Rio de Janeiro Botanical Garden: Biodiversity Conservation in a Tropical Arboretum
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef] [PubMed]
- Volis, S. Conservation utility of botanic garden living collections: Setting a strategy and appropriate methodology. Plant Divers. 2017, 39, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V. Botanic Gardens and Genetic Conservation. Sibbaldia 2009, 7, 5–18. [Google Scholar] [CrossRef]
- Antonelli, A.; Fry, C.; Smith, R.J.; Simmonds, M.S.J.; Kersey, P.J.; Pritchard, H.W.; Abbo, M.S.; Acedo, C.; Adams, J.; Ainsworth, A.M.; et al. State of the World’s Plants and Fungi 2020; Royal Botanic Gardens: Kew, UK, 2020. [Google Scholar] [CrossRef]
- Cochrane, J.A.; Crawford, A.D.; Monks, L.T. The significance of ex situ seed conservation to reintroduction of threatened plants. Aust. J. Bot. 2007, 55, 356–361. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex Situ Conservation of Plant Diversity in the World’s Botanic Gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Chen, J.; Cannon, C.H.; Hu, H. Tropical botanical gardens: At the in situ ecosystem management frontier. Trends Plant Sci. 2009, 14, 584–589. [Google Scholar] [CrossRef]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 27 September 2023).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.M.N.; Maunder, M.; Pereira, T.S.; Peixoto, A.L. Brazilian botanic gardens: An assessment of conservation capacity. Sibbaldia 2016, 14, 97–117. [Google Scholar] [CrossRef]
- Sutherland, L.; Cosgrove, C. Valuing a national collection—A work in progress at the Australian National Botanic Gardens. BG Journal 2010, 7, 7–11. Available online: https://www.jstor.org/stable/24811071 (accessed on 5 July 2024).
- Bruns, E.; Westwood, M.; Griffith, M.P.; Hipp, A.; Lobdell, M.; Meyes, A.; Rollinson, C.; Still, S.; Worcester, L.; Hoban, S. Quantifying Endangerment Value: A Promising Tool to Support Curation Decisions. Sibbaldia 2023, 22, 1–24. [Google Scholar] [CrossRef]
- Casazza, I.F. O Jardim Botânico do Rio de Janeiro: Um lugar de Ciência (1915–1931). Master’s Dissertation, Fundação Oswaldo Cruz., Fiocruz, Rio de Janeiro, 2011. Available online: https://www.arca.fiocruz.br/handle/icict/19764 (accessed on 28 September 2023).
- Sanjad, N. Os jardins botânicos luso-brasileiros. Ciência E Cult. 2010, 62, 20–22. [Google Scholar]
- Rodrigues, J.B. Hortus Fluminensis ou Breve Notícia Sobre as Plantas Cultivadas no Jardim Botânico do Rio de Janeiro; Typ. Leuzinger: Rio de Janeiro, Brazil, 1894. Available online: https://bibdigital.rjb.csic.es/records/item/14610-hortus-fluminensis?offset=1 (accessed on 9 April 2024).
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1891, Imprensa Nacional, Rio de Janeiro. 1891, pp. 25–26. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 27 September 2023).
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1907, Imprensa Nacional, Rio de Janeiro. 1907, pp. 4–5. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 27 September 2023).
- Rodriguésia. Noticiario e Actividades Varias. Rodriguésia 1936, 1, 83–84. Available online: http://memoria.bn.br/pdf/144398/per144398_1936_00004.pdf (accessed on 15 August 2023).
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1915, Imprensa Nacional, Rio de Janeiro. 1915, p. 34. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 27 September 2023).
- Conti, V.M.; Iwamoto, S.; Almeida, T.M.H.; Pereira, T.S. Revisão dos limites do Jardim Botânico do Rio de Janeiro, Brasil. Rodriguésia 2008, 59, 603–607. [Google Scholar] [CrossRef]
- JBRJ—Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available online: https://www.gov.br/jbrj/pt-br/acesso-a-informacao/institucional/156 (accessed on 20 September 2023).
- Coelho, M.A.N. O inventário da coleção. In Árvores Notáveis—200 Anos do Jardim Botânico do Rio de Janeiro; Andrea Jakobsson Estudio: Rio de Janeiro, Brazil, 2008; pp. 24–31. [Google Scholar]
- Jabot (Scientific Collections Management Software); Version 3.0; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil. Available online: http://rb.jbrj.gov.br/v2/consulta.php (accessed on 20 September 2023).
- Silva, L.A.E.; Fraga, C.N.; Almeida, T.M.H.; Gonzalez, M.; Lima, R.O.; Rocha, M.S.; Bellon, E.; Ribeiro, R.S.; Oliveira, F.A.; Clemente, L.S.; et al. Jabot—Sistema de gerenciamento de coleções botânicas: A experiência de uma década de desenvolvimento e avanços. Rodriguésia 2017, 68, 391–410. [Google Scholar] [CrossRef]
- CBD—Convention on Biological Diversity. Global Strategy for Plant Conservation 2011–2020. Available online: https://www.cbd.int/gspc/ (accessed on 16 August 2023).
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- POWO—Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 17 August 2023).
