In Vivo Dosimetry for Superficial High Dose Rate Brachytherapy with Optically Stimulated Luminescence Dosimeters: A Comparison Study with Metal-Oxide-Semiconductor Field-Effect Transistors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Calibration
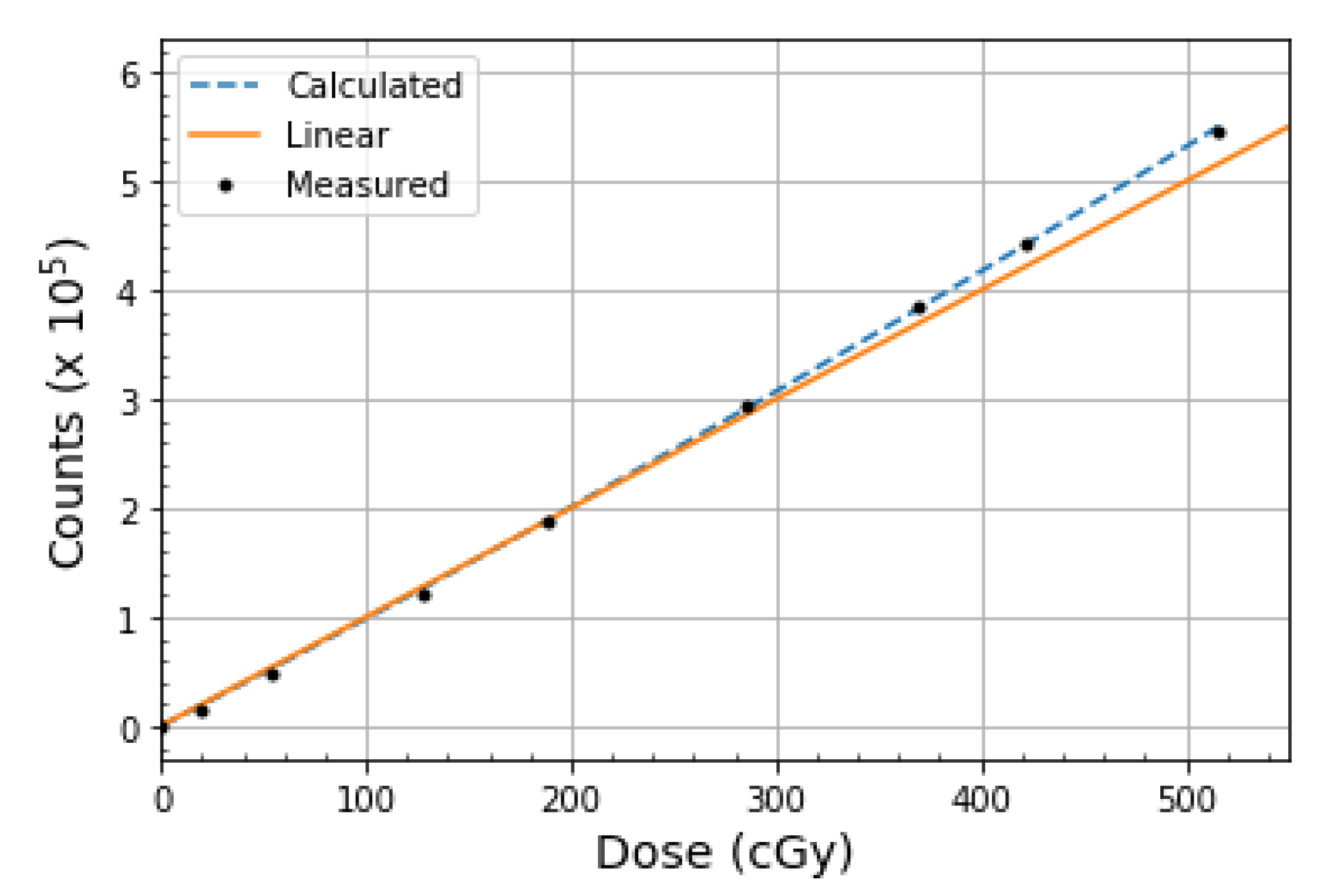
2.2. Dose Linearity
2.3. Dose Rate Dependence
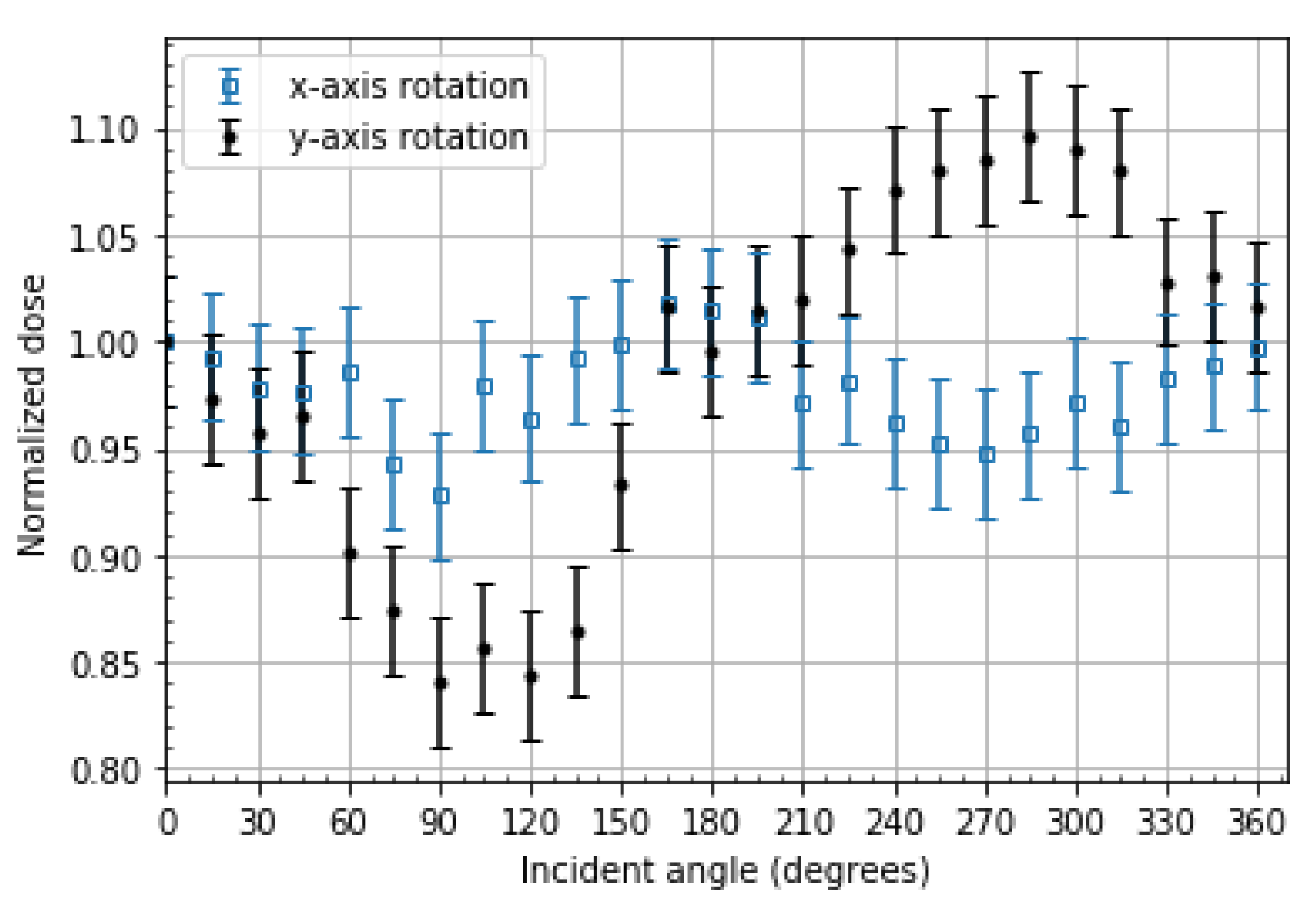
2.4. Angular Dependence
2.5. Readout Depletion
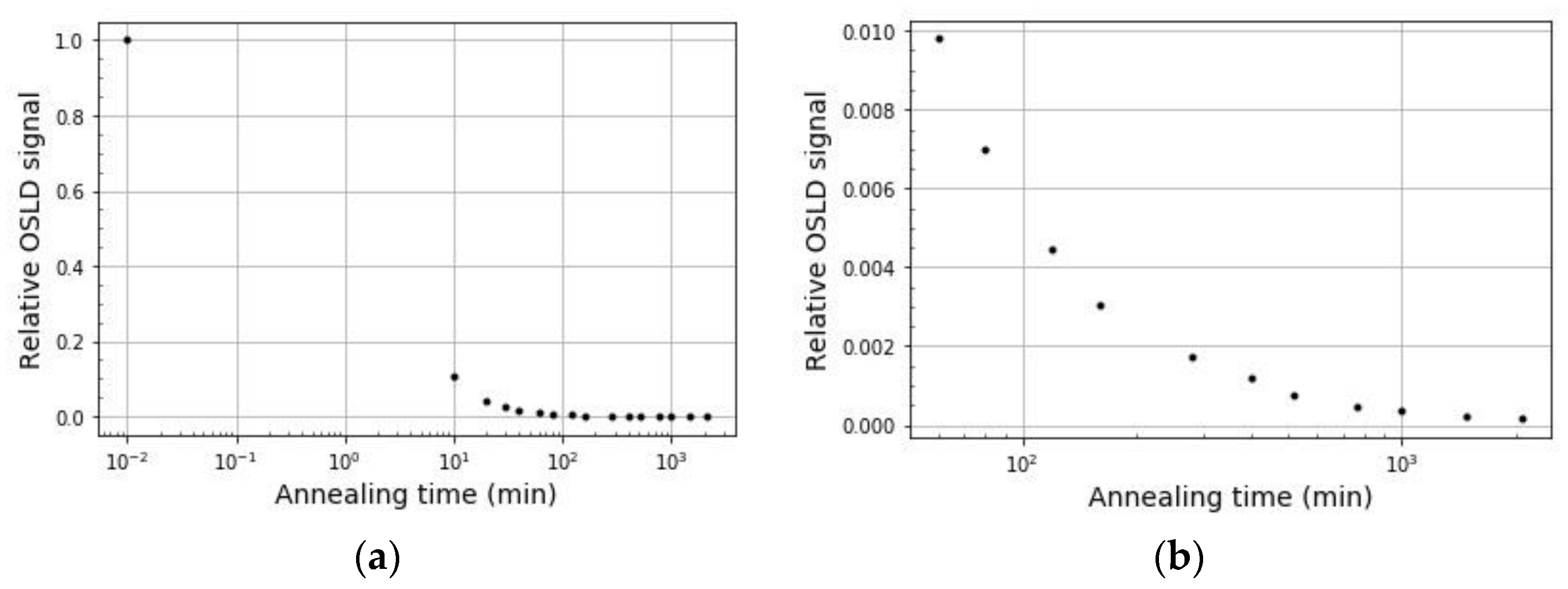
2.6. Optical Annealing
2.7. End-to-End Testing
3. Results
3.1. Calibration
3.2. Dose Linearity
3.3. Dose Rate Dependence
3.4. Angular Dependence
3.5. Readout Depletion
3.6. Optical Annealing
3.7. End-to-End Testing
4. Discussion
4.1. Calibration
4.2. Dose Linearity
4.3. Dose Rate Dependence
4.4. Angular Dependence
4.5. Readout Depletion
4.6. Optical Annealing
4.7. End-to-End Testing
