanosine, the dynamics of the release of these nucleotides, and the action of nucleoside transporters determine the ultimate effects of purinergic signaling. In particular, the hENTs (Equilibrium Nucleoside Transporters) play an important role in retrieving and regulating purinergic signaling, while others, such as the hCTN1 (Concentrative Nucleoside Transporters), are involved in signal transduction, acting as “transceptors” []. Dysfunction of the purinergic system is known to contribute to the development of several diseases, including gout, diabetes, neurological disorders, osteoporosis and cancer, as well as viral infections such as HIV [,,]. Purine metabolism is fundamental to all cells, with particular significance for cells of the immune system, which are now well-established to be influenced by microenvironments. T-lymphocytes, in particular, play a pivotal role in this connection due to two main reasons []. Firstly, their biological fate is strongly influenced by the resident microenvironment []. Secondly, T-lymphocytes are cells undergoing rapid proliferative activation, which requires a constant and sustained purine flux, as well as metabolic and genetic adaptation [,,]. The recent data suggest that future research should be conducted to determine whether purine nucleotides and their derivatives are the basis of the connections between energy metabolism and adaptive genetic response. This could lead to a greater understanding of how this affects the immune system, for example, by influencing the Th17 rather than the Treg response. Additionally, further investigation could be conducted into the role of purines in intra- and extracellular processes. The purine fluxes dependent on T-lymphocyte metabolism may constitute a system of inter-lymphocyte reciprocal regulation and influence, acting on the one hand as a method of controlling the proliferative and survival mechanisms of cells in the immune and tumor microenvironment, and on the other, as a means of inter-cellular communication [,,]. Additionally, it is conceivable that genetic and metabolic control of T lymphocytes may prove instrumental in the therapeutic reprogramming of CAR-T cells []. Furthermore, intra- and extracellular purine fluxes have the potential to perturb nuclear transport mechanisms that are largely dependent on a GTP/GDP balance []. This can influence the flux of proteins whose nucleus-cytoplasmic shuttling determines the T lymphocyte’s differentiative state and the cell’s ability to export and regulate non-coding regulatory sequences [,]. Thus, an integrated examination of purine regulatory processes is inextricably linked with the regulation of information and regulatory flux within the nuclear envelope.
2. Purine and Purino-Receptors Role
Biological purines are defined as small molecules that possess a purine ring and which may be present within or outside the cell. The most frequently mentioned purine is adenosine 5′-triphosphate (ATP), which has been identified as a critical metabolic molecule within the cell, with cellular concentrations remaining at approximately 1–5 nM []. Its role as an extracellular messenger was first described by Burnstock in 1972 []. In the extracellular space, it functions as a paracrine mediator through interactions with membrane receptors, influencing the immune environment and the activation and phenotype of T lymphocytes as well [,]. Nevertheless, GTP (guanosine triphosphate) fulfills pivotal functions within the cell, including participation in RNA and DNA synthesis [], as well as in G-protein-coupled signaling []. Intracellular guanosine triphosphate (GTP) concentrations vary widely between tissues; for example, in lymphocytes, it ranges from 0.1 to 1 millimolar, with a slight elevation in proliferating cells [,,]. The equilibrium state of the purine pool is contingent upon a dynamic equilibrium between the synthesis and degradation of purine nucleotides []. In mammals, two distinct synthesis pathways have been identified: the de novo pathway and the salvage pathway (Figure 1). The salvage pathway, employed by cells with diminished energy demands, encompasses the recycling of purine bases and degraded nucleotides through the intermediary of 5-phosphoribosyl-1-pyrophosphate (PRPP). This process is catalyzed by adenine phosphoribosyl transferases (APRTs) and hypoxanthine—guanine phosphoribosyl transferases (HPRTs) (Table 1) []. In contrast, cells with high energy requirements—such as actively dividing cancer cells—exhibit a preference for de novo synthesis []. T and B lymphocytes are highly dependent on de novo synthesis of GTP for their proliferation. However, they are unable to utilize the salvage pathway []. De novo synthesis is initiated by a dynamic complex called the purinosome. The purinosome catalyzes the conversion of PRPP to inosine monophosphate (IMP) [,]. IMP is then used for the production of AMP and GMP. In fact, IMP is then converted to adenosine monophosphate (AMP) by the enzyme adenylsuccinate synthase (ADSS) and the enzyme adenylsuccinate lyase (ADSL) or it can be converted to xanthosine 5′-monophosphate by the enzyme inosine monophosphate dehydrogenase (IMPDH1 or IMPDH2) and then to guanosine monophosphate (GMP) by the enzyme guanosine monophosphate synthetase (GMPS) (Table 1). This is followed by a series of sequential phosphorylations leading to GTP formation from GMP by the enzymes guanylate kinase (GUK) (Table 1) and nucleoside diphosphate kinase (NME) [], and ATP formation from AMP or ADP []. Extracellular purines play a critical role in a variety of processes such as cell death, phagocytosis, activation of the inflammasome, antigen presentation, and cell migration []. This is achieved through the activation of purinergic receptors, which leads to the initiation of a cascade of signal transduction pathways. These receptors are divided into two subfamilies: P1 and P2. P1 receptors are G protein-coupled, adenosine-sensing endogenous ligands and are involved in a variety of physiological responses []. They are further divided into several subtypes (A1, A2A, A2B, A3), identified in the 1990s (Table 2) []. Through these receptors, adenosine and its agonists are able to modulate the activity of adenylate cyclase, the enzyme responsible for the increase in cAMP, with stimulatory or inhibitory effects. Elevated intracellular concentrations of cAMP can have a suppressor effect on the activity of immune and inflammatory cells []. P2 receptors are divided into P2X and P2Y. The former are ion channel and consist of seven subtypes (P2X1-7) (Table 2); the latter are G-protein-coupled and are divided into eight subtypes based on agonist selectivity and signal transduction pathways: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 e P2Y14 (Table 2) [,]. P2Y receptors are activated by a variety of nucleotides, including ATP, UTP, ADP, and UDP; P2X receptors are only activated by ATP. P2Y receptors also differentially regulate downstream effectors, including adenylate cyclase and phospholipase C, by coupling to specific G proteins []. These receptors are widely expressed on almost all immune cells []. P2X and P2Y promote leukocyte chemotaxis and inflammasome activation, with production of pro-inflammatory cytokines, because much of the extracellular ATP comes from injured or dead cells and acts as a pro-inflammatory signal. Adenosine also interacts with A2A e A2B receptors, reducing leukocyte activation intensity and duration []. In T lymphocytes, the purinergic system is important in the early stages of maturation. Negative selection is enhanced by P2X7 and inhibited by A2A in the thymus and peripheral tissues [,]. In adult T lymphocytes, the ATP/adenosine ratio is important for cell fate []. In particular, P2X7 appears critical for the Th17 phenotype, inducing dendritic cells to release cytokines including IL-23 and TGFβ []. Other receptors such as P2X1, P2X4, P2X5 and P2Y2 are widely expressed by lymphocytes []. Adenosine, on the other hand, acts as an immunoregulatory mediator. Through the A2A receptor, it promotes the expansion of Tregs [].
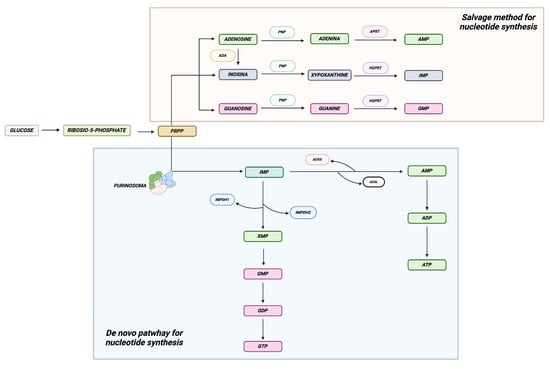
Figure 1.
Purine synthesis. The figure depicts the two principal pathways regulating the synthesis of purine nucleotides. The de novo pathway, involved in the synthesis of new purine nucleotides, serves cells with elevated energy requirements, while the salvage pathway is utilized by cells with lower energy demands. Created in BioRender.com.

Table 1.
Enzymes involved in purine metabolism: de novo and salvage pathways.

Table 2.
Purine receptors and purine transporters in immune system.
2.1. Balance Between ATP/GTP and the Adenosine/Guanosine Pool
The equilibrium between extracellular ATP and adenosine hinges on an intricate network of ectoenzymes, namely the ectonucleotidases. These enzymes, which exhibit varying subtypes, are capable of hydrolyzing tri-, di-, and monophosphate nucleosides, as well as polyphosphate dinucleosides. Additionally, they possess the ability to produce diphosphate nucleosides, monophosphate nucleosides, phosphate nucleosides, and inorganic pyrophosphate []. Extracellular ATP functions as an immunostimulatory signal, while extracellular adenosine acts as an immunoregulatory signal, modulating the function of several cellular components of the adaptive and innate immune response. Consequently, maintaining a balance between ATP and adenosine is essential for immune homeostasis []. The four principal groups of ectonucleotidases are ectonucleoside triphosphate diphosphohydrolase (E-NTPDase), ecto-5′-nucleotidase (eN), ectonucleotide pyrophosphatase/pyrophosphodiesterase (E-NPPs) and alkaline phosphatases (APs). E-NTPDases represent the primary enzymes involved in purinergic signaling. They are nucleotide-specific and catalyze the hydrolysis of triphosphate and diphosphate nucleosides into monophosphates []. The initial step in the degradation of ATP and ADP is catalyzed by the ecto-nucleotidase NTPDase-1/CD39, along with other members of this family, including NTPDases 2, 3, and 8, which are located on the cell surface. The resulting AMP is subsequently hydrolyzed by ecto-5′nucleotidase/CD73, an enzyme that is anchored to GPI (glycosylphosphatidylinositol), resulting in the conversion to adenosine and inorganic phosphate []. Extracellular adenosine derived from CD73 has immunosuppressive tissue functions, thereby modulating the extracellular microenvironment from pro- to anti-inflammatory []. This is achieved through the action of surface NTPDases in conjunction with CD73. Indeed, CD39 was initially identified in B lymphocytes and subsequently in T cells [,]. It has been demonstrated that the expression of this molecule is controlled by a number of factors, including pro-inflammatory cytokines, oxidative stress, and hypoxia [,]. CD73 has been identified in leukocytes derived from peripheral blood, spleen, lymph nodes, and thymus []. Its expression is also regulated by hypoxic conditions and proinflammatory mediators [,]. Both CD39 and CD73 are expressed in murine CD4+ Foxp3+ regulatory T cells (Tregs). Human regulatory T cells (Tregs) express CD39 on their surface, but in contrast to murine T cells, CD73 is predominantly expressed intracellularly []. Additionally, Th17 cells generated with IL-6 and TGFβ have been demonstrated to express both ectonucleotidases, a phenomenon observed in Th1 cells as well []. Murine and human memory T cells have been shown to express CD39 [], while naive CD8+ cells express CD73 []. Adenosine deaminase (ADA), in contrast, functions to catalyze the irreversible hydrolytic deamination of adenosine and deoxyadenosine, the final products of which are inosine and deoxynosine []. Two isoenzymes of ADA have been identified in humans. ADA1 regulates intracellular adenosine concentration and interacts with the extracellular adenosine receptor (also known as ADORA2A, the adenosine A2A receptor) or DPP4 (dipeptidyl peptidase-4, a protein found on the surface of immune cells) in the presence of ADA2, which is less abundant in humans (Table 1) []. ADA deficiencies, therefore, underlie one of the most important immunodeficiencies: ADA-SCID []. P2Y receptors, which are coupled to G proteins, can also be activated by guanosine triphosphate (GTP) []. The production and degradation of guanosine triphosphate (GTP) are regulated by a number of key enzymes involved in purine nucleotide metabolism, which are themselves subject to complex regulatory mechanisms. These processes play a crucial role in the control of a range of cellular functions, including cell proliferation and intracellular signaling. IMPDH1/2 represent the key limiting enzymes in the catalysis of GTP biosynthesis, as they convert IMP to XMP, a critical step in the synthesis of GMP []. In contrast, GMPR exerts a negative regulatory effect on GTP production by converting GMP to IMP (Table 1). This process results in the antagonistic interaction with IMPDH and GMPS, thereby modulating their activity (Table 1) []. The use of GEVALs (GTP Evaluators), a sensor system capable of detecting intracellular GTP, indicates that GTP production and consumption exhibit heterogeneity, resulting in the formation of gradients that influence cellular phenotypes []. Rho protein, a member of the Ras superfamily of GTPases, is widely distributed in immune cells. It functions as a “molecular switch,” controlling numerous signaling pathways in a nucleotide-dependent manner []. Indeed, this protein plays a role in modulating the immune response to infection. It is essential for the maturation of the immune system and the differentiation of peripheral cells, such as T lymphocytes, into their specialized functional forms []. De novo biosynthesis serves as the primary source of GTP in the majority of cell types, with regulation occurring through both transcriptional and post-translational mechanisms that remain to be fully elucidated [].
2.2. Nucleoside Transporters in the Regulation of Purinergic Signals
Within the field of purinergic signaling, nucleoside-transporters play a pivotal role. These are encoded by genes belonging to two distinct families of SLCs (solute carriers), namely: SLC28 and SLC29. The former category comprises the three human concentrative nucleoside transporters (hCNT1, 2 and 3), while the latter includes the four members of the human equilibrative nucleoside transporters (hENT1, 2, 3 and 4) (Table 2) []. It should be noted that while CNT members are not bidirectional transporters, ENT members are. In general, CNT1 is associated with the transport of pyrimidines, CNT2 with that of purines, and CNT3 is responsible for mediating the uptake of both. It has been observed that the equilibrium transporters, with the exception of ENT4, possess broad permeant selectivity, which enables them to transport both purines and pyrimidines []. Both transporter families exhibit common characteristics and are frequently expressed in analogous cell types and tissues, which gives rise to a certain degree of redundancy. T cells predominantly express ENT proteins, which promote their function and expansion []. ENT3 is predominantly expressed in peripheral T cells, particularly in effector populations, while ENT1 is expressed at lower levels. The absence of ENT3 affects the homeostasis of these cells by maintaining lysosomal function and regulating nucleoside availability. This results in the development of an effector-like surface phenotype, but the cells are unable to proliferate and survive after stimulation []. In particular, studies have demonstrated that the majority of CD4+ ENT3−/−cells manifest an effector phenotype, while a small subset retains a naive phenotype. Additionally, an increase in Foxp3+ regulatory T lymphocytes has also been observe []. The hypothesis has been proposed that ENTs may be related to metabolic homeostasis, while CNTs are thought to be associated with a greater range of functions, including nucleoside sensing and signal transduction, which has led to their classification as “transceptors” []. This notion postulates that certain transporters, such as hCNT1, also serve as signal transducers []. In contrast, hCNT2 is associated with energy metabolism via its interacting proteins; furthermore, both hCNT2 and hCNT3 are capable of influencing cell biology through their ability to regulate extracellular adenosine levels, which in turn affects purinergic signalling [,]. Given the importance of nucleotides in numerous physiological processes, it is reasonable to hypothesize that their alteration may contribute to the development of pathological conditions. Accordingly, these characteristics render nucleoside transporters crucial targets for the treatment of pathologies associated with purinergic signaling.
