Abstract
NK cells have traditionally been classified as effectors of innate immunity, even though they also exhibit some features of adaptive immunity such as memory. NK cells contribute to the lysis and growth inhibition of cancer, mediating direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) and regulating the functions of other immune cells, respectively. NK cells regulate the function of other immune cells via the release of inflammatory cytokines and chemokines. Currently, NK cell therapeutics in oral cancer have been less efficient due to several limitations, as follows: (a) lower percentages of NK cells in peripheral blood immune cells; (b) limited survival and decreased function of NK cells, especially in the tumor microenvironment; and (c) a lack of tools or methodologies to expand and activate NK cells to the levels that are required for the effective targeting of oral cancer. To overcome these limitations, we established and demonstrated a novel technology for activating and expanding highly functional NK cells coined as supercharged NK (sNK) cells. This review summarizes the characteristics of sNK cells and highlights their superior anti-cancer activity when compared to primary activated NK cells.
1. Introduction and Background: Oral Cancer and Natural Killer Cells
Oral cancer, which represents approximately three percent of all cancers diagnosed or about 54,000 new cases annually in the United States, is the most prevalent subset of head and neck cancer; it has a 50% overall survival rate at 5 years and there are no effective therapeutic modalities [1,2,3,4,5,6,7]. Oral cancer progression consists of a series of histopathological changes, including hyperplasia, dysplasia, carcinoma in situ, and, lastly, oral cancer [8]. Oral cancer has an epithelial origin and is found to be associated with several etiological factors, including genetic, epigenetic, microbial, habitual, geographical location, or ethical groups [9,10]. Many factors contribute to the onset of oral cancer, including low maintenance of oral hygiene, oral ulcers, human papillomavirus (HPV), mucosal leukoplakia, alcoholism, and the long-term use of tobacco [11,12,13]. Oral cancers are more prevalent in men due to their increased use of tobacco and alcohol [14]. There are very few treatment options for stage IV oral cancer, especially those associated with metastasis [15]. Also, oral cancer stem-like cells (CSCs) have downmodulated MHC-class I and are difficult to target with chemotherapeutic, radiotherapeutic, and T cell-based immunotherapeutic strategies; these CSCs also correlate negatively with oral cancer inhibition and therapeutic response [16,17]. Therefore, there is a need for effective treatment. In this regard, NK cells are known to target these aggressive tumors and can be used in patients to eliminate these tumors. This is a clear advantage of this therapy for hard-to-cure oral tumors. In T cell therapies, a graft vs. host disease can complicate the therapy. Fortunately, such a complication does not exist with NK cells, since they do not mediate graft vs. host disease [18,19].
Natural killer (NK) cells were found to play a crucial role in cancer inhibition due to their effector functions, including direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC), as well as by regulating or activating the anti-cancer function of other immune effectors via NK cell-secreted inflammatory cytokines and chemokines [20,21,22]. NK cells induce greater lysis in CSCs expressing a low surface expression of MHC-class I, CD54, and PD-L1 compared to differentiation tumors with higher surface expression levels of MHC-class I, CD54, and PD-L1 [23,24]. Several in vitro studies have classified oral tumor cell lines as stem-like and differentiated tumors. Among these cell lines, we have extensively studied OSCSCs (oral squamous carcinoma stem-like cells) and OSCCs (oral squamous cell carcinomas) [25,26]. Stem-like OSCSC oral cancer cell lines were found to express high levels of CD44, EpCAM, CD26, and CD338, as well as expressing low levels of CD166, MHC-class I, CD54, and B7H1; however, the differentiated OSCC oral cancer cell lines were found to express higher levels of B7H1, CD54, MHC-class I, and EGF-R, and low levels of CD44 and CD133 on their surface [25,27,28,29]. We have found OSCSCs to be excellent targets of NK cell-mediated cytotoxicity, whereas OSCCs are significantly resistant to NK cell-mediated cytotoxicity [25,28,29]. Cytokines secreted from NK cells, particularly IFN-γ and TNF-α, induce differentiation in CSCs. NK cell-secreted cytokines induced significant differentiation in OSCSCs, resulting in their growth inhibition [16,24,28,30,31]. Upon differentiation, tumors proliferate and metastasize at a minimal rate. To sum up, NK cell-mediated lysis directly kills oral tumors, and NK cell-mediated oral tumor differentiation inhibits tumor growth and spread. Also, NK cell-induced differentiation in oral tumors enhances the efficacy of therapeutics such as CD8+ T immunotherapies, radiation, chemotherapies, checkpoint inhibitors, and antibodies that target differentiated tumor subsets. The efficacy of NK cell-based therapeutics has been shown in several solid tumors [24,32,33,34,35,36,37,38,39,40,41,42,43,44].
Thus, the optimal function of NK cells or NK cell-based therapies directly affects the prognosis of cancer patients and also reduces the chances of tumor relapse by targeting CSCs [16,30,31,43,44,45,46,47,48]. Increased numbers and the anti-cancer activity of peripheral blood-derived NK cells, as well as increased NK cell infiltration in the tumor tissues, play significant roles in improving cancer patients’ prognosis [45,46,47,48]. Studies from our laboratory and several other studies have reported reduced numbers and anti-cancer activities in NK cells from cancer patients [49,50,51,52]. Reduced percentages and the defective function of NK cells were found in both tumor tissue and peripheral blood-derived immune subsets of cancer patients [50,51]. Several technologies have been introduced to expand and activate NK cells, which allows for the production of a large number of NK cells as therapeutics for cancer patients [53,54,55,56,57]. We have previously demonstrated superior technology to induce significant cell expansion and anti-cancer functional activation in NK cells. Due to the superior anti-cancer function of these expanded NK cells, they were named supercharged NK (sNK) cells [52,58,59,60].
In this review, we discuss the characteristics of sNK cells, including their superior anti-cancer potential compared to primary peripheral blood-derived NK cells, particularly in inducing direct cytotoxicity or ADCC, as well as inducing differentiation in oral tumors. The increased anti-cancer activity of sNK cells was seen in both in vitro and in vivo studies. A single infusion of sNK cells in vivo stopped disease progression and induced the in vivo differentiation of oral tumors. Furthermore, we demonstrate that oral tumors differentiated by sNK cells become more susceptible to chemotherapeutic drugs as compared to those differentiated by primary activated NK cells. Finally, we demonstrate that sNK cells survive and retain their function in oral tumors, whereas primary NK cells lose their function and cannot survive in tumor tissue.