4.8. OSLDs vs. MOSFETs: Clinical Relevance
5. Conclusions
- Use of screened nanoDotsTM is recommended for building calibration curves.
- The appropriate calibration must be selected before measurement.
- Using a weak beam of light, OSLDs exhibit minimal signal depletion with multiple readouts (−0.05% per readout).
- OSLDs exhibit angular dependence in edge-on cases which are 90° ± 30° and 270° ± 30° in incident angle. It is recommended to place OSLDs orthogonal or near orthogonal to the expected incident dose gradient to prevent angular dependence and volume-averaging corrections during readout.
- Optical annealing of OSLDs is a viable way to reuse OSLDs for clinical and research purposes, permitting a baseline measurement to be made after the annealing period and before the next irradiation.
- Using the light source described in this paper, OSLDs must be optically annealed for a minimum of 24 h and subsequently kept in the dark for a minimum of four minutes prior to a baseline readout.
- Precise comparisons of OSLD, MOSFET, and TPS point doses are only recommended when OSLDs and MOSFETs are positioned in low-dose gradient regions or when the OSLD irradiation geometry closely matches the geometry of the tissue of interest. Otherwise, very large discrepancies are to be expected due to positional uncertainties.
- In vivo dose measurements can successfully be made.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Derm. Pr. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Kinds of Cancer. Available online: https://www.cdc.gov/cancer/kinds.htm (accessed on 3 July 2022).
- Government of Canada. Non Melanoma Skin Cancer. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/non-melanoma-skin-cancer.html (accessed on 3 July 2022).
