Update on Current Hormonal and Non-Hormonal Contraceptive Options in Non-Human Primates
Abstract
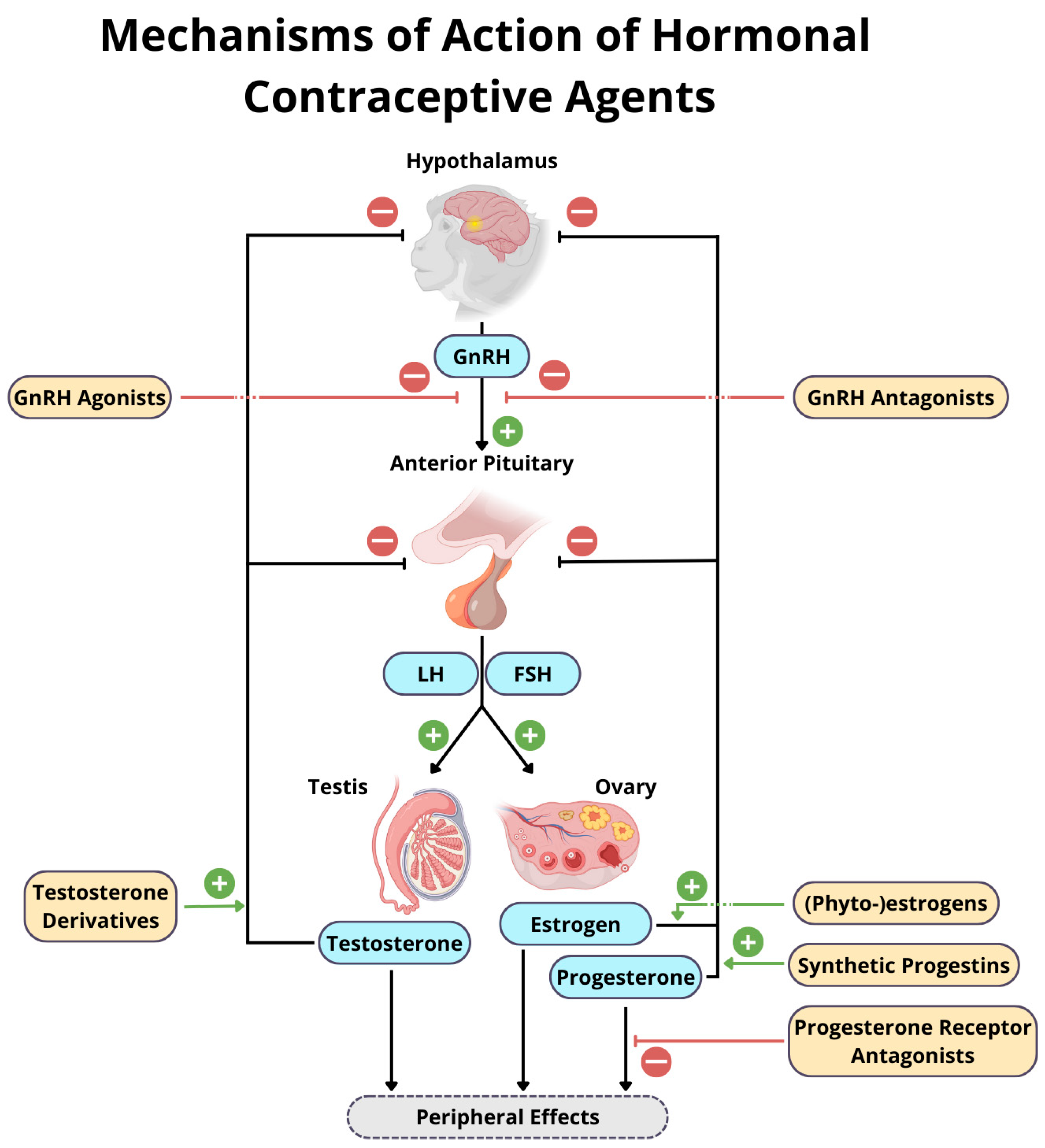
:1. Introduction
2. Hormonal Contraceptives
2.1. GnRH Agonists
2.1.1. Deslorelin Acetate (Suprelorin®)
2.1.2. Other GnRH Agonists
2.2. GnRH Antagonists
2.3. Progestins
2.3.1. Etonogestrel (Implanon®, Implanon NXT®, Nexplanon®)
2.3.2. Levonorgestrel (Norplant®, Norplant-2®, Jadelle®)
2.3.3. Medroxyprogesterone Acetate (Depo-Provera®)
2.3.4. Melengestrol Acetate
2.3.5. Progestin or Combined Progestin–Estrogen Contraceptive Pills
2.4. Progesterone Receptor Antagonists
2.5. Testosterone Derivatives
2.6. Phyto-Estrogens
3. Non-Hormonal Contraception
3.1. Immunocontraception
3.1.1. Zona Pellucida Vaccines
3.1.2. EPPIN Based Vaccines
3.1.3. GnRH Vaccines
3.2. EP055
3.3. Triptonide
3.4. Prostaglandin Receptor Antagonists
3.5. Prostaglandin F2α Analogues
3.6. COX-Inhibitors
3.7. Phosphodiesterase Inhibitors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plowman, A.B.; Jordan, N.R.; Anderson, N.; Condon, E.; Fraser, O. Welfare Implications of Captive Primate Population Management: Behavioural and Psycho-Social Effects of Female-Based Contraception, Oestrus and Male Removal in Hamadryas Baboons (Papio hamadryas). Appl. Anim. Behav. Sci. 2005, 90, 155–165. [Google Scholar] [CrossRef]
- Glatston, A.R. The Control of Zoo Populations with Special Reference to Primates. Anim. Welf. 1998, 7, 269–281. [Google Scholar] [CrossRef]
- Wallace, P.; Asa, C.; Agnew, M.; Cheyne, S. A Review of Population Control Methods in Captive-Housed Primates. Anim. Welf. 2016, 25, 7–20. [Google Scholar] [CrossRef]
- Nangia, A.K.; Myles, J.L.; Thomas AJ, J.R. Vasectomy Reversal for the Post-Vasectomy Pain Syndrome: A Clinical and Histological Evaluation. J. Urol. 2000, 164, 1939–1942. [Google Scholar] [CrossRef]
- Balena, R.; Toolan, B.C.; Shea, M.; Markatos, A.; Myers, E.R.; Lee, S.C.; Opas, E.E.; Seedor, J.G.; Klein, H.; Frankenfield, D. The Effects of 2-Year Treatment with the Aminobisphosphonate Alendronate on Bone Metabolism, Bone Histomorphometry, and Bone Strength in Ovariectomized Nonhuman Primates. J. Clin. Investig. 1993, 92, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Burkart, J.M.; Van Schaik, C.P. Cognitive Consequences of Cooperative Breeding in Primates? Anim. Cogn. 2010, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.M.; Terrell, S.P.; Savage, A. Causes of Mortality in Captive Cotton-top Tamarins (Saguinus oedipus). Zoo Biol. 2004, 23, 127–137. [Google Scholar] [CrossRef]
- Clayton, R.N. Gonadotrophin-Releasing Hormone: Its Actions and Receptors. J. Endocrinol. 1989, 120, 11–19. [Google Scholar] [CrossRef]
- Agnew, M.K.; Asa, C.S.; Franklin, A.D.; McDonald, M.M.; Cowl, V.B. Deslorelin (Suprelorin®) Use in North American and European Zoos and Aquariums: Taxonomic Scope, Dosing and Efficacy. J. Zoo Wildl. Med. 2021, 52, 427–436. [Google Scholar] [CrossRef]
- Cowl, V.B.; Walker, S.L.; Rambaud, Y.F. Assessing The Efficacy Of Deslorelin Acetate Implants (Suprelorin®) In Alternative Placement Sites. J. Zoo Wildl. Med. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Jana Carlsen, F.; de Jongh, T.; Pluháčková, J. (Eds.) EAZA Best Practice Guidelines for Chimpanzees (Pan troglodytes), 1st ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Monclus, L.; Carbonell, M.D.; Gerique, C.; Casares, M.; Lopez-Bejar, M. Effect of Deslorelin Implants on the Testicular Function in Male Ring-Tailed Lemurs (Lemur catta): Deslorelin Effect in Lemur Catta. J. Zoo Aquar. Res. 2018, 6, 37–40. [Google Scholar] [CrossRef]
- Rosenfield, D.A.; Viau, P.; Oliveira, C.A.; Pizzutto, C.S. Effectiveness of the GnRH Analogue Deslorelin as a Reversible Contraceptive in a Neotropical Primate, the Common Marmoset Callithrix jacchus (Mammalia: Primates: Callitrichidae). J. Threat. Taxa 2016, 8, 8652. [Google Scholar] [CrossRef]
- Taberer, T.R.; Mead, J.; Hartley, M.; Harvey, N.D. Impact of Female Contraception for Population Management on Behavior and Social Interactions in a Captive Troop of Guinea Baboons (Papio papio). Zoo Biol. 2023, 42, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.E.; Mackiewicz, A.L.; Ardeshir, A.; Alber, S.A.; Christe, K.L. Hormonal Suppression in Female Rhesus Macaques (Macaca mulatta) Implanted Subcutaneously with Deslorelin. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 226–233. [Google Scholar] [CrossRef]
