An Improvement in Enclosure Design Can Positively Impact Welfare, Reduce Aggressiveness and Stabilise Hierarchy in Captive Galapagos Giant Tortoises
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Experimental Design and Data Acquisition
2.3. Statistical Analyses
2.3.1. Latency to Habituation
2.3.2. Effect of the Enclosure Change on Time-Budgets and on Interactions
2.3.3. Rearrangement and Stability of the Hierarchy among Individuals
2.3.4. Determinants of the Outcome of an Interaction
3. Results
3.1. Individual Latency to Habituation
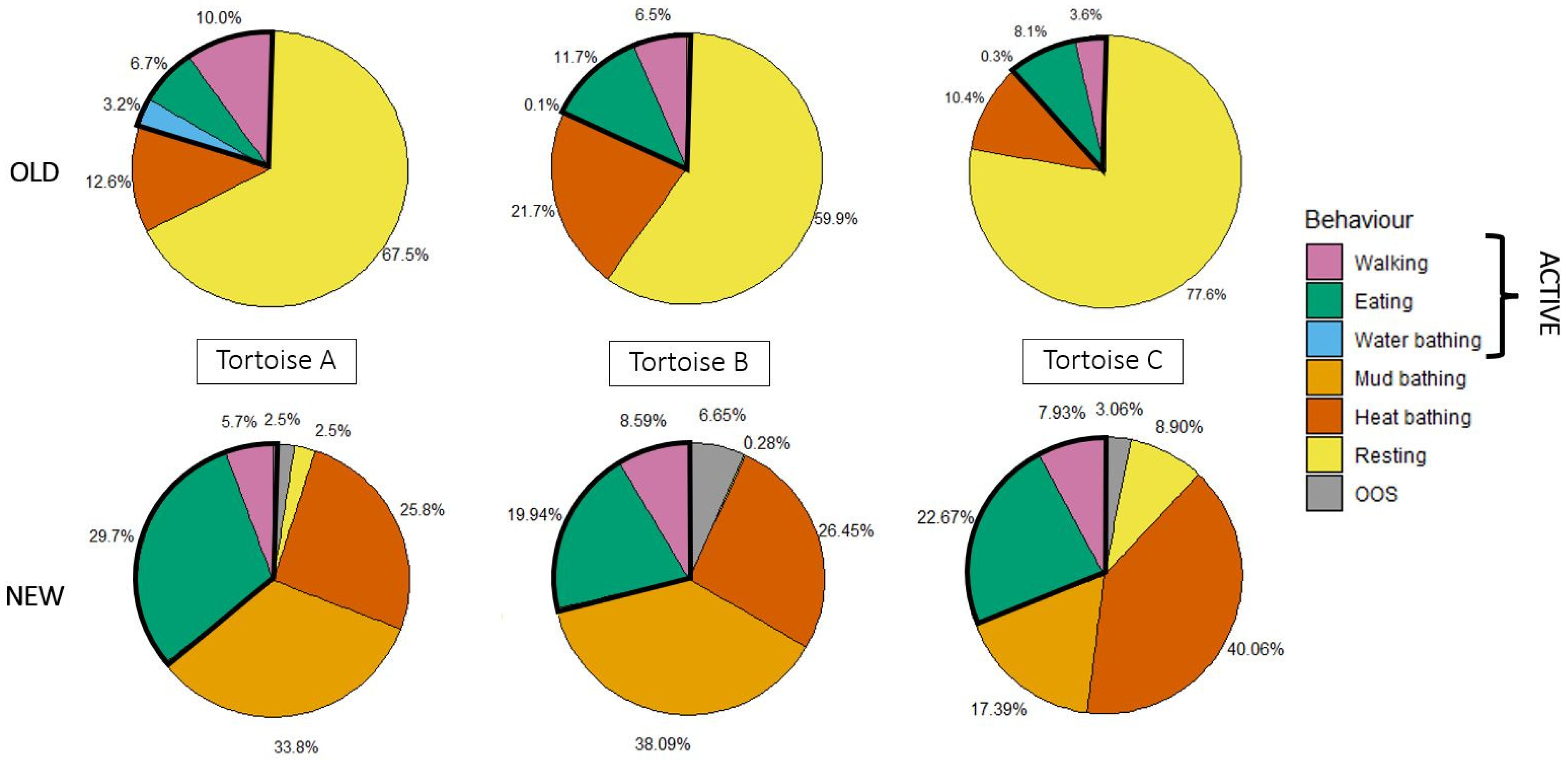
3.2. Effect of Enclosure Change on Time-Budgets and Aggressivity in Habituated Tortoises
3.3. Dynamics of Variation in the Dominance Hierarchy
3.4. Determinants of the Outcome of an Interaction
4. Discussion
4.1. Welfare Implications of Enclosure Switching
4.2. Successful Reduction in the Occurrence of Agonistic Interactions
4.3. Drivers of Aggressions and Audition in Giant Tortoises
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caccone, A.; Gibbs, J.P.; Ketmaier, V.; Suatoni, E.; Powell, J.R. Origin and evolutionary relationships of giant Galapagos tortoises. Proc. Natl. Acad. Sci. USA 1999, 96, 13223–13228. [Google Scholar] [CrossRef] [PubMed]
- Caccone, A.; Gentile, G.; Gibbs, J.P.; Fritts, T.H.; Snell, H.L.; Betts, J.; Powell, J.R. Phylogeography and History of Giant Galapagos Tortoises. Evolution 2002, 56, 2052–2066. [Google Scholar] [CrossRef] [PubMed]
- Ciofi, C.; Milinkovitch, M.; Gibbs, J.J.; Caccone, A.A.; Powell, J.J. Microsatellite analysis of genetic divergence among populations of giant Galápagos tortoises. Mol. Ecol. 2002, 11, 2265–2283. [Google Scholar] [CrossRef] [PubMed]