2.3. Effects of Purinergic Signaling in Immune Cells
Adenosine triphosphate (ATP) and other nucleotides are released by cells in response to stress or damage and interact with nearly any subgroup of immune cells through P2X and P2Y receptors, respectively []. Both apoptotic and necrotic cells release ATP and other nucleotides, which function as danger signals to the cell []. Additional mechanisms of damage include mechanical stimulation [], hypoxia, and pathogen invasion []. The release has disparate effects on immune cells. In polymorphonucleates (neutrophils, eosinophils, basophils, mast cells), adenosine and ATP exert opposing effects, influencing functions such as superoxide generation [] and degranulation []. In monocytes, macrophages, and microglia, ATP and its receptors serve a vital function in the regulation of phagocytic and migratory activities [,]. Depending on the activated receptor, ATP can either stimulate or tolerate the immune response in dendritic cells []. Adenosine has complex effects on dendritic cells via A2A or A2B receptors (Table 2). ATP, acting as a chemotaxin for immature dendritic cells, can impair CD4+ polarization toward Th1 and favor Th2. This occurs as a result of the semi-mature state of the dendritic cells induced via P2Y11 receptors (Table 2) []. This state is characterized by an upregulation of co-stimulatory molecules and an inhibition of IL-12 production. IL-10 is then produced in favor of IL-12, which ultimately results in an impairment of Th1-mediated responses and the establishment of Th2 tolerance or response []. Furthermore, it is conceivable that it may facilitate the development of murine Th17 cells by virtue of IL-6 synthesis, a process which is mediated by the A2B receptor []. At the lymphocyte level, adenosine has been shown to elevate cAMP levels, thereby exerting a potent inhibitory effect on lymphocyte proliferation as well as immune response, particularly in individuals with ADA deficiency []. The catabolism of extracellular ATP has been demonstrated to be the primary source of adenosine for lymphocytes. It can be demonstrated that this process represents a link between T and B lymphocytes. This is evidenced by the capacity of B lymphocytes to produce adenosine from the degradation of extracellular ATP, which is then utilized by T lymphocytes. This is due to the fact that B lymphocytes possess a high capacity for extracellular nucleotide degradation, a capability that T lymphocytes lack, despite the presence of ectonucleotidases in both cell types []. CD39 is predominantly expressed in CD4+ regulatory T lymphocytes (Tregs) []. In contrast, CD73 is minimally expressed in the circulating CD4+ cells of healthy individuals but exhibits a marked increase in patients with chronic inflammation []. In humans, it has been demonstrated that 90% of Foxp3+ regulatory T cells (Tregs) express the enzyme CD39, and CD73 is present in the cytoplasm but not on the cell surface [,]. Tregs exhibit particular sensitivity to the effects of extracellular adenosine []. Indeed, ATP exerts an inhibitory role on the generation and function of these cells through a receptor-mediated mechanism, which also induces the conversion of Tregs into Th17 cells []. Furthermore, human regulatory T cells (Tregs) have been demonstrated to degrade adenosine triphosphate (ATP) to adenosine via the ectoenzymes CD39 and CD73, which exerts an immunosuppressive effect []. In contrast, CD39 and CD73 have been observed to suppress the immune response in Th17 lymphocytes through adenosine production. Additionally, the expression of these ectoenzymes is known to be tightly regulated by factors that induce differentiation towards Th17, including IL-6 and TGFβ []. Additionally, A2A and A2B receptors have been observed to be expressed on T lymphocytes (Table 2). Of these receptors, A2B has been shown to play a role in the deactivation of lymphocytes by adenosine, while A2A has the ability to regulate cytokine production in activated T lymphocytes [,]. Moreover, the release of ATP by pannexin hemicanals or vesicular exocytosis autocrine has been demonstrated to amplify T cell receptor (TCR)-mediated lymphocyte activation. This amplification process is dependent on the involvement of P2X7, P2X1, and P2X4 receptors (Table 2) []. Moreover, P2X7 activation has also been demonstrated to induce T lymphocyte death []. The ultimate impact of these receptors on immune cells hinges on the specific subset of lymphocytes and the concentration of ATP. Furthermore, evidence exists indicating the involvement of P2Y receptors in lymphocytes []. Specifically, P2Y2 plays a role in ATP-induced T-cell migration, and adenine nucleotides have been demonstrated to inhibit CD4+ T-cell activation via an elevation of cyclic adenosine monophosphate (cAMP), which is induced by an unidentified P2Y receptor (Table 2) [,]. Furthermore, adenosine and A2 receptor agonists, which elevate cAMP levels, have been demonstrated to inhibit the activity and reactivity of natural killer (NK) cells []. Specifically, the killing capacity of these cells is reduced by the P2Y11 receptor, while the P2X7 receptor can result in either apoptosis or cell activation [,].
3. T Lymphocytes and Nuclear Envelope
The immune system is an integrated network of organs, cells, and biochemical cascades that serves to safeguard the host against pathogens, detrimental external stimuli, and trauma []. The immune system is comprised of two primary branches: innate and adaptive immunity. Innate immunity is mediated by myeloid cells, which elicit a rapid, non-specific response as the initial line of defense. These cells facilitate host defense and inflammation through the production of cytokines and chemokines, complement cascade activation, phagocytosis, and the presentation of antigens to adaptive immunity []. Specific adaptive immunity is mediated by CD4 and CD8 T lymphocytes and antibody production by B lymphocytes []. The nuclear envelope (NE) is a highly specialized membrane that delineates the nucleus of eukaryotic cells and serves an essential role in maintaining the structure and function of these cells. The structure consists of two nuclear membranes (inner and outer), nuclear pores, and, in the case of metazoans, the lamina (Figure 2) []. The perinuclear space is located between the outer nuclear membrane (ONM) and the inner nuclear membrane (INM) []. The outer nuclear membrane (ONM) is continuous with the endoplasmic reticulum, thereby establishing a continuous perinuclear space with the lumen of the reticulum []. Furthermore, the INM is capable of interacting directly with proteins present within the nucleoplasm []. The formation of invaginated structures within the INM results in the development of a specialized reticular structure within the nucleoplasm itself. Additionally, INM proteins interact with V-type intermediate filament proteins to form the nuclear lamina on the nucleoplasmic surface of the INM []. The nuclear lamina is subdivided based on gene sequence into type A/C and type B, and plays a pivotal role in providing mechanical support for the nucleus, organizing chromatin, and regulating gene expression []. The inner nuclear membrane (INM) and outer nuclear membrane (ONM) are permeable only to nonpolar small molecules. Polar molecules, ions, and macromolecules are able to pass between the nucleoplasm and cytoplasm through nuclear pore complexes, which are formed by the fusion of the two membranes []. The nuclear envelope is not a static entity; it serves a regulatory function beyond that of a mere physical barrier. Indeed, it plays a role in regulating cellular signals. In cells of the innate and adaptive immune system, it serves as an integrating mechanism for chemical and mechanical signals encountered by immune cells during the inflammatory process []. In addition, through its various components, such as lamins, nuclear pore complexes (NPC), and lamina-associated polypeptide 2A (LAP2) proteins, cation and anion channels, the nuclear envelope regulates immune cell functions that encompass macrophage polarization and lymphocyte differentiation, cell migration, and lifespan []. The nuclear envelope of human cells has recently been found to contain a new set of enigmatic organelles, which have been named “vaults”. These structures are predominantly located within the cytoplasm and show high levels of conservation among species []. The discovery of these structures within the NE could demonstrate their ability to transfer from the cytoplasm to the nuclear envelope, where they would be expected to associate with the nuclear pore complex (NPC) []. A potential role for major vaults protein (MVP) in a range of cellular functions has been put forth, based on its purported “shuttle” function between the cytoplasm and the nucleus. These proposed functions include the nuclear import of the onco-suppressor PTEN [], the export of nuclear hormone receptors [], as well as the potential for drug export, which may lead to drug resistance in the context of tumor treatments []. Indeed, there are hypotheses that suggest a correlation between these two factors in the context of cell survival and malignant progression []. Additionally, they may play a role in immune responses, such as antiviral defense through nuclear-cytoplasmic transport mechanisms or innate immunity against pulmonary pathogens (Figure 2) [,]. They may also support dendritic cell maturation, which in turn enables the proliferation of specific T cells []. Nevertheless, the exact role of these organelles remains a topic of ongoing investigation, as it is likely to be shaped by the varying evolutionary trajectories of different species.
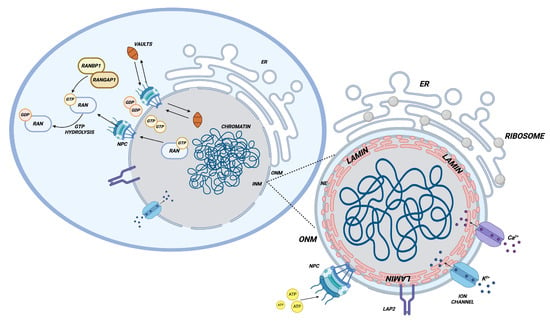
Figure 2.
Nuclear envelope and nuclear-cytoplasmic transport. The figure illustrates the structure of the nuclear envelope, delineating its various components (nuclear pores, ion channels, lamins, etc.) and the mechanisms that regulate nuclear-cytoplasmic transport. This process is mediated by the RAN-RANGAP1-RANBP1 pathway, which establishes a GDP/GTP gradient, and by cytoplasmic ribonucleoprotein “vaults”, which have recently been demonstrated to play a pivotal role in diverse biological processes. Created in BioRender.com.
3.1. The Nuclear Issue During Immune Cells Regulation
Of the nuclear lamins studied in immune cells, the NE-associated proteins are the most extensively investigated. The amount of lamin A/C present varies considerably depending on the type of immune cell being considered. Macrophages and dendritic cells express the highest levels of the protein, while T cells and B cells exhibit barely detectable amounts [,]. In comparison to other somatic cells, immune cells demonstrate highly rapid alterations in the quantity of lamins throughout differentiation, activation, and migratory processes []. In neutrophils, low levels of lamin A/C contribute to flexibility and migratory capacity, which are essential for the effective response to infection []. The aforementioned reduced levels serve to facilitate the breakdown of NE, chromatin condensation, and the formation of neutrophil extracellular traps, otherwise known as NETosis []. The reduced level of lamins A/C appears to facilitate not only the high rate of migration exhibited by neutrophils but also their relatively short lifespan []. In macrophages, high levels of lamin A/C are correlated with the development of adipose tissue inflammation and obesity-related insulin resistance, which are hallmarks of type two diabetes []. Conversely, reducing these levels has been shown to diminish the expression of pro-inflammatory genes in response to external stimuli, such as lipopolysaccharide. Moreover, the c-Fos protein exerts an influence upon tumor-associated macrophages (TAMs) []. In dendritic cells, lamin A/C plays a pivotal role in the defense against viral infection []. However, further research is necessary to elucidate the underlying mechanisms. The function of nuclear pore complexes has also been the subject of extensive study, as they regulate chromatin organization, gene expression, and DNA repair []. In particular, the role of nucleoporins has been elucidated, demonstrating that reduced levels of nucleoporin 96, an essential element for NPC assembly, lead to altered immune responses, decreased interferon-mediated expression of major histocompatibility complexes, impaired antigen presentation and subsequent reduced T-lymphocyte proliferation, and increased susceptibility to infection []. Nucleoporins 88 and 214 contribute to the nuclear accumulation of certain key factors in immune transcription, including NF-κB (Nuclear factor-kappa B), which regulates the strength and duration of the immune response []. A significant interaction between NPC and the Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes has been observed. This interaction plays a crucial role in regulating cellular processes such as cell migration, a fundamental aspect of neutrophil function []. It is finally established that ion channels created between the inner nuclear membrane (INM) and outer nuclear membrane (ONM), with potassium channels being of particular importance, regulate the phosphorylation of CREB protein in macrophages []. The blocking of these channels has the potential to influence the functionality of adjacent ion channels or the Ca2⁺ concentration in the perinuclear space, consequently modifying the membrane potential of the NE []. Ultimately, potassium channels participate in microglia response to external stimuli by promoting the production of nitric oxide and cytokines. This process may regulate calcium and potassium fluxes in the nucleus [].