- Norton, T.; Penfold, M.L.; Lessnau, B.; Jochle, W.; Staaden, S.L.; Jolliffe, A.; Bauman, J.E.; Spratt, J. Long-Acting Deslorelin Implants to Control Aggression in Male Lion-Tailed Macaques (Macaca silenus). In Proceedings of the American Association of Zoo Veterinarians Conference 2000, New Orleans, LA, USA, 17–21 September 2000. [Google Scholar]
- Young, H.; Young, H. The Use of Deslorelin, a GnRH Agonist, to Reduce Aggression in Male Chacma Baboons (Papio ursinus) to Improve Animal Welfare in a Captive Setting. Master Thesis, Oxford Brookes University, Oxford, UK, 2013. [Google Scholar]
- Martinez, G.; Lacoste, R.; Dumasy, M.; Garbit, S.; Brouillet, S.; Coutton, C.; Arnoult, C.; Druelle, F.; Molina-Vila, P. Deslorelin Acetate Implant Induces Transient Sterility and Behavior Changes in Male Olive Baboon (Papio anubis): A Case Study. J. Med. Primatol. 2020, 49, 344–348. [Google Scholar] [CrossRef]
- Penfold, L.M.; Norton, T.; Asa, C.S. Effects of GnRH Agonists on Testosterone and Testosterone-stimulated Parameters for Contraception and Aggression Reduction in Male Lion-tailed Macaques (Macaca silenus). Zoo Biol. 2021, 40, 541–550. [Google Scholar] [CrossRef]
- Baniel, A.; Cowlishaw, G.; Huchard, E. Jealous Females? Female Competition and Reproductive Suppression in a Wild Promiscuous Primate. Proc. R. Soc. B. 2018, 285, 20181332. [Google Scholar] [CrossRef]
- Dal Pesco, F.; Trede, F.; Zinner, D.; Fischer, J. Kin Bias and Male Pair-Bond Status Shape Male-Male Relationships in a Multilevel Primate Society. Behav. Ecol. Sociobiol. 2021, 75, 24. [Google Scholar] [CrossRef]
- Karaskiewicz, C.L.; Ramirez, M.; Bales, K.L. Physiological and Behavioral Effects of Hormonal Contraceptive Treatment in Captive, Pair-Bonded Primates (Plecturocebus cupreus). J. Am. Assoc. Lab. Anim. Sci. 2023, 62, 494–501. [Google Scholar] [CrossRef]
- Fuentes, A. Re-Evaluating Primate Monogamy. Am. Anthropol. 1998, 100, 890–907. [Google Scholar] [CrossRef]
- Dolotovskaya, S.; Walker, S.; Heymann, E.W. What Makes a Pair Bond in a Neotropical Primate: Female and Male Contributions. R. Soc. Open Sci. 2020, 7, 191489. [Google Scholar] [CrossRef] [PubMed]
- Bemment, N.; Abelló, M.T.; Bionda, T.; Meulen, T.; Bugg, T.; Guéry, J.P.; Lafaut, S.; Laurent, S.; Linn, S.; Liptovszky, M.; et al. EAZA Best Practice Guidelines for the Western Lowland Gorilla (Gorilla gorilla gorilla), 3rd ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Fraser, H.M.; Sandow, J.; Seidel, H.; Von Rechenberg, W. An Implant of a Gonadotropin Releasing Hormone Agonist (Buserelin) Which Suppresses Ovarian Function in the Macaque for 3–5 Months. Acta Endocrinol. 1987, 115, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Bremner, W.J.; Nestor, J.J.; Vickery, B.H.; Steiner, R.A. Suppression of Plasma Gonadotropins and Testosterone in Adult Male Monkey (Macaca fascicularis) by a Potent Inhibitory Analogue of Gonadotropin-Releasing Hormone. J. Clin. Endocrinol. Metab. 1986, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bremner, W.J.; Bagatell, C.J.; Steiner, R.A. Gonadotropin-Releasing Hormone Antagonist plus Testosterone: A Potential Male Contraceptive. J. Clin. Endocrinol. Metab. 1991, 73, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, H.B.; Mäkäräinen, L. The Pharmacodynamics and Efficacy of Implanon®1. Contraception 1998, 58, 91S–97S. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.C.; Feng, L.X.; Newton, J.R.; Van Beek, A.; Coelingh-Bennink, H.J. Release Characteristics, Ovarian Activity and Menstrual Bleeding Pattern with a Single Contraceptive Implant Releasing 3-Ketodesogestrel. Contraception 1993, 47, 251–261. [Google Scholar] [CrossRef]
- Kjos, S.L. Contraception and the Risk of Type 2 Diabetes Mellitus in Latina Women with Prior Gestational Diabetes Mellitus. JAMA 1998, 280, 533. [Google Scholar] [CrossRef]
- Varma, R.; Mascarenhas, L. Endometrial Effects of Etonogestrel (Implanon) Contraceptive Implant. Curr. Opin. Obstet. Gynecol. 2001, 13, 335–341. [Google Scholar] [CrossRef]
- Bourry, O.; Peignot, P.; Rouquet, P. Contraception in the Chimpanzee: 12-Year Experience at the CIRMF Primate Centre, Gabon. J. Med. Primatol. 2005, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Maaskant, A.; Scarsi, K.K.; Meijer, L.; Roubos, S.; Louwerse, A.L.; Remarque, E.J.; Langermans, J.A.M.; Stammes, M.A.; Bakker, J. Long-Acting Reversible Contraception with Etonogestrel Implants in Female Macaques (Macaca Mulatta and Macaca Fascicularis). Front. Vet. Sci. 2024, 10, 1319862. [Google Scholar] [CrossRef]