- Tropicos. Missouri Botanical Garden. Available online: https://tropicos.org (accessed on 17 August 2023).
- Brasil—Ministério do Meio Ambiente. Portaria N° 148, de 7 de Junho de 2022. Lista Nacional de Espécies Ameaçadas de Extinção. 2022. Available online: https://in.gov.br/en/web/dou/-/portaria-mma-n-148-de-7-de-junho-de-2022-406272733 (accessed on 14 September 2023).
- IUCN—International Union for Conservation of Nature. Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 15 August 2023).
- BGCI. ThreatSearch Online Database. Botanic Gardens Conservation International. Richmond, UK. Available online: https://tools.bgci.org/threat_search.php (accessed on 23 August 2023).
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1913, Imprensa Nacional, Rio de Janeiro. 1913, p. 85. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 21 September 2023).
- Casazza, I.F. Desenvolvimentismo e conservacionismo na Era Vargas (1930–1945): A atuação científica e política de Paulo Campos Porto. História Ciências Saúde-Manguinhos 2020, 27, 411–430. [Google Scholar] [CrossRef]
- Bediaga, B.; Drummond, R.P. Cronologia Jardim Botânico do Rio de Janeiro. Available online: https://www.gov.br/jbrj/pt-br/centrais-de-conteudo/publicacoes/cronologia.pdf (accessed on 16 August 2023).
- Aguiar, L.M.S.; Camargo, A.J.A. Cerrado: Ecologia e Caracterização; Embrapa Cerrados: Brasília, Brazil, 2004; Available online: www.embrapa.br/busca-de-publicacoes/-/publicacao/566918/cerrado-ecologia-e-caracterizacao (accessed on 5 July 2024).
- Bediaga, B.; Lima, H.C.L.; Morim, M.P.; Barros, C.F. As atividades científicas durante dois séculos. In Jardim Botânico do Rio de Janeiro: 1808–2008; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2008; pp. 33–43. [Google Scholar]
- Royal Botanic Garden Edinburgh—Arboretum (Tree Collection). Available online: https://www.rbge.org.uk/collections/living-collection/living-collection-at-the-royal-botanic-garden-edinburgh/#treecollection (accessed on 3 January 2024).
- Almeida, T.M.H.; Gonzaga, D.R.; Peixoto, A.L. Cacti in distress: How to enhance ex situ conservation strategies through living collections. Oryx 2024, accepted. [Google Scholar]
- Cavender, N.; Westwood, M.; Bechtoldt, C.; Donnelly, G.; Oldfield, S.; Gardner, M.; Rae, D.; Mcnamara, W. Strengthening the conservation value of ex situ tree collections. Oryx 2015, 49, 416–424. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Teixido, A.L.; Zanetti, M.; Pádua, J.G.; Andrade, A.C.S.; Costa, M.L.N. Ex situ conservation of threatened plants in Brazil: A strategic plan to achieve Target 8 of the Global Strategy for Plant Conservation. Rodriguésia 2018, 69, 1547–1555. [Google Scholar] [CrossRef]
- Nicholson, R. Hedging a bet against extinction: The use of shears in ex-situ conservation. Biodivers. Conserv. 1994, 3, 628–631. [Google Scholar] [CrossRef]
- Gratzfeld, J. From Idea to Realisation—BGCI’s Manual on Planning, Developing and Managing Botanic Gardens; Botanic Gardens Conservation International: Richmond, UK, 2016. [Google Scholar]
- Gardner, M.F. Managing botanic garden collections of high conservation value. Sibbaldia 2021, 20, 81–94. [Google Scholar] [CrossRef]
- BFG—The Brazil Flora Group. Brazilian Flora 2020: Leveraging the power of a collaborative scientific network. Taxon 2021, 71, 178–198. [Google Scholar] [CrossRef]
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1920, Imprensa Nacional, Rio de Janeiro. 1920, pp. 44–45. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 21 September 2023).
- Silveira, F.R. O Jardim Botânico do Rio de Janeiro. Rodriguésia 1935, 1, 15. [Google Scholar]
- Mello, R.J.F.B. Plano de Ação Nacional para Conservação das Cactáceas. Série Espécies Ameaçadas n°24; Ribeiro, S.S., Zappi, D., Taylor, N., Machado, M., Eds.; Instituto Chico Mendes de Conservação da Biodiversidade: Brasília, Brazil, 2011; p. 112. [Google Scholar]
- Brasil. Relatório do Ministério da Agricultura (RJ) do ano de 1916, Imprensa Nacional, Rio de Janeiro. 1916, p. 27. Available online: https://memoria.bn.br/docreader/DocReader.aspx?bib=873730&Pesq&pagfis=1 (accessed on 20 September 2023).