2. Supercharged NK (sNK) Cells
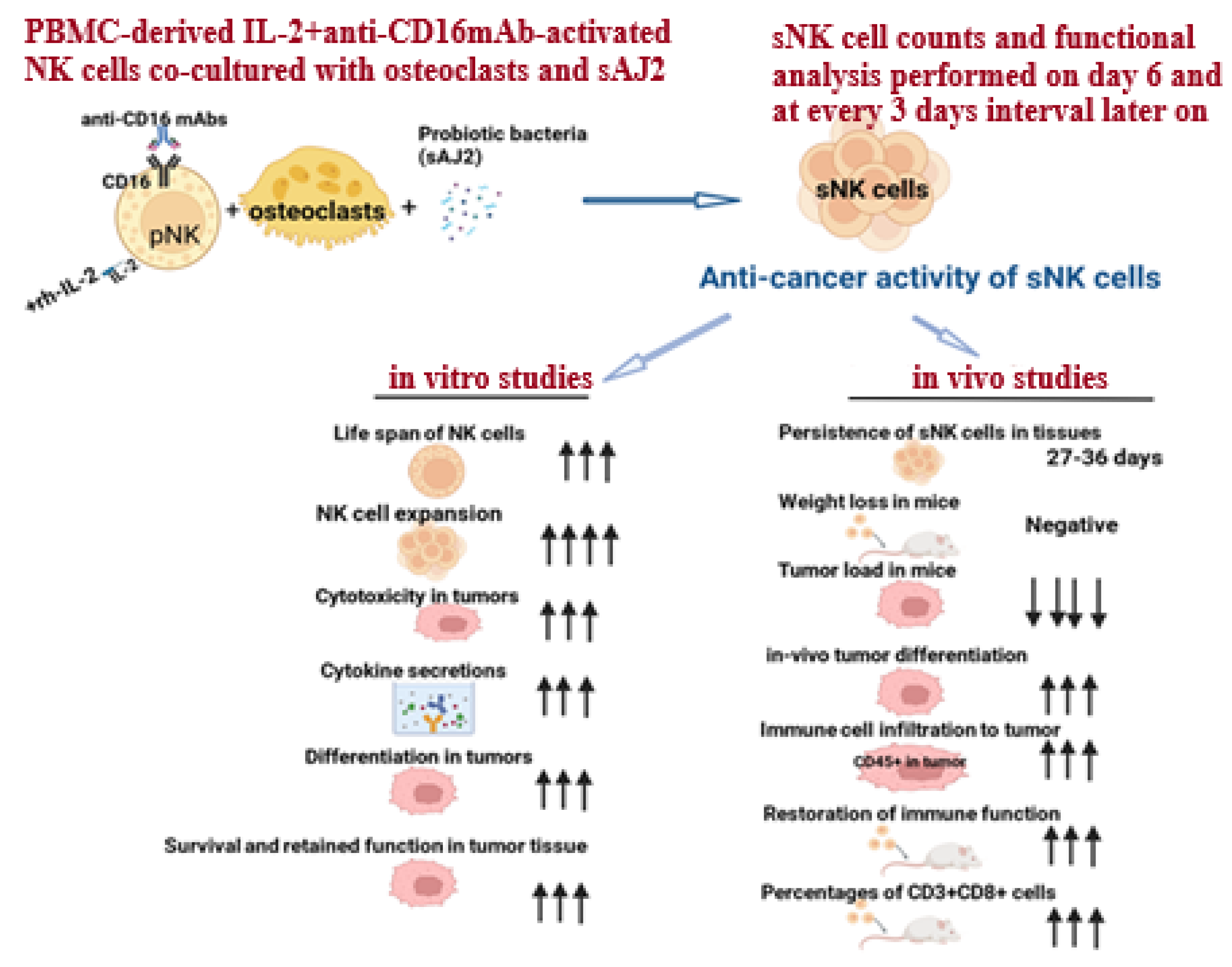
Supercharged NK (sNK) cell generation is performed by co-culturing peripheral blood-derived IL-2 and anti-CD16 mAb-activated NK cells with osteoclasts (OCs) and sonicated probiotic bacteria (sAJ2) (Figure 1). Osteoclasts were found to exhibit a positive surface expression of MICA/B, KLRG1, and ULBPs; these are ligands for NK cell-activating surface receptors. Osteoclasts also secrete a wide range of cytokines and chemokines, including IL-12, IL-15, IFN-γ, and IL-18, which are known to activate NK cells [61,62]. Thus, surface markers and the secreted factors of osteoclasts play a significant role in cell expansion and the functional activation of NK cells. Gram-positive probiotic bacteria Streptococcus thermophiles, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus bulgaricus were selected based on their activation in NK cells. The combination of these probiotic treatments in NK cells was found to increase cytokine secretion by NK cells, including IFN-γ, which could facilitate the signals required for NK cell expansion [28,39,52,63]. Therefore, a combination treatment of both probiotics and OCs in NK cells results in the induction of signals participating in cell expansion and the functional activation of NK cells, generating sNK cells. sNK cells have demonstrated increased lifespan, cell expansion, cytotoxicity, and the secretion of cytokines; these sNK cell characteristics ultimately result in the increased differentiation and killing of oral cancer both in vivo and in vitro [52,58,59]. sNK cells’ characteristics and increased functions are discussed in the next sections of this review.
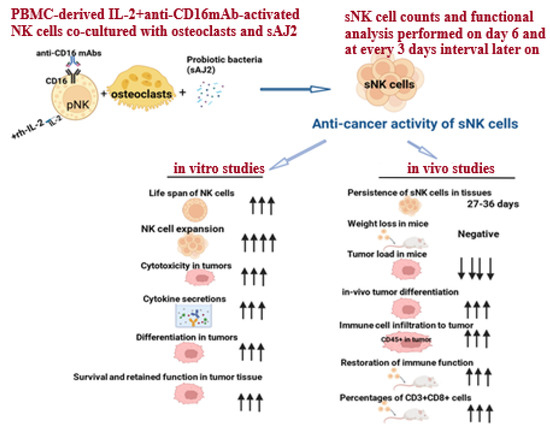
Figure 1.