- Roubos, S.; Louwerse, A.L.; Langermans, J.A.M.; Bakker, J. Retrospective Analysis of the Effectiveness and Reversibility of Long-Acting Contraception Etonogestrel (Implanon®) in Common Marmosets (Callithrix jacchus). Animals 2021, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Brandon, D.D.; Markwick, A.J.; Chrousos, G.P.; Loriaux, D.L. Glucocorticoid Resistance in Humans and Nonhuman Primates. Cancer. Res. 1989, 49, 2203s–2213s. [Google Scholar] [PubMed]
- Maijer, A.M.; Semple, S. Investigating Potential Effects of the Contraceptive Implanon on the Behavior of Free-Ranging Adult Female Barbary Macaques. J. Appl. Anim. Welf. Sci. 2016, 19, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Vickery, Z.; Madden, T.; Zhao, Q.; Secura, G.M.; Allsworth, J.E.; Peipert, J.F. Weight Change at 12 Months in Users of Three Progestin-Only Contraceptive Methods. Contraception 2013, 88, 503–508. [Google Scholar] [CrossRef]
- Amico, J.; Kumar, B.; Rosenstein, H.; Gold, M. The Contraceptive Implant: An Updated Review of the Evidence. Curr. Obstet. Gynecol. Rep. 2015, 4, 79–88. [Google Scholar] [CrossRef]
- Shutt, K.; MacLarnon, A.; Heistermann, M.; Semple, S. Grooming in Barbary Macaques: Better to Give than to Receive? Biol. Lett. 2007, 3, 231–233. [Google Scholar] [CrossRef]
- Bettinger, T.; Cougar, D.; Lee, D.R.; Lasley, B.L.; Wallis, J. Ovarian Hormone Concentrations and Genital Swelling Patterns in Female Chimpanzees with Norplant Implants. Zoo Biol. 1997, 16, 209–223. [Google Scholar] [CrossRef]
- Savage, A.; Zirofsky, D.S.; Shideler, S.E.; Smith, T.E.; Lasley, B.L. Use of Levonorgestrel as an Effective Means of Contraception in the White-Faced Saki (Pithecia pithecia). Zoo Biol. 2002, 21, 49–57. [Google Scholar] [CrossRef]
- Wheaton, C.J.; Savage, A.; Shukla, A.; Neiffer, D.; Qu, W.; Sun, Y.; Lasley, B.L. The Use of Long Acting Subcutaneous Levonorgestrel (LNG) Gel Depot as an Effective Contraceptive Option for Cotton-top Tamarins (Saguinus oedipus). Zoo Biol. 2011, 30, 498–522. [Google Scholar] [CrossRef]
- Lemus, A.E.; Vilchis, F.; Damsky, R.; Chávez, B.A.; García, G.A.; Grillasca, I.; Pérez-Palacios, G. Mechanism of Action of Levonorgestrel: In Vitro Metabolism and Specific Interactions with Steroid Receptors in Target Organs. J. Steroid Biochem. Mol. Biol. 1992, 41, 881–890. [Google Scholar] [CrossRef]
- Pereboom, Z.J.J.M.; de Laet, C.P.M.; Leus, K. The Use of Contraception In European Zoo Chimpanzees (Pan Troglodytes). In European Studbook for Chimpanzee (Pan troglodytes); Carlsen, F., Jongh, T., Eds.; Copenhagen Zoo: Copenhagen, Denmark, 2019. [Google Scholar]
- Shimizu, K.; Takenoshita, Y.; Mitsunaga, F.; Nozaki, M. Suppression of Ovarian Function and Successful Contraception in Macaque Monkeys Following a Single Injection of Medroxyprogesterone Acetate. J. Reprod. Dev. 1996, 42, 147–155. [Google Scholar] [CrossRef]
- Unger, B.; Roullet, D.; Pauly, A.; Laidebeure, S.; Lécu, A.; Morino, L. EAZA Best Practice Guidelines for Crowned Sifaka (Propithecus coronatus) and Coquerel’s Sifaka (Propithecus coquereli), 1st ed.; European Association of Zoos and Aquariums EAZA: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Asa, C.S.; Porton, I.J.; Junge, R. Reproductive Cycles and Contraception of Black Lemurs (Eulemur macaco macaco) with Depot Medroxyprogesterone Acetate during the Breeding Season. Zoo Biol. 2007, 26, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Steklis, H.D.; Linn, G.S.; Howard, S.M.; Kling, A.S.; Tiger, L. Effects of Medroxyprogesterone Acetate on Socio-Sexual Behavior of Stumptail Macaques. Physiol. Behav. 1982, 28, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.J.; Schuerch, F.S.; Heffernan, S.; Thomson, P.C.; O’Brien, J.K.; McGreevy, P.D. The Effect of Medroxyprogesterone Acetate on Behavioural Responses of Captive Female Hamadryas Baboons (Papio hamadryas). Anim. Reprod. Sci. 2008, 108, 412–424. [Google Scholar] [CrossRef]
- Mustoe, A.C.; Jensen, H.A.; French, J.A. Describing Ovarian Cycles, Pregnancy Characteristics, and the Use of Contraception in Female White-Faced Marmosets, Callithrix geoffroyi. Am. J. Primatol. 2012, 74, 1044–1053. [Google Scholar] [CrossRef]