3.2. Nuclear Envelope and Nuclear Transport Regulation in T Lymphocyte
The nuclear envelope also impacts the functionality of lymphocytes, a type of white blood cell. T lymphocytes are classified as adaptive immune cells and are further divided into two subgroups: CD4+ helper (Th) and CD8+ cytotoxic (CTL) cells. T helper lymphocytes are subdivided into the following categories: These include Th1, Th2, Th9, Th17, Th22, follicular T helpers and regulatory T lymphocytes (Tregs) []. Following antigen presentation, there is a transient increase in the expression of lamin A/C, while at rest, the level of expression is either absent or reduced []. This illustrates that the composition of NE can vary contingent on the state of cell activation. Indeed, a hypothesis has been proposed that the transient increase in the expression of lamin A/C in T lymphocytes may be associated with the requirement to accelerate the formation of synapses between T lymphocytes and antigen-presenting cells, or the necessity to migrate from the lymph node to the targeted tissue []. Nevertheless, the function of lamin A/C may be of greater significance in the differentiation of lymphocytes during an infection or autoimmune response []. Lamin A/C deficiency impairs Th1 lymphocyte differentiation without influencing Th2 lymphocyte differentiation. Indeed, a markedly diminished response to intracellular viral and parasitic infections was evidenced in animal models with laminin A/C deficiency []. Nevertheless, this resulted in enhanced differentiation of regulatory T lymphocytes with immunomodulatory properties, which demonstrated protective efficacy in a murine model of inflammatory bowel disease []. However, the precise manner by which T lymphocytes regulate the expression of lamin A/C remains uncertain. Potential mechanisms may involve the AKT/protein kinase B signaling pathway [], microRNAs [], and/or retinoic acid []. A further influence of NE is mediated by the LINC complex protein, SUN2, which regulates the proliferation, function, and viability of T lymphocytes []. The role of ion channels in lymphocytes has been the subject of only limited investigation. Thus far, anion channels have only been observed within the ONM in T lymphocytes, whereas the INM has exhibited both anion and ion channel activity [,]. Additional investigation is required to determine whether NE ion channels are capable of modulating the physiological processes of lymphocytes, such as gene transcription, proliferation, differentiation, and apoptosis []. RANBP1, a member of the RAS superfamily of small GTPases, plays a critical role in regulating nuclear transport, with its location near the cytoplasmic side of the nuclear envelope making it a crucial mediator in this process. GTPases, which are guanosine triphosphate (GTP)-binding proteins, regulate a plethora of cellular pathways []. These regulatory mechanisms are termed ‘GTPase switches’, as the protein alternates between an inactive GDP-binding conformation and an active GTP-binding conformation, in which effector molecules interact to trigger cellular responses [,,]. The inherently slow rates of nucleotide exchange and GTP hydrolysis mean that interconversion between these forms requires external factors, including guanosine nucleotide exchange factors (GEFs) for the ‘on’ conformation and GTPase-activating proteins (GAPs) for the ‘off’ conformation []. Nucleus-cytoplasmic transport (NCT) is largely dependent on the RAN/GTP-RAN/GDP gradient across the nuclear membrane. RAN, which is part of the RAS superfamily, also exhibits low GTPase activity, which is enhanced by other molecules, including RANBP1 and RANGAP1 (Ran GTPase-activating protein) []. The RANBP1 protein has a specific affinity for the GTP-bound form of RAN; as a result, it facilitates the RANGTP molecule’s accessibility to RANGAP1-mediated hydrolysis. This mechanism ensures the maintenance of the nucleus-cytoplasm gradient, thereby guaranteeing the functionality of nuclear import and export. The nuclear-cytoplasmic flux of GTP/GDP is responsible for maintaining a purine nucleotide-rich environment within both the free and RAN-bound forms. This environment is capable of regulating both the interchange processes between the nucleus and cytoplasm and the overall cellular purine flux []. Additionally, recent findings suggest its involvement in the differentiation of Th17 cells mediated by the serum and glucocorticoid-regulated kinase 1 (SGK1) []. Indeed, while SGK1 [,] phosphorylates FOXO1 (Forkhead box O1) to render it susceptible to nuclear export, it also regulates the nuclear export of FOXO1 in a RANBP1-dependent manner. Consequently, this allows the transcription factor RORγt (Retinoic acid receptor-related-orphan-receptor-γt), a key regulator of Th17 lymphocytes, to be expressed in the cytoplasm [].
3.3. Metabolic Activated T Lymphocytes Produce Chromatin Change
Before activation, T lymphocytes are in what is known as a quiescent state, and become active when they encounter a pathogen. This stimulation occurs via the T lymphocyte receptor (TCR) and the major histocompatibility complex (MHC). Following the activation process, these cells proceed to undergo proliferation, differentiation, and the production of cytokines, which collectively serve to stimulate the immune response []. Furthermore, this activation results in alterations to the spatial configuration of the nuclear architecture, which in turn influence transcriptional activities []. It has been demonstrated that lymphocyte activation results in transcriptome amplification [], histone acetylation and chromatin decondensation []. By means of STORM microscopy, the disruption of the nuclear envelope was evidenced, accompanied by the release of double-stranded DNA and the presence of nuclear pore complexes and nuclear lamina proteins in the cytoplasm. This process occurs in order to promote gene expression and transcription of genes that are crucial for T cell function in response to an activating stimulus []. Cytosolic DNA can be detected by cyclic GMP-AMP synthase (cGAS), a pivotal enzyme in the innate immune system. Once bound to cytoplasmic DNA, cGAS goes through a conformational change that activates it, which in turn results in the conversion of GTP and ATP into a second messenger known as cGAMP. This serves as a ligand for the STING (Stimulator of interferon response CGAMP interactor 1) receptor. Consequently, a signaling cascade is triggered, which results in the synthesis of interferons and pro-inflammatory cytokines. The latter also stimulate T lymphocyte proliferation []. The presence of cGAS demonstrates the interaction between cellular metabolic activity, the organization of chromatin within the cell nucleus, and immune signalling during the activation of lymphocytes [,]. It is evident, therefore, that the process of lymphocyte activation and the subsequent chromatin decondensation are influenced by metabolism; indeed, the inhibition of metabolic activity has been shown to significantly increase chromatin compaction, while reducing the release of DNA into the cytoplasm []. Normally, during lymphocyte activation, due to clonal expansion, the cells adopt anaerobic metabolism, which requires a high intake of nutrients and significant ATP consumption []. Indeed, during the quiescent state, metabolism is predominantly dependent on oxidative phosphorylation (OXPHOS) and β-oxidation of fatty acids []. However, upon activation, lymphocytes increase glucose uptake and aerobic glycolysis, even in the presence of oxygen, a process known as the Warburg effect. This allows for the rapid synthesis of lipids, proteins, and nucleic acids, which are necessary for cell proliferation [,]. The process is regulated by the PI3K/AKT pathway (Phosphatidylinositol 3-kinase/Serina-threonina kinase 1), which in turn depends on pathways regulated by mTOR (Mammalian target of rapamycin) and the proto-oncogene MYC [,]. In addition to glucose, there is an increase in glutamine uptake during clonal expansion. This has been observed to promote Th17 differentiation [].
4. Purine Metabolism and Reorganization of Nuclear/Cytoplasmic Transport Under Physiological and Pathological Conditions
Purines and their receptors play a role in a number of physiological functions. ATP, ADP, and adenosine, in addition to the other purine compounds, play an active role in energy metabolism and intercellular signalling. Purinergic receptors are expressed in all tissues and are critical in controlling physiological functions through their interaction with purine compounds. At the level of the central nervous system, they are expressed on neurons and glial cells, and regulate the release of neurotransmitters and neuronal excitability []. In the cardiovascular system they regulate the contractile processes of the heart and blood vessels. This occurs via the actions of these peptides on cardiac muscle cells, vascular smooth muscle cells and endothelial cells [,]. With respect to the digestive system, these substances are expressed on the cells of the intestinal musculature and enteric neurons, where they exert a controlling influence over the processes of gastrointestinal motility, digestive fluid secretion, and intestinal absorption []. In the respiratory system, they facilitate the maintenance of airway and alveolar homeostasis in bronchial epithelial cells and alveolar and smooth muscle cells [,]. The effects on immune cells are also multifaceted, encompassing both stimulatory and inhibitory mechanisms []. Imbalances in purine metabolism and purinergic signalling are implicated in a number of pathological processes, including gout, inflammation, neurological disorders, viral infections, immunodeficiencies and cancer. For instance, the deposition of monosodium urate (MSU), a monosodium salt of uric acid, in the joints is the primary cause of gout, which represents a final catabolic product of purine metabolism []. Furthermore, genetic and mechanistic links have been established between several inflammatory diseases and ectonucleotidases CD39 and CD38 []. Nucleocytoplasmic transport (NCT) across the nuclear envelope is a vital process in maintaining cellular homeostasis. Dysregulation of NCT has been linked to the aging process and the development of neurodegenerative diseases, including Alzheimer’s and Huntington’s diseases []. Defects in nuclear pore proteins have been identified in numerous cancer cell types. These defects affect the transfer of factors such as p53 and β-catenin between the nucleus and cytoplasm []. Moreover, a considerable number of viruses have been observed to utilize the nuclear transport apparatus to facilitate their life cycle within host cells. In some instances, this process may also lead to the suppression of the host immune response, a phenomenon that can be attributed to the impairment of nuclear transport [,]. From a pathological and purely speculative standpoint, it remains of paramount importance to gain a deeper understanding of the mechanisms through which the intra-nuclear purine pool, which is evidently indispensable for the formation of genetic information, is capable of regulating the structure of the nuclear envelope and, consequently, the processes of nuclear-cytoplasmic transport. Furthermore, it is crucial to elucidate the manner in which dysregulation of this process may lead to alterations in nuclear communication and the establishment of a novel metabolic configuration of T lymphocytes. From a pathological perspective, this could provide an explanation for some aberrant T-dependent responses observed in diseases with complex traits, particularly in the context of immune dysregulation in neoplastic processes and in diseases characterized by an aberrant inflammatory or autoinflammatory response.
4.1. Nervous System
The brain and immune system interact in order to maintain homeostasis through the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system []. Nucleotides are released by exocytosis during synaptic transmission. For instance, the autonomic, sensory, and motor nerves have been shown to innervate immune cells, releasing ATP as a co-transmitter in close proximity to said immune cells []. Similarly, ATP released by activated mast cells has been demonstrated to serve as an indispensable mediator in the activation of nerves []. However, compelling evidence has emerged indicating that nerve fibers also establish intimate associations with a diverse range of immune cells, including eosinophils [], macrophages [], and T and B lymphocytes []. This has sparked a surge of interest in neuroimmunology, which has the potential to illuminate hitherto unrecognized roles of ATP and purinergic signaling. The sympathetic nervous system, when active and innervating immune organs, releases a number of signalling molecules in the proximity of immune cells. These include classic neurotransmitters such as norepinephrine and epinephrine, as well as co-transmitters such as ATP and adenosine. The former has been well characterized and their role in immune function well documented; the latter have received rather little attention to date. Consequently, immune cells express a variety of adrenergic and purinergic receptors that are sensitive to these molecules. Upon activation of these receptors, immune or inflammatory mediators such as cytokines and chemokines are produced []. Any alterations to this system have the potential to result in the development of a number of different diseases. For instance, purine nucleoside phosphorylase (PNP) deficiency is characterized by immunological aberrations, and approximately half of the patients present with neurological abnormalities. These can be attributed to the enzyme defect or may arise as a consequence of the underlying immune defects [].
4.2. Viral Infections: HIV
In recent years, purine metabolism has also been observed in several viral diseases, including human immunodeficiency virus (HIV). Human immunodeficiency virus (HIV) is a type of disease that affects some of the cells that comprise the immune system, particularly CD4+ lymphocytes. In the absence of treatment, the gradual weakening of this system causes the infected person to become unable to organize an adequate immune response against viruses, bacteria, and other pathogens []. Indeed, in recent years it has been observed that viral entry has a profound effect on purine nucleotide metabolism within infected cells. This is evidenced by studies into the pathophysiology of this disease. Indeed, studies conducted by Carlucci et al. on PBLs from normal, asymptomatic, AIDS-infected subjects have indicated that purine metabolism plays an important role in this viral disease and is significantly altered [,]. In particular, in subjects presenting no clinical symptoms, the levels of NAD, ADP, and ATP were significantly reduced, whereas in symptomatic patients, NAD and ADP were lower, while AMP and GTP showed significantly increased levels. Significant alterations in the ATP/ADP and GTP/GDP ratios, along with a reduction in the A/G ratio, were evident in HIV-1-positive subjects when compared with the control group. Both patient groups exhibited a block in total nucleic acid formation, which was attributed to a defect in de novo synthesis [,]. Such alterations may be attributed to modifications in RNA and DNA metabolism within infected cells. As a case in point, the rise in guanine nucleotides may be attributable to the replication of viral RNA in host cells, given that HIV RNA has previously been demonstrated to be abundant in guanine nucleotides []. In light of this information, purine metabolism may prove to be a pivotal area of investigation in the study of this viral disease. It is a requisite that reverse transcription complexes (RTCs), which are responsible for the retroviral replication process, gain access to the nuclear environment in order for HIV to penetrate the host cell. Lentiviruses, including HIV, are capable of exploiting nuclear transport mechanisms through the evolution of retroviral reverse transcription complexes (RTCs). This is, in part, due to the specific interactions between the viral capsid protein and nucleoporins such as Nup358 and Nup153 []. Indeed, studies have demonstrated that the HIV nucleocapsid protein serves as a macromolecular nuclear transport receptor (NTR), utilizing host factors to regulate the requirements of nuclear pore complexes (NPCs) during the process of nuclear invasion []. Imp7 (importin 7) also appears to be involved in this mechanism. Depletion of Imp7 in CD4 cells has been shown to limit HIV-1 infection []. Nuclear export of HIV RNA is also mediated by the viral Rev protein. In addition to an RNA binding domain specific for the Rev response element (RRE), the Rev protein also contains a nuclear export signal (NES). CRM1 (exportin 1) acts as a nuclear export receptor. This process requires the GTP-bound form of Ran []. This results in the formation of a Rev/CRM1/RanGTP complex that interacts with a number of nucleoporins []. Correlation has been found between RANBP1 NESs and HIV-1 Rev NESs. Indeed, the NES signals of RANBP1 and Rev functionally interfere with each other. This indicates competition for a common export pathway and the formation of a RANBP1/RanGTP/CRM1 complex. RANBP1 interferes with Rev-mediated expression of HIV-1, whereas RANBP1 inactivation of the nuclear export signal abrogates Rev []. Given that Th17 are known to be susceptible and permissive to HIV entry and support viral replication and that RANBP1 is involved in the differentiation of these lymphocytes, this is very important [].