Illustration showing the generation process and characteristics of sNK cells. Arrows reflect the comparison between sNK cells and IL-2-activated primary NK cells in both in vitro and in vivo studies.
3. Superior Anti-Cancer Activity in Oral Tumors by sNK Cells
We have previously identified and classified primary oral tumor cell lines based on stem-like or differentiated phenotypes as OSCSCs (oral squamous carcinoma stem-like cells) and OSCCs (oral squamous cell carcinomas) [25]. The stem-like oral tumor OSCSCs were found to exhibit high expression levels of CD338, CD44, CD26, and EpCAM, as well as low expression levels of CD54, CD166, MHC-class I, and PD-L1 on their surface; they also expressed an increased sensitivity to NK cell-mediated killing [25]. In contrast, the differentiated counterpart OSCCs were found to express lower levels of CD133 and CD44 and higher levels of CD54, EGF-R, PD-L1, and MHC-class I on their surface, as well as being resistant to NK cell-mediated killing.
sNK cells induce cytotoxicity in oral CSCs as well in differentiated oral tumors. We observed significantly higher levels of cytotoxicity by sNK cells when compared with primary IL-2-activated NK cell-mediated cytotoxicity against OSCSCs. Increased levels of ADCC against oral tumors were also seen in sNK cells. It was found that differentiation in tumors results in their resistance to NK cell-mediated cytotoxicity [25]; however, significantly higher levels of cytotoxicity were seen when sNK cells were used as effectors against OSCCs. These sNK cell characteristics are critical to target the heterogenous population of tumors, especially tumors expressing higher MHC-class I, which are known to escape primary NK cell-mediated killing.
sNK cells induce higher levels of differentiation in oral tumors (Figure 1). Primary NK cell-secreted cytokines, such as IFN-γ and TNF-α, were found to play a key role in inducing differentiation in oral tumors. Significantly low levels of tumor growth and metastasis were seen in differentiated tumors compared to stem-like tumors; additionally, differentiation in tumors increases their sensitivity to chemotherapy drugs [24,64]. sNK cells were found to release significantly increased levels of cytokines compared to primary NK cells [52,60,65]. Primary NK cells express cytokine release or cell survival for 5–7 days, whereas sNKs survived and maintained cytokine secretion for 27–36 days [52,60,65]. sNK cell-secreted cytokines are superior at inducing differentiation in oral tumors compared to cytokines secreted by primary NK cells [52,60]. Since 90% of head and neck malignant neoplasms express differentiated phenotypes, sNK cells could effectively eradicate the majority of oral cancers [66]. Several studies have demonstrated that cancer therapeutics like CD8+ T cell immunotherapies, antibodies, checkpoint inhibitors, radiation, and chemotherapies have failed to treat CSC tumors, but are efficient against differentiated tumors [24,38,39,40,41]. This suggests that sNK cell-based immunotherapy alone or its combination with other therapeutics is the future of oral cancer therapeutics.
4. Infusion of sNK Cells Reversed Disease Progression in Oral Tumor-Bearing Humanized Mice
The sNK cells’ in vivo efficacy against oral tumors was investigated using humanized-BLT (hu-BLT) mice; this mouse model was found to be one of the best humanized pre-clinical models, representing a full repertoire of the human immune system [67,68]. Hu-BLT mice provide a platform to validate human therapeutics when interactions are involved between human immune cells and the tumor microenvironment. Oral CSC OSCSCs were implanted in the oral cavity of hu-BLT mice one week after tumor implantation (once the tumor was established); then, mice were infused with sNK cells. Oral tumor-bearing mice infused with sNK cells therapeutics expressed slight/no tumor burden and demonstrated an increased lifespan compared to those with no treatment. sNK cell therapeutics induced a significant reduction in oral tumors, whereby the tumors dissected from sNK-treated groups were significantly smaller in size compared to the untreated group [39].
sNK cells demonstrated the in vivo differentiation of oral tumors, as tumors dissected from the sNK therapy group grew at a very minimal rate ex vivo; expressed higher levels of PD-L1, CD54, and MHC-class I on the surface; and were resistant to primary NK cell-mediated cytotoxicity [24,25,39]. sNK therapeutics resulted in an up to ninefold increase in the percentages of immune cells in the tumor microenvironment of the infused group as compared to tumors from the untreated group [39]. sNK therapy also increased the percentages of CD3+CD8+ T cells in the immune cells of the spleen, peripheral blood, and bone marrow of sNK cell-treated mice [39].
It was found that immune function is reduced or defective in cancer patients [49,50,51,52]. Similarly, an inhibition in the immune cell function was seen in oral tumor hu-BLT mice. We observed a 60–90% restoration in the cytotoxic and cytokine secretion levels of immune cells in various tissue compartments of oral tumor hu-BLT mice treated with sNK cells [39]. These findings suggest that the mechanisms involved in sNK cell therapy are as follows: (a) selection/direct killing of the tumor, (b) differentiation of tumors, (c) recruiting immune cells or increasing immune infiltration in the tumor, and (d) activation in CD8+ T cells [39]. We observed a similar efficacy of sNK cell therapy irrespective of whether they were autologous or allogeneic sNK cells [60].
sNK cell therapy combined with chemotherapy against oral tumors in hu-BLT mice demonstrated a slightly better efficacy compared to sNK cells alone [60]. A further reduction in tumor load and a greater restoration of immune cell function was observed when sNK therapy was combined with chemotherapy [60]. When checkpoint inhibitor PD-1 antibody was combined with sNK cells, a reduced tumor burden and an increased immune cell function restoration was seen in tumor-bearing hu-BLT mice [60]. Combining sNK cells with chemo-drugs or check-point inhibitors could be highly effective in treating oral cancer, as sNK cells could directly kill CSCs and differentiated tumors in addition to inducing differentiation in tumors, allowing sNK and chemotherapy combined effects against those tumors [60]. This concludes the significant potential of sNK cells to treat oral cancer; combining sNK cell treatment with other therapies will provide successful therapeutics in oral cancer patients.