- Lopez, L.M.; Ramesh, S.; Chen, M.; Edelman, A.; Otterness, C.; Trussell, J.; Helmerhorst, F.M. Progestin-Only Contraceptives: Effects on Weight. Cochrane Database Syst. Rev. 2016, 2016, CD008815. [Google Scholar] [CrossRef]
- Labrie, C.; Cusan, L.; Plante, M.; Lapointe, S.; Labrie, F. Analysis of the Androgenic Activity of Synthetic “Progestins” Currently Used for the Treatment of Prostate Cancer. J. Steroid. Biochem. 1987, 28, 379–384. [Google Scholar] [CrossRef]
- Linn, G.S.; Steklis, H.D. The Effects of Depo-Medroxyprogesterone Acetate (DMPA) on Copulation-Related and Agonistic Behaviors in an Island Colony of Stumptail Macaques (Macaca arctoides). Physiol. Behav. 1990, 47, 403–408. [Google Scholar] [CrossRef]
- Zumpe, D.; Bonsall, R.W.; Kutner, M.H.; Michael, R.P. Medroxyprogesterone Acetate, Aggression, and Sexual Behavior in Male Cynomolgus Monkeys (Macaca fascicularis). Horm. Behav. 1991, 25, 394–409. [Google Scholar] [CrossRef]
- Pazol, K.; Wilson, M.E.; Wallen, K. Medroxyprogesterone Acetate Antagonizes the Effects of Estrogen Treatment on Social and Sexual Behavior in Female Macaques. J. Clin. Endocrinol. Metab. 2004, 89, 2998–3006. [Google Scholar] [CrossRef]
- Crawford, J.C.; Boulet, M.; Drea, C.M. Smelling Wrong: Hormonal Contraception in Lemurs Alters Critical Female Odour Cues. Proc. R. Soc. B. 2011, 278, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Palagi, E. Beyond Odor Discrimination: Demonstrating Individual Recognition by Scent in Lemur Catta. Chem. Senses 2006, 31, 437–443. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Agnew, M.K.; Asa, C.S.; Simms, B.; Wiley, J.N.; Powell, D.M. Reproductive Potential and Implant Loss in Female Hamadryas Baboons (Papio Hamadryas) Previously Contracepted with Melengestrol Acetate Contraceptive Implants at AZA Institutions. Zoo Biol. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vaglio, S.; Minicozzi, P.; Kessler, S.E.; Walker, D.; Setchell, J.M. Olfactory Signals and Fertility in Olive Baboons. Sci. Rep. 2021, 11, 8506. [Google Scholar] [CrossRef] [PubMed]
- Möhle, U.; Heistermann, M.; Einspanier, A.; Hodges, J.K. Efficacy and Effects of Short- and Medium-Term Contraception in the Common Marmoset (Callithrix jacchus) Using Melengestrol Acetate Implants. J. Med. Primatol. 1999, 28, 36–47. [Google Scholar] [CrossRef]
- Asa, C.S.; Porton, I.J. Contraception in Nonhuman Primates. In Wildlife Contraception: Issues, Methods, and Applications, 1st ed.; Asa, C.S., Porton, I.J., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 119–149. ISBN 978-0-8018-8304-0. [Google Scholar]
- de Vleeschouwer, K.; Leus, K.; Elsacker, L. An Evaluation of the Suitability of Contraceptive Methods in Golden-Headed Lion Tamarins (Leontopithecus chrysomelas), with Emphasis on Melengestrol Acetate (MGA) Implants: (I) Effectiveness, Reversibility and Medical Side-Effects. Anim. Welf. 2000, 9, 251–271. [Google Scholar] [CrossRef]
- McDonald, M.M.; Agnew, M.K.; Asa, C.S.; Powell, D.M. Melengestrol Acetate Contraceptive Implant Use in Colobus Monkeys (Colobus Guereza): Patterns through Time and Differences in Reproductive Potential and Live Births. Zoo Biol. 2021, 40, 124–134. [Google Scholar] [CrossRef]
- Wood, C.; Ballou, J.D.; Houle, C.S. Restoration of Reproductive Potential Following Expiration or Removal of Melengestrol Acetate Contraceptive Implants in Golden Lion Tamarins (Leontopithecus rosalia). J. Zoo Wildl. Med. 2001, 32, 417–425. [Google Scholar] [CrossRef]
- Murnane, R.D.; Zdziarski, J.M.; Walsh, T.F.; Kinsel, M.J.; Meehan, T.P.; Kovarik, P.; Briggs, M.; Raverty, S.A.; Phillips, L.G. Melengestrol Acetate-Induced Exuberant Endometrial Decidualization in Goeldi’s Marmosets (Callimico goeldii) and Squirrel Monkeys (Saimiri sciureus). J. Zoo Wildl. Med. 1996, 27, 315–324. [Google Scholar]
- Portugal, M.M.; Asa, C.S. Effects of Chronic Melengestrol Acetate Contraceptive Treatment on Perineal Tumescence, Body Weight, and Sociosexual Behavior of Hamadryas Baboons (Papio hamadryas). Zoo Biol. 1995, 14, 251–259. [Google Scholar] [CrossRef]
- Agnew, M.K.; Asa, C.S.; Clyde, V.L.; Keller, D.L.; Meinelt, A. A Survey of Bonobo (Pan paniscus) Oral Contraceptive Pill Use in North American Zoos: Oral Contraceptive Use in Bonobos. Zoo Biol. 2016, 35, 444–453. [Google Scholar] [CrossRef]
