CFD Modeling of a Lab-Scale Microwave Plasma Reactor for Waste-to-Energy Applications: A Review
Abstract
:1. Introduction
2. Materials and Methods
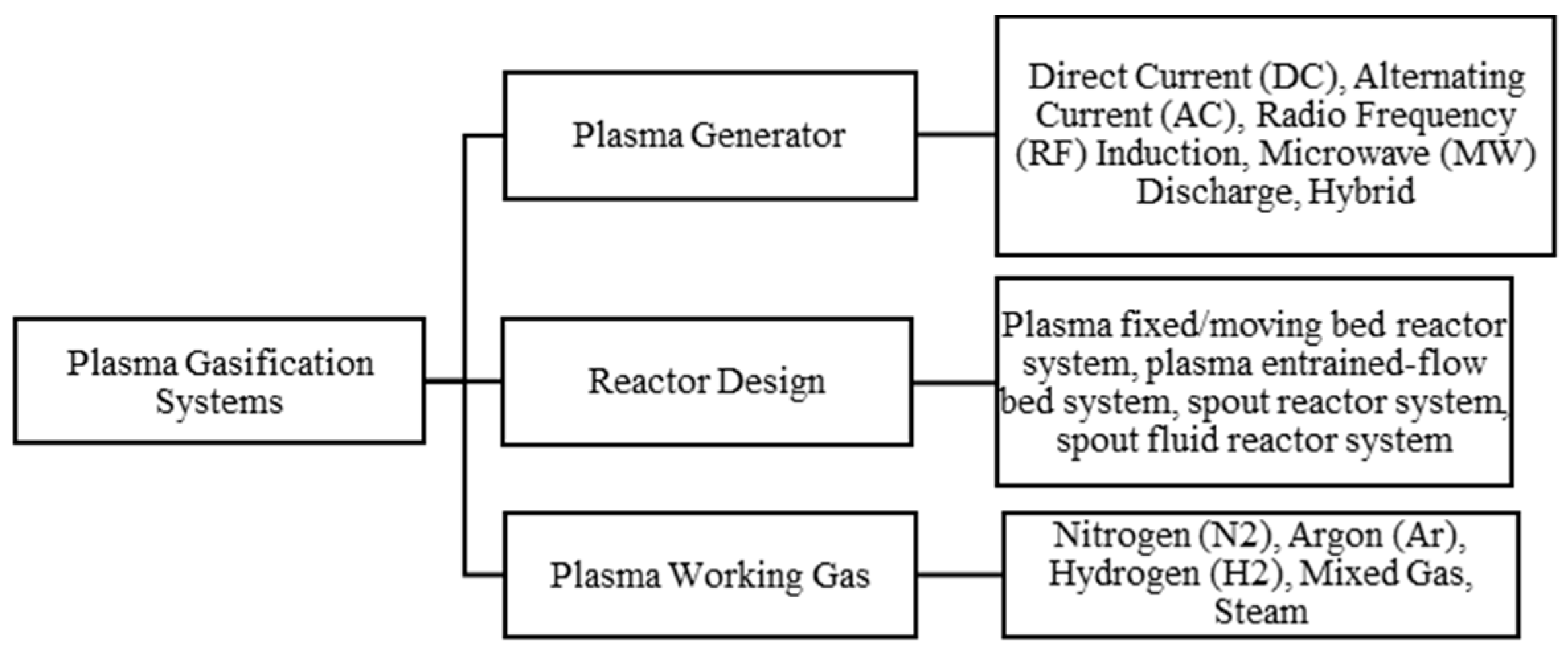
3. EMIPG System and Process Description
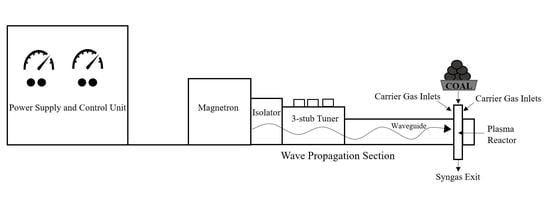
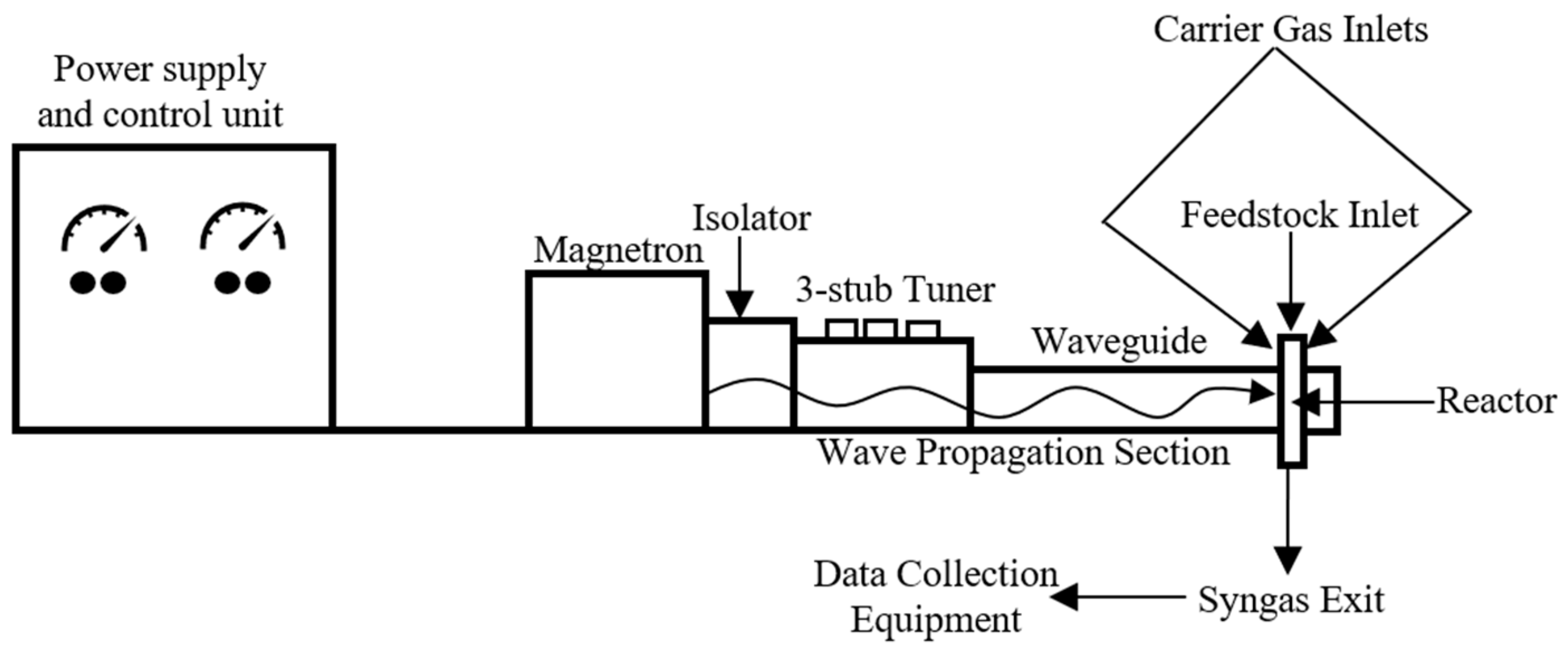
3.1. EMIPG System Physical Description
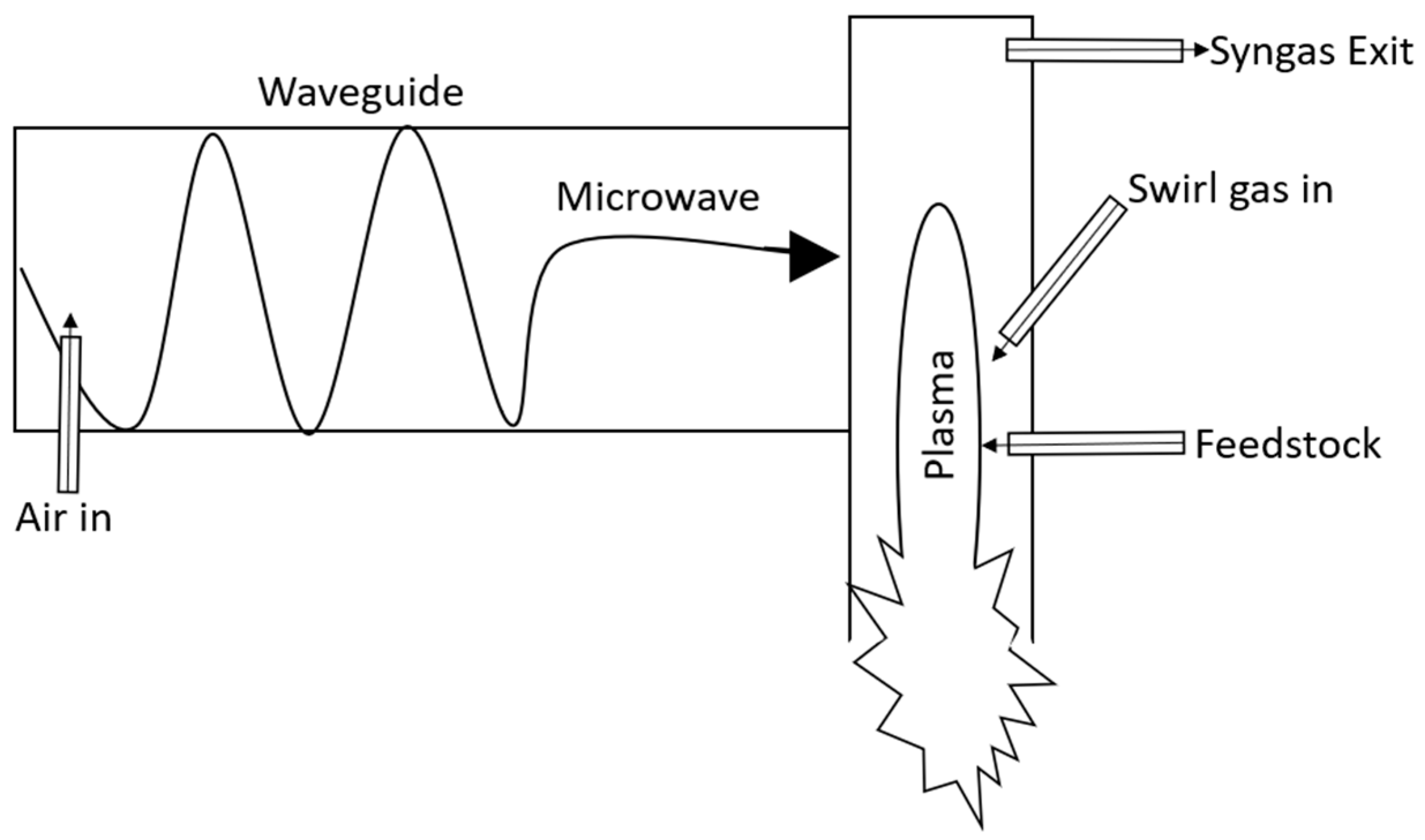
3.2. EMIPG Reactor Physical Description
3.3. Reaction Kinetics within an EMIPG Reactor
3.4. Governing Equations within an EMIPG Reactor
3.5. Modeling Tools/Software for an EMIPG Reactor
4. Forward Look and Conclusions
4.1. Forward Look
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Disclaimer
References