5. Increased Survival and Augmented Function of sNK Cells in Oral Tumor Microenvironments
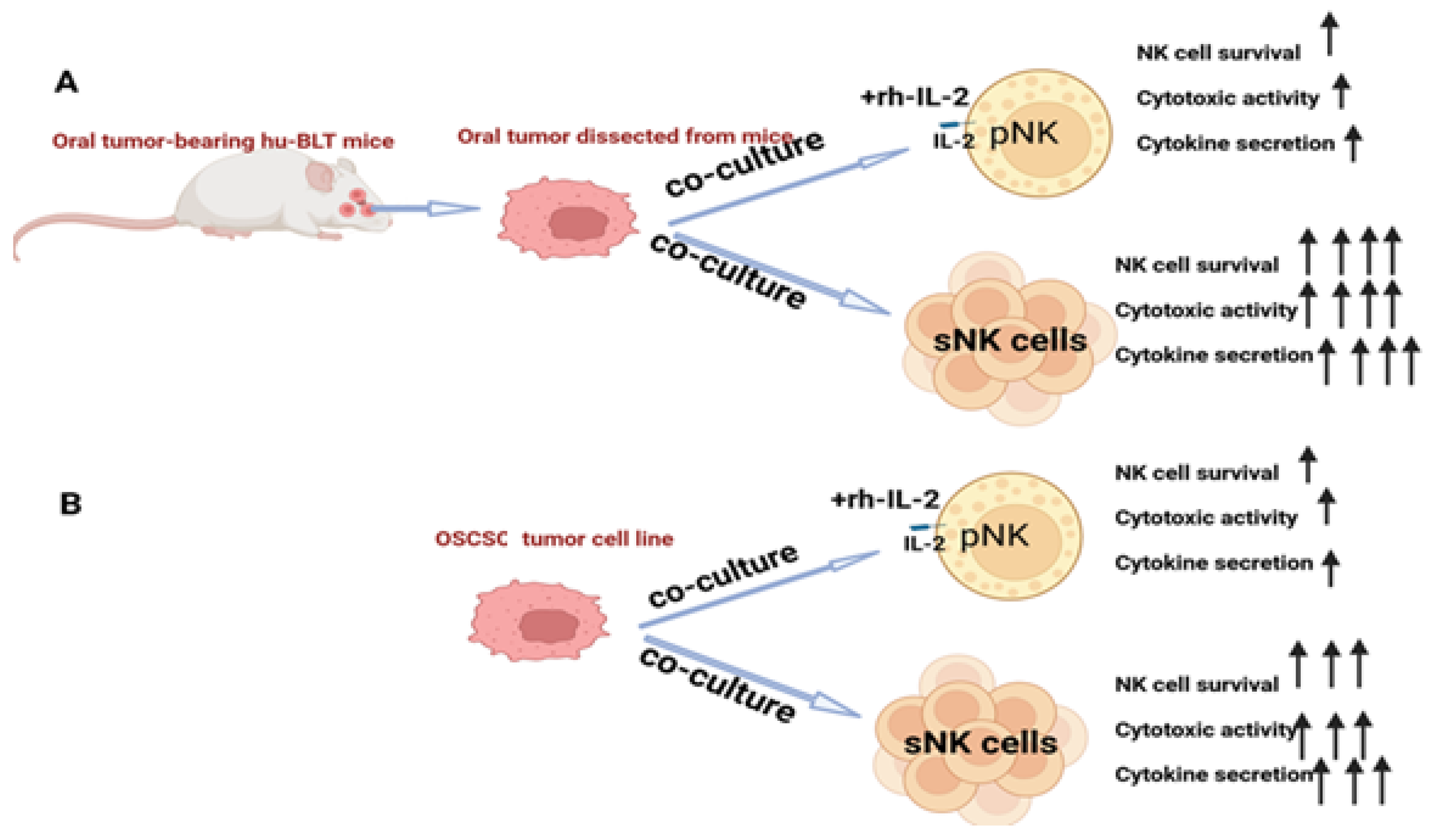
Tumor microenvironment-induced suppressed function or cell death in primary NK cells was demonstrated, and reduced NK cell function was also seen when NK cells were co-cultured with tumors in vitro [69]. When primary NK cells and sNK cells were exposed to oral tumors dissected from hu-BLT mice or with OSCSCs, in both cases, primary NK cells expressed minimal survival and lost function, but sNK cells survived longer and retained their function (Figure 2). This indicates the persistence or continued anti-cancer effect of sNK cells in the tumor microenvironment, validating the fact that this approach is best for treating oral tumors.
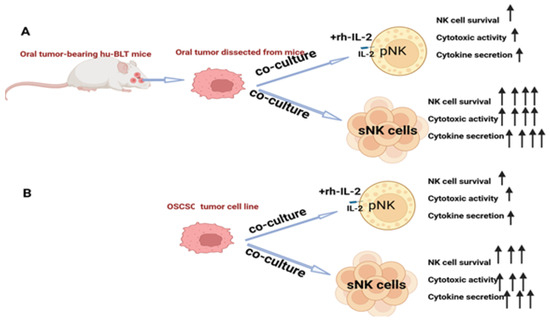
Figure 2.
The increased survival and augmented function of sNK cells in the tumor microenvironment. (A) Illustration showing that primary IL-2-activated NK cells or sNK cells were co-cultured with oral tumor tissue dissected from hu-BLT mice before cell survival and the function of primary or sNK cells were assessed. (B) Illustration showing that primary IL-2-activated NK cells or sNK cells were co-cultured with OSCSC tumors before cell survival and the function of primary or sNK cells were assessed.