4.3. Cancer
Purine metabolism has been shown to regulate malignant behavior and response to immune checkpoint inhibitors in several tumours. Due to the increased growth rate of neoplastic cells, purine demand is highly regulated at the tumour level. In the tumour microenvironment (TME), adenosine is recognized as an important modulator of immune cell function [,,]. It is evident that purinergic receptors on immune cells exert a pivotal influence on the modulation of immune responses []. Additionally, the ectonucleotidases CD39 and CD73 have the potential to enhance the concentration of anti-inflammatory adenosine and diminish proinflammatory ATP within the microenvironment (Figure 3) []. A considerable increase in the expression of both CD39 and CD73 has been observed in a range of blood-related neoplasms, including leukemias and lymphomas []. Consequently, the generation of adenosine in the tumour microenvironment represents one of the mechanisms employed by the tumour in order to create an immunosuppressive environment, which is mediated by a number of mechanisms, including the inhibition of cytokine production and activation by T cells [], the impairment of the cytotoxic activity of T cells and NK cells [], the induction of Treg cells [], the inhibition of the maturation and activation of dendritic cells [] and the conversion of TAMs into the M2 subtype (Figure 3) []. Cancer cells frequently demonstrate elevated nuclear translocation speeds and capacities in response to rapid signals and metabolic stresses []. A considerable number of nuclear transport proteins are overexpressed in malignant cells, including XPO1 (Exportin-1), which is responsible for the relocation of numerous suppressor genes to the cytoplasm, thereby rendering them inactive and contributing to drug resistance [,]. Additionally, other nuclear import factors, such as Impβ1 and Impβ2, are more highly expressed in tumours. This may facilitate the entry of numerous oncogenic transcription factors into the nucleus, thereby promoting tumourigenesis [,]. To illustrate, the transcription factor NF-κB is imported from the cytoplasm into the nucleus of T cells, where it regulates the expression of genes that promote proliferation, cytokine production, and differentiation of these cells. It has been demonstrated that the inhibition of nuclear transport of NF-κB can result in a reduction in the immune response against tumours []. Indeed, T-cell immunity frequently fails to develop adequately in cancer patients, with altered proliferation and function of effector cells and tumour-infiltrating lymphocytes (TILs) [,]. Consequently, there is mounting evidence that human tumours express antigenic peptides that are recognized by cytotoxic T lymphocytes. The gene encoding the small GTPase Ran was discovered to be exposed in epitopes that are recognized by HLA-A33-restricted CTLs. These CTLs were established by T cells that were found to be infiltrating gastric adenocarcinoma. Ran is known to play a role in controlling the cell cycle, including by regulating nucleocytoplasmic transport, mitotic spindle organization, and nuclear envelope formation [,].
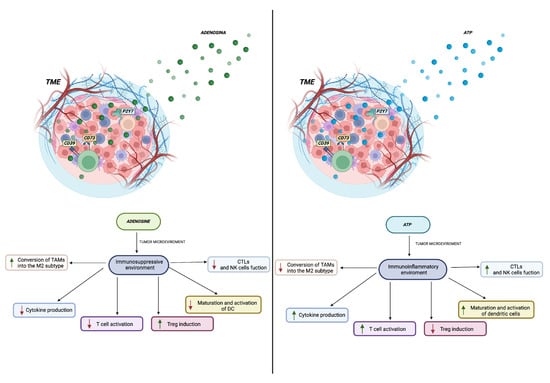
Figure 3.
The role of ATP and adenosine in the tumour microenvironment is illustrated in the accompanying figure. It demonstrates how an immunosuppressive and/or inflammatory environment is established with the increase (green arrow) or decrease (red arrow) of different cell populations within the tumour microenvironment. These changes are dependent on the presence and concentration of adenosine and adenosine triphosphate, which have the ability to regulate the functions of the immune cells present. Created in BioRender.com.
4.4. Immunodeficiency
Adenosine deaminase (ADA) is a pivotal enzyme in the purine recovery pathway, facilitating the irreversible deamination of adenosine and 2′-deoxyadenosine into inosine and 2′-deoxyinosine, respectively []. A deficiency of this enzyme, resulting from a mutation in the ADA gene, is a primary cause of autosomal recessive severe combined immunodeficiency (SCID), one of the most prevalent genetic disorders. In the absence or impairment of ADA function, there is an accumulation of toxic metabolites, including adenosine, 2′-deoxyadenosine, and deoxyadenosine triphosphate (dATP) []. This disease is typified by severe lymphocytopenia, affecting T and B lymphocytes and NK cells. However, given the ubiquitous nature of this enzyme, non-immunological manifestations also occur, including neurodevelopmental deficiencies, sensorineural hearing impairment, and skeletal abnormalities []. Indeed, 2′-deoxyadenosine, as a cytotoxic metabolite, has been demonstrated to exert lymphotoxic effects, while dATP accumulates primarily in erythrocytes and lymphocytes [,]. In contrast, elevated adenosine concentrations have been demonstrated to promote apoptosis and impede the differentiation of thymocytes, ultimately leading to a significant reduction in lymphocyte numbers in mouse and human models []. Furthermore, the enzyme is involved in T-cell receptor signalling and the inflammatory response via G-protein-coupled receptors [], and it plays a role in neurotransmission []. It is possible for immunodeficiency and autoimmunity to occur simultaneously in the same individual, and there have been numerous instances in which autoimmune dysregulation has been observed in individuals with ADA-SCID [,]. However, autoimmunity in these individuals also occurs as a result of specific alterations caused by the accumulation of metabolites []. The autoimmune manifestations associated with ADA deficiency may result from altered purine metabolism, which could interfere with the normal function of regulatory T cells []. It is evident that Tregs primarily oversee the peripheral regulation of autoreactive T cells and generate and sustain high concentrations of adenosine. This is due to their capacity to express both CD39 and CD73, which enables them to produce extracellular adenosine. Conversely, they display low expression of ADA [].
5. Conclusions
An in-depth comprehension of the molecular mechanisms underlying purinergic signalling paves the way for the development of novel approaches to the management of a range of human diseases, including neurological conditions, viral infections, immune deficiencies and cancer. The critical role played by purinergic signalling in regulating the immune system and inflammatory response is evident from the expression of purinergic receptors in the majority of immune cells. It is plausible that purine metabolism can be employed as a prognostic biomarker and as a potential therapeutic target to enhance the efficacy of immunotherapy in cancer patients. This hypothesis is supported by the observation that analysis of the immune microenvironment has revealed a correlation between gene expression related to purine metabolism and an increase in immune cell infiltration, including TILs, and elevated immune checkpoint expression, including PD-1 and PD-L1 (Programmed cell death protein/ligand 1) []. Furthermore, elucidating the function of purines may prove valuable in identifying biomarkers for the diagnosis of viral infections, such as Human Immunodeficiency Virus 1 (HIV-1) []. Given that a metabolism-chromatin interaction directly regulates the transcriptional mechanism that is crucial for T-cell proliferation, it is evident that metabolic inhibitors could potentially modulate chromatin structure and function. Consequently, this could provide a novel avenue for modulating the response of these cells in a therapeutic manner, which is an area that warrants further investigation []. The use of purine-rich foods or purine-enriched food supplements has been the subject of extensive investigation in the context of various diseases, including gout. In this context, the relationship between purine balance and intracellular metabolism has been identified as a particularly significant area of interest. Recent fundamental studies have demonstrated, contrary to long-held medical concepts, that while higher levels of meat and seafood consumption are associated with an increased risk of gout, higher levels of dairy consumption have been shown to be associated with a decreased risk. The consumption of vegetables or protein sources rich in purines in moderation does not appear to be associated with an elevated risk of gout. This novel finding may potentially facilitate a more rational utilisation of purine products in the modulation of intracellular metabolism in complex diseases, where the precise role of cellular metabolism and the immune system remains elusive []. The majority of studies have focused on the understanding and targeting of the ectonucleotidase enzymes CD39 and CD73. A number of humanized anti-CD39 antibodies are currently undergoing clinical trials for the treatment of lymphomas, as are a multitude of attempts to develop novel anti-CD73 antibodies that selectively target the immunosuppressive adenosinergic pathway [,,]. Moreover, a strategy that specifically addresses nucleocytoplasmic transport and NE components is crucial for optimal efficacy. For instance, a viable therapeutic target may be IPO7, a gene that encodes Imp7. There is a negative correlation between the expression of IPO7 and the infiltration of CD8 cells within the tumor microenvironment []. Furthermore, Ran-associated tumor antigenic peptides may also serve as target molecules in the context of specific immunotherapy targeting CTLs []. From these results, it can be concluded that purine metabolism and nucleocytoplasmic transport are crucial elements in the control of immune cells, notably T lymphocytes. This is especially the case with regard to purine receptors, ectonucleotidases, the nuclear envelope, and importins/exporters such as karyopherins and all factors involved in regulating and integrating purine metabolism with the formation and regulation of envelope selectivity as a dynamic structure in nuclear-cytoplasmic interactions. This may have dramatic implications for a novel approach to the diagnosis, prognosis and treatment of a variety of diseases.
Author Contributions
Conceptualization, N.T., C.B., F.T. and R.A.; methodology, F.T. and R.A.; software, C.B.; validation, F.T. and R.A.; formal analysis, N.T., C.B., F.T. and R.A.; investigation, N.T.; resources, N.T., C.B., E.C. and S.A.; data curation, N.T., C.B., E.C. and S.A.; writing—original draft preparation, R.A.; writing—review and editing, N.T., C.B., F.T. and R.A.; visualization, N.T., C.B., E.C., S.A., F.T. and R.A.; supervision, F.T. and R.A.; project administration, R.A.; funding acquisition, F.T. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a generous donation from the Stillitani family, in memory of Carmelo.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ADA | Adenosine Deaminase |
| ADSL | Adenylosuccinate lyase |
| ADSS | Adenylosuccinate synthase |
| AKT | Serina-threonina kinase 1/Protein kinase B |
| APRT | Adenine phosphoribosyltransferase |
| APs | Alkaline phosphatases |
| cGAMP | Cyclic guanosine monophosphateadenosine monophosphate |
| cGAS | Cyclic GMP-AMP synthase |
| CMR1 | Exportin 1 |
| DDP4 | Dipeptidyl Peptidase-4 |
| EMT | Epithelial-mesenchymal transition |
| eN | Ecto-5′-nucleotidase |
| E-NPP | Ecto-nucleotide pyrophosphatase/phosphodiesterase |
| E-NTPDase | Ecto-nucleoside triphosphate diphosphohydrolase |
| EPHA4 | Ephrin type-A receptor 4 |
| FOXO1 | Forkhead box O1 |
| GAP | GTPase-activating protein |
| GEF | Guanine nucleotide exchange factors |
| GEVALs | GTP-Evaluators |
| GMPR | Guanosine monophosphate reductase |
| GMPS | Guanosine monophosphate synthetase |
| GPI | Glycosylphosphatidylinositol |
| GUK | Guanylate kinase |
| hCTN | Human concentrative nucleoside transporter |
| hENT | Human equilibrative nucleoside transporter |
| HPRT | Hypoxanthine-guanine phosphoribosyl transferase |
| HSC | Hematopoietic stem cell |
| Impβ1/β2 | Importin β1/β2 |
| Imp7 | Importin 7 |
| IMPDH | Inosine-5′-monophosphate dehydrogenase |
| INM | Inner nuclear membrane |
| LINC | Linker of nucleoskeleton and cytoskeleton complex |
| MSU | Monosodium urate |
| mTOR | Mammalian target of rapamycin |
| MVP | Major vault protein |
| NCT | Nucleocytoplasmatic trasnsport |
| NE | Nuclear Envelope |
| NES | Nuclear export signal |
| NF-kB | Nuclear factor-kappa B |
| NME | Nucleoside diphosphate kinase |
| NPC | Nuclear pore complex |
| NTR | Nuclear transport receptors |
| ONM | Outer nuclear membrane |
| PBL | Peripheral blood lymphocyte |