- Caccone, A. Evolution and phylogenetics. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 117–138. [Google Scholar] [CrossRef]
- Kehlmaier, C.; Albury, N.A.; Steadman, D.W.; Graciá, E.; Franz, R.; Fritz, U. Ancient mitogenomics elucidates diversity of extinct West Indian tortoises. Sci. Rep. 2021, 11, 3224. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. 2021. Available online: https://www.iucnredlist.org/es (accessed on 30 May 2022).
- Cayot, L.J. The history of Galapagos tortoise conservation. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 333–353. [Google Scholar] [CrossRef]
- Toral-Granda, M.V.; Causton, C.E.; Jäger, H.; Trueman, M.; Izurieta, J.C.; Araujo, E.; Cruz, M.; Zander, K.; Izurieta, A.; Garnett, S.T. Alien species pathways to the Galapagos Islands, Ecuador. PLoS ONE 2017, 12, e0184379. [Google Scholar] [CrossRef]
- Frazier, J. The Galapagos: Island home of giant tortoises. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–21. [Google Scholar] [CrossRef]
- Galapagos Conservancy 2021 Outrage at Massacre of Giant Tortoises in Galápagos. Published 13 October 2021. Available online: https://www.galapagos.org/newsroom/outrage-at-massacre-of-giant-tortoises-in-galapagos/ (accessed on 30 May 2022).
- Charney, N.D. Galapagos tortoises in a changing climate. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 317–330. [Google Scholar] [CrossRef]
- Cayot, L.J. The Restoration of Giant Tortoise and Land Iguana Populations in Galapagos. Galapagos Res. 2008, 65, 39–43. Available online: http://hdl.handle.net/1834/36138 (accessed on 30 May 2022).