- LeFauve, M.K.; Margulis, S.W. Chemical Contraceptive Impacts on Cyclic Progesterone and Sexual Behavior in Zoo-housed Western Lowland Gorillas. Am. J. Primatol. 2023, 85, e23418. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, M.; Edelman, A.; Nichols, M.D.; Jensen, J.T. Bleeding Patterns and Patient Acceptability of Standard or Continuous Dosing Regimens of a Low-Dose Oral Contraceptive: A Randomized Trial. Contraception 2003, 67, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, L. Menstrual Reduction with Extended Use of Combination Oral Contraceptive Pills: Randomized Controlled Trial. Obstet. Gynecol. 2001, 98, 771–778. [Google Scholar] [CrossRef] [PubMed]
- de Waal, F.B.M. Tension Regulation and Nonreproductive Functions of Sex in Captive Bonobos (Pan paniscus). Nat. Geogr. Res. 1987, 3, 318–335. [Google Scholar]
- Ryu, H.; Hill, D.A.; Furuichi, T. Prolonged Maximal Sexual Swelling in Wild Bonobos Facilitates Affiliative Interactions between Females. Behaviour 2015, 152, 285–311. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Perdue, B.M.; Legg, A.M. Sexual Behavior in Female Western Lowland Gorillas (Gorilla gorilla gorilla): Evidence for Sexual Competition. Am. J. Primatol. 2009, 71, 587–593. [Google Scholar] [CrossRef]
- Vervaecke, H.; Van Elsacker, L.; Möhle, U.; Heistermann, M.; Verheyen, R.F. Inter-Menstrual Intervals in Captive Bonobos (Pan paniscus). Primates 1999, 40, 283–289. [Google Scholar] [CrossRef]
- Paoli, T.; Palagi, E.; Tacconi, G.; Tarli, S.B. Perineal Swelling, Intermenstrual Cycle, and Female Sexual Behavior in Bonobos (Pan paniscus). Am. J. Primatol. 2006, 68, 333–347. [Google Scholar] [CrossRef]
- Sitruk-Ware, R. New Progestagens for Contraceptive Use. Hum. Reprod. Update 2006, 12, 169–178. [Google Scholar] [CrossRef]
- Dinger, J.C.; Heinemann, L.A.J.; Kühl-Habich, D. The Safety of a Drospirenone-Containing Oral Contraceptive: Final Results from the European Active Surveillance Study on Oral Contraceptives Based on 142,475 Women-Years of Observation. Contraception 2007, 75, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Q.; Grandi, S.M.; Filion, K.B.; Abenhaim, H.A.; Joseph, L.; Eisenberg, M.J. Drospirenone-Containing Oral Contraceptive Pills and the Risk of Venous and Arterial Thrombosis: A Systematic Review. BJOG 2013, 120, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.F.; Nanda, K.; Grimes, D.A.; Lopez, L.M.; Schulz, K.F. 20 mg versus > 20 mg Estrogen Combined Oral Contraceptives for Contraception. Cochrane Database Syst. Rev. 2013, 2013, CD003989. [Google Scholar] [CrossRef] [PubMed]
- Gerstman, B.B.; Piper, J.M.; Tomita, D.K.; Ferguson, W.J.; Stadel, B.V.; Lundin, F.E. Oral Contraceptive Estrogen Dose and the Risk of Deep Venous Thromboembolic Disease. Am. J. Epidemiol. 1991, 133, 32–37. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grey, T.W.; Stuebe, A.M.; Chen, M.; Truitt, S.T.; Gallo, M.F. Combined Hormonal versus Nonhormonal versus Progestin-Only Contraception in Lactation. Cochrane Database Syst. Rev. 2015, 2015, CD003988. [Google Scholar] [CrossRef]
- Sarfaty, A.; Margulis, S.W.; Atsalis, S. Effects of Combination Birth Control on Estrous Behavior in Captive Western Lowland Gorillas (Gorilla gorilla gorilla). Zoo Biol. 2012, 31, 350–361. [Google Scholar] [CrossRef]
- Nadler, R.D.; Dahl, J.F.; Gould, K.G.; Collins, D.C. Effects of an Oral Contraceptive on Sexual Behavior of Chimpanzees (Pan troglodytes). Arch. Sex. Behav. 1993, 22, 477–500. [Google Scholar] [CrossRef]
- Shively, C.A.; Manuck, S.B.; Kaplan, J.R.; Koritnik, D.R. Oral Contraceptive Administration, Interfemale Relationships, and Sexual Behavior in Macaca fascicularis. Arch. Sex. Behav. 1990, 19, 101–117. [Google Scholar] [CrossRef]
- van Vliet, H.A.; Grimes, D.A.; Lopez, L.M.; Schulz, K.F.; Helmerhorst, F.M. Triphasic versus Monophasic Oral Contraceptives for Contraception. Cochrane Database Syst. Rev. 2011, 11, CD003553. [Google Scholar] [CrossRef]
- Henderson, J.A.; Shively, C.A. Triphasic Oral Contraceptive Treatment Alters the Behavior and Neurobiology of Female Cynomolgus Monkeys. Psychoneuroendocrinology 2004, 29, 21–34. [Google Scholar] [CrossRef]