- Abreu, R.C.R.; Rodrigues, P.J.F.P. Exotic tree Artocarpus heterophyllus (Moraceae) invades the Brazilian Atlantic Rainforest. Rodriguésia 2010, 61, 677–688. [Google Scholar] [CrossRef]
- Frachon, N.; Gardner, M.; Rae, D. Data Capture Project at the Royal Botanic Garden Edinburgh. Sibbaldia 2009, 7, 77–82. [Google Scholar] [CrossRef]
- Molinaro, L.C.; Costa, D.P. Briófitas do arboreto do Jardim Botânico do Rio de Janeiro. Rodriguésia 2001, 52, 107–124. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Ramsay, M.M. Ex situ conservation of bryophytes: Progress and potential of a pilot project. Bol. Sociedad Española Briologia 2005, 26–27, 17–22. [Google Scholar]
- Spirina, U. Conservation ex situ of bryophytes. In: Botanic Garden of Tver State University (Middle part of European Russia). In Proceedings of the 4th Global Botanic Gardens Congress, Dublin, Ireland, 13–18 June 2010; Available online: https://www.bgci.org/files/Dublin2010/papers/Spirina-Uliana.pdf (accessed on 15 August 2023).
- Arnold Arboretum. Developing an Exemplary Collection. Available online: http://arboretum.harvard.edu/stories/developing-an-exemplary-collection-a-vision-for-the-next-century-at-the-arnold-arboretum-of-harvard-university/ (accessed on 3 July 2023).


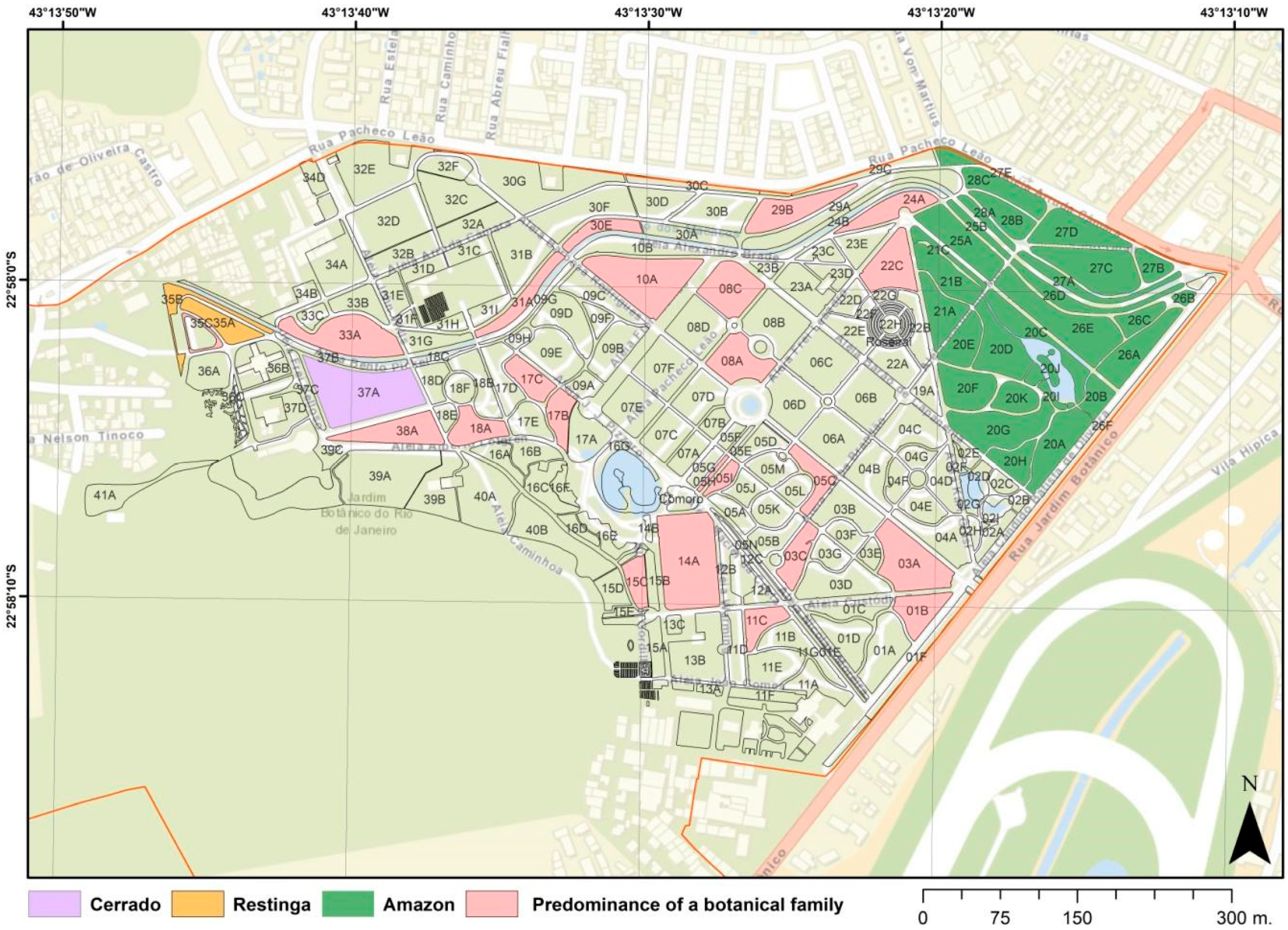
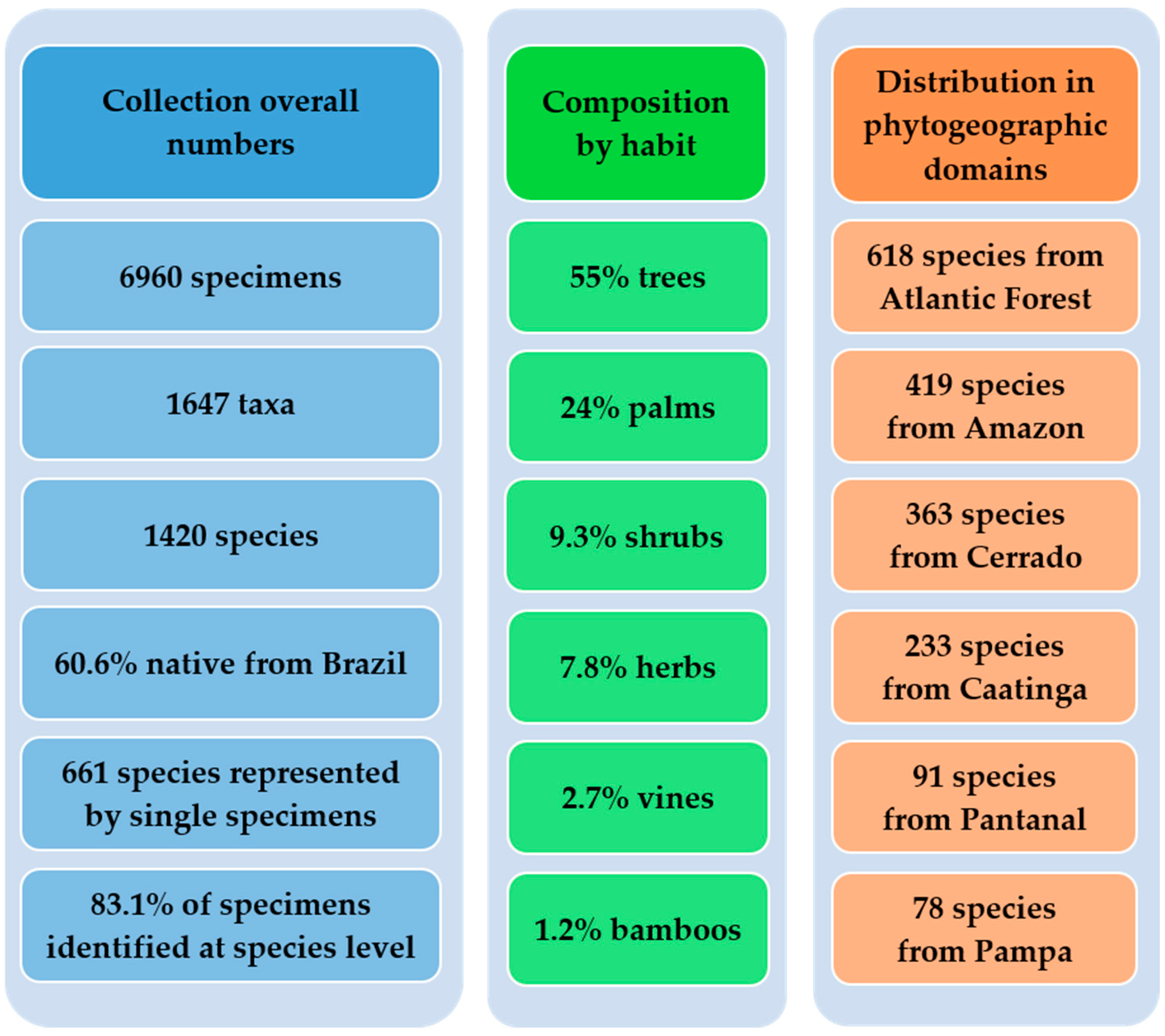
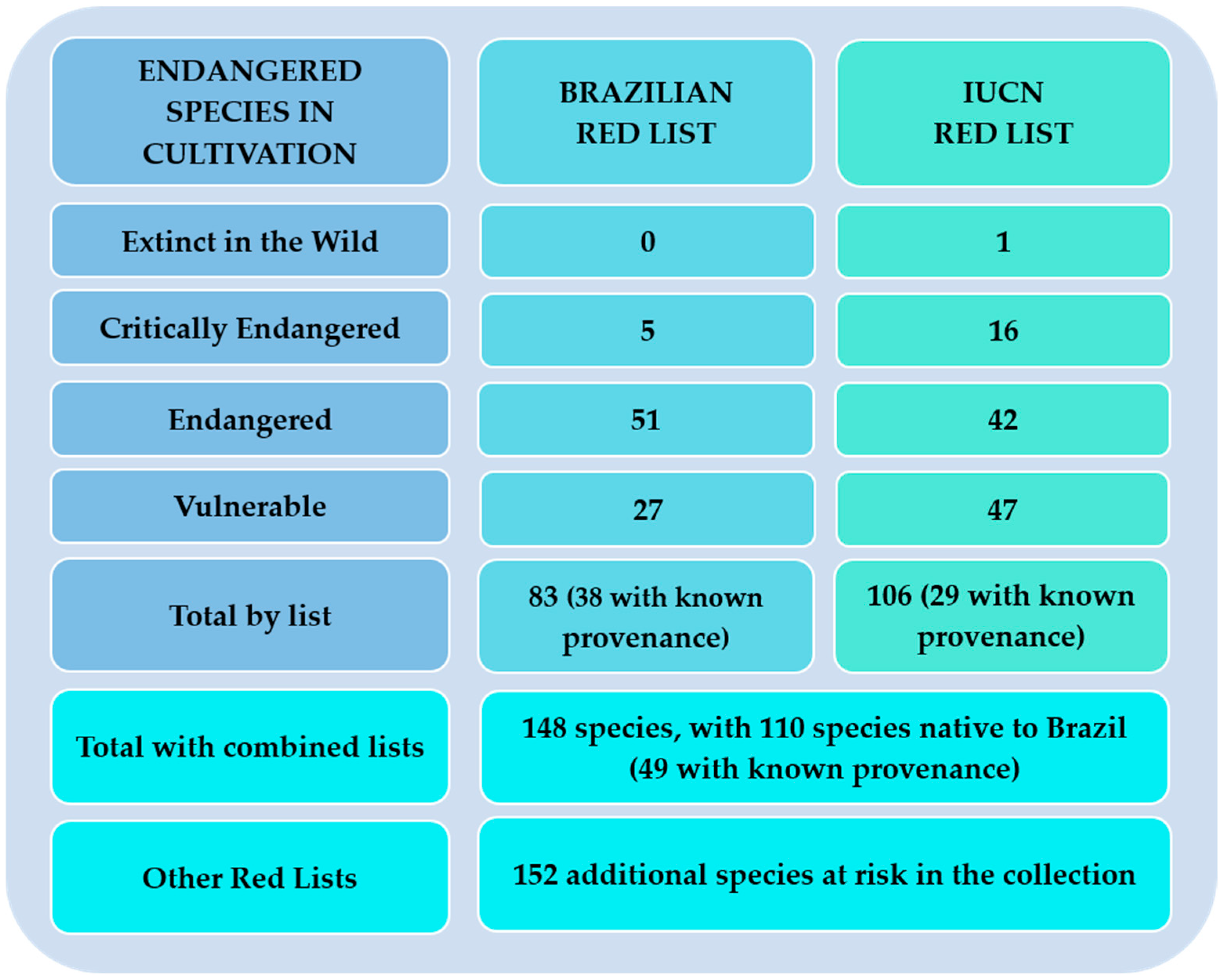
| Botanical Family | Total spp. in Cultivation | Native spp. in Cultivation | Species Richness in Brazil | Percentage of Representation of Brazilian Species |
|---|---|---|---|---|
| Leguminosae | 252 | 195 | 2899 | 6.73% |
| Arecaceae | 154 | 55 | 416 | 13.22% |
| Myrtaceae | 78 | 61 | 1063 | 5.74% |
| Malvaceae | 72 | 51 | 864 | 5.90% |
| Bignoniaceae | 54 | 36 | 414 | 8.69% |
| Apocynaceae | 39 | 19 | 814 | 2.33% |
| Araceae | 36 | 26 | 524 | 4.96% |
| Rubiaceae | 34 | 18 | 1475 | 1.22% |
| Euphorbiaceae | 31 | 18 | 972 | 1.85% |
| Sapotaceae | 31 | 25 | 247 | 10.12% |
| Botanical Family | Percentage of the Family in the Planting Bed | Planting Bed with Greater Family Diversity | Number of Species (Number of Specimens) in the Bed from the Predominant Family |
|---|---|---|---|
| Arecaceae | 87.30% | 3A | 48 (98) |
| Leguminosae | 84.00% | 14A | 84 (118) |
| Myrtaceae | 78.60% | 8C | 33 (45) |
| Moraceae | 72.20% | 29B | 13 (23) |
| Rubiaceae | 69.60% | 1B | 16 (21) |
| Apocynaceae | 68.2% | 11C | 15 (24) |