6. Molecular Mechanism Underlying Superior Anti-Cancer Function of sNK Cells
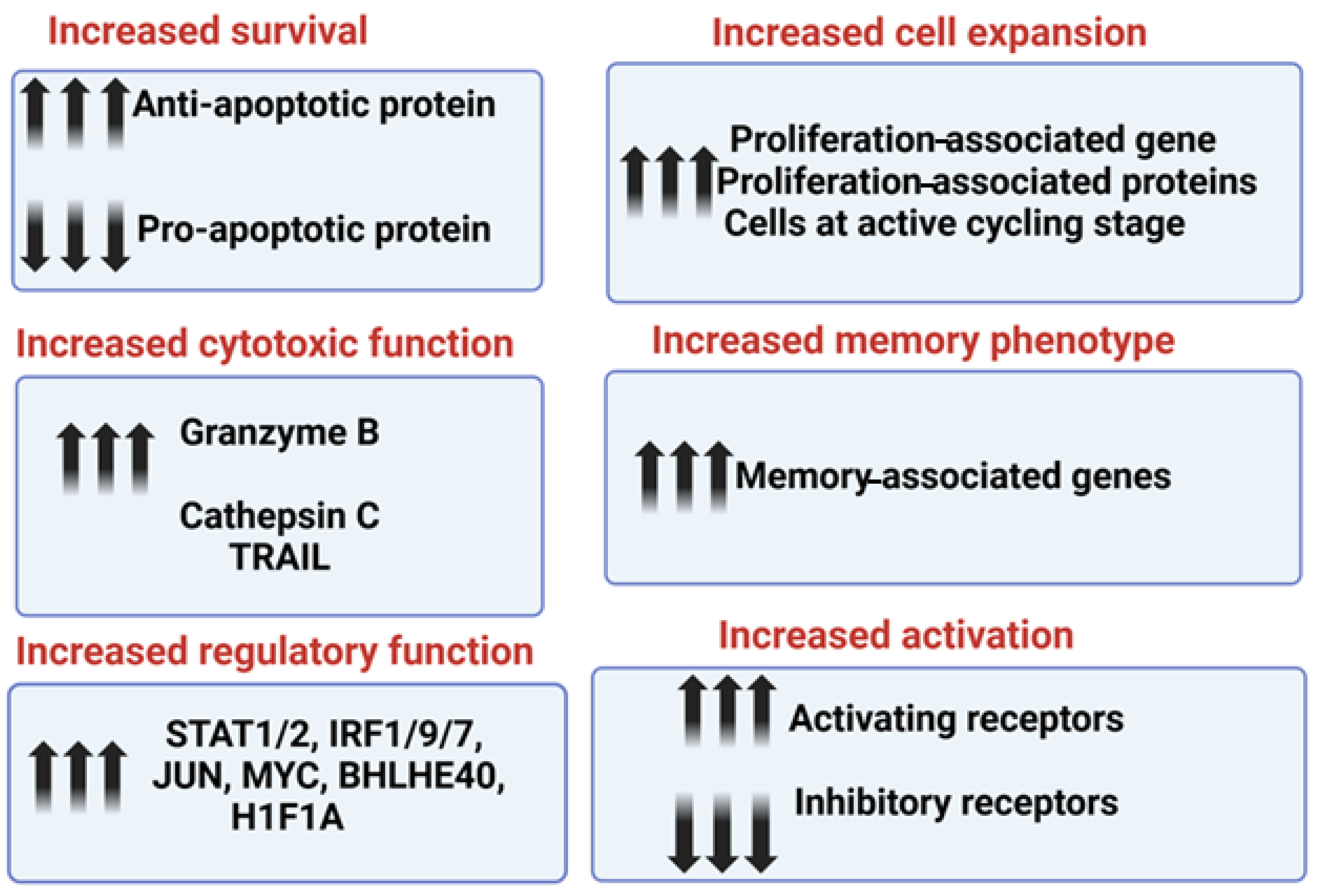
The increased survival and retained function of sNK cells in the tumor microenvironment is due to higher expression levels of anti-apoptotic proteins, including BCL2, and decreased expression levels of pro-apoptotic proteins; this allows sNK cells to resist the induction of cell death and a loss of cytotoxicity within the tumor microenvironment (Figure 3). In addition to oral cancer, sNK cells induce significant levels of anti-cancer activity against hematological malignancies and pancreatic, hepatic, and glioblastoma tumors [39,52,60,61,65]. The increased cytotoxic function in sNK cells is due to the increased expression levels of cytotoxic-associated granules granzyme B and cathepsin C. Also, the level of Trail expression was found to be elevated in the sNK cells from the single-cell transcriptomic analysis (Figure 3). The increased regulatory function of sNK cells is due to the increased gene expression levels of STAT1, STAT2, IRF1, IRF7, IRF9, JUN, MYC, BHLHE40, and H1F1A (Figure 3). Increased sNK cell expansion is maintained because of increased proliferation-associated genes and proteins; the majority of cells are at the active cycling stage (Figure 3). sNK cells express higher levels of memory-associated genes. sNK cells demonstrated a higher expression of activating receptors CD16, CD56, Nkp30, Nkp44, Nkp46, NKG2D, and CD54, and a downmodulation of inhibitory receptor NKG2A ([52] and manuscript in press). Thus, the increased secretion of IFN-γ and TNF-α [60], as well as cytotoxic function, cell survival/expansion, regulatory function, and memory phenotype, contribute to the increased activity of sNK cells to lyse both stem-like and differentiated tumors [70]. A summary of mechanisms contributing to their superior anti-cancer activity, highlighting the enhanced characteristics of sNK cells and illustrating how these advanced traits make them more effective in targeting and eliminating tumor cells, is shown in Figure 3.
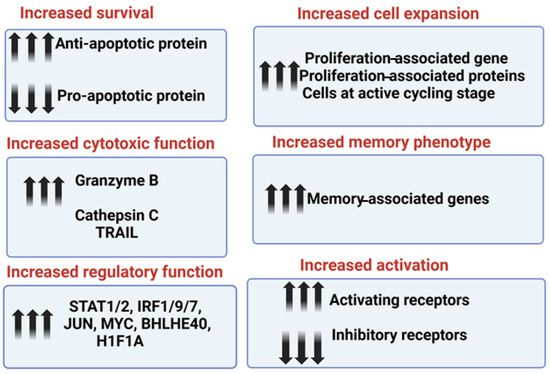
Figure 3.