| PD1/L1 | Programmed cell death protein 1/ligand 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PNP | Purine nucleotide phosphorylase |
| PRPP | Phosphoribosyl pyrophosphate |
| PTEN | Phosphatase and tensin homolog |
| RAN | Ran-related nuclear protein |
| RANBP1 | Ran-binding protein 1 |
| RANGAP1 | Ran GTPase-activating protein |
| RORγt | Retinoic acid receptor-related-orphan-receptor-γt |
| RRE | Rev response element |
| RTC | Replication-transcription complex |
| SCID | Severe combined immunodeficiency |
| SGK1 | Serum and glucocorticoid-regulated kinase 1 |
| SLC | Solute carriers |
| STING | Stimulator of interferon response CGAMP interactor 1 |
| TAM | Tumor-associated macrophages |
| TCR | T-cell receptor |
| TILs | Tumour-infiltrating lymphocytes |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| XO | Xanthine oxidase |
| XPO1 | Exportin-1 |
References
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From Purines to Purinergic Signalling: Molecular Functions and Human Diseases. Signal Transduct. Target Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.N.; Szent-Györgyi, A. The Physiological Activity of Adenine Compounds with Especial Reference to Their Action upon the Mammalian Heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Boeynaems, J.-M. Purinergic Signalling and Immune Cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef] [PubMed]
- Deaglio, S.; Robson, S.C. Ectonucleotidases as Regulators of Purinergic Signaling in Thrombosis, Inflammation, and Immunity. Adv. Pharmacol. 2011, 61, 301. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for Purines and Pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Khakh, B.S.; North, R.A. Neuromodulation by Extracellular ATP and P2X Receptors in the CNS. Neuron 2012, 76, 51–69. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. Purinergic Receptors in Thrombosis and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef]
- Scott, K.L.; Halfmann, C.T.; Hoefakker, A.D.; Purkayastha, P.; Wang, T.C.; Lele, T.P.; Roux, K.J. Nucleocytoplasmic Transport Rates Are Regulated by Cellular Processes That Modulate GTP Availability. bioRxiv 2023. bioRxiv:2023.12.29.573651. [Google Scholar] [CrossRef]
- Seegmiller, J.E.; Watanabe, T.; Shreier, M.H.; Waldmann, T.A. Immunological Aspects of Purine Metabolism. In Purine Metabolism in Man—II: Regulation of Pathways and Enzyme Defects; Müller, M.M., Kaiser, E., Seegmiller, J.E., Eds.; Springer US: Boston, MA, USA, 1977; pp. 412–433. ISBN 978-1-4613-4223-6. [Google Scholar]
- Di Virgilio, F.; Vuerich, M. Purinergic Signaling in the Immune System. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic Regulation of the Immune System. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Selezneva, A.; Gibb, A.J.; Willis, D. The Nuclear Envelope as a Regulator of Immune Cell Function. Front. Immunol. 2022, 13, 840069. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Somovilla-Crespo, B.; Rius, C.; Gonzalez-Granado, J.M. Lamin A/C and the Immune System: One Intermediate Filament, Many Faces. Int. J. Mol. Sci. 2020, 21, 6109. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Perugini, V.; González-Granado, J.M. Nuclear Envelope Lamin-A as a Coordinator of T Cell Activation. Nucleus 2014, 5, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hetzer, M.W.; Walther, T.C.; Mattaj, I.W. PUSHING THE ENVELOPE: Structure, Function, and Dynamics of the Nuclear Periphery. Annu. Rev. Cell. Dev. Biol. 2005, 21, 347–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, X.; Chen, Z.; Ma, H.; Liu, Y. Super-Resolution Imaging of T Lymphocyte Activation Reveals Chromatin Decondensation and Disrupted Nuclear Envelope. Commun. Biol. 2024, 7, 717. [Google Scholar] [CrossRef]
- Kalab, P.; Heald, R. The RanGTP Gradient—A GPS for the Mitotic Spindle. J. Cell Sci. 2008, 121, 1577–1586. [Google Scholar] [CrossRef]
- Audia, S.; Brescia, C.; Dattilo, V.; D’Antona, L.; Calvano, P.; Iuliano, R.; Trapasso, F.; Perrotti, N.; Amato, R. RANBP1 (RAN Binding Protein 1): The Missing Genetic Piece in Cancer Pathophysiology and Other Complex Diseases. Cancers 2023, 15, 486. [Google Scholar] [CrossRef]
- El-Tanani, M.; Nsairat, H.; Mishra, V.; Mishra, Y.; Aljabali, A.A.A.; Serrano-Aroca, Á.; Tambuwala, M.M. Ran GTPase and Its Importance in Cellular Signaling and Malignant Phenotype. Int. J. Mol. Sci. 2023, 24, 3065. [Google Scholar] [CrossRef]
- Bischoff, F.R.; Görlich, D. RanBP1 Is Crucial for the Release of RanGTP from Importin β-Related Nuclear Transport Factors. FEBS Lett. 1997, 419, 249–254. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The Role of Purinergic P2X7 Receptor in Inflammation and Cancer: Novel Molecular Insights and Clinical Applications. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, F.; Tabucchi, A.; Perrett, D.; Pizzichini, M.; Rosi, F.; Pagani, R.; Marinello, E. Purine Metabolism in HIV-1 Virus-Infected T Lymphocyte Population. Biomed. Pharmacother. 1996, 50, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, D.R.; Fisher, E.L.; Rathmell, J.C. Microenvironmental Influences on T Cell Immunity in Cancer and Inflammation. Cell. Mol. Immunol. 2022, 19, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Brescia, C.; Audia, S.; Pugliano, A.; Scaglione, F.; Iuliano, R.; Trapasso, F.; Perrotti, N.; Chiarella, E.; Amato, R. Metabolic Drives Affecting Th17/Treg Gene Expression Changes and Differentiation: Impact on Immune-Microenvironment Regulation. APMIS, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Quéméneur, L.; Gerland, L.-M.; Flacher, M.; Ffrench, M.; Revillard, J.-P.; Genestier, L. Differential Control of Cell Cycle, Proliferation, and Survival of Primary T Lymphocytes by Purine and Pyrimidine Nucleotides1. J. Immunol. 2003, 170, 4986–4995. [Google Scholar] [CrossRef]
- Conley, J.M.; Gallagher, M.P.; Berg, L.J. T Cells and Gene Regulation: The Switching On and Turning Up of Genes after T Cell Receptor Stimulation in CD8 T Cells. Front. Immunol. 2016, 7, 76. [Google Scholar] [CrossRef]
- Schneider, E.; Winzer, R.; Rissiek, A.; Ricklefs, I.; Meyer-Schwesinger, C.; Ricklefs, F.L.; Bauche, A.; Behrends, J.; Reimer, R.; Brenna, S.; et al. CD73-Mediated Adenosine Production by CD8 T Cell-Derived Extracellular Vesicles Constitutes an Intrinsic Mechanism of Immune Suppression. Nat. Commun. 2021, 12, 5911. [Google Scholar] [CrossRef]
- Wang, T.; Gnanaprakasam, J.N.R.; Chen, X.; Kang, S.; Xu, X.; Sun, H.; Liu, L.; Rodgers, H.; Miller, E.; Cassel, T.A.; et al. Inosine Is an Alternative Carbon Source for CD8+-T-Cell Function under Glucose Restriction. Nat. Metab. 2020, 2, 635–647. [Google Scholar] [CrossRef]
- Saveljeva, S.; Sewell, G.W.; Ramshorn, K.; Cader, M.Z.; West, J.A.; Clare, S.; Haag, L.-M.; de Almeida Rodrigues, R.P.; Unger, L.W.; Iglesias-Romero, A.B.; et al. A Purine Metabolic Checkpoint That Prevents Autoimmunity and Autoinflammation. Cell Metab. 2022, 34, 106–124.e10. [Google Scholar] [CrossRef]
- Giuffrida, L.; Sek, K.; Henderson, M.A.; Lai, J.; Chen, A.X.Y.; Meyran, D.; Todd, K.L.; Petley, E.V.; Mardiana, S.; Mølck, C.; et al. CRISPR/Cas9 Mediated Deletion of the Adenosine A2A Receptor Enhances CAR T Cell Efficacy. Nat. Commun. 2021, 12, 3236. [Google Scholar] [CrossRef]
- Brescia, C.; Dattilo, V.; D’Antona, L.; Chiarella, E.; Tallerico, R.; Audia, S.; Rocca, V.; Iuliano, R.; Trapasso, F.; Perrotti, N.; et al. RANBP1, a Member of the Nuclear-Cytoplasmic Trafficking-Regulator Complex, Is the Terminal-Striking Point of the SGK1-Dependent Th17+ Pathological Differentiation. Front. Immunol. 2023, 14, 1213805. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Xiao, S.; Thalhamer, T.; Madi, A.; Han, T.; Kuchroo, V. SGK1 Governs the Reciprocal Development of Th17 and Regulatory T Cells. Cell Rep. 2018, 22, 653–665. [Google Scholar] [CrossRef]
- Traut, T.W. Physiological Concentrations of Purines and Pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Junger, W.G. Immune Cell Regulation by Autocrine Purinergic Signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Castillo-Leon, E.; Dellepiane, S.; Fiorina, P. ATP and T-Cell-Mediated Rejection. Curr. Opin. Organ Transplant. 2018, 23, 34–43. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.-M. Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.-Y. Regulation, Signaling and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef]
- Zala, D.; Schlattner, U.; Desvignes, T.; Bobe, J.; Roux, A.; Chavrier, P.; Boissan, M. The Advantage of Channeling Nucleotides for Very Processive Functions. F1000Res 2017, 6, 724. [Google Scholar] [CrossRef]
- Kopra, K.; Mahran, R.; Yli-Hollo, T.; Tabata, S.; Vuorinen, E.; Fujii, Y.; Vuorinen, I.; Ogawa-Iio, A.; Hirayama, A.; Soga, T.; et al. Homogeneous Luminescent Quantitation of Cellular Guanosine and Adenosine Triphosphates (GTP and ATP) Using QT-LucGTP&ATP Assay. Anal. Bioanal. Chem. 2023, 415, 6689–6700. [Google Scholar] [CrossRef]
- Liu, J.; Hong, S.; Yang, J.; Zhang, X.; Wang, Y.; Wang, H.; Peng, J.; Hong, L. Targeting Purine Metabolism in Ovarian Cancer. J. Ovarian Res. 2022, 15, 93. [Google Scholar] [CrossRef]
- Natsumeda, Y.; Prajda, N.; Donohue, J.P.; Glover, J.L.; Weber, G. Enzymic Capacities of Purine de Novo and Salvage Pathways for Nucleotide Synthesis in Normal and Neoplastic Tissues. Cancer Res. 1984, 44, 2475–2479. [Google Scholar] [PubMed]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, L.D.; Bofill, M.; Ruckemann, K.; Simmonds, H.A. Importance of Ribonucleotide Availability to Proliferating T-Lymphocytes from Healthy Humans. Disproportionate Expansion of Pyrimidine Pools and Contrasting Effects of de Novo Synthesis Inhibitors. J. Biol. Chem. 1995, 270, 29682–29689. [Google Scholar] [CrossRef]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de Novo Purine Biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef]
- Pedley, A.M.; Benkovic, S.J. A New View into the Regulation of Purine Metabolism—The Purinosome. Trends. Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; French, J.B.; Fang, Y.; Benkovic, S.J. The Purinosome, a Multi-Protein Complex Involved in the de Novo Biosynthesis of Purines in Humans. Chem. Commun. 2013, 49, c3cc41437j. [Google Scholar] [CrossRef]
- Knowles, J.R. Enzyme-Catalyzed Phosphoryl Transfer Reactions. Annu. Rev. Biochem. 1980, 49, 877–919. [Google Scholar] [CrossRef]
- Stagg, J.; Golden, E.; Wennerberg, E.; Demaria, S. The Interplay between the DNA Damage Response and Ectonucleotidases Modulates Tumor Response to Therapy. Sci. Immunol. 2023, 8, eabq3015. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. In Adenosine Receptors in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 161–188. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Klotz, K.-N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Livingston, M.; Heaney, L.G.; Ennis, M. Adenosine, Inflammation and Asthma—A Review. Inflamm. Res. 2004, 53, 171–178. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Boison, D. ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2022, 74, 799–824. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 Purinergic Signalling in the Tumour Microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Ayna, G.; Krysko, D.V.; Kaczmarek, A.; Petrovski, G.; Vandenabeele, P.; Fésüs, L. ATP Release from Dying Autophagic Cells and Their Phagocytosis Are Crucial for Inflammasome Activation in Macrophages. PLoS ONE 2012, 7, e40069. [Google Scholar] [CrossRef] [PubMed]
- Lépine, S.; Le Stunff, H.; Lakatos, B.; Sulpice, J.C.; Giraud, F. ATP-Induced Apoptosis of Thymocytes Is Mediated by Activation of P2X7 Receptor and Involves de Novo Ceramide Synthesis and Mitochondria. Biochim. Biophys. Acta (BBA)—Mol. Cell. Biol. Lipids 2006, 1761, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Romio, M.; Reinbeck, B.; Bongardt, S.; Hüls, S.; Burghoff, S.; Schrader, J. Extracellular Purine Metabolism and Signaling of CD73-Derived Adenosine in Murine Treg and Teff Cells. Am. J. Physiol.-Cell Physiol. 2011, 301, C530–C539. [Google Scholar] [CrossRef]
- Gorini, S.; Callegari, G.; Romagnoli, G.; Mammi, C.; Mavilio, D.; Rosano, G.; Fini, M.; Di Virgilio, F.; Gulinelli, S.; Falzoni, S.; et al. ATP Secreted by Endothelial Cells Blocks CX3CL1-Elicited Natural Killer Cell Chemotaxis and Cytotoxicity via P2Y11 Receptor Activation. Blood 2010, 116, 4492–4500. [Google Scholar] [CrossRef]
- Woehrle, T.; Yip, L.; Elkhal, A.; Sumi, Y.; Chen, Y.; Yao, Y.; Insel, P.A.; Junger, W.G. Pannexin-1 Hemichannel–Mediated ATP Release Together with P2X1 and P2X4 Receptors Regulate T-Cell Activation at the Immune Synapse. Blood 2010, 116, 3475–3484. [Google Scholar] [CrossRef]