- Griffin, L.L.; Ali, F.R.; Lear, J.T. Non-melanoma skin cancer. Clin. Med. J. 2016, 16, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.M.; Dasgeb, B.; Liem, S.; Ali, A.; Harrison, A.; Finkelstein, M.; Cha, J.; Anne, R.; Greenbaum, S.; Sherwin, W.; et al. High-dose-rate brachytherapy for the treatment of basal and squamous cell carcinomas on sensitive areas of the face: A report of clinical outcomes and acute and subacute toxicities. Adv. Radiat. Oncol. 2020, 6, 100616. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.M.; Xia, N.X.; Yang, L.; Li, Z.; Yang, H.; Yu, H.J.; Liu, Y.; Lei, H.; Zhou, F.X.; Xie, C.H.; et al. CTC1 increases the radioresistance of human melanoma cells by inhibiting telomere shortening and apoptosis. Int. J. Mol. Med. 2014, 33, 1484–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronek, J. Brachytherapy in the treatment of skin cancer: An overview. Postep. Derm. Alergol. 2015, 32, 326–367. [Google Scholar] [CrossRef] [Green Version]
- Delishaj, D.; Rembielak, A.; Manfredi, B.; Ursino, S.; Pasqualetti, F.; Laliscia, C.; Orlandi, F.; Morganti, R.; Fabrini, M.G.; Paiar, F. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: A review of literature. J. Contemp. Brachytherapy 2016, 8, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Amendola, B.E.; Perez, N.; Amendola, M.A.; Fowler, J. High-dose-rate (HDR) brachytherapy for facial non-melanoma skin cancer (NMSC) using custom-made molds and a shortened fractionation schedule. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, S616. [Google Scholar] [CrossRef]
- Laliscia, C.; Fuentes, T.; Coccia, N.; Mattioni, R.; Perrone, F.; Paiar, F. High-dose-rate brachytherapy for non-melanoma skin cancer using tailored custom moulds—A single center experience. Contemp. Oncol. 2021, 25, 12–16. [Google Scholar] [CrossRef]
- Casey, S.; Awotwi-Pratt, J.; Bahl, G. Surface mould brachytherapy for skin cancers: The British Columbia cancer experience. Cureus 2019, 11, e6412. [Google Scholar] [CrossRef]
- Fonseca, G.P.; Johansen, J.G.; Smith, R.L.; Beaulieu, L.; Beddar, S.; Kertzscher, G.; Verhaegen, F.; Tanderup, K. In vivo dosimetry in brachytherapy: Requirements and future directions for research, development, and clinical practice. Phys. Imaging Radiat. Oncol. 2020, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Granero, D.; Pérez-Calatayud, J.; Casal, E.; Ballester, F.; Venselaar, J. A dosimetryic study on the Ir-192 high dose rate Flexisource. Med. Phys. 2006, 33, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Kertzscher, G.; Rosenfeld, A.; Tanderup, K.; Cygler, J.E. In vivo dosimetry: Trends and prospects for brachytherapy. Br. J. Radiol. 2014, 87, 206. [Google Scholar] [CrossRef] [Green Version]
- Tanderup, K.; Beddar, S.; Andersen, C.E.; Kertzscher, G.; Cygler, J.E. In vivo dosimetry in brachytherapy. Med. Phys. 2013, 40, 070902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Jursinic, P.A. In vivo measurements for high dose rate brachytherapy with optically stimulated luminescent dosimeters. Med. Phys. 2013, 40, 071730. [Google Scholar] [CrossRef] [PubMed]
- Shablonin, E.; Popov, A.; Prieditis, G.; Vasil’Chenko, E.; Lushchik, A. Thermal annealing and transformation of dimer F centers in neutron-irradiated Al2O3 single crystals. J. Nucl. Mater. 2021, 543, 152600. [Google Scholar] [CrossRef]
- Evans, B.D.; Pogatshnik, G.J.; Chen, Y. Optical properties of lattice defects in α-Al2O3. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1994, 91, 258–262. [Google Scholar] [CrossRef]
- Lushchik, A.; Lushchik, C.; Schwartz, K.; Savikhin, F.; Shablonin, E.; Shugai, A.; Vasil’Chenko, E. Creation and clustering of Frenkel defects at high density of electronic excitations in wide-gap materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 277, 40–44. [Google Scholar] [CrossRef]
- Jursinic, P.A. Characterization of optically stimulated luminescent dosimeters, OSLDs, for clinical dosimetric measurements. Med. Phys. 2007, 34, 4594–4604. [Google Scholar] [CrossRef]
- Jursinic, P.A. Changes in optically stimulated luminescent dosimeter (OSLD) dosimetric characteristics with accumulated dose. Med. Phys. 2010, 37, 132–140. [Google Scholar] [CrossRef]
- Ponmalar, Y.R.; Manickam, R.; Sathiyan, S.; Ganesh, K.M.; Arun, R.; Godson, H.F. Response of nanodot optically stimulated luminescence dosimeters to therapeutic electron beams. J. Med. Phys. 2017, 42, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kutcher, G.J.; Coia, L.; Gillin, M.; Hanson, W.F.; Leibel, S.; Morton, R.J.; Palta, J.R.; Purdy, J.A.; Reinstein, L.E.; Svensson, G.K.; et al. Comprehensive QA for radiation oncology: Report of AAPM radiation therapy committee Task Group 40. Med. Phys. 1994, 21, 581–618. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Sharma, S.D.; Ravindran, B.P. Characteristics of mobile MOSFET dosimetry system for megavoltage photon beams. J. Med. Phys. 2014, 39, 142–149. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Wu, C.-H.; Lee, J.-H.; Hsieh, Y.-J.; Yu, C.-Y.; Liao, Y.-J.; Kuo, L.-C.; Liang, J.-A.; Huang, D.Y.C. A Study on the dose distributions in various materials from an Ir-192 HDR brachytherapy source. PLoS ONE 2012, 7, e44528. [Google Scholar] [CrossRef] [Green Version]
- Sun Nuclear: A Mirion Medical Company. Solid Water® HE. Available online: https://www.sunnuclear.com/products/solid-water-he (accessed on 10 October 2022).