- Zapata, L.B.; Steenland, M.W.; Brahmi, D.; Marchbanks, P.A.; Curtis, K.M. Effect of Missed Combined Hormonal Contraceptives on Contraceptive Effectiveness: A Systematic Review. Contraception 2013, 87, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Im, A.; Appleman, L.J. Mifepristone: Pharmacology and Clinical Impact in Reproductive Medicine, Endocrinology and Oncology. Expert Opin. Pharmacother. 2010, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Teutsch, G.; Philibert, D. History and Perspectives of Antiprogestins from the Chemist’s Point of View. Hum. Reprod. 1994, 9, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Dhawan, L.; Lalitkumar, P.G.L.; Ghosh, D. A Multiparametric Study of the Action of Mifepristone Used in Emergency Contraception Using the Rhesus Monkey as a Primate Model. Contraception 2003, 68, 453–469. [Google Scholar] [CrossRef]
- Heikinheimo, O.; Gordon, K.; Lähteenmäki, P.; Williams, R.F.; Hodgen, G.D. Antiovulatory Actions of RU 486: The Pituitary Is Not the Primary Site of Action in Vivo. J. Clin. Endocrinol. Metab. 1995, 80, 1859–1868. [Google Scholar] [CrossRef]
- Nayak, N.R.; Ghosh, D.; Lasley, B.L.; Sengupta, J. Anti-Implantation Activity of Luteal Phase Mifepristone Administration Is Not Mimicked by Prostaglandin Synthesis Inhibitor or Prostaglandin Analogue in the Rhesus Monkey. Contraception 1997, 55, 103–114. [Google Scholar] [CrossRef]
- Micks, E.; Shekell, T.; Stanley, J.; Zelinski, M.; Martin, L.; Riefenberg, S.; Adevai, T.; Jensen, J. Medical Termination of Pregnancy in Cynomolgus Macaques. J. Med. Primatol. 2012, 41, 394–402. [Google Scholar] [CrossRef]
- Tarantal, A.F.; Hendrickx, A.G.; Matlin, S.A.; Lasley, B.L.; Gu, Q.-Q.; Thomas, C.A.A.; Vince, P.M.; Look, P.F.A.V. Effects of Two Antiprogestins on Early Pregnancy in the Long-Tailed Macaque (Macaca fascicularis). Contraception 1996, 54, 107–115. [Google Scholar] [CrossRef]
- Borman, S.M.; Schwinof, K.M.; Niemeyer, C.; Chwalisz, K.; Stouffer, R.L.; Zelinski-Wooten, M.B. Low-Dose Antiprogestin Treatment Prevents Pregnancy in Rhesus Monkeys and Is Reversible after 1 Year of Treatment. Hum. Reprod. 2003, 18, 69–76. [Google Scholar] [CrossRef]
- Wistuba, J.; Luetjens, C.M.; Ehmcke, J.; Redmann, K.; Damm, O.S.; Steinhoff, A.; Sandhowe-Klaverkamp, R.; Nieschlag, E.; Simoni, M.; Schlatt, S. Experimental Endocrine Manipulation by Contraceptive Regimen in the Male Marmoset (Callithrix jacchus). Reproduction 2013, 145, 439–451. [Google Scholar] [CrossRef]
- Weinbauer, G.F.; Schlatt, S.; Walter, V.; Nieschlag, E. Testosterone-Induced Inhibition of Spermatogenesis Is More Closely Related to Suppression of FSH than to Testicular Androgen Levels in the Cynomolgus Monkey Model (Macaca fascicularis). J. Endocrinol. 2001, 168, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Trisomboon, H.; Malaivijitnond, S.; Watanabe, G.; Taya, K. Ovulation Block by Pueraria mirifica: A Study of Its Endocrinological Effect in Female Monkeys. Endocrine 2005, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Trabert, B. Hormone Therapy: Short-Term Relief, Long-Term Consequences. Lancet 2015, 385, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Saver, A.E. Immunocontraception for Animals: Current Status and Future Perspective. Am. J. Reprod. Immunol. 2016, 75, 426–439. [Google Scholar] [CrossRef]
- van de Voort, C.A.; Schwoebel, E.D.; Dunbar, B.S. Immunization of Monkeys with Recombinant Complimentary Deoxyribonucleic Acid Expressed Zona Pellucida Proteins. Fertil. Steril. 1995, 64, 838–847. [Google Scholar] [CrossRef]
- Bagavant, H.; Sharp, C.; Kurth, B.; Tung, K.S.K. Induction and Immunohistology of Autoimmune Ovarian Disease in Cynomolgus Macaques (Macaca fascicularis). Am. J. Pathol. 2002, 160, 141–149. [Google Scholar] [CrossRef]
- Paterson, M.; Koothan, P.T.; Morris, K.D.; O’Byrne, K.T.; Braude, P.; Williams, A.; Aitken, R.J. Analysis of the Contraceptive Potential of Antibodies against Native and Deglycosylated Porcine ZP3 in Vivo and in Vitro. Biol. Reprod. 1992, 46, 523–534. [Google Scholar] [CrossRef]
- Gray, M.E.; Cameron, E.Z. Does Contraceptive Treatment in Wildlife Result in Side Effects? A Review of Quantitative and Anecdotal Evidence. Reproduction 2010, 139, 45–55. [Google Scholar] [CrossRef]
- Dunbar, B.S.; Avery, S.; Lee, V.; Prasad, S.; Schwahn, D.; Schwoebel, E.; Skinner, S.; Wilkins, B. The Mammalian Zona Pellucida: Its Biochemistry, Immunochemistry, Molecular Biology, and Developmental Expression. Reprod. Fertil. Dev. 1994, 6, 331–347. [Google Scholar] [CrossRef]