| Convolvulaceae | 66.60% | 5H | 4 (6) |
| Marantaceae | 61.90% | 15C | 26 (81) |
| Euphorbiaceae | 57.10% | 30E | 8 (18) |
| Malvaceae | 52.40% | 22C | 11 (14) |
| Araceae | 46.20% | 17B | 24 (44) |
| Asparagaceae | 45.20% | 38A | 14 (50) |
| Rutaceae | 40.60% | 8A | 13 (17) |
| Acanthaceae | 38.90% | 31A | 7 (13) |
| Lamiaceae | 37.50% | 5C | 6 (11) |
| Clusiaceae | 36.80% | 24A | 7 (13) |
| Sapotaceae | 35.20% | 10A | 19 (25) |
| Melastomataceae | 34.60% | 17C | 9 (15) |
| Aquifoliaceae | 33.30% | 5J | 5 (11) |
| Nymphaeaceae | 33.30% | 35C | 4 (4) |
| Simaroubaceae | 33.30% | 5I | 4 (4) |
| Bignoniaceae | 30.80% | 33A | 12 (22) |
| Asteraceae | 30.00% | 18A | 9 (9) |
| Pandanaceae | 30.00% | 3C | 6 (15) |
| Species | Native to Brazil | Brazilian Red List | IUCN Red List | Known Provenance in Database |
|---|---|---|---|---|
| Apocynaceae Aspidosperma parvifolium A. DC. | yes | EN | no | |
| Apocynaceae Aspidosperma polyneuron Müll. Arg. | yes | EN | no | |
| Araceae Philodendron gloriosum André | no | VU | no | |
| Araucariaceae Araucaria angustifolia (Bertol.) Kuntze | yes | EN | CR | yes |
| Arecaceae Acanthophoenix rubra (Bory) H. Wendl. | no | CR | no | |
| Arecaceae Adonidia merrillii (Becc.) Becc. | no | VU | no | |
| Arecaceae Bentinckia nicobarica (Kurz.) Becc. | no | EN | no | |
| Arecaceae Butia capitata (Mart.) Becc. | yes | VU | no | |
| Arecaceae Butia purpurascens Glassman | yes | EN | VU | yes |
| Arecaceae Butia yatay (Mart.) Becc. | yes | VU | no | |
| Arecaceae Calamus ciliaris Blume | no | VU | no | |
| Arecaceae Dictyosperma album var. conjugatum H. E. Moore and Guého | no | CR | no | |
| Arecaceae Dypsis decaryi (Jum.) Beentje and J. Dransf. | no | VU | no | |
| Arecaceae Euterpe edulis Mart. | yes | VU | no | |
| Arecaceae Hyophorbe lagenicaulis (L.H.Bailey) H. E. Moore | no | CR | no | |
| Arecaceae Hyophorbe verschaffeltii H. Wendl. | no | CR | no | |
| Arecaceae Latania lontaroides (Gaertn.) H. E. Moore | no | EN | no | |
| Arecaceae Ravenea rivularis Jum. and H. Perrier | no | VU | no | |
| Arecaceae Sabal bermudana L. H. Bailey | no | EN | no | |
| Arecaceae Sabal causiarum (O. F. Cook) Becc. | no | VU | no | |
| Arecaceae Syagrus botryophora (Mart.) Mart. | yes | VU | yes | |
| Arecaceae Syagrus macrocarpa Barb. Rodr. | yes | EN | EN | yes |
| Arecaceae Syagrus picrophylla Barb. Rodr. | yes | VU | yes | |
