1. Introduction
The Association of Zoos and Aquariums defines enrichment as a process that improves and/or enhances animal environments and care, with the goal of increasing behavioral choices and providing opportunities for species-appropriate behaviors [
1]. Enrichment is a principle that is meant to “enhance the quality of captive animal care by identifying and providing the environmental stimuli necessary for optimal psychological and physiological well-being” (p. 1) [
2]. There are various types of enrichment, with environmental enrichment being the most common and often just referred to plainly as enrichment, given how routine it is for animals in human care [
3]. Environmental enrichment can include foraging tasks, toys and objects that can be manipulated by the animal, physically altering the environment, and sensory stimuli. Various other subsets of enrichment include food-based enrichment (the animal gains food as part of the enrichment), structural enrichment (changes or additions are made to the environment for enrichment purposes), sensory enrichment (engages one or more senses as part of enrichment), and cognitive enrichment.
Cognitive enrichment promotes well-being by engaging cognitive skills in animals but is not frequently used in captive settings [
3]. For example, computer game tasks that test cognitive skills using discrimination tasks via a computer touchscreen serve as cognitive enrichment [
4]. Any task that requires training, such as a two-alternative forced-choice task or a match to sample task, can also be viewed as cognitive enrichment because the animals must learn behaviors by interacting with a human trainer and follow conceptual rules to gain a reward. Cognitive enrichment has been gaining more attention; however, it is likely not used as much due to the increased amount of time and effort from human caretakers and staff this type of enrichment can necessitate. Without proper enrichment, animals residing in human care can face negative consequences. For example, carnivores such as otters can display abnormal behaviors, such as pacing or hair plucking, when under human care if they expend too little effort to obtain their food [
5]. However, when appropriate enrichment devices or techniques are used for the species, abnormal behavior patterns can decrease in otters [
6,
7].
When considering what kind of enrichment to use for an animal, it is important to understand their habitat and the naturalistic behaviors that species would display in that environment. North American river otters are members of the subfamily Lutrinae within the family Mustelidae. There are 13 otter species that vary in their habitat, diet, size, and foraging skills [
8]. North American river otters are found throughout most of North America from the Atlantic to Pacific coasts in a wide range of habitats including rivers, streams, lakes, bogs, and rocky seacoasts [
9]. North American river otters normally reside in social groups of females and their young or unrelated males that have overlapping home ranges with other males showing their gregariousness [
9]. Their primary food source tends to be small, bottom-dwelling fish, but they also consume crustaceans and insects along with a variety of other species in more opportunistic situations such as birds, mammals, reptiles, and amphibians [
10,
11,
12,
13,
14,
15,
16]. Therefore, otters forage both underwater and on land, depending on their prey type. North American river otters usually find their den sites on land, utilizing available structures such as tree logs and abandoned dens made by other animals [
17]. Female otters will also prepare natal nests on land near small streams in a secluded location [
17]. As semi-aquatic mammals, North American river otters spend time both in the water as well as on land, so they frequently use their senses both underwater and in the air.
Enrichment is frequently used in zoos to encourage species-specific, naturalistic behaviors and to reduce stereotypical behaviors, which is important for carnivores like river otters that can capture a diverse array of prey items [
18]. There have been few studies examining enrichment practices with otters, and they have largely focused on foraging tasks and food-based enrichment. For example, Ross [
7] introduced a simple feeding enrichment technique for Asian small-clawed otters (
Aonyx cinerea) where two of their three daily meals were inserted into grapevine balls and then placed in a pool. Various stereotypical behaviors, including auto hair-plucking and door manipulations (trying to move/open doors), significantly decreased in the two otters when the feeding enrichment was used. Foster-Turley and Markowitz [
19] also used a simple feeding enrichment task where Asian small-clawed otters were given the opportunity to hunt live crickets. Compared to when the otters were given dead crickets, cat food, and gelatin capsules, the otters were most engaged with hunting the live crickets. This foraging opportunity increased the activity of the otters, and they still chose to engage in the foraging activities even when other food items were easily available. In this study, the otters were very inquisitive and would seek out a challenge rather than have easy access to their food [
19].
In another study, Nelson [
6] introduced multiple environmental enrichment initiatives to a male North American river otter. These included live fish, frozen fish, a swim tube (a large tube the otter could swim through), and PVC scent tubes placed throughout the exhibit. The two food-based enrichment initiatives (live fish and frozen fish) were the most effective at decreasing stereotyped behaviors, and increasing species-appropriate behaviors, compared to the other two enrichment initiatives [
6]. These three studies [
6,
7,
19] show how effective food-based enrichment tasks appear to be for Asian small-clawed otters and river otters but do not explore other types of enrichment extensively.
Cognitive challenges are important for captive animals to experience and may be especially important for otters. Even when they fail to solve the task, the cognitive challenge itself may be enriching for otters [
18]. This concept was supported in a study where two sea otters (
Enhydra lutris) were given a novel tool-use task. The sea otters were not successful in solving the tool-use task; however, for one of the otters it seemed that the task was still enriching. The otter spent 55.4% of her time during each trial with the apparatus, compared to the other otter that spent only 6.2% of his time with the apparatus [
18]. This study also highlights how it can be important for enrichment to be individualized. Some tasks may be engaging and enriching for certain individuals but not for others.