- Govind, C.K.; Gupta, S.K. Failure of Female Baboons (Papio anubis) to Conceive Following Immunization with Recombinant Non-Human Primate Zona Pellucida Glycoprotein-B Expressed in Escherichia coli. Vaccine 2000, 18, 2970–2978. [Google Scholar] [CrossRef]
- O’Rand, M.G.; Hamil, K.G.; Adevai, T.; Zelinski, M. Inhibition of Sperm Motility in Male Macaques with EP055, a Potential Non-Hormonal Male Contraceptive. PLoS ONE 2018, 13, e0195953. [Google Scholar] [CrossRef] [PubMed]
- O’Rand, M.G.; Widgren, E.E.; Sivashanmugam, P.; Richardson, R.T.; Hall, S.H.; French, F.S.; VandeVoort, C.A.; Ramachandra, S.G.; Ramesh, V.; Jagannadha Rao, A. Reversible Immunocontraception in Male Monkeys Immunized with Eppin. Science 2004, 306, 1189–1190. [Google Scholar] [CrossRef]
- Levy, J.K.; Miller, L.A.; Cynda Crawford, P.; Ritchey, J.W.; Ross, M.K.; Fagerstone, K.A. GnRH Immunocontraception of Male Cats. Theriogenology 2004, 62, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Sealfon, S.C.; Weinstein, H.; Millar, R.P. Molecular Mechanisms of Ligand Interaction with the Gonadotropin-Releasing Hormone Receptor. Endocr. Rev. 1997, 18, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Boedeker, N.C.; Hayek, L.-A.C.; Murray, S.; de Avila, D.M.; Brown, J.L. Effects of a Gonadotropin-Releasing Hormone Vaccine on Ovarian Cyclicity and Uterine Morphology of an Asian Elephant (Elephas maximus). J. Zoo Wildl. Med. 2012, 43, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Killian, G.; Kreeger, T.J.; Rhyan, J.; Fagerstone, K.; Miller, L. Observations on the Use of GonaCon in Captive Female Elk (Cervus elaphus). J. Wildl. Dis. 2009, 45, 184–188. [Google Scholar] [CrossRef]
- Killian, G.; Miller, L.; Rhyan, J.; Doten, H. Immunocontraception of Florida Feral Swine with a Single-Dose GnRH Vaccine. Am. J. Reprod. Immunol. 2006, 55, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Friary, J.A.; Miller, L.A.; Tucker, S.J.; Fagerstone, K.A. Long-Term Fertility Control in Female Cats with GonaConTM, a GnRH Immunocontraceptive. Theriogenology 2011, 76, 1517–1525. [Google Scholar] [CrossRef]
- Vargas-Pino, F.; Gutiérrez-Cedillo, V.; Canales-Vargas, E.J.; Gress-Ortega, L.R.; Miller, L.A.; Rupprecht, C.E.; Bender, S.C.; García-Reyna, P.; Ocampo-López, J.; Slate, D. Concomitant Administration of GonaConTM and Rabies Vaccine in Female Dogs (Canis familiaris) in Mexico. Vaccine 2013, 31, 4442–4447. [Google Scholar] [CrossRef]
- Chappel, S.C.; Ellinwood, W.E.; Huckins, C.; Herbert, D.C.; Spies, H.G. Active Immunization of Male Rhesus Monkeys against Luteinizing Hormone Releasing Hormone. Biol. Reprod. 1980, 22, 333–342. [Google Scholar] [CrossRef]
- Moresco, A.; Feltrer-Rambaud, Y.; Wolfman, D.; Agnew, D.W. Reproductive One Health in Primates. Am. J. Primatol. 2022, 84, e23325. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.; Smith, G.C.; Gomm, M.; Brash, M.; Bellamy, F.; Massei, G.; Conwell, R.; Vial, F. Evaluation of a Single-Shot Gonadotropin-Releasing Hormone (GnRH) Immunocontraceptive Vaccine in Captive Badgers. Eur. J. Wildl. Res. 2019, 65, 59. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D.P.; Coats, J.; Bellamy, F.; Quy, R.; Pietravalle, S.; Brash, M.; Miller, L.A. Long-Term Effects of Immunocontraception on Wild Boar Fertility, Physiology and Behaviour. Wildl. Res. 2012, 39, 378. [Google Scholar] [CrossRef]
- Ruan, Q.-L.; Hua, Y.-Q.; Wang, M.; Duan, J.-A. Reproductive toxicity of Triptolide on male Caenorhabditis elegans, a model organism. Chin. Tradit. Pat. Med. 2018, 2018, 1009–1014. [Google Scholar]
- Chang, Z.; Qin, W.; Zheng, H.; Schegg, K.; Han, L.; Liu, X.; Wang, Y.; Wang, Z.; McSwiggin, H.; Peng, H.; et al. Triptonide Is a Reversible Non-Hormonal Male Contraceptive Agent in Mice and Non-Human Primates. Nat. Commun. 2021, 12, 1253. [Google Scholar] [CrossRef]
- Peluffo, M.C.; Stanley, J.; Braeuer, N.; Rotgeri, A.; Fritzemeier, K.-H.; Fuhrmann, U.; Buchmann, B.; Adevai, T.; Murphy, M.J.; Zelinski, M.B.; et al. A Prostaglandin E2 Receptor Antagonist Prevents Pregnancies during a Preclinical Contraceptive Trial with Female Macaques. Hum. Reprod. 2014, 29, 1400–1412. [Google Scholar] [CrossRef]
- Fox, J.G. Creating Genetically Modified Marmosets. In The Common Marmoset in Captivity and Biomedical Research, 1st ed.; Marini, R.P., Wachtman, L.M., Tardif, S.D., Mansfield, K., Eds.; Academic Press, an Imprint of Elsevier: London, UK, 2019; p. 338. [Google Scholar]