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Huq, M.S.; Ibbott, G.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 2004, 31, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, I.H.; Ye, S.; Kim, K. Evaluation of treatment plans using various treatment techniques for the radiotherapy of cutaneous Kaposi’s sarcoma developed on the skin of feet. J. Appl. Clin. Med. Phys. 2014, 15, 4970. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.J.; Ebeling, R.; Hiatt, J.R.; Curran, B.; Sternick, E. Optically stimulated luminescent dosimetry for high dose rate brachytherapy. Front. Oncol. 2012, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- DeWerd, L.A.; Ibbott, G.S.; Meigooni, A.S.; Mitch, M.G.; Rivard, M.J.; Stump, K.E.; Thomadsen, B.R.; Venselaar, J.L.M. A dosimetric uncertainty analysis for photon-emitting brachytherapy sources: Report of AAPM Task Group No. 138 and GEC-ESTRO. Med. Phys. 2011, 38, 782–801. [Google Scholar] [CrossRef] [Green Version]
- Ponmalar, R.; Manickam, R.; Ganesh, K.M.; Saminathan, S.; Raman, A.; Godson, H.F. Dosimetric characterization of optically stimulated luminescence dosimeter with therapeutic photon beams for use in clinical radiotherapy measurements. J. Cancer Res. Ther. 2017, 13, 304–312. [Google Scholar] [CrossRef]
- Liu, K. Preliminary investigation into the regeneration of luminescent signal in nanoDot OSLDs. J. Appl. Clin. Med. Phys. 2020, 21, 256–262. [Google Scholar] [CrossRef]
- Raj, L.J.S.; Pearlin, B.; Peace, T.; Isiah, R.; Singh, I.R.R. Characterisation and use of OSLD for In vivo dosimetry in head and neck intensity-modulated radiation therapy. J. Radiother Pract. 2020, 20, 448–454. [Google Scholar] [CrossRef]