- Jensen, E.L.; Tapia, W.; Caccone, A.; Russello, M.A. Genetics of a head-start program to guide conservation of an endangered Galápagos tortoise (Chelonoidis ephippium). Conserv. Genet. 2015, 16, 823–832. [Google Scholar] [CrossRef]
- Russello, M.A.; Hyseni, C.; Gibbs, J.P.; Cruz, S.; Marquez, C.; Tapia, W.; Velensky, P.; Powell, J.R.; Caccone, A. Lineage identification of Galápagos tortoises in captivity worldwide. Anim. Conserv. 2007, 10, 304–311. [Google Scholar] [CrossRef]
- Furrer, S.; Hatt, J.; Snell, H.; Marquez, C.; Honegger, R.; Rübel, A. Comparative study on the growth of juvenile Galapagos giant tortoises (Geochelone nigra) at the Charles Darwin Research Station (Galapagos Islands, Ecuador) and Zoo Zurich (Zurich, Switzerland). Zoo Biol. 2004, 23, 177–183. [Google Scholar] [CrossRef]
- Shaw, C.E. Breeding the Galapagos Tortoise—Success Story. Oryx 1967, 9, 119–126. [Google Scholar] [CrossRef]
- Warwick, C. Reptilian ethology in captivity: Observations of some problems and an evaluation of their aetiology. Appl. Anim. Behav. Sci. 1990, 26, 1–13. [Google Scholar] [CrossRef]
- Freeland, L.; Ellis, C.; Michaels, C.J. Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting. Animals 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.F.; Krekorian, C.O. Agonistic Behavior of the Galapagos Tortoise, Geochelone elephantopus, with Emphasis on Its Relationship to Saddle-Backed Shell Shape. Herpetologica 1983, 39, 448–456. [Google Scholar]
- Townsend, C.H. The Galapagos tortoises in their relation to the Whaling Industry—A study of old logbooks. Zool. Sci. Contrib. N. Y. Zool. Soc. 1925, 4, 55–135. [Google Scholar] [CrossRef]
- Cooper, W.E.; Dimopoulos, I.; Pafilis, P. Sex, Age, and Population Density Affect Aggressive Behaviors in Island Lizards Promoting Cannibalism. Ethology 2015, 121, 260–269. [Google Scholar] [CrossRef]
- Schuett, G.W. Fighting dynamics of male copperheads, Agkistrodon contortrix (Serpentes, Viperidae): Stress-induced inhibition of sexual behavior in losers. Zoo Biol. 1996, 15, 209–221. [Google Scholar] [CrossRef]
- McMillan, D.M.; Irschick, D.J. Experimental Test of Predation and Competition Pressures on the Green Anole (Anolis carolinensis) in Varying Structural Habitats. S. Am. J. Herpetol. 2010, 44, 272–278. [Google Scholar] [CrossRef]
- Silvestre, A.M. How to Assess Stress in Reptiles. J. Exot. Pet Med. 2014, 23, 240–243. [Google Scholar] [CrossRef]
- Warwick, C.; Arena, P.; Lindley, S.; Jessop, M.; Steedman, C. Assessing reptile welfare using behavioural criteria. In Practice 2013, 35, 123–131. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Ward, S.J.; Sherwen, S.; Clark, F.E. Advances in Applied Zoo Animal Welfare Science. J. Appl. Anim. Welf. Sci. 2018, 21 (Suppl. S1), 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hoehfurtner, T.; Wilkinson, A.; Nagabaskaran, G.; Burman, O.H.P. Does the provision of environmental enrichment affect the behaviour and welfare of captive snakes? Appl. Anim. Behav. Sci. 2021, 239, 105324. [Google Scholar] [CrossRef]
- Mellen, J.; Macphee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Berger, A. Activity patterns, chronobiology and the assessment of stress and welfare in zoo and wild animals. Int. Zoo Yearb. 2011, 45, 80–90. [Google Scholar] [CrossRef]
- Cayot, L.J. Ecology of Giant Tortoises (Geochelone elephantopus) in the Galapagos Islands. Ph.D. Dissertation, Syracuse University, Syracuse, NY, USA, 1987. [Google Scholar]
- Blake, S.; Tapia, P.I.; Safi, K.; Ellis-Soto, D. Diet, Behavior, and Activity Patterns. In Galapagos Giant Tortoises; Gibbs, J.P., Cayot, L.J., Aguilera, W.T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 207–239. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 29 June 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, R Package version 3.3.6; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 30 May 2022).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots; R Package Version 0.2.5; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 30 May 2022).