| Arecaceae Tahina spectabilis J. Dransf. and Rakotoarin. | no | CR | no | |
| Asparagaceae Beaucarnea recurvata Lem. | no | CR | no | |
| Asparagaceae Dracaena umbraculifera Jacq. | no | CR | no | |
| Asteraceae Stifftia fruticosa (Vell.) D. J. N.Hind and Semir | yes | VU | no | |
| Bignoniaceae Ekmanianthe longiflora (Griseb.) Urban | no | EM | no | |
| Bignoniaceae Handroanthus arianeae (A. H. Gentry) S.Grose | yes | EN | yes | |
| Bignoniaceae Handroanthus cristatus (A. H. Gentry) S.Grose | yes | EN | yes | |
| Bignoniaceae Handroanthus incanus (A. H. Gentry) S.Grose | yes | VU | no | |
| Bignoniaceae Handroanthus riodocensis (A. H. Gentry) S.Grose | yes | EN | yes | |
| Bignoniaceae Handroanthus serratifolius (Vahl) S. Grose | yes | EN | no | |
| Bignoniaceae Jacaranda mimosifolia D. Don | no | VU | no | |
| Bignoniaceae Paratecoma peroba (Record) Kuhlm. | yes | EN | no | |
| Bignoniaceae Zeyheria tuberculosa (Vell.) Bureau ex Verl. | yes | VU | no | |
| Bromeliaceae Aechmea castanea L. B. Sm. | yes | EN | yes | |
| Bromeliaceae Alcantarea glaziouana (Leme) J. R. Grant | yes | EN | yes | |
| Burseraceae Aucoumea klaineana Pierre | no | VU | no | |
| Calophyllaceae Kielmeyera aureovinosa M. Gomes | yes | EN | yes | |
| Calophyllaceae Kielmeyera rizziniana Saddi | yes | EN | EN | no |
| Canellaceae Cinnamodendron axillare Endl. ex Walp. | yes | EN | EN | no |
| Chrysobalanaceae Couepia schottii Fritsch | yes | EN | VU | yes |
| Clusiaceae Clusia diamantina Bittrich | yes | EN | EN | no |
| Combretaceae Terminalia acuminata (Allemão) Eichler | yes | EN | EN | no |
| Combretaceae Terminalia hoehneana (N.F.Mattos) Gere and Boatwr. | yes | VU | yes | |
| Cycadaceae Cycas circinalis L. | no | EN | no | |
| Dichapetalaceae Tapura follii Prance | yes | CR | CR | yes |
| Dichapetalaceae Tapura wurdackiana Prance | yes | EN | EN | yes |
| Dioscoreaceae Dioscorea pseudomacrocapsa G. M. Barroso et al. | yes | EN | no | |
| Erythroxylaceae Erythroxylum ovalifolium Peyr. | yes | VU | yes | |
| Euphorbiaceae Joannesia princeps Vell. | yes | VU | no | |
| Ginkgoaceae Ginkgo biloba L. | no | EN | yes | |
| Iridaceae Neomarica northiana (Schneev.) Sprague | yes | EN | yes | |
| Lamiaceae Tectona grandis L.f. | no | EN | no | |
| Lauraceae Aniba rosiodora Ducke | yes | EN | EN | no |
| Lecythidaceae Bertholletia excelsa Bonpl. | yes | VU | VU | no |