- Klein, E.E.; Hanley, J.; Bayouth, J.; Yin, F.-F.; Simon, W.; Dresser, S.; Serago, C.; Aguirre, F.; Ma, L.; Arjomandy, B.; et al. Task Group 142 report: Quality assurance of medical accelerators. Med. Phys. 2009, 36, 4197–7212. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Dunn, L.; Lye, J.E.; Kenny, J.W.; Alves, A.D.C.; Cole, A.; Asena, A.; Kron, T.; Williams, I.M. Angular dependence of the response of the nanoDot OSLD system for measurements at depth in clinical megavoltage beams. Med. Phys. 2014, 41, 64712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, J.R.; Kry, S.F.; Sahoo, N.; Followill, D.S.; Ibbott, G.S. Angular dependence of the nanoDot OSL dosimeter. Med. Phys. 2011, 38, 3955–3962. [Google Scholar] [CrossRef] [Green Version]
- Jursinic, P.A. Angular dependence of dose sensitivity of nanoDot optically stimulated luminescent dosimeters in different radiation geometries. Med. Phys. 2015, 42, 5633–5641. [Google Scholar] [CrossRef]
- Rejab, M.; Wong, J.H.D.; Jamalludin, Z.; Jong, W.L.; Malik, R.A.; Ishak, W.Z.W.; Ung, N.M. Dosimetric characterisation of the optically-stimulated luminescence dosimeter in cobalt-60 high dose rate brachytherapy system. Australas. Phys. Eng. Sci. Med. 2018, 41, 475–485. [Google Scholar] [CrossRef]
- Dunn, L.; Lye, J.; Kenny, J.; Lehmann, J.; Williams, I.; Kron, T. Commissioning of optically stimulated luminescence dosimeters for use in radiotherapy. Radiat. Meas. 2013, 51–52, 31–39. [Google Scholar] [CrossRef]
- Scarboro, S.B.; Cody, D.; Alvarez, P.; Followill, D.; Court, L.; Stingo, F.C.; Zhang, D.; Gray, M.M.N.I.; Kry, S.F. Characterization of the nanoDot OSLD dosimeter in CT. Med. Phys. 2015, 42, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Yukihara, E.G.; Whitley, V.H.; McKeever, S.W.S.; Akselrod, A.E.; Akselrod, M.S. Effect of high-dose irradiation on the optically stimulated luminescence of Al2O3:C. Radiat. Meas. 2004, 38, 317–330. [Google Scholar] [CrossRef]
- Yukihara, E.G.; McKeever, S.W.S. Optically stimulated luminescence (OSL) dosimetry in medicine. Phys. Med. Biol. 2008, 53, R351. [Google Scholar] [CrossRef]