- Killick, R.; Haynes, K.; Eckley, I.A. Changepoint: An R Package for Changepoint Analysis; R Package Version 2.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=changepoint (accessed on 30 May 2022).
- Neumann, C.; Kulik, L. EloRating: Animal Dominance Hierarchies by Elo Rating; R Package Version 0.46.11; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=EloRating (accessed on 30 May 2022).
- Bolker, B.M. Linear and Generalized Linear Mixed Models. In Ecological Statistics: Contemporary Theory and Application; Fox, G.A., Negrete-Yankelevich, S., Sosa, V.J., Eds.; Oxford University Press: New York, NY, USA, 2015; pp. 309–333. [Google Scholar] [CrossRef]
- Eagan, T. Evaluation of Enrichment for Reptiles in Zoos. J. Appl. Anim. Welf. Sci. 2019, 22, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.M.; Rose, P.E. Concepts, applications, uses and evaluation of environmental enrichment: Perceptions of zoo professionals. J. Zoo Aquar. Res. 2020, 8, 18–28. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Brent, L.Y.; Schapiro, S.J. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab. Anim. Sci. 1991, 41, 372–377. [Google Scholar]
- Young, R.J. Environmental Enrichment for Captive Animals; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wells, A.; Terio, K.A.; Ziccardi, M.H.; Munson, L. The Stress Response to Environmental Change in Captive Cheetahs (Acinonyx jubatus). J. Zoo Wildl. Med. 2004, 35, 8–14. [Google Scholar] [CrossRef]
- Ferreira, V.H.B.; Fonseca, E.D.P.; Das Chagas, A.C.C.S.; Pinheiro, L.G.M.; de Sousa, M.B.C.; da Silva, H.P.A.; Galvão-Coelho, N.L.; Ferreira, R.G. Personality traits modulate stress responses after enclosure change of captive capuchin monkeys (Sapajus libidinosus). Appl. Anim. Behav. Sci. 2020, 232, 105111. [Google Scholar] [CrossRef]
- Little, K.A.; Sommer, V. Change of enclosure in langur monkeys: Implications for the evaluation of environmental enrichment. Zoo Biol. 2002, 21, 549–559. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, M.S. Time budgets in Red Junglefowl as a baseline for the assessment of welfare in domestic fowl. Appl. Anim. Behav. Sci. 1989, 24, 77–80. [Google Scholar] [CrossRef]
- Veasey, J.; Waran, N.; Young, R. On Comparing the Behaviour of Zoo Housed Animals with Wild Conspecifics as a Welfare Indicator, Using the Giraffe (Giraffa camelopardalis) as a Model. Anim. Welf. 1996, 5, 139–153. [Google Scholar]
- Rose, P.; Roper, A.; Banks, S.; Giorgio, C.; Timms, M.; Vaughan, P.; Hatch, S.; Halpin, S.; Thomas, J.; O’Brien, M. Evaluation of the time-activity budgets of captive ducks (Anatidae) compared to wild counterparts. Appl. Anim. Behav. Sci. 2022, 251, 105626. [Google Scholar] [CrossRef]
- Johnson, B.; Langton, J. Behaviour change in Amur tigers Panthera tigris altaica after an enclosure move. J. Zoo Aquar. Res. 2021, 9, 186–192. [Google Scholar] [CrossRef]
- Ryan, J.C.; Litchfield, C.A. Impact of an enclosure rotation on the activity budgets of two zoo-housed giant pandas (Ailuropoda melanoleuca): An observational case study. Eat Sleep Work 2020, 1, 26–38. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Pardo-Sanchez, J.; Weise, C. The establishment and maintenance of dominance hierarchies. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200450. [Google Scholar] [CrossRef]