In addition to tasks that are designed with enrichment as the main goal, cognitive tasks designed to study the perceptual or cognitive abilities of animals can also be enriching. The animal must use their cognitive skills to engage in the task they are participating in, which could be classified as cognitive enrichment [
3]. Two studies used a two-alternative forced-choice task to study the perceptual and cognitive abilities of North American river otters [
20,
21]. In this task, the otter is trained to choose between two different visual stimuli on each trial. A correct choice results in a food reward, whereas an incorrect choice results in the otter not receiving food for that trial (note that the otters were never deprived of food; they always received their full diet each day). The otters were often motivated to participate in this cognitive task even when it could be quite challenging for the otters to learn a conceptual rule. For example, although one otter’s performance was not significantly different than chance during the first test of the second experiment [
20], the otter would still readily participate on every trial (performance on later tests was approximately 70% correct, with the other otter achieving approximately 80% correct in the first experiment). In addition to these cognitive tasks being enriching for the animal subject, it also allows researchers to learn more about the cognitive and perceptual abilities of many understudied species that are kept in zoos. For example, DeLong et al. [
20] found preliminary evidence of categorization (the ability to group objects together based on defining features) in North American river otters. These scientific findings can in turn be applied to aid in creating better enrichment initiatives for the species studied across many zoos.
Various types of enrichment can be used to study cognitive phenomena in otters, such as memory. Saliveros et al. [
22] studied spatial and long-term memory of Asian short-clawed otters (
Aonyx cinereus) in a foraging context. Three groups of otters (
n = 25 individuals) were presented with five novel extractive foraging tasks (which could be classified as either food-based or cognitive enrichment). The otters solved all five foraging puzzles significantly faster the second time they were introduced (
M = 65.23 s) compared to the first time (
M = 160.64 s), even when this time interval between sessions was greater than 100 days. This study shows support for otters possessing long-term memory capabilities in a foraging context. A simulated foraging task provided cognitive enrichment while investigating spatial memory in Asian small-clawed otters [
23]. Thus far, no studies have examined memory of any kind in North American river otters (
Lontra canadensis).
In the current study, we sought to examine long-term object recognition memory (the ability to recognize a familiar object over varying lengths of time; [
24]) in a North American river otter using a technique that involved sensory enrichment. It is important to study memory in otters to gain knowledge about the species as well as inform enrichment programs for zoos with otters. The ability to remember objects could be useful and adaptive for otters, such as while foraging. There is evidence that otters use sight, as their primary sensory modality when foraging, as well as touch to catch their prey [
25]. A foraging otter could use multiple sensory modalities along with object recognition memory to find the prey that will result in the greatest metabolic payoff [
26]. Otters could also potentially remember aversive events, including conflicts with predators such as wolves, coyotes, bobcats, alligators, and/or killer whales [
9], depending on where the otters reside. Otters could use object recognition memory to identify predators and avoid future conflicts.
In order to develop proper sensory enrichment, it is important to understand the perceptual abilities of the species the enrichment is being designed for. However, not much is known about the visual acuity of North American river otters. Balliet and Schusterman [
27] found that in bright conditions, Asian small-clawed otters had equivalent visual acuity in the air (14–15 min of arc) as they did underwater (15–16 min of arc). However, in dim light conditions Asian small-clawed otters showed better visual acuity in the air (38–39 min of arc) than underwater (57–58 min of arc; [
28]). Regarding color vision, two anatomical studies found that both sea otters and European river otters (
Lutra lutra) have dichromatic color vision with S and M/L cones [
28,
29,
30]. In a behavioral study, European river otters were able to discriminate blue and green from multiple shades of gray (Kasprzyk, 1990, cited in [
31]). Svoke et al. [
32] suggests that Asian small-clawed otters likely have dichromatic vision as well. Additionally, DeLong et al. [
21] found preliminary evidence of dichromatic vision in North American river otters, though further research is necessary.
There have only been two studies that examined visual object discrimination in any species of otter and both investigated North American river otters [
21,
33]. Slack [
33] presented two North American river otters, a male and female, with a variety of 2D stimulus pairs. In a two-alternative forced-choice task, one otter succeeded with all eleven stimulus pairs while the second otter succeeded with only two of the stimulus pairs. The features used by the otters to complete the task were not investigated. DeLong et al. [
21] presented two North American river otters with 2D objects to explore salient visual features, including shape and color. Both otters successfully learned to discriminate between training stimuli using multiple features, including shape and color in combination with other possible features such as the size and brightness of the stimuli. One of the otters was able to discriminate between test stimuli equally well using either shape (when color was removed as a cue) or color (when shape was removed as a cue). The DeLong et al. [
21] study showed that North American river otters are able to discriminate between 2D objects using multiple features and are potentially equally likely to rely on color or shape.
In addition to vision, olfaction is an important sensory modality in otters which can be engaged during sensory enrichment. Rostain et al. [
34] showed that river otters perceive olfactory signals that communicate both sex and species identity. Signaling territory through scent cues is a behavior that otters frequently engage in. North American river otters tend to leave spraint sites, mostly consisting of large piles of scat, outside of their dens, next to freshwater pools along seacoasts, at junctions of streams, and near trees [
9]. To date, there is no published research to suggest how often otters use olfaction to identify objects other than animals or individual otters. Similarly lacking is psychophysical research on olfactory detection and discrimination in otters.
We chose to investigate object recognition memory using sensory enrichment involving both visual and olfactory elements. Object recognition memory is a type of memory that may aid non-human animals, including otters, in avoiding predators, locating food, choosing mates, and selecting an appropriate habitat. Object recognition memory can be defined as the ability to discriminate between familiar and novel objects [
35]. Object recognition memory has been studied in a variety of non-human animals such as dogs [
36], domestic pigs [
35], rodents [
37,
38,
39], monkeys [
40], and fish [
41,
42] as well as human infants (e.g., [
43,
44]). To date, there has not been any research examining object recognition memory in any species of otter using any of these tasks.