- Menniti, D.; Burgio, A.; Scordino, N. Population growth, sustainable development, energy resources and environmental protection: The nuclear option. In Proceedings of the 2007 IEEE Lausanne POWERTECH, Lausanne, Switzerland, 1–5 July 2007; pp. 1812–1816. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. WHAT A WASTE 2.0 A Global Snapshot of Solid Waste Management to 2050 OVERVIEW; U.S. Army Research Laboratory: Aldephi, MD, USA, Reprint from Journal of Intelligence Community Research and Development; 21 September 2012. [Google Scholar]
- Charis, G.; Danha, G.; Muzenda, E.; Muzenda, E.; Patel, B.; Mateescu, C.; Muzenda, E. Waste to Energy Opportunities in Botswana: A Case Study Review. In Proceedings of the 2019 7th International Renewable and Sustainable Energy Conference, IRSEC 2019, Agadir, Morocco, 27–30 November 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2019. [Google Scholar]
- Frimpong, S. Global Energy Security: The Case for a Multifaceted Solution Strategy. J. Energy Eng. 2008, 134, 109–110. [Google Scholar] [CrossRef]
- Shittu, O.S.; Williams, I.D.; Shaw, P.J. Global E-waste management: Can WEEE make a difference? A review of e-waste trends, legislation, contemporary issues and future challenges. Waste Manag. 2021, 120, 549–563. [Google Scholar] [CrossRef]
- Haque, M.S.; Uddin, S.; Sayem, S.M.; Mohib, K.M. Coronavirus disease 2019 (COVID-19) induced waste scenario: A short overview. J. Environ. Chem. Eng. 2021, 9, 104660. [Google Scholar] [CrossRef]
- US Department of Defense. Task Force on Energy Systems for Forward/Remote Operating Bases; US Department of Defense: Washington, DC, USA, 2016.