- Kinsey, G.R.; Huang, L.; Jaworska, K.; Khutsishvili, K.; Becker, D.A.; Ye, H.; Lobo, P.I.; Okusa, M.D. Autocrine Adenosine Signaling Promotes Regulatory T Cell–Mediated Renal Protection. J. Am. Soc. Nephrol. 2012, 23, 1528–1537. [Google Scholar] [CrossRef]
- King, A.E.; Ackley, M.A.; Cass, C.E.; Young, J.D.; Baldwin, S.A. Nucleoside Transporters: From Scavengers to Novel Therapeutic Targets. Trends Pharmacol. Sci. 2006, 27, 416–425. [Google Scholar] [CrossRef]
- Bono, M.R.; Fernández, D.; Flores-Santibáñez, F.; Rosemblatt, M.; Sauma, D. CD73 and CD39 Ectonucleotidases in T Cell Differentiation: Beyond Immunosuppression. FEBS Lett. 2015, 589, 3454–3460. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular Function and Molecular Structure of Ecto-Nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Csóka, B.; Németh, Z.H.; Törő, G.; Koscsó, B.; Kókai, E.; Robson, S.C.; Enjyoji, K.; Rolandelli, R.H.; Erdélyi, K.; Pacher, P.; et al. CD39 Improves Survival in Microbial Sepsis by Attenuating Systemic Inflammation. FASEB J. 2015, 29, 25–36. [Google Scholar] [CrossRef]
- Dwyer, K.M.; Deaglio, S.; Gao, W.; Friedman, D.; Strom, T.B.; Robson, S.C. CD39 and Control of Cellular Immune Responses. Purinergic Signal. 2007, 3, 171–180. [Google Scholar] [CrossRef]
- Maliszewski, C.R.; Delespesse, G.J.; Schoenborn, M.A.; Armitage, R.J.; Fanslow, W.C.; Nakajima, T.; Baker, E.; Sutherland, G.R.; Poindexter, K.; Birks, C. The CD39 Lymphoid Cell Activation Antigen. Molecular Cloning and Structural Characterization. J. Immunol. 1994, 153, 3574–3583. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Köhler, D.; Eckle, T.; Kong, T.; Robson, S.C.; Colgan, S.P. Central Role of Sp1-Regulated CD39 in Hypoxia/Ischemia Protection. Blood 2009, 113, 224. [Google Scholar] [CrossRef]
- Thompson, L.F.; Eltzschig, H.K.; Ibla, J.C.; Wiele, C.J.V.D.; Resta, R.; Morote-Garcia, J.C.; Colgan, S.P. Crucial Role for Ecto-5′-Nucleotidase (CD73) in Vascular Leakage during Hypoxia. J. Exp. Med. 2004, 200, 1395. [Google Scholar] [CrossRef]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-Nucleotidase (CD73) Regulation by Hypoxia-Inducible Factor-1 Mediates Permeability Changes in Intestinal Epithelia. J. Clin. Investg. 2002, 110, 993–1002. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Howie, D.; Nolan, K.F.; Agorogiannis, E.I.; Greaves, D.R.; Cobbold, S.P.; Waldmann, H. Generation of Anti-Inflammatory Adenosine Byleukocytes Is Regulated by TGF-β. Eur. J. Immunol. 2011, 41, 2955–2965. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine Generation Catalyzed by CD39 and CD73 Expressed on Regulatory T Cells Mediates Immune Suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Moncrieffe, H.; Nistala, K.; Kamhieh, Y.; Evans, J.; Eddaoudi, A.; Eaton, S.; Wedderburn, L.R. High Expression of the Ectonucleotidase CD39 on T Cells from the Inflamed Site Identifies Two Distinct Populations, One Regulatory and One Memory T Cell Population. J. Immunol. 2010, 185, 134–143. [Google Scholar] [CrossRef]
- Chalmin, F.; Mignot, G.; Bruchard, M.; Chevriaux, A.; Végran, F.; Hichami, A.; Ladoire, S.; Derangère, V.; Vincent, J.; Masson, D.; et al. Stat3 and Gfi-1 Transcription Factors Control Th17 Cell Immunosuppressive Activity via the Regulation of Ectonucleotidase Expression. Immunity 2012, 36, 362–373. [Google Scholar] [CrossRef]
- Lindley, E.R.; Pisoni, R.L. Demonstration of Adenosine Deaminase Activity in Human Fibroblast Lysosomes. Biochem. J. 1993, 290, 457–462. [Google Scholar] [CrossRef]
- Franco, R.; Pacheco, R.; Gatell, J.M.; Gallart, T.; Lluis, C. Enzymatic and Extraenzymatic Role of Adenosine Deaminase 1 in T-Cell-Dendritic Cell Contacts and in Alterations of the Immune Function. Crit. Rev. Immunol. 2007, 27, 459–509. [Google Scholar] [CrossRef]
- Bradford, K.L.; Moretti, F.A.; Carbonaro-Sarracino, D.A.; Gaspar, H.B.; Kohn, D.B. Adenosine Deaminase (ADA)-Deficient Severe Combined Immune Deficiency (SCID): Molecular Pathogenesis and Clinical Manifestations. J. Clin. Immunol. 2017, 37, 626–637. [Google Scholar] [CrossRef]
- Sander, S.; Müller, I.; Garcia-Alai, M.M.; Nicke, A.; Tidow, H. New Insights into P2X7 Receptor Regulation: Ca2+-Calmodulin and GDP Bind to the Soluble P2X7 Ballast Domain. J. Biol. Chem. 2022, 298, 102495. [Google Scholar] [CrossRef]
- Jain, J.; Almquist, S.J.; Ford, P.J.; Shlyakhter, D.; Wang, Y.; Nimmesgern, E.; Germann, U.A. Regulation of Inosine Monophosphate Dehydrogenase Type I and Type II Isoforms in Human Lymphocytes. Biochem. Pharmacol. 2004, 67, 767–776. [Google Scholar] [CrossRef]
- Spector, T.; Jones, T.E.; Miller, R.L. Reaction Mechanism and Specificity of Human GMP Reductase. Substrates, Inhibitors, Activators, and Inactivators. J. Biol. Chem. 1979, 254, 2308–2315. [Google Scholar] [CrossRef]
- Bianchi-Smiraglia, A.; Rana, M.S.; Foley, C.E.; Paul, L.M.; Lipchick, B.C.; Moparthy, S.; Moparthy, K.; Fink, E.E.; Bagati, A.; Hurley, E.; et al. Internally Ratiometric Fluorescent Sensors for Evaluation of Intracellular GTP Levels and Distribution. Nat. Methods 2017, 14, 1003–1009. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J. Role of Rho GTPases in the immune regulation of infection and inflammation. Chin. J. Schistosomiasis Control 2017, 29, 807–813. [Google Scholar] [CrossRef]
- Mzali, R.; Seguin, L.; Liot, C.; Auger, A.; Pacaud, P.; Loirand, G.; Thibault, C.; Pierre, J.; Bertoglio, J. Regulation of Rho Signaling Pathways in Interleukin-2-stimulated Human T-lymphocytes. FASEB J. 2005, 19, 1911–1913. [Google Scholar] [CrossRef]
- Wolff, D.W.; Bianchi-Smiraglia, A.; Nikiforov, M.A. Compartmentalization and Regulation of GTP in Control of Cellular Phenotypes. Trends Mol. Med. 2022, 28, 758–769. [Google Scholar] [CrossRef]
- Young, J.D.; Yao, S.Y.M.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The Human Concentrative and Equilibrative Nucleoside Transporter Families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Abdul Rahman, A. Equilibrative Nucleoside Transporter 2: Properties and Physiological Roles. Biomed. Res. Int. 2020, 2020, 5197626. [Google Scholar] [CrossRef]
- Wei, C.-W.; Lee, C.-Y.; Lee, D.-J.; Chu, C.-F.; Wang, J.-C.; Wang, T.-C.; Jane, W.-N.; Chang, Z.-F.; Leu, C.-M.; Dzhagalov, I.L.; et al. Equilibrative Nucleoside Transporter 3 Regulates T Cell Homeostasis by Coordinating Lysosomal Function with Nucleoside Availability. Cell Rep. 2018, 23, 2330–2341. [Google Scholar] [CrossRef]
- Donaton, M.C.V.; Holsbeeks, I.; Lagatie, O.; Van Zeebroeck, G.; Crauwels, M.; Winderickx, J.; Thevelein, J.M. The Gap1 General Amino Acid Permease Acts as an Amino Acid Sensor for Activation of Protein Kinase A Targets in the Yeast Saccharomyces Cerevisiae. Mol. Microbiol. 2003, 50, 911–929. [Google Scholar] [CrossRef]
- Duflot, S.; Riera, B.; Fernández-Veledo, S.; Casadó, V.; Norman, R.I.; Casado, F.J.; Lluís, C.; Franco, R.; Pastor-Anglada, M. ATP-Sensitive K+ Channels Regulate the Concentrative Adenosine Transporter CNT2 Following Activation by A1 Adenosine Receptors. Mol. Cell. Biol. 2004, 24, 2710–2719. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Pinilla-Macua, I.; Torres, G.; Casado, F.J.; Pastor-Anglada, M. Link between High-Affinity Adenosine Concentrative Nucleoside Transporter-2 (CNT2) and Energy Metabolism in Intestinal and Liver Parenchymal Cells. J. Cell. Physiol. 2010, 225, 620–630. [Google Scholar] [CrossRef]
- Idzko, M.; Hammad, H.; van Nimwegen, M.; Kool, M.; Willart, M.A.M.; Muskens, F.; Hoogsteden, H.C.; Luttmann, W.; Ferrari, D.; Di Virgilio, F.; et al. Extracellular ATP Triggers and Maintains Asthmatic Airway Inflammation by Activating Dendritic Cells. Nat. Med. 2007, 13, 913–919. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides Released by Apoptotic Cells Act as a Find-Me Signal for Phagocytic Clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Homolya, L.; Steinberg, T.H.; Boucher, R.C. Cell to Cell Communication in Response to Mechanical Stress via Bilateral Release of Atp and Utp in Polarized Epithelia. J. Cell Biol. 2000, 150, 1349–1360. [Google Scholar] [CrossRef]
- Hazleton, J.E.; Berman, J.W.; Eugenin, E.A. Purinergic Receptors Are Required for HIV-1 Infection of Primary Human Macrophages. J. Immunol. 2012, 188, 4488–4495. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Kramer, S.B.; Weissmann, G.E.; Hirschhorn, R.O. Adenosine: A Physiological Modulator of Superoxide Anion Generation by Human Neutrophils. J. Exp. Med. 1983, 158, 1160–1177. [Google Scholar] [CrossRef]
- Schrier, D.J.; Imre, K.M. The Effects of Adenosine Agonists on Human Neutrophil Function. J. Immunol. 1986, 137, 3284–3289. [Google Scholar] [CrossRef]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A Rare Functional Haplotype of the P2RX4 and P2RX7 Genes Leads to Loss of Innate Phagocytosis and Confers Increased Risk of Age-Related Macular Degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar] [CrossRef]
- Ohsawa, K.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. Adenosine A3 Receptor Is Involved in ADP-Induced Microglial Process Extension and Migration. J. Neurochem. 2012, 121, 217–227. [Google Scholar] [CrossRef]
- Wilkin, F.; Stordeur, P.; Goldman, M.; Boeynaems, J.-M.; Robaye, B. Extracellular Adenine Nucleotides Modulate Cytokine Production by Human Monocyte-derived Dendritic Cells: Dual Effect on IL-12 and Stimulation of IL-10. Eur. J. Immunol. 2002, 32, 2409–2417. [Google Scholar] [CrossRef]
- La Sala, A.; Ferrari, D.; Corinti, S.; Cavani, A.; Di Virgilio, F.; Girolomoni, G. Extracellular ATP Induces a Distorted Maturation of Dendritic Cells and Inhibits Their Capacity to Initiate Th1 Responses1. J. Immunol. 2001, 166, 1611–1617. [Google Scholar] [CrossRef]
- Panther, E.; Corinti, S.; Idzko, M.; Herouy, Y.; Napp, M.; La Sala, A.; Girolomoni, G.; Norgauer, J. Adenosine Affects Expression of Membrane Molecules, Cytokine and Chemokine Release, and the T-Cell Stimulatory Capacity of Human Dendritic Cells. Blood 2003, 101, 3985–3990. [Google Scholar] [CrossRef]
- Wilson, J.M.; Kurtz, C.C.; Black, S.G.; Ross, W.G.; Alam, M.S.; Linden, J.; Ernst, P.B. The A2B Adenosine Receptor Promotes Th17 Differentiation via Stimulation of Dendritic Cell IL-6. J. Immunol. 2011, 186, 6746–6752. [Google Scholar] [CrossRef]
- Wolberg, G.; Zimmerman, T.P.; Hiemstra, K.; Winston, M.; Chu, L.C. Adenosine Inhibition of Lymphocyte-Mediated Cytolysis: Possible Role of Cyclic Adenosine Monophosphate. Science 1975, 187, 957–959. [Google Scholar] [CrossRef]
- Barankiewicz, J.; Dosch, H.M.; Cohen, A. Extracellular Nucleotide Catabolism in Human B and T Lymphocytes. The Source of Adenosine Production. J. Biol. Chem. 1988, 263, 7094–7098. [Google Scholar] [CrossRef]
- Dwyer, K.M.; Hanidziar, D.; Putheti, P.; Hill, P.A.; Pommey, S.; McRae, J.L.; Winterhalter, A.; Doherty, G.; Deaglio, S.; Koulmanda, M.; et al. Expression of CD39 by Human Peripheral Blood CD4+CD25+ T Cells Denotes a Regulatory Memory Phenotype. Am. J. Transpl. 2010, 10, 2410–2420. [Google Scholar] [CrossRef]
- Doherty, G.A.; Bai, A.; Hanidziar, D.; Longhi, M.S.; Lawlor, G.O.; Putheti, P.; Csizmadia, E.; Nowak, M.; Cheifetz, A.S.; Moss, A.C.; et al. CD73 Is a Phenotypic Marker of Effector Memory Th17 Cells in Inflammatory Bowel Disease. Eur. J. Immunol. 2012, 42, 3062–3072. [Google Scholar] [CrossRef]
- Mandapathil, M.; Hilldorfer, B.; Szczepanski, M.J.; Czystowska, M.; Szajnik, M.; Ren, J.; Lang, S.; Jackson, E.K.; Gorelik, E.; Whiteside, T.L. Generation and Accumulation of Immunosuppressive Adenosine by Human CD4+CD25highFOXP3+ Regulatory T Cells. J. Biol. Chem. 2010, 285, 7176–7186. [Google Scholar] [CrossRef]
- Aswad, F.; Kawamura, H.; Dennert, G. High Sensitivity of CD4+CD25+ Regulatory T Cells to Extracellular Metabolites Nicotinamide Adenine Dinucleotide and ATP: A Role for P2X7 Receptors. J. Immunol. 2005, 175, 3075–3083. [Google Scholar] [CrossRef]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Westendorf, A.M.; Grassi, F. ATP Inhibits the Generation and Function of Regulatory T Cells Through the Activation of Purinergic P2X Receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef]
- Mirabet, M.; Herrera, C.; Cordero, O.J.; Mallol, J.; Lluis, C.; Franco, R. Expression of A2B Adenosine Receptors in Human Lymphocytes: Their Role in T Cell Activation. J. Cell Sci. 1999, 112 Pt 4, 491–502. [Google Scholar] [CrossRef]
- Koshiba, M.; Rosin, D.L.; Hayashi, N.; Linden, J.; Sitkovsky, M.V. Patterns of A2A Extracellular Adenosine Receptor Expression in Different Functional Subsets of Human Peripheral T Cells. Flow Cytometry Studies with Anti-A2A Receptor Monoclonal Antibodies. Mol. Pharmacol. 1999, 55, 614–624. [Google Scholar]