Object recognition memory is frequently assessed in non-human animals using the novel object recognition (NOR) task [
42], which was the task used in the current study. The NOR task was originally developed for rats [
45] but has been adapted and used to investigate object recognition memory in many different non-human animal species (e.g., monkeys, horses, birds, cats, and pigs). In this task, an animal is first exposed to two identical objects in the familiarization trial that they have never seen before (e.g., two balls that are exactly the same in shape, size, color, material, etc.). The animal is then removed from the test area for the memory interval that the researcher wants to test. The memory interval is the time between the end of the familiarization trial and the test trial. Finally, the animals reenter the test area where this time they are exposed to one familiar object from the previous familiarization trial, as well as a novel object (e.g., one of the balls they were shown in the familiarization trial and a water bottle that they have never seen before). The animal is then observed, and their behavior is recorded in both the familiarization and the test trials to see if they show a significant preference for either object, usually based on the amount of time spent close to and/or interacting with the object [
24,
41,
42,
46]. Significantly more time spent near or interacting with either the familiar or the novel object would demonstrate that the animal remembered the familiar object and thus is evidence of object recognition memory [
42]. If no difference is found in the time spent with the familiar or novel object, this would demonstrate that the animal did not remember the familiar object, which would not provide evidence of object recognition memory.
The NOR task has many advantages that make it suitable to collect data on memory in otters while also serving as sensory enrichment. The NOR task is relatively simple in that it does not involve training (e.g., training the animal via operant conditioning to approach or avoid any stimulus for a food reward), so there is less involvement from zookeepers, and the results can be obtained relatively quickly. The NOR task is also easily adapted to a variety of species, making it quite a useful task in comparative psychology research [
47]. Since the NOR task has been used in such a wide variety of species, is relatively simple to perform, and has ecological relevance for many species [
47] this was a good task to use as a contrast to a highly time-intensive cognitive enrichment training task (as in [
21]). The presentations of numerous different stimuli during the NOR task allow the otters to explore new objects using vision and olfaction, which provides opportunities for sensory enrichment.
The current study was the first study to utilize the NOR task with any species of otter. One goal of this study was to explore the usefulness of a relatively simple, less time-intensive sensory enrichment task in otters, and contrast the engagement and attention of the otters with highly time-intensive cognitive enrichment training tasks. Another goal was to search for evidence of object recognition memory in otters. When conducting cognitive research with zoo animals, it is best to provide tasks that can serve as some form of enrichment. It is beneficial to the animals participating in the research, and it fulfills a mission of the AZA by enhancing the welfare of animals under human care [
48].
The current study provided sensory enrichment by utilizing multimodal stimulus pairs. The stimulus pairs in this study were 3D objects that were visually accessible paired with odorants that were contained in plastic bottles and presented next to the 3D objects. Visual stimuli are frequently used in studies examining object recognition memory, especially when the NOR task is used, e.g., [
24,
35,
36,
37,
41,
42]. However, olfaction seems to be a primary sensory modality in otters that is used throughout their daily lives [
49]. In fact, olfaction is said to be one of the most frequently used senses in mustelids [
50]. Since otters may frequently use both vision and olfaction, it seemed best to use multimodal stimulus pairs in this study to ensure that the otter could discriminate between the objects as well as encode multiple cues during the familiarization trial. Therefore, multiple cues were provided in two (potentially three if the otter chose to touch the stimulus pairs) different sensory modalities to maximize potential discrimination and enrichment opportunities.
The objects and the odorants were novel to the otter. New objects that the otter had never encountered were used for the visual component of the stimulus pairs. Odorants were selected based on several criteria: a food item the otter does not eat (e.g., no meat scents were used), the otter was unlikely to have encountered it before (e.g., artificial floral scents), and the zookeeper reported it was not used in any previous enrichment experiences. Another reason for using multimodal stimuli is multisensory facilitation, which is the phenomena where organisms benefit from using multiple senses to complete cognitive tasks [
51]. The otter subject in our study could potentially use vision, olfaction, or both to remember the stimulus pairs.
This study investigated object recognition memory at three memory intervals (10 min, 1 h, and 24 h). These memory intervals were chosen to test a range of intervals while limiting the total number of sessions (10 sessions per interval) to fit in a constrained testing period. The familiarization trials were recorded in the current study to observe whether time spent with the stimuli and explorations of the stimuli decreased over time and then restrict analyses for the test trials to the appropriate time period. We hypothesized that the otter would show evidence of object recognition memory across all the memory intervals tested since Saliveros et al. [
22] found evidence of long-term memory within a foraging context of Asian short-clawed otters after memory intervals of over 100 days. If the NOR task provides effective sensory enrichment it could inform future enrichment programs for the otters. In contrast, if this task does not provide effective sensory enrichment, it suggests other forms of enrichment may be more optimal for river otters.