- Al-Senan, R.M.; Hatab, M.R. Characteristics of an OSLD in the diagnostic energy range. Med. Phys. 2011, 38, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Guo, F.; Zhu, D.; Stryker, S.; Trumpore, S.; Roberts, K.; Higgins, S.; Nath, R.; Chen, Z.; Liu, W. On the use of bolus for pacemaker dose measurement and reduction in radiation therapy. J. Appl. Clin. Med. Phys. 2018, 19, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peet, S.C.; Wilks, R.; Kairn, T.; Crowe, S.B. Measuring dose from radiotherapy treatments in the vicinity of a cardiac pacemaker. Phys. Med. 2016, 32, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.F.; Young, C.; Gelblum, D.; Shi, C.; Rincon, C.; Hipp, E.; Li, J.; Wang, D. A review and analysis of managing commonly seen implanted devices for patients undergoing radiation therapy. Adv. Radiat. Oncol. 2021, 6, 100732. [Google Scholar] [CrossRef] [PubMed]
- Gopiraj, A.; Billimagga, R.S.; Ramasubramanian, V. Performance characteristics and commissioning of MOSFET as an in-vivo dosimeter for high energy photon external beam radiation therapy. Rep. Pract. Oncol. Radiother. 2008, 13, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.; Butson, M.J.; Yu, P.K.N. Energy dependence corrections to MOSFET dosimetric sensitivity. Australas. Phys. Eng. Sci. Med. 2009, 32, 16–20. [Google Scholar] [CrossRef]
- Consorti, R.; Petrucci, A.; Fortunato, F.; Soriani, A.; Marzi, S.; Iaccarino, G.; Landoni, V.; Benassi, M. In vivo dosimetry with MOSFETs: Dosimetric characterization and first clinical results in intraoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 952–960. [Google Scholar] [CrossRef]
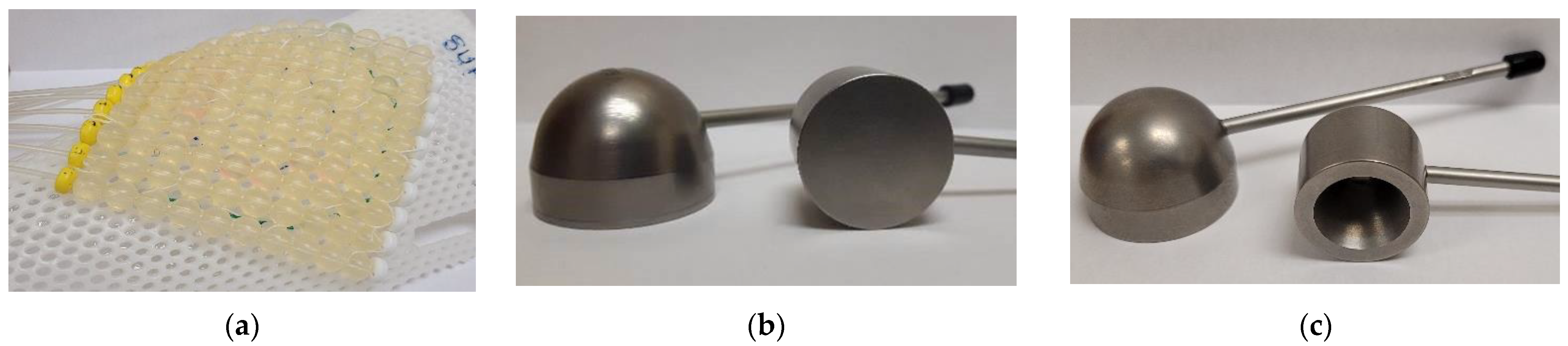





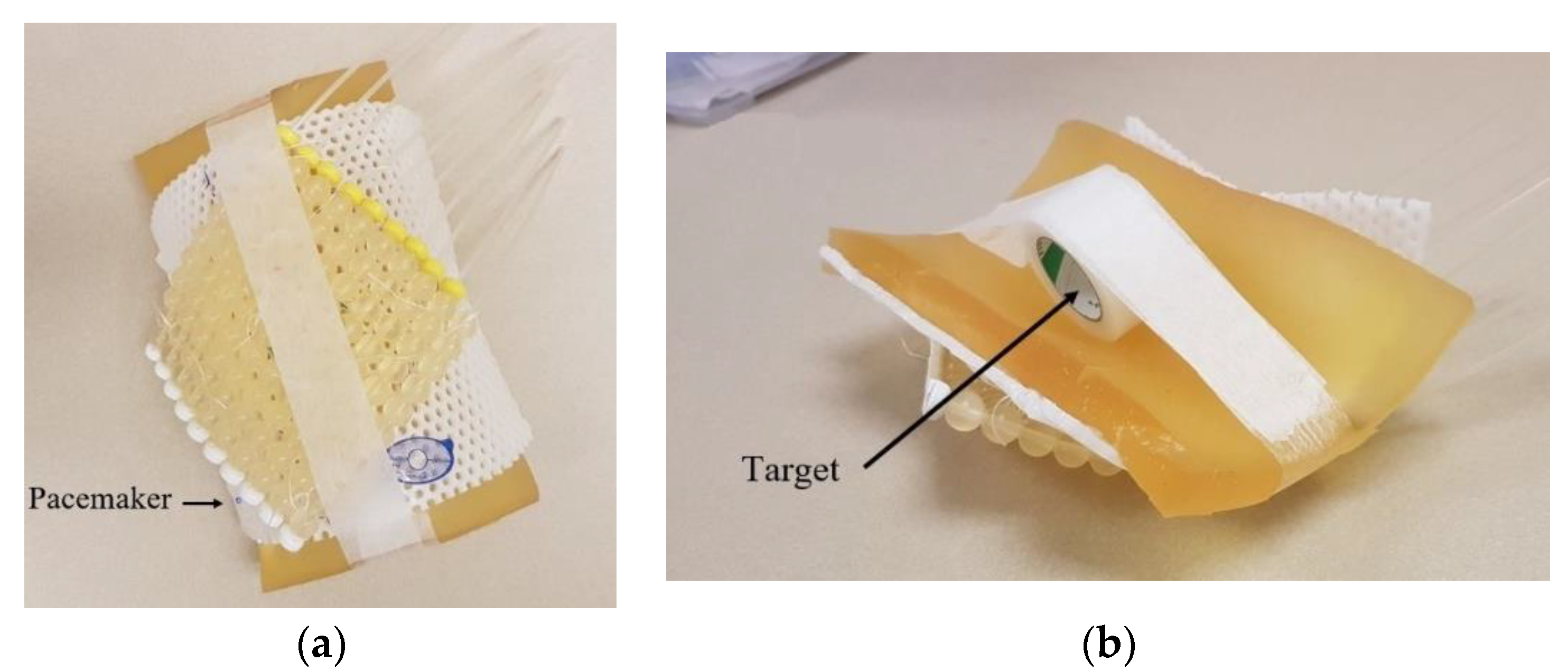
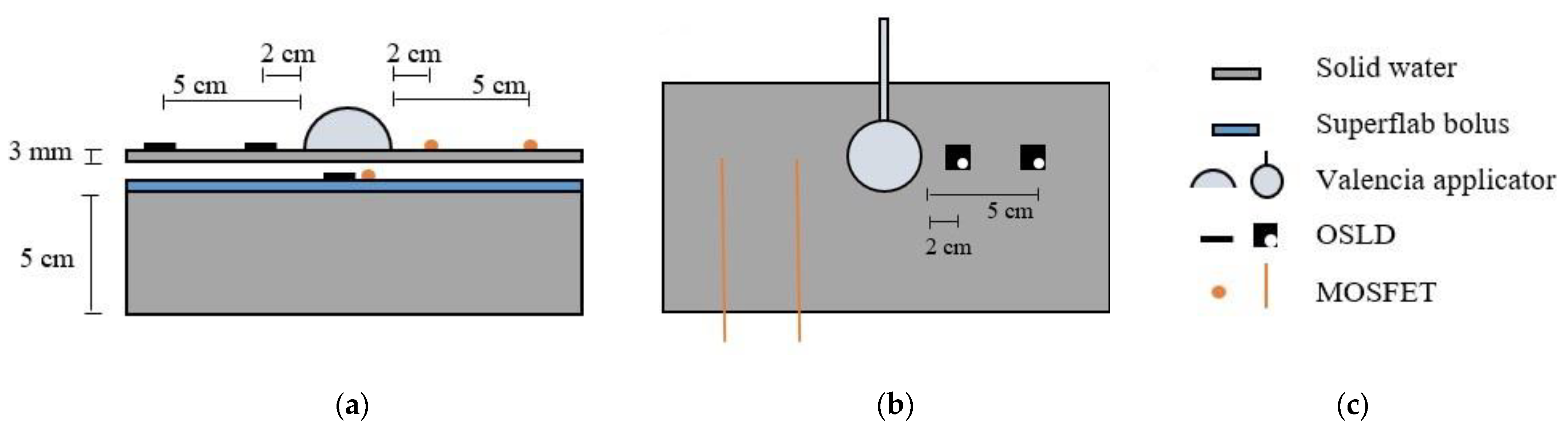
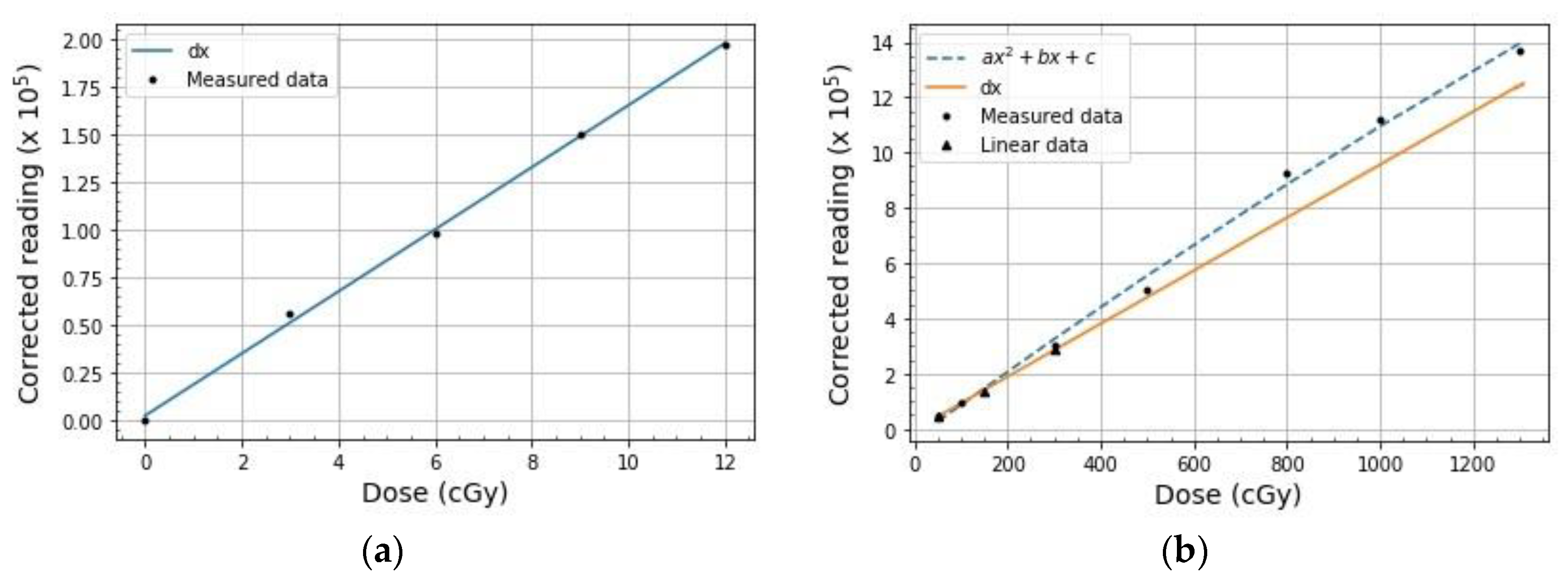


| Calibration Type | Dose Range (cGy) | Doses Used to Build the Curve (cGy) |