- Evans, L.T.; Evans, L.T.; Quaranta, J.V. A study of the social behavior of a captive herd of giant tortoises. Zoologica 1951, 36, 171–181. [Google Scholar] [CrossRef]
- Alberts, A.C. Dominance hierarchies in male lizards: Implications for zoo management programs. Zoo Biol. 1994, 13, 479–490. [Google Scholar] [CrossRef]
- Gutnick, T.; Weissenbacher, A.; Kuba, M.J. The underestimated giants: Operant conditioning, visual discrimination and long-term memory in giant tortoises. Anim. Cogn. 2020, 23, 159–167. [Google Scholar] [CrossRef]
- Mafli, A.; Wakamatsu, K.; Roulin, A. Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Anim. Behav. 2011, 81, 859–863. [Google Scholar] [CrossRef]
- Radzio, T.A.; Cox, J.A.; Spotila, J.R.; O’Connor, M.P. Aggression, Combat, and Apparent Burrow Competition in Hatchling and Juvenile Gopher Tortoises (Gopherus polyphemus). Chelonian Conserv. Biol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Waters, R.M.; Bowers, B.B.; Burghardt, G.M. Personality and Individuality in Reptile Behavior. In Personality in Nonhuman Animals; Vonk, J., Weiss, A., Kuczaj, S.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 153–184. [Google Scholar] [CrossRef]
- Carlson, B.E.; Tetzlaff, S.J. Long-term behavioral repeatability in wild adult and captive juvenile turtles (Terrapene carolina): Implications for personality development. Ethology 2020, 126, 668–678. [Google Scholar] [CrossRef]
- Jacobs, J.A.; Coe, J.B.; Widowski, T.M.; Pearl, D.L.; Niel, L. Defining and Clarifying the Terms Canine Possessive Aggression and Resource Guarding: A Study of Expert Opinion. Front. Vet. Sci. 2018, 5, 115. [Google Scholar] [CrossRef]
- Quadros, S.; Goulart, V.D.L.R.; Passos, L.; Vecci, M.A.; Young, R.J. Zoo visitor effect on mammal behaviour: Does noise matter? Appl. Anim. Behav. Sci. 2014, 156, 78–84. [Google Scholar] [CrossRef]
- Jackson, C.G.; Awbrey, F.T. Mating Bellows of the Galapagos Tortoise, Geochelone elephantopus. Herpetologica 1978, 34, 134–136. [Google Scholar]
- Carter, K.C.; Keane, I.A.T.; Clifforde, L.M.; Rowden, L.J.; Fieschi-Méric, L.; Michaels, C.J. The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. J. Zoöl. Bot. Gard. 2021, 2, 664–676. [Google Scholar] [CrossRef]



| State Behaviour | Definition | Classification |
|---|---|---|
| Eating | Consuming food or water | Active |
| Walking | Moving, in locomotion | |
| Water bathing | Being at least partially in the pond | |
| Mud bathing | Being at least partially in the mud wallow | Inactive |
| Heat bathing | Resting under a heat station | |
| Resting | Lying on the floor, stationary | |
| Out-of-sight | Not visible from any camera | |
| Interaction | Two animals face each other, close enough to touch | Classification |
| Peaceful | Both individuals keeping their head out at resting level, no biting attempt | Non-agonistic |
| Intimidation | One individual raising head higher than resting level, the other retracting head in submission, no biting attempt | Agonistic |
| Fight | Both individuals raising head higher than resting level and trying to bite each other |
| Characteristic | Old Enclosure | New Enclosure |
|---|---|---|