5. Video Coding
Video coding was completed by three independent coders who were all blind to the identity of the stimulus pairs (whether they were novel or familiar) while coding. Since they were blind, the coders just referred to the stimulus pairs as right stimulus pair and left stimulus pair when coding. The experimenter trained the coders using two familiarization session videos as examples, though the experimenter did not serve as one of the three video coders. All of the test trials and the familiarization trials were coded by a primary coder. There were also two secondary coders. Each secondary coder coded 50% of the data, one of each memory interval within each test block (
Table 1), instead of 100% of the data like the primary coder. The secondary coders did not have access to the primary coder’s data. The two secondary coders did have access to each other’s data, but they coded the opposite 50% of the videos (e.g., they never coded the same video). Therefore, 100% of the videos were coded by one of the secondary coders. The criterion for inter-rater reliability was set at 80% or higher [
54]. This was not met at first when the familiarization videos were coded, so the five instances where there were large discrepancies between coders (differences in timing greater than 10 s, or any differences in the number of explorations) were highlighted. The procedures for video coding were reviewed with all three coders in a re-training meeting with the experimenter. The main coder and the reliability coder for that video then recoded that part of the video. After recoding the criterion was met for all familiarization sessions.
There were two dependent variables that were coded in each video of test trials: (1) the amount of time spent in each section of the enclosure, and (2) the number of explorations made to each stimulus pair with the nose or paw on either the 3D object or the odorant bottle. The time spent near each stimulus pair is almost always examined as the primary dependent variable in the NOR task, (e.g., [
35,
41,
42]), and the number of touches (explorations) has been used as a secondary dependent variable [
24]. To code the amount of time spent on each side of the enclosure, there were visible lines on the floor separating the areas close to each stimulus pair from the area not in close proximity to the stimulus pairs at the back of the enclosure (
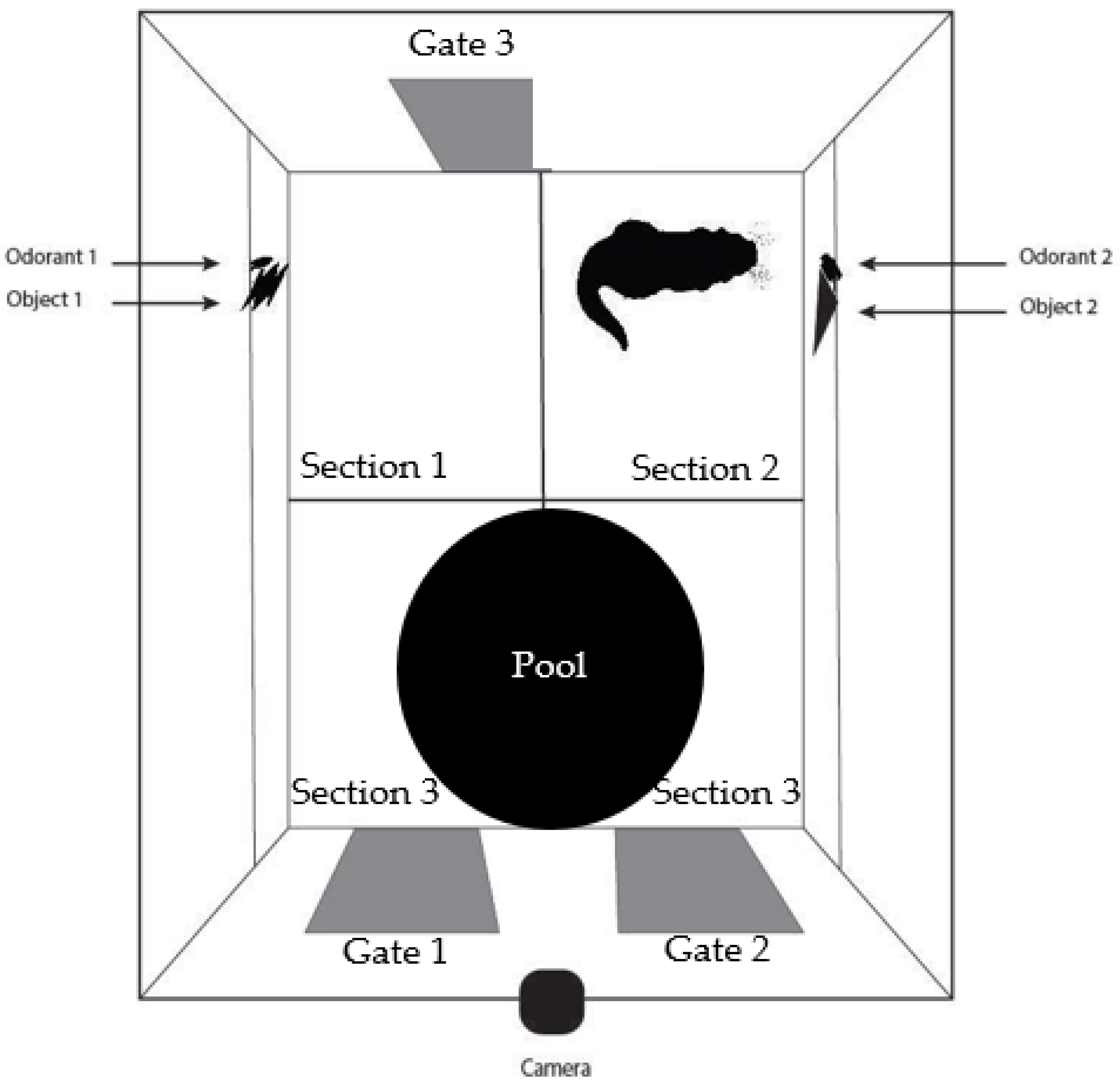
Figure 1). The video was watched in its entirety twice; once to code the time spent close to the right stimulus pair, and once to code the time spent close to the left stimulus pair. During the first time watching the video, the coder kept a tally of the number of explorations to both stimulus pairs. This was repeated the second time watching the video to ensure the correct number of explorations was coded. If necessary, it was permitted for the coders to watch the video for a third time, skipping to a certain section of the video where there was a potential exploration. This was done on occasion because it was sometimes difficult to determine if the otter was close enough to the stimulus pair (within a few centimeters) to count as an exploration.