| Lecythidaceae Cariniana ianeirensis R. Knuth | yes | EN | EN | yes |
| Lecythidaceae Cariniana legalis (Mart.) Kuntze | yes | EN | VU | no |
| Lecythidaceae Couratari asterotricha Prance | yes | EN | CR | yes |
| Lecythidaceae Couratari pyramidata (Vell.) Kunth | yes | EN | EN | yes |
| Lecythidaceae Gustavia gracillima Miers | no | VU | yes | |
| Leguminosae Amburana acreana (Ducke) A. C. Sm. | yes | VU | VU | no |
| Leguminosae Amburana cearensis (Allemão) A. C. Sm. | yes | EN | no | |
| Leguminosae Apuleia leiocarpa (Vogel) J. F. Macbr. | yes | VU | no | |
| Leguminosae Arapatiella psilophylla (Harms) R. S. Cowan | yes | VU | no | |
| Leguminosae Browneopsis macrofoliolata Klitg. | no | CR | no | |
| Leguminosae Centrolobium paraense Tul. | yes | EN | no | |
| Leguminosae Chloroleucon tortum (Mart.) Pittier | yes | CR | no | |
| Leguminosae Dalbergia nigra (Vell.) Allemão ex Benth. | yes | VU | VU | no |
| Leguminosae Dimorphandra exaltata Schott | yes | EN | EN | no |
| Leguminosae Dimorphandra wilsonii Rizzini | yes | EN | CR | yes |
| Leguminosae Dinizia jueirana-facao G. P. Lewis and G. S. Siqueira | yes | CR | CR | yes |
| Leguminosae Dipteryx alata Vogel | yes | VU | yes | |
| Leguminosae Elizabetha speciosa Ducke | yes | VU | VU | no |
| Leguminosae Gleditsia amorphoides (Griseb.) Taub. | yes | VU | no | |
| Leguminosae Grazielodendron rio-docensis H. C. Lima | yes | EN | yes | |
| Leguminosae Harleyodendron unifoliolatum R. S. Cowan | yes | EN | EN | no |
| Leguminosae Inga cordistipula Mart. | yes | VU | VU | no |
| Leguminosae Inga hispida Schott ex Benth. | yes | VU | yes | |
| Leguminosae Inga maritima Benth. | yes | EN | EN | yes |
| Leguminosae Luetzelburgia trialata (Ducke) Ducke | yes | EN | no | |
| Leguminosae Machaerium legale (Vell.) Benth. | yes | CR | yes | |
| Leguminosae Machaerium obovatum Kuhlm. and Hoehne | yes | VU | no | |
| Leguminosae Machaerium villosum Vogel | yes | VU | no | |
| Leguminosae Martiodendron fluminense Lombardi | yes | EN | no | |
| Leguminosae Muellera filipes (Benth.) M. J. Silva and A. M. G. Azevedo | yes | VU | VU | no |
| Leguminosae Muellera virgilioides (Vogel) M. J. Silva and A. M. G. Azevedo | yes | VU | VU | yes |
| Leguminosae Paubrasilia echinata (Lam.) Gagnon, H.C.Lima and G.P.Lewis | yes | EN | EN | yes |
| Leguminosae Peltogyne discolor Vogel | yes | VU | no | |
| Leguminosae Peltogyne mattosiana Rizzini | yes | EN | no | |