- Koshiba, M.; Rosin, D.L.; Hayashi, N.; Linden, J.; Sitkovsky, M.V. Extracellular ATP Exerts Opposite Effects on Activated and Regulatory CD4+ T Cells via Purinergic P2 Receptor Activation. The J. Immunol. 2012, 189, 1303–1310. [Google Scholar]
- Duhant, X.; Schandené, L.; Bruyns, C.; Gonzalez, N.S.; Goldman, M.; Boeynaems, J.-M.; Communi, D. Extracellular Adenine Nucleotides Inhibit the Activation of Human CD4+ T Lymphocytes1. J. Immunol. 2002, 169, 15–21. [Google Scholar] [CrossRef]
- Priebe, T.; Platsoucas, C.D.; Nelson, J.A. Adenosine Receptors and Modulation of Natural Killer Cell Activity by Purine Nucleosides. Cancer Res. 1990, 50, 4328–4331. [Google Scholar] [PubMed]
- Kawamura, H.; Aswad, F.; Minagawa, M.; Govindarajan, S.; Dennert, G. P2X7 Receptors Regulate NKT Cells in Autoimmune Hepatitis1. The J. Immunol. 2006, 176, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Bassler, K.; Schulte-Schrepping, J.; Warnat-Herresthal, S.; Aschenbrenner, A.C.; Schultze, J.L. The Myeloid Cell Compartment-Cell by Cell. Annu. Rev. Immunol. 2019, 37, 269–293. [Google Scholar] [CrossRef]
- Ruterbusch, M.; Pruner, K.B.; Shehata, L.; Pepper, M. In Vivo CD4+ T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu. Rev. Immunol. 2020, 38, 705–725. [Google Scholar] [CrossRef]
- Watson, M.L. The nuclear envelope. J. Biophys. Biochem. Cytol. 1955, 1, 257–270. [Google Scholar] [CrossRef]
- Malhas, A.; Goulbourne, C.; Vaux, D.J. The Nucleoplasmic Reticulum: Form and Function. Trends Cell Biol. 2011, 21, 362–373. [Google Scholar] [CrossRef]
- Prokocimer, M.; Davidovich, M.; Nissim-Rafinia, M.; Wiesel-Motiuk, N.; Bar, D.Z.; Barkan, R.; Meshorer, E.; Gruenbaum, Y. Nuclear Lamins: Key Regulators of Nuclear Structure and Activities. J. Cell. Mol. Med. 2009, 13, 1059–1085. [Google Scholar] [CrossRef]
- Patil, S.; Sengupta, K. Role of A- and B-type Lamins in Nuclear Structure–Function Relationships. Biol. Cell 2021, 113, 295–310. [Google Scholar] [CrossRef]
- Hampoelz, B.; Andres-Pons, A.; Kastritis, P.; Beck, M. Structure and Assembly of the Nuclear Pore Complex. Annu. Rev. Biophys. 2019, 48, 515–536. [Google Scholar] [CrossRef]
- van Zon, A.; Mossink, M.H.; Scheper, R.J.; Sonneveld, P.; Wiemer, E.A.C. The Vault Complex. Cell. Mol. Life Sci. 2003, 60, 1828–1837. [Google Scholar] [CrossRef]
- Chugani, D.C.; Rome, L.H.; Kedersha, N.L. Evidence That Vault Ribonucleoprotein Particles Localize to the Nuclear Pore Complex. J. Cell Sci. 1993, 106 Pt1, 20–29. [Google Scholar] [CrossRef]
- Chung, J.-H.; Eng, C. Nuclear-Cytoplasmic Partitioning of Phosphatase and Tensin Homologue Deleted on Chromosome 10 (PTEN) Differentially Regulates the Cell Cycle and Apoptosis. Cancer Res. 2005, 65, 8096–8100. [Google Scholar] [CrossRef]
- Abbondanza, C.; Rossi, V.; Roscigno, A.; Gallo, L.; Belsito, A.; Piluso, G.; Medici, N.; Nigro, V.; Molinari, A.M.; Moncharmont, B.; et al. Interaction of Vault Particles with Estrogen Receptor in the MCF-7 Breast Cancer Cell. J. Cell Biol. 1998, 141, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Kitazono, M.; Okumura, H.; Ikeda, R.; Sumizawa, T.; Furukawa, T.; Nagayama, S.; Seto, K.; Aikou, T.; Akiyama, S. Reversal of LRP-Associated Drug Resistance in Colon Carcinoma Sw-620 Cells. Int. J. Cancer 2001, 91, 126–131. [Google Scholar] [CrossRef]
- Berger, W.; Spiegl-Kreinecker, S.; Buchroithner, J.; Elbling, L.; Pirker, C.; Fischer, J.; Micksche, M. Overexpression of the Human Major Vault Protein in Astrocytic Brain Tumor Cells. Int. J. Cancer 2001, 94, 377–382. [Google Scholar] [CrossRef]
- Mrázek, J.; Kreutmayer, S.B.; Grässer, F.A.; Polacek, N.; Hüttenhofer, A. Subtractive Hybridization Identifies Novel Differentially Expressed ncRNA Species in EBV-Infected Human B Cells. Nucleic Acids Res. 2007, 35, e37. [Google Scholar] [CrossRef]
- Kowalski, M.P.; Dubouix-Bourandy, A.; Bajmoczi, M.; Golan, D.E.; Zaidi, T.; Coutinho-Sledge, Y.S.; Gygi, M.P.; Gygi, S.P.; Wiemer, E.A.C.; Pier, G.B. Host Resistance to Lung Infection Mediated by Major Vault Protein in Epithelial Cells. Science 2007, 317, 130–132. [Google Scholar] [CrossRef]
- Berger, W.; Steiner, E.; Grusch, M.; Elbling, L.; Micksche, M. Vaults and the Major Vault Protein: Novel Roles in Signal Pathway Regulation and Immunity. Cell. Mol. Life Sci. 2008, 66, 43. [Google Scholar] [CrossRef]
- Gieseler, R.K.; Xu, H.; Schlemminger, R.; Peters, J.H. Serum-Free Differentiation of Rat and Human Dendritic Cells, Accompanied by Acquisition of the Nuclear Lamins A/C as Differentiation Markers. Adv. Exp. Med. Biol. 1993, 329, 287–291. [Google Scholar] [CrossRef]
- González-Granado, J.M.; Silvestre-Roig, C.; Rocha-Perugini, V.; Trigueros-Motos, L.; Cibrián, D.; Morlino, G.; Blanco-Berrocal, M.; Osorio, F.G.; Freije, J.M.P.; López-Otín, C.; et al. Nuclear Envelope Lamin-A Couples Actin Dynamics with Immunological Synapse Architecture and T Cell Activation. Sci. Signal. 2014, 7, ra37. [Google Scholar] [CrossRef]
- Olins, A.L.; Zwerger, M.; Herrmann, H.; Zentgraf, H.; Simon, A.J.; Monestier, M.; Olins, D.E. The Human Granulocyte Nucleus: Unusual Nuclear Envelope and Heterochromatin Composition. Eur. J. Cell Biol. 2008, 87, 279–290. [Google Scholar] [CrossRef]
- Manley, H.R.; Keightley, M.C.; Lieschke, G.J. The Neutrophil Nucleus: An Important Influence on Neutrophil Migration and Function. Front. Immunol. 2018, 9, 2867. [Google Scholar] [CrossRef]
- Raab, M.; Gentili, M.; de Belly, H.; Thiam, H.-R.; Vargas, P.; Jimenez, A.J.; Lautenschlaeger, F.; Voituriez, R.; Lennon-Duménil, A.-M.; Manel, N.; et al. ESCRT III Repairs Nuclear Envelope Ruptures During Cell Migration to Limit DNA Damage and Cell Death. Science 2016, 352, 359–362. [Google Scholar] [CrossRef]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.-J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 696. [Google Scholar] [CrossRef]
- Bottazzi, B.; Nobili, N.; Mantovani, A. Expression of C-Fos Proto-Oncogene in Tumor-Associated Macrophages. J. Immunol. 1990, 144, 4878–4882. [Google Scholar] [CrossRef]
- Niedergang, F. Dendritic Cells Mature to Resist Lamin Degradation and Herpes Virus Release. J. Cell Biol. 2019, 218, 387–388. [Google Scholar] [CrossRef]
- Faria, A.M.C.; Levay, A.; Wang, Y.; Kamphorst, A.O.; Rosa, M.L.P.; Nussenzveig, D.R.; Balkan, W.; Chook, Y.M.; Levy, D.E.; Fontoura, B.M.A. The Nucleoporin Nup96 Is Required for Proper Expression of Interferon-Regulated Proteins and Functions. Immunity 2006, 24, 295–304. [Google Scholar] [CrossRef]
- Xylourgidis, N.; Roth, P.; Sabri, N.; Tsarouhas, V.; Samakovlis, C. The Nucleoporin Nup214 Sequesters CRM1 at the Nuclear Rim and Modulates NFκB Activation in Drosophila. J. Cell Sci. 2006, 119, 4409–4419. [Google Scholar] [CrossRef]
- Olins, A.L.; Hoang, T.V.; Zwerger, M.; Herrmann, H.; Zentgraf, H.; Noegel, A.A.; Karakesisoglou, I.; Hodzic, D.; Olins, D.E. The LINC-Less Granulocyte Nucleus. Eur. J. Cell Biol. 2009, 88, 203–214. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Li, B.; Jie, W.; Huang, L.; Wei, P.; Li, S.; Luo, Z.; Friedman, A.K.; Meredith, A.L.; Han, M.-H.; Zhu, X.-H.; et al. Nuclear BK Channels Regulate Gene Expression via the Control of Nuclear Calcium Signaling. Nat. Neurosci. 2014, 17, 1055–1063. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Cao, T.; Zhang, L.; Ma, Y.; Jiang, S.; Teng, X.; Sun, X. Large-Conductance Calcium-Activated Potassium Channels Mediate Lipopolysaccharide-Induced Activation of Murine Microglia. J. Biol. Chem. 2019, 294, 12921–12932. [Google Scholar] [CrossRef]
- Rønningen, T.; Shah, A.; Oldenburg, A.R.; Vekterud, K.; Delbarre, E.; Moskaug, J.Ø.; Collas, P. Prepatterning of Differentiation-Driven Nuclear Lamin A/C-Associated Chromatin Domains by GlcNAcylated Histone H2B. Genome Res. 2015, 25, 1825–1835. [Google Scholar] [CrossRef]
- Toribio-Fernández, R.; Zorita, V.; Rocha-Perugini, V.; Iborra, S.; Martínez del Hoyo, G.; Chevre, R.; Dorado, B.; Sancho, D.; Sanchez-Madrid, F.; Andrés, V.; et al. Lamin A/C Augments Th1 Differentiation and Response against Vaccinia Virus and Leishmania Major. Cell. Death Dis. 2018, 9, 9. [Google Scholar] [CrossRef]
- Toribio-Fernández, R.; Herrero-Fernandez, B.; Zorita, V.; López, J.A.; Vázquez, J.; Criado, G.; Pablos, J.L.; Collas, P.; Sánchez-Madrid, F.; Andrés, V.; et al. Lamin A/C Deficiency in CD4+ T-cells Enhances Regulatory T-cells and Prevents Inflammatory Bowel Disease. J. Pathol. 2019, 249, 509–522. [Google Scholar] [CrossRef]
- Bauer, B.; Baier, G. Protein Kinase C and AKT/Protein Kinase B in CD4+ T-Lymphocytes: New Partners in TCR/CD28 Signal Integration. Mol. Immunol. 2002, 38, 1087–1099. [Google Scholar] [CrossRef]
- Zuo, B.; Yang, J.; Wang, F.; Wang, L.; Yin, Y.; Dan, J.; Liu, N.; Liu, L. Influences of Lamin A Levels on Induction of Pluripotent Stem Cells. Biol. Open 2012, 1, 1118–1127. [Google Scholar] [CrossRef]
- Pino-Lagos, K.; Benson, M.J.; Noelle, R.J. Retinoic Acid in the Immune System. Ann. N. Y. Acad. Sci. 2008, 1143, annals.1443.017. [Google Scholar] [CrossRef]
- Donahue, D.A.; Porrot, F.; Couespel, N.; Schwartz, O. SUN2 Silencing Impairs CD4 T Cell Proliferation and Alters Sensitivity to HIV-1 Infection Independently of Cyclophilin A. J. Virol. 2017, 91, e02303-16. [Google Scholar] [CrossRef]
- Franco-Obregón, A.; Wang, H.W.; Clapham, D.E. Distinct Ion Channel Classes Are Expressed on the Outer Nuclear Envelope of T- and B-Lymphocyte Cell Lines. Biophys. J. 2000, 79, 202–214. [Google Scholar] [CrossRef]
- Fedorenko, O.A.; Volkova, T.M.; Marchenko, S.M. New cation channel of the T-lymphocyte nuclear membrane. Fiziol. Zh. 2006, 52, 17–21. [Google Scholar]
- Shan, S. ATPase and GTPase Tangos Drive Intracellular Protein Transport. Trends. Biochem. Sci. 2016, 41, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Sepehrimanesh, M. Nucleocytoplasmic Transport: Regulatory Mechanisms and the Implications in Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4165. [Google Scholar] [CrossRef]
- Binger, K.J.; Linker, R.A.; Muller, D.N.; Kleinewietfeld, M. Sodium Chloride, SGK1, and Th17 Activation. Pflugers Arch. Eur. J. Physiol. 2015, 467, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; Dattilo, V.; D’Antona, L.; Menniti, M.; Bianco, C.; Ortuso, F.; Alcaro, S.; Schenone, S.; Perrotti, N.; Amato, R. SGK1, the New Player in the Game of Resistance: Chemo-Radio Molecular Target and Strategy for Inhibition. Cell. Physiol. Biochem. 2016, 39, 1863–1876. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Catalogna, G.; Gallo, E.; di Martino, S.; Mileo, A.M.; Carosi, M.; Dattilo, V.; Schenone, S.; Musumeci, F.; Lavia, P.; et al. The Small Molecule SI113 Synergizes with Mitotic Spindle Poisons in Arresting the Growth of Human Glioblastoma Multiforme. Oncotarget 2017, 8, 110743–110755. [Google Scholar] [CrossRef][Green Version]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent Insights of T Cell Receptor-Mediated Signaling Pathways for T Cell Activation and Development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Kouzine, F.; Wojtowicz, D.; Yamane, A.; Resch, W.; Kieffer-Kwon, K.-R.; Bandle, R.; Nelson, S.; Nakahashi, H.; Awasthi, P.; Feigenbaum, L.; et al. Global Regulation of Promoter Melting in Naïve Lymphocytes. Cell 2013, 153, 988–999. [Google Scholar] [CrossRef]
- Kieffer-Kwon, K.-R.; Nimura, K.; Rao, S.S.P.; Xu, J.; Jung, S.; Pekowska, A.; Dose, M.; Stevens, E.; Mathe, E.; Dong, P.; et al. Myc Regulates Chromatin Decompaction and Nuclear Architecture during B Cell Activation. Mol. Cell 2017, 67, 566–578.e10. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type-I Interferon Pathway. Science 2013, 339, 1232458. [Google Scholar] [CrossRef]