To code the time spent with a stimulus pair, when the otter’s entire body crossed one of the lines (both his front and back legs, but not his tail), the coder would start a stopwatch. As soon as the otter’s head and front paws touched the line again as he was heading to the opposite side or the back of the enclosure, the coder would pause the stopwatch.
Section 1 and
Section 2 were coded separately and
Section 3 was not coded as it was not necessary for any of the planned analyses. In the familiarization trials, the amount of time spent in
Section 1 and
Section 2 was coded in 5-min increments (
Figure 1). The average amount of time spent near the stimulus pairs and number of explorations was compared across each 5-min increment during the familiarization trial. Lucon-Xiccato and Dadda (2014) only coded the first five minutes of their test trials after they found a decrease in time spent close to the stimulus after the first five minutes in the familiarization trials, and a similar pattern was found in the current study. After the first five minutes in the familiarization sessions, Sailor spent very little time with the stimulus pair on the right and hardly made any explorations (
Figure 5). Therefore, when coding the test videos only the first five minutes were coded and included in the analyses.
To code the explorations, the coder kept a tally of the number of times the otter touched each stimulus pair while they were coding the time spent on each side. The video was coded for the number of explorations in one complete sitting. It was sometimes difficult to determine in the video if the otter made direct contact with the stimulus, or if he was just very close to the stimulus (within a few centimeters) but not actually touching it. Therefore, an exploration was defined as the otter’s nose being within a few centimeters from the stimulus. Sailor has been previously trained to touch stimuli with his nose, so whenever any touches were made to the stimuli he used his nose not his paws. This is why his nose was specified in the definition of an exploration for the purpose of the current study. Additionally, during video coding none of the coders ever saw Sailor touch the stimulus pairs with his paws.
6. Data Analyses
Statistical analyses were performed using SPSS Statistics for Mac, version 28 (SPSS Corp., Armonk, NY, USA). All of the data were checked manually to ensure no data entry errors were made. There was no missing data since all sessions were completed in full. Since this was a single subject design, determining the normality of the familiarization and test data was important for choosing a statistical method. Normality of the familiarization data was assessed using a Shapiro–Wilk test and showed that the overall time spent near the two stimulus pairs did depart significantly from normality (
W = 0.95,
p = 0.004). The Normal Q-Q plot and histogram were examined to further assess normality. The Q-Q plot provided evidence of normality, but the histogram seemed to show that the data were of a more uniform distribution. Finally, the skewness was 0.27 (values between −1 and 1 are generally considered excellent and indicate the shape of the data are fairly normal, while values between −2 and 2 are generally acceptable; George & Mallery, 2020), indicating that the distribution was approximately symmetric, and the kurtosis was −1.12, indicating the distribution was more heavy-tailed compared to the normal distribution (the general rules for interpreting kurtosis follow the same as for skewness; [
55]. We decided to use paired-samples
t-tests to analyze the familiarization data because the familiarization data were only going to be used to determine the amount of time to code the test videos and not to form any conclusions regarding memory and because there was some evidence of normality.
Multiple paired-samples
t-tests were used to analyze the familiarization sessions to determine the length of time test videos should be coded. Therefore, a Bonferroni correction was performed to ensure the alpha stayed at 0.05. Seven total
t-tests were completed; therefore, the corrected alpha was 0.007. One paired-samples
t-test was completed to compare the overall average time in
Section 1 (near the left stimulus pair) to
Section 2 (the area near the right stimulus pair). The remaining six paired-samples
t-tests compared each of the four 5-min intervals to each other using the proportion of time spent in
Section 2 (
Figure 1) versus the time spent outside of that section. Only the time spent in
Section 2, not
Section 1, was used due to the otter’s side bias.
Normality of the test sessions was assessed using a Shapiro–Wilk test and showed that the dependent variable of overall time spent near each stimulus pair did not depart significantly from normality (
W = 0.97,
p = 0.54). A second Shapiro–Wilk test was performed and showed that the distribution of the dependent variable of overall explorations did significantly depart from normality (
W = 0.92,
p = 0.02). The Normal Q-Q plot and histogram were examined to further assess the normality of both variables. These plots for both overall time and explorations provided evidence that both variables were normally distributed. Finally, the skewness of the overall times was −0.49, indicating that the distribution was approximately symmetric, and the kurtosis was −0.02, indicating the distribution was less heavy-tailed compared to the normal distribution. The skewness of the overall explorations was 0.38, indicating that the distribution was approximately symmetric, and the kurtosis was 0.72, indicating the distribution was less heavy-tailed compared to the normal distribution. Based on the preponderance of evidence, we decided that the data for both the overall time and explorations approximated the normal distribution closely enough to proceed in using paired-samples
t-tests in the analyses of test sessions instead of nonparametric tests. Additionally, much of the previous research with the NOR task used
t-tests in at least some parts of their data analyses for memory, (e.g., [
24,
39,
41,
42]).