| Leguminosae Pericopsis elata (Harms) Meeuwen | no | EN | no | |
| Leguminosae Pterocarpus indicus Willd. | no | EN | no | |
| Lythraceae Lafoensia replicata Pohl | yes | VU | no | |
| Malpighiaceae Stigmaphyllon vitifolium A. Juss. | yes | CR | no | |
| Malvaceae Abutilon anodoides A. St.-Hil. and Naudin | yes | EN | yes | |
| Malvaceae Adansonia grandidieri Baill. | no | EN | no | |
| Malvaceae Pseudobombax petropolitanum A. Robyns | yes | EN | EN | no |
| Marantaceae Goeppertia tuberosa (Vell.) Borchs. and S. Suárez | yes | EN | no | |
| Marantaceae Goeppertia widgrenii (Körn.) Borchs. and S. Suárez | yes | EN | no | |
| Marantaceae Ischnosiphon ovatus Körn. | yes | EN | no | |
| Melastomataceae Merianthera pulchra Kuhlm. | yes | VU | yes | |
| Meliaceae Cedrela fissilis Vell. | yes | VU | VU | yes |
| Meliaceae Cedrela odorata L. | yes | VU | VU | no |
| Meliaceae Khaya senegalensis (Desr.) A. Juss. | no | VU | no | |
| Meliaceae Swietenia humilis Zucc. | no | EN | no | |
| Meliaceae Swietenia macrophylla King | yes | VU | VU | yes |
| Meliaceae Trichilia casaretti C. DC. | yes | VU | no | |
| Moraceae Ficus cyclophylla (Miq.) Miq. | yes | EN | no | |
| Moraceae Sorocea guilleminiana Gaudich. | yes | VU | yes | |
| Musaceae Musa coccinea Andrews | no | EN | no | |
| Myristicaceae Virola bicuhyba (Schott ex Spreng.) Warb. | yes | EN | no | |
| Myristicaceae Virola surinamensis (Rol. ex Rottb.) Warb. | yes | VU | EN | no |
| Myrtaceae Campomanesia hirsuta Gardner | yes | EN | EN | yes |
| Myrtaceae Campomanesia phaea (O.Berg) Landrum | yes | VU | no | |
| Myrtaceae Eucalyptus deglupta Blume | no | VU | no | |
| Myrtaceae Eugenia itaguahiensis Nied. | yes | EN | no | |
| Myrtaceae Eugenia mattosii D. Legrand | yes | EN | EN | yes |
| Myrtaceae Eugenia pulcherrima Kiaersk. | yes | VU | VU | no |
| Myrtaceae Myrcia aethusa (O.Berg) N. Silveira | yes | VU | yes | |
| Myrtaceae Myrcia carioca A.R.Lourenço and E. Lucas | yes | VU | VU | no |
| Myrtaceae Myrcia ovata Cambess. | yes | VU | yes | |
| Myrtaceae Plinia edulis (Vell.) Sobral | yes | VU | no | |
| Myrtaceae Plinia renatiana G.M.Barroso and Peixoto | yes | EN | yes | |
| Myrtaceae Plinia spiritosantensis (Mattos) Mattos | yes | EN | yes | |
| Podocarpaceae Podocarpus sellowii Klotzsch ex Endl. | yes | EN | no | |
| Polygonaceae Coccoloba gigantifolia Melo, Cid Ferreira and Gribel | yes | EN | yes | |