- Meier, A.; Shah, M.; Engeling, K.; Quinn, K. Demonstration of Plasma Assisted Waste Conversion to Gas. In Proceedings of the 49th International Conference on Environmental Systems, Boston, MA, USA, 7–11 July 2019; pp. 1–13. [Google Scholar]
- Tang, L.; Huang, H.; Hao, H.; Zhao, K. Development of plasma pyrolysis/gasification systems for energy efficient and environmentally sound waste disposal. J. Electrostat. 2013, 71, 839–847. [Google Scholar] [CrossRef]
- Perkins, G. Production of electricity and chemicals using gasification of municipal solid wastes. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–39. [Google Scholar]
- Blaisi, N.I.; Roessler, J.G.; Watts, B.E.; Paris, J.; Ferraro, C.C.; Townsend, T.G. Construction material properties of high temperature arc gasification slag as a portland cement replacement. J. Clean. Prod. 2018, 196, 1266–1272. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Breeze, P. Advanced Waste-to-Energy Technologies. In Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–75. [Google Scholar]
- Sekiguchi, H.; Orimo, T. Gasification of polyethylene using steam plasma generated by microwave discharge. Thin Solid Films 2004, 457, 44–47. [Google Scholar] [CrossRef]
- Uhm, H.S.; Na, Y.H.; Hong, Y.C.; Shin, D.H.; Cho, C.H. Production of hydrogen-rich synthetic gas from low-grade coals by microwave steam-plasmas. Int. J. Hydrogen Energy 2014, 39, 4351–4355. [Google Scholar] [CrossRef]
- Sanlisoy, A.; Carpinlioglu, M.O. A review on plasma gasification for solid waste disposal. Int. J. Hydrogen Energy 2017, 42, 1361–1365. [Google Scholar] [CrossRef]
- Ho, G.S.; Faizal, H.M.; Ani, F.N. Microwave induced plasma for solid fuels and waste processing: A review on affecting factors and performance criteria. Waste Manag. 2017, 69, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Delikonstantis, E.; Sturm, G.; Stankiewicz, A.I.; Bosmans, A.; Scapinello, M.; Dreiser, C.; Lade, O.; Brand, S.; Stefanidis, G.D. Biomass gasification in microwave plasma: An experimental feasibility study with a side stream from a fermentation reactor. Chem. Eng. Process. Process Intensif. 2019, 141, 107538. [Google Scholar] [CrossRef]
- Wu, T.N. Environmental perspectives of microwave applications as remedial alternatives: Review. Pract. Period. Hazardous Toxic Radioact. Waste Manag. 2008, 12, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma gasification of municipal solid waste for waste-to-value processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Saleem, F.; Harris, J.; Zhang, K.; Harvey, A. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Saleem, F.; Rehman, A.; Abbas, A.; Hussain Khoja, A.; Ahmad, F.; Liu, L.; Zhang, K.; Harvey, A. A comparison of the decomposition of biomass gasification tar compound in CO, CO2, H2 and N2 carrier gases using non-thermal plasma. J. Energy Inst. 2021, 97, 161–168. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Ashokkumar, V.; Pandiyan, R.; Halimatul Munawaroh, H.S.; Chew, K.W.; Chen, W.H.; Ngamcharussrivichai, C. Pyrolysis: An effective technique for degradation of COVID-19 medical wastes. Chemosphere 2021, 275, 130092. [Google Scholar] [CrossRef] [PubMed]
- Chicone, C. Problems and Projects: Waveguides, Lord Kelvin’s Model. In An Invitation to Applied Mathematics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 775–791. [Google Scholar]
- Hong, Y.C.; Lee, S.J.; Shin, D.H.; Kim, Y.J.; Lee, B.J.; Cho, S.Y.; Chang, H.S. Syngas production from gasification of brown coal in a microwave torch plasma. Energy 2012, 47, 36–40. [Google Scholar] [CrossRef]
- Ellison, C.R.; Hoff, R.; Mărculescu, C.; Boldor, D. Investigation of microwave-assisted pyrolysis of biomass with char in a rectangular waveguide applicator with built-in phase-shifting. Appl. Energy 2020, 259, 114217. [Google Scholar] [CrossRef]
- Sun, H.; Lee, J.; Bak, M.S. Experiments and modeling of atmospheric pressure microwave plasma reforming of a methane-carbon dioxide mixture. J. CO2 Util. 2021, 46, 101464. [Google Scholar] [CrossRef]
- Vecten, S.; Wilkinson, M.; Martin, A.; Dexter, A.; Bimbo, N.; Dawson, R.; Herbert, B. Experimental study of steam and carbon dioxide microwave plasma for advanced thermal treatment application. Energy 2020, 207, 118086. [Google Scholar] [CrossRef]
- Vecten, S.; Wilkinson, M.; Bimbo, N.; Dawson, R.; Herbert, B.M.J. Experimental investigation of the temperature distribution in a microwave-induced plasma reactor. Fuel Process. Technol. 2021, 212, 106631. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, J.G. Syngas Production from Coal through Microwave Plasma Gasification: Influence of Oxygen, Steam, and Coal Particle Size. Energy Fuels 2011, 26, 524–529. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yun, Y.M.; Seo, M.W.; Kim, Y.K.; Ra, H.W.; Lee, J.G. Hydrogen and syngas production from glycerol through microwave plasma gasification. Int. J. Hydrogen Energy 2013, 38, 14559–14567. [Google Scholar] [CrossRef]
- Shin, D.H.; Hong, Y.C.; Lee, S.J.; Kim, Y.J.; Cho, C.H.; Ma, S.H.; Chun, S.M.; Lee, B.J.; Uhm, H.S. A pure steam microwave plasma torch: Gasification of powdered coal in the plasma. Surf. Coat. Technol. 2013, 228, S520–S523. [Google Scholar] [CrossRef]