Multiple paired-samples t-tests were used to assess for evidence of memory and side bias in the test sessions, so again a Bonferroni correction was performed to ensure alpha stayed at 0.05. There were five t-tests completed for each dependent variable (including one for each of the three memory intervals, one for overall assessment across all intervals, and one testing for the side bias), so the corrected alpha was 0.01. For each memory interval, the data were pooled (e.g., there were ten 24-h memory interval sessions that were pooled together) for time spent with the familiar stimulus pair and novel stimulus pair. This was done to see if there was a significant difference in either the amount or time spent with, or the number of explorations with the novel versus the familiar stimulus pairs. The time spent with the familiar versus the novel stimulus pair in each of the three memory intervals were compared across three paired-samples t-tests. Additionally, all the times in all the memory intervals were pooled together and then a fourth paired-samples t-test was conducted to assess overall memory. This was all repeated for the dependent variable of explorations. If the otter spent significantly more time or made significantly more explorations with either the familiar or the novel stimulus pair, this would show evidence of object recognition memory.
To check for side biases in the test sessions, two total paired-samples t-tests (accounted for in the Bonferroni correction) were conducted for the dependent measures of overall time spent with each stimulus pair and overall explorations. A side bias occurs when the otter spends significantly more time or explorations with the stimulus pair on one side consistently over the other simply based on the side of the enclosure not based on the stimulus pairs themselves. A side bias would not indicate object preference just a side preference without regard for the stimulus pair. The amount of time spent on the right and left sides was compared. If a significant difference is found consistently for the same side (either right or left) this would indicate a side bias.
Exploration and discrimination of the stimulus pairs were assessed using formulas created by May et al. [
42] (
Table 2). Exploration in the familiarization trials (E
F) was to be used to determine how long data should be coded in the test trials. Discrimination (D1) was calculated for each of the three memory intervals to determine if the otter explored one object significantly more than the other. D1 was compared to a theoretical mean of 0 in a total of three unpaired two-sample
t-tests, as performed by May et al. [
42]. A Bonferroni correction was performed, and the corrected alpha was 0.02. A significant difference from the theoretical means would indicate a preference for either the novel or the familiar object in the test phase. A positive value for D1 would indicate a preference for the familiar stimulus pair, while a negative value would indicate a preference for the novel stimulus pair (
Table 2).
A Pearson correlation was computed to check the inter-rater reliability between the three independent coders by comparing both of the secondary coders together to the primary coder. For the familiarization sessions, a value of 0.8 or higher was acceptable and met. Initially, the criterion was not met so the three coders individually reviewed their training and recoded some of the familiarization sessions. For the time spent with the right or left stimulus pair, if there was a discrepancy greater than 10 s between the primary coder and one of the secondary coders, both coders recoded that session. For the explorations, if there was a discrepancy greater than 1 (usually where one coder said there was one exploration and the other coder said there were zero explorations) between the coders, both the main coder and secondary coder recoded that session. Once the recoding was complete, the criterion was met. Pearson correlations coefficients were between 0.84 and 0.97 (time on the left, r(71) = 0.93; time on the right, r(71) = 0.84; explorations on the left, r(71) = 0.89; and explorations on the right, r(71) = 0.97). For the test sessions, a value of 0.8 or higher was acceptable and met. Only minor recoding was necessary for the test sessions (16.67% of the test sessions were recoded). There were three sessions where the time spent close to the right stimulus pair was recoded, and two sessions where the number of explorations with the right stimulus pair was recoded. The same recoding procedures were used for test sessions as was used for familiarization sessions. Pearson correlation coefficients were between 0.93 and 0.99 (time on the left, r(29) = 0.98; time on the right, r(29) = 0.99; explorations on the left, r(29) = 0.94; and explorations on the right, r(29) = 0.93).
8. Discussion
The NOR task was not an effective form of sensory enrichment in the current study. Sailor spent approximately 15% of his time near both stimulus pairs during the test sessions and conducted very few explorations of the stimulus pairs. In about one third of the test sessions, Sailor did not explore either stimulus pair at all, showing very little engagement in the task. He spent most of his time in
Section 3 of the enclosure (
Figure 1). Sailor was allowed to move around the enclosure as he pleased, and he often spent much of his time swimming in the small pool that was present. Therefore, Sailor was active during these test sessions (he was not sleeping or sitting still) he just was not engaging with the multimodal stimuli.
Additionally, there was no evidence for memory retention in the otter for any of the three memory intervals (10 min, 1 h, and 24 h) in this study. Sailor did not spend more time with the familiar stimulus pair compared to the novel object pair, or vice versa. Sailor spent significantly more time on the left side of the enclosure, regardless of the location of the familiar or novel stimulus pair, showing a side bias. There was no side bias regarding the number of explorations to the familiar vs. novel stimulus pair, but Sailor did not explore the familiar stimulus pair significantly more often than the novel object pair (or vice versa). The lack of evidence for memory retention is likely a product of Sailor’s lack of interaction with and interest in the sensory stimuli in this task.
Many studies on enrichment in otters utilize food to some extent to keep the otter engaged or as a reward for participating in the task [
7,
19,
20,
21,
22,
56]. In comparison, the NOR task relies on the sensory stimuli alone to engage the animal. While this may be suitable for other species, it is possible that sensory enrichment may not be an engaging form of enrichment for otters. It is also possible that the NOR task and sensory enrichment may have simply not been effective for the individual otter that participated in the current study but could be effective for a different otter. This was the case for the two sea otters that participated in Hanna et al.’s [
18] tool use task. Despite both otters being unable to complete the task, one otter was very engaged with the apparatus while the other otter was not. It is possible that another otter could be very engaged in the NOR task or other sensory enrichment unlike the otter that participated in the current study.