| Proteaceae Macadamia ternifolia F. Muell. | no | EN | no | |
| Rubiaceae Coffea arabica L. | no | EN | no | |
| Rubiaceae Riodocea pulcherrima Delprete | yes | EN | yes | |
| Rubiaceae Simira eliezeriana Peixoto | yes | EN | EN | yes |
| Rutaceae Esenbeckia leiocarpa Engl. | yes | VU | no | |
| Salicaceae Xylosma glaberrima Sleumer | yes | VU | no | |
| Santalaceae Acanthosyris paulo-alvinii G. M. Barroso | yes | CR | no | |
| Sapotaceae Chrysophyllum imperiale (Linden ex K. Koch and Fintelm.) Benth. and Hook. | yes | EN | EN | yes |
| Sapotaceae Chrysophyllum paranaense T. D. Penn. | yes | VU | yes | |
| Sapotaceae Chrysophyllum splendens Spreng. | yes | VU | no | |
| Sapotaceae Manilkara bella Monach. | yes | EN | yes | |
| Sapotaceae Manilkara elata (Allemão ex Miq.) Monach. | yes | EN | no | |
| Sapotaceae Pouteria pachycalyx T. D. Penn. | yes | CR | yes | |
| Sapotaceae Pradosia kuhlmannii Toledo | yes | EN | EN | no |
| Solanaceae Brugmansia suaveolens (Willd.) Sweet | no | EW | no | |
| Solanaceae Brunfelsia jamaicensis Griseb. | no | VU | no | |
| Urticaceae Coussapoa curranii S. F. Blake | yes | EN | VU | no |
| Zamiaceae Ceratozamia kuesteriana Regel | no | CR | no | |
| Zamiaceae Dioon purpusii Rose | no | EN | no | |
| Zamiaceae Encephalartos altensteinii Lehm. | no | VU | no | |
| Zamiaceae Zamia pumila L. | no | VU | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, T.M.H.d.; Coelho, M.A.N.; Peixoto, A.L. Rio de Janeiro Botanical Garden: Biodiversity Conservation in a Tropical Arboretum. J. Zool. Bot. Gard. 2024, 5, 378-394. https://doi.org/10.3390/jzbg5030026
Almeida TMHd, Coelho MAN, Peixoto AL. Rio de Janeiro Botanical Garden: Biodiversity Conservation in a Tropical Arboretum. Journal of Zoological and Botanical Gardens. 2024; 5(3):378-394. https://doi.org/10.3390/jzbg5030026
Chicago/Turabian StyleAlmeida, Thaís Moreira Hidalgo de, Marcus Alberto Nadruz Coelho, and Ariane Luna Peixoto. 2024. "Rio de Janeiro Botanical Garden: Biodiversity Conservation in a Tropical Arboretum" Journal of Zoological and Botanical Gardens 5, no. 3: 378-394. https://doi.org/10.3390/jzbg5030026
APA StyleAlmeida, T. M. H. d., Coelho, M. A. N., & Peixoto, A. L. (2024). Rio de Janeiro Botanical Garden: Biodiversity Conservation in a Tropical Arboretum. Journal of Zoological and Botanical Gardens, 5(3), 378-394. https://doi.org/10.3390/jzbg5030026