- Su, L.; Kumar, R.; Ogungbesan, B.; Sassi, M. Experimental investigation of gas heating and dissociation in a microwave plasma torch at atmospheric pressure. Energy Convers. Manag. 2014, 78, 695–703. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, Y.C.; Hsiao, Y.H. Microwave plasma studies of Spirulina algae pyrolysis with relevance to hydrogen production. Energy 2014, 64, 567–574. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, K.T. Production of hydrogen and nano carbon powders from direct plasmalysis of methane. Int. J. Hydrogen Energy 2009, 34, 833–838. [Google Scholar] [CrossRef]
- Wang, Y.F.; You, Y.S.; Tsai, C.H.; Wang, L.C. Production of hydrogen by plasma-reforming of methanol. Int. J. Hydrogen Energy 2010, 35, 9637–9640. [Google Scholar] [CrossRef]
- Sturm, G.S.J.; Munoz, A.N.; Aravind, P.V.; Stefanidis, G.D. Microwave-Driven Plasma Gasification for Biomass Waste Treatment at Miniature Scale. IEEE Trans. Plasma Sci. 2016, 44, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Hrycak, B.; Czylkowski, D.; Miotk, R.; Dors, M.; Jasinski, M.; Mizeraczyk, J. Application of atmospheric pressure microwave plasma source for hydrogen production from ethanol. Int. J. Hydrog. Energy 2014, 39, 14184–14190. [Google Scholar] [CrossRef]
- Su, X.; Jin, H.; Guo, L.; Guo, S.; Ge, Z. Experimental study on Zhundong coal gasification in supercritical water with a quartz reactor: Reaction kinetics and pathway. Int. J. Hydrogen Energy 2015, 40, 7424–7432. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.T.; Khan, M.M.K.; Oo, A.M.T. Performance assessment of gasification reactors for sustainable management of municipal solid waste. J. Environ. Manag. 2021, 291, 112661. [Google Scholar] [CrossRef]
- Okino, A.; Miyahara, H.; Yabuta, H.; Mizusawa, Y.; Doi, T.; Watanabe, M.; Hotta, E. Development of a new multi-plasma gas inductively coupled plasma torch. In Proceedings of the IEEE International Conference on Plasma Science, Baltimore, MD, USA, 1 July 2004; p. 306. [Google Scholar]
- Pintsuk, G.; Hasegawa, A. Tungsten as a Plasma-Facing Material. In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–53. [Google Scholar]
- Ebeling, W. Coulomb interaction and ionization equilibrium in partially ionized plasmas. Physica 1969, 43, 293–306. [Google Scholar] [CrossRef]
- Makonnen, Y.; Beauchemin, D. The inductively coupled plasma as a source for optical emission spectrometry and mass spectrometry. In Sample Introduction Systems in ICPMS and ICPOES; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–55. [Google Scholar]
- Pattiya, A. Direct Thermochemical Liquefaction for Energy Applications Fast Pyrolysis; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, J.G. Hydrogen-rich syngas production through coal and charcoal gasification using microwave steam and air plasma torch. Int. J. Hydrog. Energy 2012, 37, 17093–17100. [Google Scholar] [CrossRef]
- Ibrahimoglu, B.; Cucen, A.; Yilmazoglu, M.Z. Numerical modeling of a downdraft plasma gasification reactor. Int. J. Hydrogen Energy 2017, 42, 2583–2591. [Google Scholar] [CrossRef]
- Silva, V.B.R.E.; Cardoso, J. Introduction and overview of using computational fluid dynamics tools. In Computational Fluid Dynamics Applied to Waste-to-Energy Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar]
- Kobayashi, H.; Howard, J.B.; Sarofim, A.F. Coal devolatilization at high temperatures. Symp. Combust. 1977, 16, 411–425. [Google Scholar] [CrossRef]
- Kuo, P.C.; Illathukandy, B.; Wu, W.; Chang, J.S. Plasma gasification performances of various raw and torrefied biomass materials using different gasifying agents. Bioresour. Technol. 2020, 314, 123740. [Google Scholar] [CrossRef]
- Park, D.C.; Day, S.J.; Nelson, P.F. Formation of N-containing gas-phase species from char gasification in steam. Fuel 2008, 87, 807–814. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. Distribution of sulfur species in gaseous and condensed phase during downdraft gasification of corn straw. Energy 2014, 64, 248–258. [Google Scholar] [CrossRef]
- Kuo, P.C.; Wu, W.; Chen, W.H. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 2014, 117, 1231–1241. [Google Scholar] [CrossRef]
- Ibrahimoglu, B.; Yilmazoglu, M.Z. Numerical modeling of a downdraft plasma coal gasifier with plasma reactions. Int. J. Hydrogen Energy 2020, 45, 3532–3548. [Google Scholar] [CrossRef]
- Silva, V.B.R.E.; Cardoso, J. How to approach a real CFD problem—A decision-making process for gasification. In Computational Fluid Dynamics Applied to Waste-to-Energy Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–83. [Google Scholar]
- Badzioch, S.; Hawksley, P.G.W. Kinetics of Thermal Decomposition of Pulverized Coal Particles. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 521–530. [Google Scholar] [CrossRef]
- Fan, F.; Wang, S.; Yang, S.; Hu, J.; Wang, H. Numerical investigation of gas thermal property in the gasification process of a spouted bed gasifier. Appl. Therm. Eng. 2020, 181, 115917. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.B.; Bispo, C.; Rouboa, A. From laboratorial to pilot fluidized bed reactors: Analysis of the scale-up phenomenon. Energy Convers. Manag. 2016, 119, 177–186. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Teixeira, S.; Chacartegui, R.; Bouziane, K.; Brito, P.S.D.; Rouboa, A. Numerical and experimental analysis of municipal solid wastes gasification process. Appl. Therm. Eng. 2015, 78, 185–195. [Google Scholar] [CrossRef]
- Reactor Design & Simulation | Ansys. Available online: https://www.ansys.com/solutions/solutions-by-industry/materials-and-chemical-processing/reactor-design (accessed on 22 February 2021).