Personality may be relevant for selecting the right type of enrichment for an individual animal. Different otters could have responded differently to a sensory enrichment task compared to Sailor. For example, aspects of Sailor’s personality, such as curiosity, could have impacted his performance in the current study [
57]. Curiosity has been defined as “the motivation towards acquisition of novel information” (p. 1) [
58]. This can be determined by an animal’s exploration of novel stimuli when there is no immediate reward, as was the case in the current study. Given Sailor’s few explorations of the stimulus pairs, it appears that his curiosity was low (at least during this study), and perhaps he would have low curiosity in other contexts. Boldness is another factor that is often studied when investigating animal personality [
57]). Boldness is characterized by animals that are more active, take more risks, and learn more quickly [
59]. It is often measured by how much the animal is exploring their environment. During this study, Sailor spent most of his time in the pool that was in the enclosure and not much time with the stimulus pairs. Sailor only spent about 15% of his time during the test trials near the stimulus pairs. This is in comparison to another study that used the NOR task with non-laboratory mammals, pigs, where they spent over 25% of their time during test trials touching the stimuli [
35]. Sailor may be low in boldness. If Sailor was low in curiosity and boldness, he may not have engaged much with stimuli or his environment compared to other otters. Having multiple subjects in a future study could ameliorate this issue since there would likely be a range of personality types.
Sailor participated in two previous studies [
20,
21] where he was motivated and engaged in a two-alternative forced-choice task which provided effective cognitive enrichment. During these tasks there was no issue getting Sailor to start a session, follow learned behavioral cues during multiple trials (up to 40 trials per day), and engage with the stimuli (viewing the stimuli and making a choice by touching one stimulus). This previous experience with a different type of task may have impacted Sailor’s behavior in the NOR task and his willingness to engage with sensory enrichment. In the two-alternative forced-choice task, Sailor would approach two stimuli, rear up onto his back legs, and touch one stimulus with his nose or paw. Sailor received a food reward each time he made a correct choice by touching the reinforced stimulus. In the current study with the NOR task, Sailor was only provided a food reward for entering the enclosure during test sessions (and for targeting the stimulus pairs in the beginning of the familiarization sessions). This was done to encourage Sailor to enter the enclosure promptly, but we did not want to bias Sailor during test sessions by feeding him for making his own explorations of the stimulus pairs (which is not a part of the classic NOR task). The majority of Sailor’s explorations occurred during the first two blocks of the study (twelve sessions). Therefore, it is possible that Sailor may have eventually realized he was not going to receive food for touching the stimuli (after the beginning a familiarization sessions) like he did in the previous study [
21]. Sailor’s behavior of exploring and touching stimuli could have eventually been extinguished because he was not receiving an anticipated food reward. The food reward in the previous study was Sailor’s external motivation for touching the stimuli, but during the current study he would have had only his internal motivation to explore the stimuli throughout the sessions. It is possible that a different otter without this past reinforcement history may be more internally motivated to make explorations of the sensory stimuli in the NOR task.
When using sensory enrichment, the animals must be able to perceive the stimuli. Since there is limited data on otter visual and olfactory perception, we did our best to utilize objects that otters can perceive. All the objects were chosen for maximum visual discriminability based on size, shape, and color based on what we know about vision in otters [
21,
60]. In a previous study [
21], Sailor himself was able to discriminate between two-dimensional stimuli when he had both shape and color as a cue. In that same study, another otter was able to further discriminate between stimuli using either shape or color alone as a cue when the other cue was removed. Therefore, there is evidence North American river otters can not only perceive but also discriminate between stimuli using shape and color as cues. Such cues were present with the three-dimensional objects used in the current study. There is a strong likelihood that Sailor could see the objects in the current study and perceive differences among them.
However, we do not know if the otter was able to perceive the odorants used in the current study. To date, there are no published psychophysical studies on odor detection and discrimination abilities in otters. The literature is focused on otter olfaction for identifying scents of other animals [
34,
49] not artificial scents like the essential oils and food extracts used in this study. Due to the large number of sessions (30) and thus the need for at least 60 unique odorants, it was not feasible to use scents of other animals for this study. It is possible Sailor did not detect some portion of our odorants. This is why multimodal stimuli (visual, olfactory, and tactile) were used to create stimulus pairs in the hopes of maximizing perception discriminability via multisensory facilitation. Another possibility is that Sailor detected the odorants but since they were not ecologically valid, they were unimportant and unengaging for him. Additionally, Sailor could touch the objects but not manipulate them. Therefore, tactile perception may not have been used much to help with recognition of the objects. Any sensory enrichment used for otters must be informed by gaining more knowledge about visual, olfactory, and tactile perception in otters.
The main limitation in this study was utilizing a single subject. There was a second otter (a 5-year-old female, Ashkii) residing at the zoo, and we planned to have her participate in the current study. This otter participated in a previous study on visual object categorization [
20] similar to the DeLong et al. [
21] study where a two-alternative forced-choice task was used. Ashkii was motivated in the previous study that involved training and performed well. During the pilot test of the current study, after viewing Ashkii in the experimental enclosure, the zoo staff decided that it would not be possible for Ashkii to participate. She exhibited problematic behaviors (scratching at and climbing on top of the gate, as well as pacing) after only a few minutes inside the experimental area. Therefore, it was necessary to exclude Ashkii from the study. This sensory enrichment task did not suit Ashkii.
Our single subject Sailor was both a male and elderly (otters older than 13 years are considered elderly; [
8]). These factors could have impacted our results, such as by affecting his sensory systems, memory, motivation, physical abilities, etc. In otters, some studies have reported sex differences in cognitive abilities [
33,
60] while others have not found such differences [
23]. One cognitive study on three otter species found an age difference where younger otters were more neophilic [
61]. We do not know if sex or age played a role in the current study since Sailor was our only subject. There was no evidence that Sailor had issues seeing or smelling, and we ensured that the stimulus pairs were easily accessible for Sailor to touch. Additionally, Sailor was successfully visually categorizing two-dimensional objects approximately two weeks prior to the current study [
20]. Stimulus pairs were hung at approximately the same height as in DeLong et al. [
21], and Sailor easily touched those stimuli.