This illustration highlights the molecular mechanism contributing to the superior anti-cancer function of sNK cells.
7. Conclusions
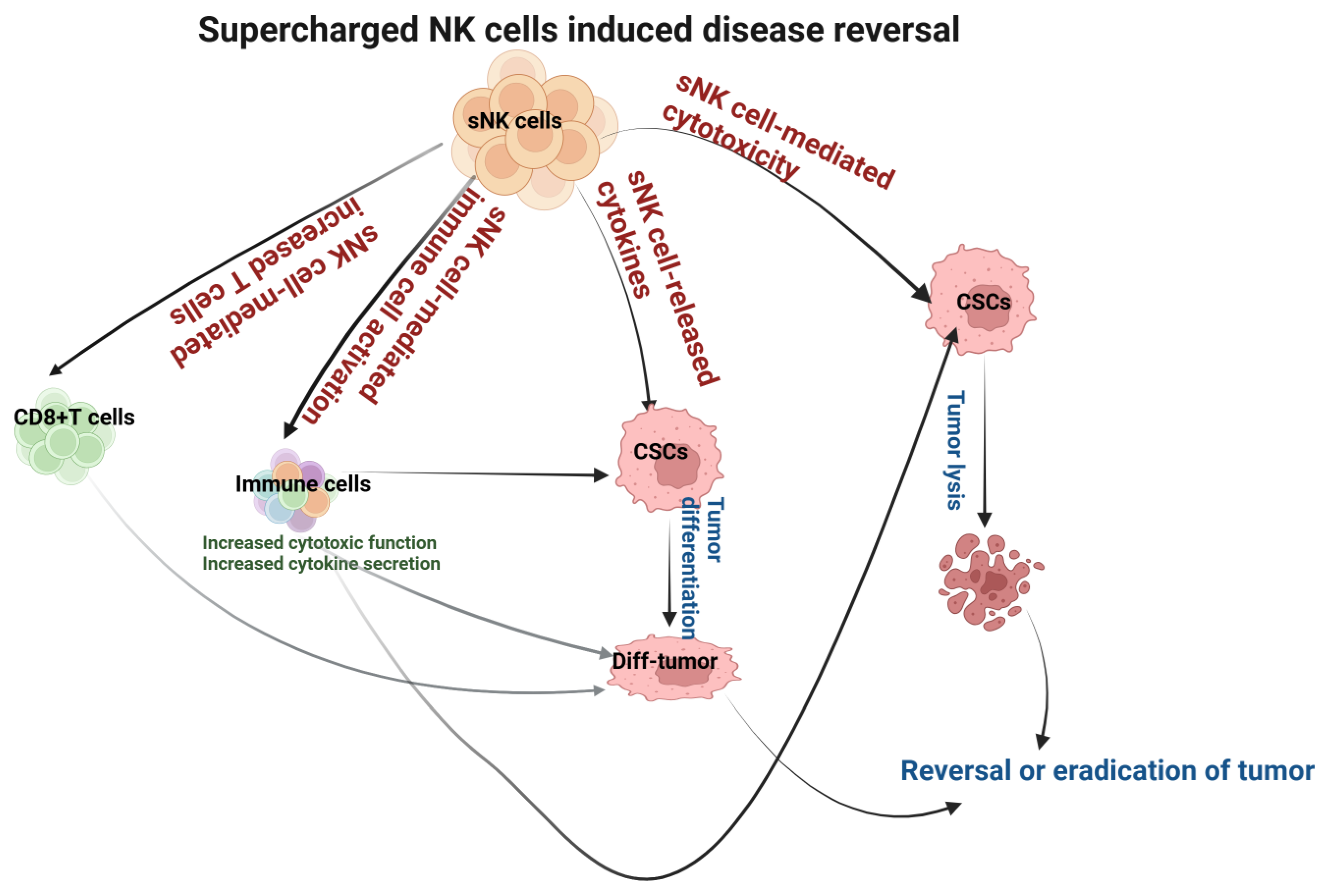
sNK cells induce significantly higher cytotoxicity against oral tumors compared to primary NK cells, as well as both CSCs and differentiated tumors. Also, sNK cells induce higher differentiation in oral tumors. The differentiation of oral tumors makes them sensitive to chemotherapeutic drugs. sNK cell efficacy as a cancer therapeutic was seen in oral tumor-bearing hu-BLT mice. Tumors resected from the group that received sNK cell therapy expressed a differentiated phenotype and grew minimal ex vivo tumors. Mice that received sNK cell therapy developed significantly small tumors. Our pre-clinical studies relating to sNK cell therapy in oral cancers have demonstrated the promising anti-cancer effects of this immunotherapy (Figure 4). sNK cells are very safe, as determined by the clinical trials of patients both in adult and pediatric populations. Indeed, the patients have reported an increase in energy, having a clear mind, and the loss of aches or pains after receiving sNK therapy. Because sNK cells can target both MHC-deficient and -competent tumors, they behave like CD8+ T cells [71,72]. The only disadvantage we can find for sNK therapy is the discomfort at the site of injection. The studies reviewed in this article suggest that sNK cell-based therapy alone or combined with other therapeutics is the future of oral cancer therapeutics.
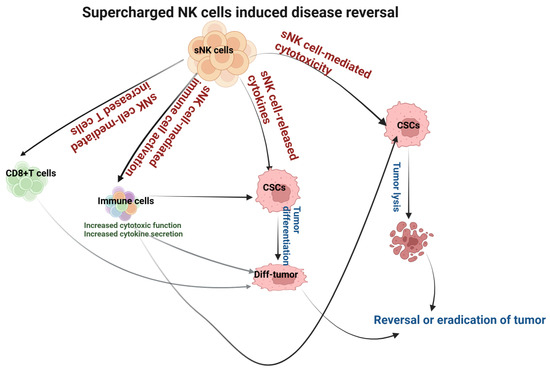
Figure 4.
Illustration showing the mechanisms involved in sNK cell-mediated disease reversal or the eradication of the oral tumor.