- Summers, P.M.; Wennink, C.J.; Hodges, J.K. Cloprostenol-Induced Luteolysis in the Marmoset Monkey (Callithrix jacchus). Reproduction 1985, 73, 133–138. [Google Scholar] [CrossRef]
- Nievergelt, C.; Pryce, C.R. Monitoring and Controlling Reproduction in Captive Common Marmosets on the Basis of Urinary Oestrogen Metabolites. Lab. Anim. 1996, 30, 162–170. [Google Scholar] [CrossRef]
- Corrada, Y.; Rodríguez, R.; Tortora, M.; Arias, D.; Gobello, C. A Combination of Oral Cabergoline and Double Cloprostenol Injections to Produce Third-Quarter Gestation Termination in the Bitch. J. Am. Anim. Hosp. Assoc. 2006, 42, 366–370. [Google Scholar] [CrossRef]
- García Mitacek, M.C.; Stornelli, M.C.; Tittarelli, C.M.; Nuñez Favre, R.; De La Sota, R.L.; Stornelli, M.A. Cloprostenol Treatment of Feline Open-Cervix Pyometra. J. Feline Med. Surg. 2014, 16, 177–179. [Google Scholar] [CrossRef]
- Sirois, J.; Sayasith, K.; Brown, K.A.; Stock, A.E.; Bouchard, N.; Doré, M. Cyclooxygenase-2 and Its Role in Ovulation: A 2004 Account. Hum. Reprod. Update 2004, 10, 373–385. [Google Scholar] [CrossRef]
- Hester, K.E.; Harper, M.J.K.; Duffy, D.M. Oral Administration of the Cyclooxygenase-2 (COX-2) Inhibitor Meloxicam Blocks Ovulation in Non-Human Primates When Administered to Simulate Emergency Contraception. Hum. Reprod. 2010, 25, 360–367. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.C.; Lynch, T.J.; Kim, S.O.; Duffy, D.M. The COX-2 Inhibitor Meloxicam Prevents Pregnancy When Administered as an Emergency Contraceptive to Nonhuman Primates. Contraception 2013, 88, 744–748. [Google Scholar] [CrossRef]
- Jensen, J.T.; Schwinof, K.M.; Zelinski-Wooten, M.B.; Conti, M.; DePaolo, L.V.; Stouffer, R.L. Phosphodiesterase 3 Inhibitors Selectively Block the Spontaneous Resumption of Meiosis by Macaque Oocytes in Vitro. Hum. Reprod. 2002, 17, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Zelinski, M.B.; Stanley, J.E.; Fanton, J.W.; Stouffer, R.L. The Phosphodiesterase 3 Inhibitor ORG 9935 Inhibits Oocyte Maturation in the Naturally Selected Dominant Follicle in Rhesus Macaques. Contraception 2008, 77, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.T.; Stouffer, R.L.; Stanley, J.E.; Zelinski, M.B. Evaluation of the Phosphodiesterase 3 Inhibitor ORG 9935 as a Contraceptive in Female Macaques: Initial Trials. Contraception 2010, 81, 165–171. [Google Scholar] [CrossRef]
| Section | Active Substance | ||
|---|---|---|---|
| 2 | Hormonal Contraception | ||
| 2.1 | GnRH Agonists | ||
| 2.1.1 | Deslorelin Acetate | ||
| 2.1.2 | Leuprolide Acetate and Buserelin | ||
| 2.2 | GnRH Antagonists | ||
| 2.3 | Progestins | ||
| 2.3.1 | Etonogestrel | ||
| 2.3.2 | Levonorgestrel | ||
| 2.3.3 | Medroxyprogesterone Acetate | ||
| 2.3.4 | Melengestrol Acetate | ||
| 2.3.5 | Progestin or Combined Progestin–estrogen Contraceptive Pills | ||
| 2.4 | Progesterone Receptor Antagonists | ||
| 2.5 | Testosterone Derivates | ||
| 2.6 | Phyto-estrogens | ||
| 3 | Non-hormonal Contraception | ||
| 3.1 | Immunocontraception | ||
| 3.1.1 | Zona Pellucida Vaccines | ||
| 3.1.2 | EPPIN Based Vaccines | ||
| 3.1.3 | GnRH Vaccines | ||
| 3.2 | EP055 | ||
| 3.3 | Triptonide | ||
| 3.4 | Prostaglandin Receptor Antagonists | ||
| 3.5 | Prostaglandin F2α Analogs | ||
| 3.6 | COX Inhibitors | ||
| 3.7 | Phosphodiesterase Inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederlof, R.A.; Bruins-van Sonsbeek, L.G.R.; Stumpel, J.B.G.; Bakker, J. Update on Current Hormonal and Non-Hormonal Contraceptive Options in Non-Human Primates. J. Zool. Bot. Gard. 2024, 5, 606-629. https://doi.org/10.3390/jzbg5040041
Nederlof RA, Bruins-van Sonsbeek LGR, Stumpel JBG, Bakker J. Update on Current Hormonal and Non-Hormonal Contraceptive Options in Non-Human Primates. Journal of Zoological and Botanical Gardens. 2024; 5(4):606-629. https://doi.org/10.3390/jzbg5040041
Chicago/Turabian StyleNederlof, Remco A., Linda G. R. Bruins-van Sonsbeek, Job B. G. Stumpel, and Jaco Bakker. 2024. "Update on Current Hormonal and Non-Hormonal Contraceptive Options in Non-Human Primates" Journal of Zoological and Botanical Gardens 5, no. 4: 606-629. https://doi.org/10.3390/jzbg5040041
APA StyleNederlof, R. A., Bruins-van Sonsbeek, L. G. R., Stumpel, J. B. G., & Bakker, J. (2024). Update on Current Hormonal and Non-Hormonal Contraceptive Options in Non-Human Primates. Journal of Zoological and Botanical Gardens, 5(4), 606-629. https://doi.org/10.3390/jzbg5040041