- Thompson, M.K.; Thompson, J.M. Introduction to ANSYS and Finite Element Modeling. In ANSYS Mechanical APDL for Finite Element Analysis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–9. [Google Scholar]
- About Ansys. Available online: https://www.ansys.com/about-ansys (accessed on 22 February 2021).
- Liu, T.; Zhao, D. Numeric simulation and analysis of H 2-O 2 premixed combustion based on OpenFOAM. In Proceedings of the 2012 IEEE Symposium on Robotics and Applications, ISRA 2012, Kuala Lumpur, Malaysia, 3–5 June 2012; pp. 27–30. [Google Scholar]
- OpenFOAM. Available online: https://www.openfoam.com/ (accessed on 18 May 2021).
- Ansys CFX | Industry-Leading CFD Software. Available online: https://www.ansys.com/products/fluids/ansys-cfx (accessed on 18 May 2021).
- Barbu, B.; Iturregi, A.; Berger, F.; Torres, E. Numerical analysis of the electric arc simulation using ansys CFX. In IET Conference Publications; IET: London, UK, 2012; Volume 2012, pp. 311–316. [Google Scholar]
- Yadav, H.N.S.; Kumar, M.; Kumar, A.; Das, M. COMSOL simulation of microwave plasma polishing on different surfaces. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- COMSOL Multiphysics® Software–Understand, Predict, and Optimize. Available online: https://www.comsol.com/comsol-multiphysics (accessed on 18 May 2021).
- Krusch, S.; Scherer, V.; Solimene, R.; Senneca, O. Assessment of coal pyrolysis kinetics for Barracuda or Ansys Fluent. Energy Procedia 2019, 158, 1999–2004. [Google Scholar] [CrossRef]
- Research and General Fluidization|CPFD Software. Available online: https://cpfd-software.com/applications/general-fluidization/ (accessed on 18 May 2021).
| Source | Advantage |
|---|---|
| [16] | Lower voltage requirement than other plasma generator methods. |
| [17] | Lower setup cost due to its ability to operate under atmospheric conditions, also allowing the system to be much more compact in size. |
| [16,18] | Works without an electrode arrangement so that it avoids operational problems specific to electrode utilization. |
| [19] | Microwave energy has already shown its ability to safely combust a variety of hazardous wastes through previous remedial applications. |
| Source | Power Setting | Magnetron | Waveguide | MFC | Three-Stub Tuner | Data Collection Equipment | Other Equipment |
|---|---|---|---|---|---|---|---|
| [28] | 1–6 kW | 2.45 GHz (Sairem GMP G4 60 K T400) | WR-340 | Alicat Scientific, Tucson AZ, USA | Yes | 3 thermocouples, HR 2000+ES spectrometer (Ocean Optics Inc., Largo, FL, USA) | E-3000 precision steam generators |
| [29] | 2–5 kW | 2.45 GHz (Sairem GMP G4 60 K T400) | WR-340 | Alicat Scientific, Tucson AZ, USA | Yes | 4 type K thermocouples, HR2000+ ES spectrometer (Ocean Optics Inc., Largo, FL, USA) | E-3000 precision steam generators |
| [18] | Up to 6 kW | 2.45 GHz (N.S.) | WR-340 | Bronkhorst F-210 AV-50 K | N.S. | Offline micro-gas chromatograph (micro-GC, Varian CP-4900), sampling bags (Tedlar, 15 L) | Impedance tuner, solid feeder |
| [30,31] | 1–1.8 kW | 2.45 GHz (SM 745, Richardson Electronics) | N.S. | Brooks 5850 | Yes | 2 R-type and 5 K-type thermocouples, GC HP 6890, TCD Carbosphere 80/100 packed column, Alltech | Glycerol preheater and feeder, steam supplier, gear pump (Cole Parmer, 74014-750), syringe pump, band heater |
| [25] | 4 kW | 2.45 GHz (N.S.) | WR-340 | N.S. | Yes | Gas analyzer (N.S.) | Quartz plate installed in the end of tapered waveguide |
| [32] | 5 kW | 2.45 GHz (N.S.) | Twisted Waveguide | N.S. | Yes | Gas analyzer (N.S.) | Quartz plate installed in the end of tapered waveguide |