Author Contributions
A.J. was the principal investigator, obtained the funding, designed the study, and wrote the manuscript along with K.K. K.K. performed the literature search and wrote the manuscript. All figures were created with BioRender.com. All authors have read and agreed to the published version of the manuscript.
Funding
NIH-NIDCR RO1-DE022552; RO1 DE12880; UCLA Academic senate grant and School of Dentistry Seed grant.
Conflicts of Interest
The authors declare that the work reviewed in the article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Abbreviations
| NK cells | Natural killer cells |
| sNK cells | Supercharged NK cells |
| IFN-γ | Interferon-gamma |
| OCs | Osteoclasts |
| MHC-class I | Major histocompatibility complex molecule class I |
| ADCC | Antibody-dependent cellular cytotoxicity |
| CSCs | Cancer stem-like cells |
| OSCSCs | Oral squamous carcinoma stem-like cells |
| OSCCs | Oral squamous cell carcinomas |
| Hu-BLT | Humanized bone marrow, liver, thymus |
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer Statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Forastiere, A.A. Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol. 2006, 7, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Dholariya, S.; Singh, R.D.; Sonagra, A.; Yadav, D.; Vajaria, B.N.; Parchwani, D. Integrating Cutting-Edge Methods to Oral Cancer Screening, Analysis, and Prognosis. Crit. Rev. Oncog. 2023, 28, 11–44. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, J. Targeting anticancer immunity in oral cancer: Drugs, products, and nanoparticles. Environ. Res. 2023, 239 Pt 1, 116751. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Al-Swiahb, J.N.; Chen, C.H.; Chuang, H.C.; Fang, F.M.; Tasi, H.T.; Chien, C.Y. Clinical, pathological and molecular determinants in squamous cell carcinoma of the oral cavity. Future Oncol. 2010, 6, 837–850. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- Gupta, P.C.; Bhonsle, R.B.; Murti, P.R.; Daftary, D.K.; Mehta, F.S.; Pindborg, J.J. An epidemiologic assessment of cancer risk in oral precancerous lesions in India with special reference to nodular leukoplakia. Cancer 1989, 63, 2247–2252. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L. The World Cancer Report and the burden of oral cancer. Eur. J. Cancer Prev. 2004, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Costantino, A.; Mercante, G.; Petruzzi, G.; Sebastiani, D.; Franzese, C.; Scorsetti, M.; Pellini, R.; Malvezzi, L.; Spriano, G. Present and Future of De-intensification Strategies in the Treatment of Oropharyngeal Carcinoma. Curr. Oncol. Rep. 2020, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Sathish, N.; Wang, X.; Yuan, Y. Human Papillomavirus (HPV)-associated Oral Cancers and Treatment Strategies. J. Dent. Res. 2014, 93 (Suppl. S7), 29s–36s. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Magrin, J.; Kowalski, L.P. Sites of recurrence in oral and oropharyngeal cancers according to the treatment approach. Oral Dis. 2003, 9, 112–118. [Google Scholar] [CrossRef]
- Grandis, J.R.; Falkner, D.M.; Melhem, M.F.; Gooding, W.E.; Drenning, S.D.; Morel, P.A. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: Clinical and immunogenetic consequences. Clin. Cancer Res. 2000, 6, 2794–2802. [Google Scholar]
- Cabrera, C.M.; Nieto, A.; Cortes, J.L.; Montes, R.M.; Catalina, P.; Cobo, F.; Barroso-del-Jesus, A.; Concha, A. The low rate of HLA class I molecules on the human embryonic stem cell line HS293 is associated with the APM components’ expression level. Cell Biol. Int. 2007, 31, 1072–1078. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Becker, P.S.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef]
- Fildes, J.E.; Yonan, N.; Leonard, C.T. Natural killer cells and lung transplantation, roles in rejection, infection, and tolerance. Transpl. Immunol. 2008, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, A.K.; Topchyan, P.; Kaur, K.; Tseng, H.C.; Teruel, A.; Hiraga, T.; Jewett, A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer 2017, 8, 537–554. [Google Scholar] [CrossRef]
- Tseng, H.C.; Arasteh, A.; Paranjpe, A.; Teruel, A.; Yang, W.; Behel, A.; Alva, J.A.; Walter, G.; Head, C.; Ishikawa, T.O.; et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE 2010, 5, e11590. [Google Scholar] [CrossRef]
- Markiewicz, M.R.; Callahan, N.; Morlandt, A. Oral Cancer: Classification, Diagnosis, and Staging. In Peterson’s Principles of Oral and Maxillofacial Surgery; Miloro, M., Ghali, G.E., Larsen, P.E., Waite, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 965–1008. [Google Scholar]
- Tseng, H.C.; Bui, V.; Man, Y.G.; Cacalano, N.; Jewett, A. Induction of Split Anergy Conditions Natural Killer Cells to Promote Differentiation of Stem Cells through Cell-Cell Contact and Secreted Factors. Front. Immunol. 2014, 5, 269. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Tseng, H.C.; Cacalano, N.; Jewett, A. Split anergized Natural Killer cells halt inflammation by inducing stem cell differentiation, resistance to NK cell cytotoxicity and prevention of cytokine and chemokine secretion. Oncotarget 2015, 6, 8947–8959. [Google Scholar] [CrossRef]
- Uppaluri, R.; Dunn, G.P.; Lewis, J.S., Jr. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun. 2008, 8, 16. [Google Scholar]
- Staveley-O’Carroll, K.; Sotomayor, E.; Montgomery, J.; Borrello, I.; Hwang, L.; Fein, S.; Pardoll, D.; Levitsky, H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA 1998, 95, 1178–1183. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, J.M.; Pittari, G.; Gooley, T.A.; Chewning, J.H.; Spellman, S.; Haagenson, M.; Gallagher, M.M.; Malkki, M.; Petersdorf, E.; Dupont, B.; et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 2012, 367, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, E.G.; Kountourakis, P.; Karamouzis, M.V.; Doufexis, D.; Ardavanis, A.; Baxevanis, C.N.; Rigatos, G.; Papamichail, M.; Perez, S.A. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol. Immunother. 2010, 59, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Re, F.; Staudacher, C.; Zamai, L.; Vecchio, V.; Bregni, M. Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer 2006, 107, 640–648. [Google Scholar] [CrossRef]