| Total area | ~68 m2 | ~350 m2 |
| Humidity | Not regulated | Regulated |
| Temperature | Not regulated | Regulated |
| Possibility to hide from visitors | No | Yes |
| Number of sand pits | 0 | 1 |
| Number of mud wallows | 0 | 1 |
| Number of heat stations | 1 | 3 |
| Number of water ponds | 1 | 2 |
| Live plants | No | Yes |
| Behaviour | t | df | p-Value | Cohen’s d |
|---|---|---|---|---|
| Walking | 0.269 | 2 | 0.813 | 0.313 |
| Eating | 3.592 | 2 | 0.069 | 1.682 |
| Water bathing | −1.208 | 2 | 0.350 | −1.000 |
| Mud bathing | 4.696 | 2 | 0.042 | 1.680 |
| Heat bathing | 2.187 | 2 | 0.160 | 1.494 |
| Resting | −24.19 | 2 | 0.002 | −1.799 |
| Active | 5.839 | 2 | 0.028 | 1.667 |
| Inactive | −12.481 | 2 | 0.006 | −1.753 |
| Interaction | t | df | p-Value | Cohen’s d |
|---|---|---|---|---|
| Fight | −0.886 | 7 | 0.405 | −0.602 |
| Intimidation | −1.005 | 5 | 0.361 | −0.840 |
| Peaceful | −10.258 | 7 | <0.01 | −1.837 |
| Total interactions | −7.350 | 7 | <0.01 | −1.136 |
| Individual | Old Enclosure | New Enclosure [D0-D6] | New Enclosure [D7-D10] |
|---|---|---|---|
| Tortoise A | 1062 | 991 | 1105 |
| Tortoise B | 773 | 1250 | 1129 |
| Tortoise C | 1165 | 759 | 766 |
| Stability | 0.250 | 0.923 | 0.667 |
| Fixed Effect | Estimate | z-Value | p-Value | Odds-Ratio [95%CI] |
|---|---|---|---|---|
| Intercept | −47.13 | −0.019 | 0.985 | |
| Daytime (Morning) | −0.647 | −0.491 | 0.624 | 0.524 [0.040; 6.933] |
| Daytime (Mid-day) | 0.006 | 0.007 | 0.995 | 0.994 [0.144; 6.852] |
| Pair (B–C) | −1.033 | −0.977 | 0.328 | 0.356 [0.045; 2.826] |
| Pair (C–A) | −1.849 | −1.443 | 0.149 | 0.157 [0.013; 1.942] |
| LZeq | 0.425 | 1.970 | 0.049 | 1.530 [1.002; 2.336] |
| Visitors | 0.172 | 0.842 | 0.400 | 1.188 [0.796; 1.772] |
| Resource | 18.700 | 0.008 | 0.994 | 13.22 × 107 [0; Inf] |
| Date | −0.034 | −0.074 | 0.941 | 0.967 [0.392; 2.381] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fieschi-Méric, L.; Ellis, C.; Servini, F.; Tapley, B.; Michaels, C.J. An Improvement in Enclosure Design Can Positively Impact Welfare, Reduce Aggressiveness and Stabilise Hierarchy in Captive Galapagos Giant Tortoises. J. Zool. Bot. Gard. 2022, 3, 499-512. https://doi.org/10.3390/jzbg3040037
Fieschi-Méric L, Ellis C, Servini F, Tapley B, Michaels CJ. An Improvement in Enclosure Design Can Positively Impact Welfare, Reduce Aggressiveness and Stabilise Hierarchy in Captive Galapagos Giant Tortoises. Journal of Zoological and Botanical Gardens. 2022; 3(4):499-512. https://doi.org/10.3390/jzbg3040037
Chicago/Turabian StyleFieschi-Méric, Léa, Charlotte Ellis, Francesca Servini, Benjamin Tapley, and Christopher J. Michaels. 2022. "An Improvement in Enclosure Design Can Positively Impact Welfare, Reduce Aggressiveness and Stabilise Hierarchy in Captive Galapagos Giant Tortoises" Journal of Zoological and Botanical Gardens 3, no. 4: 499-512. https://doi.org/10.3390/jzbg3040037
APA StyleFieschi-Méric, L., Ellis, C., Servini, F., Tapley, B., & Michaels, C. J. (2022). An Improvement in Enclosure Design Can Positively Impact Welfare, Reduce Aggressiveness and Stabilise Hierarchy in Captive Galapagos Giant Tortoises. Journal of Zoological and Botanical Gardens, 3(4), 499-512. https://doi.org/10.3390/jzbg3040037