We compensated for having a single subject by conducting 30 sessions with the otter. Smith and Little [
62]) discuss this aspect of low
n (subject) designs as beneficial, as the individual becomes a replication unit when large samples of data are collected from individuals. Since there was just a single otter in this study, Smith and Little [
62] would equate this to running a single study with 30 different otters that all completed one session (as is often the case in many psychology studies on humans). However, the results of the current study should still be interpreted with caution. It would be beneficial to present additional otters at other facilities with opportunities for sensory and cognitive enrichment to further develop a strategy for presenting optimal enrichment for this species.
It is possible that this sensory enrichment task may have more successfully engaged the otter’s attention if the stimuli had been presented in the larger on-exhibit area instead of in the off-exhibit area we used. Since we were using a traditional NOR task, it had to be located in a confined space where the otter could be closely monitored in marked areas near each stimulus pair. The placement of the gate within this area on the left side of the enclosure near where the left stimulus pair was placed (gate 3 in
Figure 1) was an issue. The otter used this gate multiple times per day to access the on-exhibit area. In our experimental setup, this gate remained closed during a session. This resulted in Sailor sitting over by the gate for extended periods of time, likely because he wanted to leave the enclosure of his own accord as he typically would be able to do. It may be useful in the future with otters to use a sensory enrichment task similar to the modified NOR task, such as those used with baboons [
63] and marmosets [
64]. In this modified task, stimuli were presented in specific spots throughout the animals’ enclosure over a period of multiple days, where a novel stimulus was added each day. This way, the animals were not confined to a smaller space and could engage with the stimuli within their whole enclosure and not just an off-exhibit space.
One potential conclusion that could be drawn from this study is that the effectiveness of sensory enrichment depends on the personality, reinforcement history, and behavioral traits of the subject, along with the features of the stimuli and the placement of the stimuli within the animal’s enclosure. Providing novel objects and odorants for an individual to explore during sensory enrichment is not always as effective for otters as it may be for other species (e.g., olfactory enrichment in elephants; [
65], and lions; [
66], and multi-sensory enrichment in lemurs; [
67]). Another potential conclusion is that despite all these factors, cognitive enrichment (and food-based enrichment) is simply more effective and engaging than sensory enrichment for otters. A previous study that investigated enrichment for a North American river otter found food-based enrichment to be the most effective at reducing stereotyped behaviors [
6]. There was a sensory enrichment device used in Nelson’s [
6] study, unfortunately conclusions about its effectiveness could not be properly compared to the food-based enrichment due to a low number of presentations of the sensory enrichment device.
We suggest that future research examining memory and other phenomena in otters utilize tasks that provide cognitive enrichment rather than sensory enrichment. The results of the current study are not in line with previous studies that successfully used the NOR task to show evidence of memory in many other animals (e.g., birds: [
68]; fish: [
42]; pigs: [
35]; and rats: [
24]). While it is possible that our results suggest that North American river otters do not retain long-term memories, it is more likely that this sensory enrichment task was not ideal for otters. Saliveros and colleagues (2020) used a task with more puzzle feeders (a well-known form of cognitive enrichment) baited with food and found some evidence of long-term memory in Asian short-clawed otters. Taken along with previous studies using cognitive enrichment successfully in North American river otters [
20,
21,
32], this seems to be a more promising approach. We explored using simple and less time-intensive sensory enrichment in the current study, but it appeared to be inferior to cognitive enrichment. Although very beneficial to animals in zoos, laboratories, and farms, cognitive enrichment (enrichment that promotes wellbeing by engaging cognitive skills in animals) seems to be used the least of any of the enrichment types [
3].
It is important to continue studying otters using different types of enrichment because there are many otters residing under human care who can benefit from effective enrichment practices. There are approximately 500 freshwater otters (4 species) in 162 Association of Zoos and Aquariums (AZA) accredited facilities across the United States (D. Hamilton, personal communication). This includes an estimated 274 North American river otters in 107 AZA accredited facilities in the United States, which are the most numerous of all species of otters in zoos, and the focal species in the current study (D. Hamilton, personal communication). Advancing animal welfare is a part of AZA’s mission [
47], and providing appropriate and engaging enrichment is one of the main ways facilities often promote animal welfare. It is also important to remember that enrichment is not one-size-fits-all for every species within a zoo. The current study not only examined a new type of enrichment that had not been used with otters before, but it also pursued new scientific discoveries about these animals regarding their memory abilities.
Another reason it is beneficial to study cognitive phenomena in otters using various types of enrichment is because it can benefit conservation research and efforts for otters. North American river otters nearly went extinct in many parts of the United States by the mid-20th century, including western New York, until reintroduction efforts began in the 1990s [
9]. Over the past 18 years, states have reported either increasing or stable river otter populations throughout the US [
69]. While some river otter populations are increasing, it does seem that the species is approaching their maximum geographic distribution in the US. Otters still face issues today such as pollution, trapping, and degradation of their environments [
8], so they may not have as many suitable habitats to continue to spread throughout the US in order to continue increasing their population numbers. Conservation efforts can be better informed about how best to support these animals if we know more about their cognitive abilities through utilizing tasks that provide cognitive enrichment and are engaging to this species.