| [33] | 1.2–1.6 kW | 2.45 GHz (N.S.) | WR-248 | N.S. | Yes | Optical emission spectroscopy system, transmission stage, optical fiber bundle, spectrometer, CCD camera, data acquisition unit | Forward and backward power meter controller |
| [34] | 0.8, 0.9, and 1 kW | Not specified | N.S. | N.S. | Yes | GC/TCD, RGA, ESEM, EA (N.S.) | Voltage regulator, cooling water |
| [35] | 0.8–1.8 kW | 2.45 GHz (National Electronics YJ-1600) | WR-340 | N.S. | Yes | GC, FTIR | Cavity resonator |
| [36] | 0.8–1.4 kW | 2.45 GHz (National Electronics YJ-1600) | ASTEX WR-340 | N.S. | Yes | GC/TCD, FTIR, MS | Cavity resonator |
| [37] | Up to 6 kW | 2.45 GHz (N.S.) | WR-340 | Bronkhorst F-201 AV-50 K | Yes | GC, collection bags (N.S.) | Variable reflector, Sairem SAS for all microwave circuits, impedance transformer |
| [38] | Up to 6 kW | 915 MHz, 2.45 GHz | WR-975, WR-430 | N.S. | GC (Shimadzu GC-2014 and SRI 8610 C), FTIR (Thermo Nicolet 380), optical emission spectroscopy (CVI DK-480), CCD camera | Water cooling, ferrite circulator with water load, directional coupler, moveable plunger |
| Source | Feedstock | Rate of Feedstock Input | Reactor Geometry | Operating Pressure | Carrier Gases/Plasma-Forming Gases | Rate of Carrier Gas/Plasma-Forming Gases Input | Ignition Source | Reactor Temperature |
|---|---|---|---|---|---|---|---|---|
| [28] | None | None | Quartz tube (L: 450 mm, OD: 25.6 mm, ID: 30 mm) | Atmospheric | H2O, CO2 | 20–50 g/min, 20–80 SLPM | Inserted tungsten rod | Up to 6300 °C |
| [29] | None | None | Quartz tube (L: 35 cm, OD: 25.6 mm, ID: 30 mm) | Atmospheric | H2O, CO2, Air | 10–50 g/min (up to 200 °C), 0–100 SLPM, 0–100 SLPM | Inserted tungsten rod | Up to 6300 °C |
| [18] | CH1.5O.49 | 09–13 g/s | Quartz Tube (L: 50 mm, OD: 34 mm, ID: 30 mm) | Atmospheric | Air, N2 | 8.5–10 NL/min, 17.9–25 NL/min | Used plasma-forming gas (N2) | 973–2173 K |
| [30,31] | Coal | 1 g/min | Quartz Tube (L:100 cm, ID: 5.8 cm) | Atmospheric | N2, O2, steam | 15 L/min, 0–1.0 L/min, 0–1.5 mL/min | Used plasma-forming gas (N2) | Above 3000 °C |
| [31] | Glycerol | 3 g/min | Quartz Tube (L:100cm, ID: 5.8 cm) | Atmospheric | N2, O2, steam | 15 L/min, 0–2.6 L/min, 0–7.2 mL/min | Used plasma-forming gas (N2) | N.S. |
| [25] | Coal | 0–3.75 kg/h | Quartz tube (L: N.S., OD: 30 mm, thickness: 1.5 mm) | Atmospheric | O2, air | 20 L/min, 15 L/min | Inserted tungsten rod | 2000–6500 K |
| [32] | Coal | 160 mol coal powder/h | Quartz tube (L: N.S., OD: 30 mm, thickness: 1.5 mm) | Atmospheric | O2 | 14 mol/h | N.S. | 5000 °C |
| [33] | None | None | Quartz tube (2.54 cm in diameter and 22.5 cm in length) | Atmospheric | Air, N2, Ar | 30 L/min-60 L/min | Inserted tungsten rod | 5446–6100 K |
| [34] | Spirulina algae | 1 g of dry Spirulina algae | Quartz tube (L: 35 cm, OD: 3.3 cm, ID: 2.9 cm) | Atmospheric | N2 | 12 L/min | N.S. | 1063–1121 K |
| [35] | CH4 | 12–18 SLPM | Quartz tube (OD: 3.3 cm) | Atmospheric | N2 | 12–18 SLPM | N.S. | N.S. |
| [36] | Methanol | 12.4 SLPM | Quartz tube (ID: 2.9 cm) | Atmospheric | N2 | N.S. | N.S. | 1500 K |
| [37] | Cellulose | 0.5 g/s | Quartz tube (ID: 31 mm, wall thickness: 2 mm) | Atmospheric | Air | 15–20 NL/min | Inserted ignition electrode system | 4000–5000 K |
| [38] | Ethanol | Introduced into system via bubbler @ 20 °C and 3% v/v | Quartz tube (N.S.) | Atmospheric | CO2, N2, Ar | 1500–3900 NL/h | N.S. | Up to 6000 K |
| Relationship | Effect |
|---|---|
| O2-to-feedstock ratio |
|
| Steam-to-feedstock ratio |
|
| Gasification efficiency |
|
| Microwave power |
|
| Rate of feedstock input |
|
| Reaction Name | Stoichiometric Description |
|---|---|
| Devolatilization | |
| Oxidation | |
| Water gas reaction | |
| Water gas shift | |
| Boudouard | |
| Methanation | |