- Song, J.-X.; Villagomes, D.; Zhao, H.; Zhu, M. cGAS in Nucleus: The Link Between Immune Response and DNA Damage Repair. Front. Immunol. 2022, 13, 1076784. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, F. cGAS–STING Signaling and Function in Metabolism and Kidney Diseases. J. Mol. Cell Biol. 2021, 13, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L. Metabolism in T Cell Activation and Differentiation. Curr. Opin. Immunol. 2010, 22, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; McGuire, P.J.; Jones, R.G.; Sparwasser, T.; Berod, L. Fatty Acid Metabolism in CD8+ T Cell Memory: Challenging Current Concepts. Immunol. Rev. 2018, 283, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Menk, A.V.; Scharping, N.E.; Moreci, R.S.; Zeng, X.; Guy, C.; Salvatore, S.; Bae, H.; Xie, J.; Young, H.A.; Wendell, S.G.; et al. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep. 2018, 22, 1509–1521. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Ma, S.; Kaech, S.M. T Cell Metabolism in a State of Flux. Immunity 2019, 51, 783–785. [Google Scholar] [CrossRef]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine Uptake and Metabolism Are Coordinately Regulated by ERK/MAPK During T Lymphocyte Activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling and Neurological Diseases: An Update. CNS Neurol. Disord. Drug Targets 2017, 16, 257–265. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- Burnstock, G.; Jacobson, K.A.; Christofi, F.L. Purinergic Drug Targets for Gastrointestinal Disorders. Curr. Opin. Pharmacol. 2017, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Abohalaka, R. Bronchial Epithelial and Airway Smooth Muscle Cell Interactions in Health and Disease. Heliyon 2023, 9, e19976. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T. Gout. Lancet 2010, 375, 318–328. [Google Scholar] [CrossRef]
- Kau, T.R.; Way, J.C.; Silver, P.A. Nuclear Transport and Cancer: From Mechanism to Intervention. Nat. Rev. Cancer 2004, 4, 106–117. [Google Scholar] [CrossRef]
- Martin, A.J.; Jans, D.A. Antivirals that Target the Host IMPα/Β1-Virus Interface. Biochem. Soc. Trans. 2021, 49, 281–295. [Google Scholar] [CrossRef]
- Uddin, M.H.; Zonder, J.A.; Azmi, A.S. Exportin 1 Inhibition as Antiviral Therapy. Drug Discov. Today 2020, 25, 1775. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The Sympathetic Nerve—An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar]
- Haskó, G.; Szabó, C. Regulation of Cytokine and Chemokine Production by Transmitters and Co-Transmitters of the Autonomic Nervous System. Biochem. Pharmacol. 1998, 56, 1079–1087. [Google Scholar] [CrossRef]
- Suzuki, R.; Furuno, T.; Okamoto, K.; Teshima, R.; Nakanishi, M. ATP Plays a Role in Neurite Stimulation with Activated Mast Cells. J. Neuroimmunol. 2007, 192, 49–56. [Google Scholar] [CrossRef]
- Arizono, N.; Matsuda, S.; Hattori, T.; Kojima, Y.; Maeda, T.; Galli, S.J. Anatomical Variation in Mast Cell Nerve Associations in the Rat Small Intestine, Heart, Lung, and Skin. Similarities of Distances between Neural Processes and Mast Cells, Eosinophils, or Plasma Cells in the Jejunal Lamina Propria. Lab. Investg. 1990, 62, 626–634. [Google Scholar]
- Chelmicka-Schorr, E.; Kwasniewski, M.N.; Czlonkowska, A. Sympathetic Nervous System Modulates Macrophage Function. Int. J. Immunopharmacol. 1992, 14, 841–846. [Google Scholar] [CrossRef]
- Madden, K.S.; Moynihan, J.A.; Brenner, G.J.; Felten, S.Y.; Felten, D.L.; Livnat, S. Sympathetic Nervous System Modulation of the Immune System. III. Alterations in T and B Cell Proliferation and Differentiation in Vitro Following Chemical Sympathectomy. J. Neuroimmunol. 1994, 49, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Jinnah, H.A.; Sabina, R.L.; Van Den Berghe, G. Chapter 187—Metabolic Disorders of Purine Metabolism Affecting the Nervous System. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Pediatric Neurology Part III; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1827–1836. [Google Scholar]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV Infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef]
- Tabucchi, A.; Carlucci, F.; Re, M.C.; Furlini, G.; Consolmagno, E.; Leoncini, R.; Pizzichini, M.; Marinello, E.; Rubino, M.; Pagani, R. The Behavior of Free Purine Nucleotides in Lymphocytes Infected with HIV-1 Virus. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 1993, 1182, 317–322. [Google Scholar] [CrossRef]
- Ratner, L.; Fisher, A.; Jagodzinski, L.L.; Mitsuya, H.; Liou, R.S.; Gallo, R.C.; Wong-Staal, F. Complete Nucleotide Sequences of Functional Clones of the AIDS Virus. AIDS Res. Hum. Retrovir. 1987, 3, 57–67. [Google Scholar] [CrossRef]
- Engelman, A.N. HIV Capsid and Integration Targeting. Viruses 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Yu, H.J.; Buffone, C.; Huang, S.-W.; Lee, K.; Goh, S.L.; Gres, A.T.; Guney, M.H.; Sarafianos, S.G.; Luban, J.; et al. The HIV-1 Capsid Core Is an Opportunistic Nuclear Import Receptor. Nat. Commun. 2023, 14, 3782. [Google Scholar] [CrossRef]
- Fassati, A.; Görlich, D.; Harrison, I.; Zaytseva, L.; Mingot, J.-M. Nuclear Import of HIV-1 Intracellular Reverse Transcription Complexes Is Mediated by Importin 7. EMBO J. 2003, 22, 3675–3685. [Google Scholar] [CrossRef]
- Askjaer, P.; Jensen, T.H.; Nilsson, J.; Englmeier, L.; Kjems, J. The Specificity of the CRM1-Rev Nuclear Export Signal Interaction Is Mediated by RanGTP. J. Biol. Chem. 1998, 273, 33414–33422. [Google Scholar] [CrossRef]
- Floer, M.; Blobel, G. Putative Reaction Intermediates in Crm1-Mediated Nuclear Protein Export*. J. Biol. Chem. 1999, 274, 16279–16286. [Google Scholar] [CrossRef] [PubMed]
- Zolotukhin, A.S.; Felber, B.K. Mutations in the Nuclear Export Signal of Human Ran-Binding Protein RanBP1 Block the Rev-Mediated Posttranscriptional Regulation of Human Immunodeficiency Virus Type 1. J. Biol. Chem. 1997, 272, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Zayas, J.P.; Mamede, J.I. HIV Infection and Spread between Th17 Cells. Viruses 2022, 14, 404. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Wang, Z.; Li, J.; Yuan, Y.; Li, T.; Zuo, M.; Feng, W.; Li, W.; Chen, M.; et al. Purine Metabolism-Related Gene Expression Signature Predicts Survival Outcome and Indicates Immune Microenvironment Profile of Gliomas. Front. Pharmacol. 2022, 13, 1038272. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef]
- Bastid, J.; Cottalorda-Regairaz, A.; Alberici, G.; Bonnefoy, N.; Eliaou, J.-F.; Bensussan, A. ENTPD1/CD39 Is a Promising Therapeutic Target in Oncology. Oncogene 2013, 32, 1743–1751. [Google Scholar] [CrossRef]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Anti-Tumor T Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef]
- Ohta, A.; Ohta, A.; Madasu, M.; Kini, R.; Subramanian, M.; Goel, N.; Sitkovsky, M. A2A Adenosine Receptor May Allow Expansion of T Cells Lacking Effector Functions in Extracellular Adenosine-Rich Microenvironments. J. Immunol. 2009, 183, 5487–5493. [Google Scholar] [CrossRef]
- Zarek, P.E.; Huang, C.-T.; Lutz, E.R.; Kowalski, J.; Horton, M.R.; Linden, J.; Drake, C.G.; Powell, J.D. A2A Receptor Signaling Promotes Peripheral Tolerance by Inducing T-Cell Anergy and the Generation of Adaptive Regulatory T Cells. Blood 2008, 111, 25–259. [Google Scholar] [CrossRef]
- Wilson, J.M.; Ross, W.G.; Agbai, O.N.; Frazier, R.; Figler, R.A.; Rieger, J.; Linden, J.; Ernst, P.B. The A2B Adenosine Receptor Impairs the Maturation and Immunogenicity of Dendritic Cells. J. Immunol. 2009, 182, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Selmeczy, Z.; Koscsó, B.; Németh, Z.H.; Pacher, P.; Murray, P.J.; Kepka-Lenhart, D.; Morris, S.M.; Gause, W.C.; Leibovich, S.J.; et al. Adenosine Promotes Alternative Macrophage Activation via A2A and A2B Receptors. FASEB J. 2012, 26, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Mahipal, A.; Malafa, M. Importins and Exportins as Therapeutic Targets in Cancer. Pharmacol. Ther. 2016, 164, 135–143. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Chen, L.; Gong, B.; Jia, D.; Sun, Q. Nuclear Transport Proteins: Structure, Function and Disease Relevance. Signal. Transduct. Target Ther. 2023, 8, 425. [Google Scholar] [CrossRef]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The Nuclear Export Protein XPO1—From Biology to Targeted Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Cagatay, T.; Chook, Y.M. Karyopherins in Cancer. Curr. Opin. Cell Biol. 2018, 52, 30–42. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Clark, P.E.; Rayman, P.; Bloom, T.; Rybicki, L.; Novick, A.C.; Bukowski, R.M.; Finke, J.H. Alterations in NFκB Activation in T Lymphocytes of Patients with Renal Cell Carcinoma. J. Natl. Cancer Inst. 1999, 91, 718–721. [Google Scholar] [CrossRef]
- Finke, J.H.; Rayman, P.; Hart, L.; Alexander, J.P.; Edinger, M.G.; Tubbs, R.R.; Klein, E.; Tuason, L.; Bukowski, R.M. Characterization of Tumor-Infiltrating Lymphocyte Subsets from Human Renal Cell Carcinoma: Specific Reactivity Defined by Cytotoxicity, Interferon-Gamma Secretion, and Proliferation. J. Immunother. Emphas. Tumor. Immunol. 1994, 15, 91–104. [Google Scholar] [CrossRef]
- Alexander, J.P.; Kudoh, S.; Melsop, K.A.; Hamilton, T.A.; Edinger, M.G.; Tubbs, R.R.; Sica, D.; Tuason, L.; Klein, E.; Bukowski, R.M. T-Cells Infiltrating Renal Cell Carcinoma Display a Poor Proliferative Response Even Though They Can Produce Interleukin 2 and Express Interleukin 2 Receptors. Cancer Res. 1993, 53, 1380–1387. [Google Scholar]
- Rosenberg, S.A. A New Era for Cancer Immunotherapy Based on the Genes That Encode Cancer Antigens. Immunity 1999, 10, 281–287. [Google Scholar] [CrossRef]
- Weis, K. Regulating Access to the Genome: Nucleocytoplasmic Transport throughout the Cell Cycle. Cell 2003, 112, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Flinn, A.M.; Gennery, A.R. Adenosine Deaminase Deficiency: A Review. Orphanet. J. Rare Dis. 2018, 13, 65. [Google Scholar] [CrossRef]
- Bobby Gaspar, H. Bone Marrow Transplantation and Alternatives for Adenosine Deaminase DeficiencyImmunol. Allergy Clin. N. Am. 2010, 30, 221–236. [Google Scholar]
- Sauer, A.V.; Brigida, I.; Carriglio, N.; Aiuti, A. Autoimmune Dysregulation and Purine Metabolism in Adenosine Deaminase Deficiency. Front. Immunol. 2012, 3, 265. [Google Scholar] [CrossRef]
- Hirschhorn, R.; Candotti, F. Immunodeficiency Due to Defects of Purine Metabolism. In Primary Immunodeficiency Diseases; A Molecular And Genetic Approach; Oxford Academic: Oxford, UK, 2006; Available online: https://academic.oup.com/book/50949/chapter-abstract/421420621?redirectedFrom=fulltext (accessed on 3 October 2024).
- Hirschhorn, R.; Nicknam, M.N.; Eng, F.; Yang, D.R.; Borkowsky, W. Novel Deletion and a New Missense Mutation (Glu 217 Lys) at the Catalytic Site in Two Adenosine Deaminase Alleles of a Patient with Neonatal Onset Adenosine Deaminase- Severe Combined Immunodeficiency. J. Immunol. 1992, 149, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Apasov, S.G.; Blackburn, M.R.; Kellems, R.E.; Smith, P.T.; Sitkovsky, M.V. Adenosine Deaminase Deficiency Increases Thymic Apoptosis and Causes Defective T Cell Receptor Signaling. J. Clin. Investig. 2001, 108, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Apasov, S.; Koshiba, M.; Sitkovsky, M. Role of A2a Extracellular Adenosine Receptor-Mediated Signaling in Adenosine-Mediated Inhibition of T-Cell Activation and Expansion. Blood 1997, 90, 1600–1610. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Dunwiddie, T.V. How Does Adenosine Inhibit Transmitter Release? Trends Pharmacol. Sci. 1988, 9, 130–134. [Google Scholar] [CrossRef]
- Etzioni, A. Immune Deficiency and Autoimmunity. Autoimmun. Rev. 2003, 2, 364–369. [Google Scholar] [CrossRef]
- Ozsahin, H.; Arredondo-Vega, F.X.; Santisteban, I.; Fuhrer, H.; Tuchschmid, P.; Jochum, W.; Aguzzi, A.; Lederman, H.M.; Fleischman, A.; Winkelstein, J.A.; et al. Adenosine Deaminase Deficiency in Adults. Blood 1997, 89, 2849–2855. [Google Scholar] [CrossRef]
- Sauer, A.V.; Brigida, I.; Carriglio, N.; Jofra Hernandez, R.; Scaramuzza, S.; Clavenna, D.; Sanvito, F.; Poliani, P.L.; Gagliani, N.; Carlucci, F.; et al. Alterations in the Adenosine Metabolism and CD39/CD73 Adenosinergic Machinery Cause Loss of Treg Cell Function and Autoimmunity in ADA-Deficient SCID. Blood 2012, 119, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism—Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Perrot, I.; Michaud, H.-A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Déjou, C.; Jecko, D.; Becquart, O.; et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019, 27, 2411–2425.e9. [Google Scholar] [CrossRef]
- Hay, C.M.; Sult, E.; Huang, Q.; Mulgrew, K.; Fuhrmann, S.R.; McGlinchey, K.A.; Hammond, S.A.; Rothstein, R.; Rios-Doria, J.; Poon, E.; et al. Targeting CD73 in the Tumor Microenvironment with MEDI9447. Oncoimmunology 2016, 5, e1208875. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Teng, Y.; Yang, B. Increased Nuclear Transporter Importin 7 Contributes to the Tumor Growth and Correlates With CD8 T Cell Infiltration in Cervical Cancer. Front. Cell Dev. Biol. 2021, 9, 732786. [Google Scholar] [CrossRef]
- Azuma, K.; Sasada, T.; Takedatsu, H.; Shomura, H.; Koga, M.; Maeda, Y.; Yao, A.; Hirai, T.; Takabayashi, A.; Shichijo, S.; et al. Ran, a Small GTPase Gene, Encodes Cytotoxic T Lymphocyte (CTL) Epitopes Capable of Inducing HLA-A33–Restricted and Tumor-Reactive CTLs in Cancer Patients. Clin. Cancer Res. 2004, 10, 6695–6702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).