- Geller, M.A.; Cooley, S.; Judson, P.L.; Ghebre, R.; Carson, L.F.; Argenta, P.A.; Jonson, A.L.; Panoskaltsis-Mortari, A.; Curtsinger, J.; McKenna, D.; et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011, 13, 98–107. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, II; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.H.; Ko, M.W.; Fang, C.; Nishimura, I.; et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology 2018, 7, e1426518. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, T.; Deng, S.C.; Wei, J.C.; Yang, P.; Wang, Q.; Chen, Z.P.; Li, W.L.; Chen, H.C.; Hu, H.; et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019, 450, 1–13. [Google Scholar] [CrossRef]
- Ou, Z.L.; Luo, Z.; Wei, W.; Liang, S.; Gao, T.L.; Lu, Y.B. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: Role of circ_0000977/miR-153 axis. RNA Biol. 2019, 16, 1592–1603. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Tsai, L.L.; Wang, M.L.; Yu, C.H.; Lo, W.L.; Chang, Y.C.; Chiou, G.Y.; Chou, M.Y.; Chiou, S.H. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013, 73, 3425–3440. [Google Scholar] [CrossRef]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef]
- Burke, S.; Lakshmikanth, T.; Colucci, F.; Carbone, E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.K.; Gao, Y.; Basse, P.H. NK cells in the tumor microenvironment. Crit. Rev. Oncog. 2014, 19, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Türkseven, M.R.; Oygür, T. Evaluation of natural killer cell defense in oral squamous cell carcinoma. Oral Oncol. 2010, 46, e34–e37. [Google Scholar] [CrossRef]
- Accomando, W.P.; Wiencke, J.K.; Houseman, E.A.; Butler, R.A.; Zheng, S.; Nelson, H.H.; Kelsey, K.T. Decreased NK cells in patients with head and neck cancer determined in archival DNA. Clin. Cancer Res. 2012, 18, 6147–6154. [Google Scholar] [CrossRef]
- Mickel, R.A.; Kessler, D.J.; Taylor, J.M.; Lichtenstein, A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer Res. 1988, 48, 5017–5022. [Google Scholar]
- Kaur, K.; Cook, J.; Park, S.H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Wynberg, J.; Srinivasan, R.; Becknell, B.; McCoy, J.P., Jr.; Takahashi, Y.; Suffredini, D.A.; Linehan, W.M.; Caligiuri, M.A.; Childs, R.W. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004, 104, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Alici, E.; Sutlu, T.; Björkstrand, B.; Gilljam, M.; Stellan, B.; Nahi, H.; Quezada, H.C.; Gahrton, G.; Ljunggren, H.G.; Dilber, M.S. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood 2008, 111, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Berg, M.; Lundqvist, A.; McCoy, P., Jr.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef]
- Shah, N.; Martin-Antonio, B.; Yang, H.; Ku, S.; Lee, D.A.; Cooper, L.J.; Decker, W.K.; Li, S.; Robinson, S.N.; Sekine, T.; et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS ONE 2013, 8, e76781. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Ohanian, N.; Cook, J.; Jewett, A. Osteoclast-expanded super-charged NK-cells preferentially select and expand CD8+ T cells. Sci. Rep. 2020, 10, 20363. [Google Scholar] [CrossRef]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.-W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.-H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2020, 12, 63. [Google Scholar] [CrossRef]
- Kaur, K.; Chen, P.C.; Ko, M.W.; Mei, A.; Senjor, E.; Malarkannan, S.; Kos, J.; Jewett, A. Sequential therapy with supercharged NK cells with either chemotherapy drug cisplatin or anti-PD-1 antibody decreases the tumor size and significantly enhances the NK function in Hu-BLT mice. Front. Immunol. 2023, 14, 1132807. [Google Scholar] [CrossRef]
- Tseng, H.C.; Kanayama, K.; Kaur, K.; Park, S.H.; Park, S.; Kozlowska, A.; Sun, S.; McKenna, C.E.; Nishimura, I.; Jewett, A. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: Role in osteoclast-mediated NK cell activation. Oncotarget 2015, 6, 20002–20025. [Google Scholar] [CrossRef]
- Li, H.; Hong, S.; Qian, J.; Zheng, Y.; Yang, J.; Yi, Q. Cross talk between the bone and immune systems: Osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood 2010, 116, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.; Chen, P.C.; Pham, J.; Nguyen, C.Q.; Kaur, K.; Raman, S.S.; Jewett, A. Characterizing hepatocellular carcinoma stem markers and their corresponding susceptibility to NK-cell based immunotherapy. Front. Immunol. 2023, 14, 1284669. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.F.; Almangush, A.; Salo, T.; da Silva, S.D.; Kujan, O.; Coletta, R.D. Emerging histopathological parameters in the prognosis of oral squamous cell carcinomas. Histol. Histopathol. 2024, 39, 1–12. [Google Scholar]
- Shimizu, S.; Hong, P.; Arumugam, B.; Pokomo, L.; Boyer, J.; Koizumi, N.; Kittipongdaja, P.; Chen, A.; Bristol, G.; Galic, Z.; et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 2010, 115, 1534–1544. [Google Scholar] [CrossRef]
- Vatakis, D.N.; Bristol, G.C.; Kim, S.G.; Levin, B.; Liu, W.; Radu, C.G.; Kitchen, S.G.; Zack, J.A. Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J. Vis. Exp. 2012, e4181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riggan, L.; Shah, S.; O’Sullivan, T.E. Arrested development: Suppression of NK cell function in the tumor microenvironment. Clin. Transl. Immunol. 2021, 10, e1238. [Google Scholar] [CrossRef]
- Kaur, K.; Safaie, T.; Ko, M.W.; Wang, Y.; Jewett, A. ADCC against MICA/B Is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers 2021, 13, 239. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Qian, H.; Shi, Y.; Tao, Z. CD8(+) T cell-based cancer immunotherapy. J. Transl. Med. 2024, 22, 394. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).