| Steam methane reforming | |
| Nitrogenous species | |
| Sulfur species |
| Source | Reactor Type | Modeling Software | Model Used | Devolatilization Considered | Equations/Models Implemented |
|---|---|---|---|---|---|
| [47] | Downdraft Plasma Coal and Biomass Gasifier Reactor | ANSYS Fluent | FRC/EDM | Yes | FRC/EDM Devolatilization: single rate model |
| [54] | Downdraft plasma coal gasifier reactor | ANSYS Fluent | FRC/EDM | Yes | FRC/EDM Devolatilization: single rate model |
| [55] | Pilot-scale plasma bubbling fluidized bed reactor | ANSYS Fluent | FRC/EDM | Yes | FRC/EDM Devolatilization: user-defined function (UDF) using single rate model developed by Badzioch and Hawsley [56]. |
| [6] | Updraft plasma gasifier reactor | ANSYS Fluent | FRC/EDM | Yes | FRC/EDM Devolatilization: UDF |
| [50] | Downdraft plasma gasifier reactor | Aspen Plus | N.S. | Yes | HCOALGEN model: used to estimate the heat of combustion, heat of formation, and heat capacity of feedstock. DCOALIGT model: used to calculate the density of the feedstock. |
| [57] | Plasma spouted bed gasifier | OpenFOAM | N.S. | Yes | Multiphase particle-in-cell approach (MPPICFoam) CoalChemistryFoam |
| Source | Mass Balance Model | Momentum Model | Energy Conservation Model | Turbulence Model |
|---|---|---|---|---|
| [55] | Solid phase: Gas phase: Supporting equations: | Solid phase: Gas phase: | Gas and solid phases: Supporting equations: | model: |
| Variable | Term | Variable | Term |
|---|---|---|---|
| Density | Gas-phase stress tensor | ||
| Instantaneous velocity of gas/solid phase | Gas–solid interphase drag coefficient | ||
| Solid-phase subscript | Mean velocity of solid | ||
| Gas-phase subscript | Generation of turbulence kinetic energy due to the mean velocity gradients | ||
| S | Mass source term | Generation of turbulence kinetic energy due to buoyancy | |
| Reaction rate | Contribution of fluctuating dilatation in compressible turbulence to the overall dissipation rate | ||
| Stoichiometric coefficient | User-defined source term | ||
| Molecular weight | User-defined source term | ||
| R | Universal gas constant | Heat transfer intensity between fluid phase and solid phase | |
| T | Temperature of gas mixture | Heat flux | |
| Gas pressure | Source term due to chemical reactions | ||
| Mass fraction | Enthalpy of the interface | ||
| Molecular weight of each species | Thermal conductivity for phase | ||
| Reynolds number based on diameter of solid phase and relative velocity | Prandtl number of the gas phase |
| Sources | CFD Software | Developer | Quick Specifications |
|---|---|---|---|
| [55,61,62] | Fluent | ANSYS |
|
| [55,63,64] | OpenFoam | Open CFD Ltd. |
|
| [55,65,66] | CFX | ANSYS |
|
| [55,67,68] | COMSOL Multiphysics | COMSOL Inc. |
|
| [55,69,70] | Barracuda | CPFD Software LLC. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedej, O.; Mbonimpa, E. CFD Modeling of a Lab-Scale Microwave Plasma Reactor for Waste-to-Energy Applications: A Review. Gases 2021, 1, 133-147. https://doi.org/10.3390/gases1030011
Sedej O, Mbonimpa E. CFD Modeling of a Lab-Scale Microwave Plasma Reactor for Waste-to-Energy Applications: A Review. Gases. 2021; 1(3):133-147. https://doi.org/10.3390/gases1030011
Chicago/Turabian StyleSedej, Owen, and Eric Mbonimpa. 2021. "CFD Modeling of a Lab-Scale Microwave Plasma Reactor for Waste-to-Energy Applications: A Review" Gases 1, no. 3: 133-147. https://doi.org/10.3390/gases1030011
APA StyleSedej, O., & Mbonimpa, E. (2021). CFD Modeling of a Lab-Scale Microwave Plasma Reactor for Waste-to-Energy Applications: A Review. Gases, 1(3), 133-147. https://doi.org/10.3390/gases1030011