|---|---|---|
| Low dose (linear) | 0–10 | 0, 3, 6, 9, 12 |
| High dose (linear) | 10–300 | 50, 150, 300 |
| High dose (non-linear) | >300 | 50, 100, 300, 500, 800, 1000, 1300 |
| Calibration Curve | Validation Dose (cGy) | Measured Dose (cGy) | % Difference from Calibration Curve |
|---|---|---|---|
| Low dose (linear) | 10 | 10.23 | 2.3 |
| 10 | 9.998 | 1.2 | |
| High dose (linear) | 200 | 203.0 | 1.5 |
| 200 | 203.8 | 1.9 | |
| High dose (non-linear) | 400 | 402.8 | 0.070 |
| 650 | 637.7 | 1.9 | |
| 900 | 912.6 | 1.4 |
| Distance (cm) | Average Counts Normalized | Inverse-Square | % Difference |
|---|---|---|---|
| 4 | 1.00 | 1.00 | - |
| 6 | 0.436 | 0.444 | 1.8 |
| 8 | 0.252 | 0.250 | 0.8 |
| 10 | 0.157 | 0.160 | 1.9 |
| 12 | 0.110 | 0.111 | 0.9 |
| Measurement Site | Measured OSLD Dose (cGy) | TPS Dose (cGy) | OSLD/TPS % Difference | OSLD/Lead % Difference |
|---|---|---|---|---|
| Target | 132.3 | 135 | 2.0 | - |
| Pacemaker | 38.40 | 40.3 | 4.7 | - |
| Pacemaker (with lead) | 37.42 | - | - | 2.6 |
| Measurement Site | Measured OSLD Dose (cGy) | Measured MOSFET Dose (cGy) | OSLD/MOSFET % Difference |
|---|---|---|---|
| Target | 599.5 | 602 | 0.42 |
| Measurement Site | Measured OSLD Dose (cGy) | Measured MOSFET Dose (cGy) | OSLD/MOSFET % Difference |
|---|---|---|---|
| OAR | 11.15 | 11.4 | 2.17 |
| OAR (with lead) | 7.861 | 9.36 | 16.0 |
| Surface | 4.946 | 5.57 | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.; Sabondjian, E.; Baltazar, A.R. In Vivo Dosimetry for Superficial High Dose Rate Brachytherapy with Optically Stimulated Luminescence Dosimeters: A Comparison Study with Metal-Oxide-Semiconductor Field-Effect Transistors. Radiation 2022, 2, 338-356. https://doi.org/10.3390/radiation2040026
Lopes A, Sabondjian E, Baltazar AR. In Vivo Dosimetry for Superficial High Dose Rate Brachytherapy with Optically Stimulated Luminescence Dosimeters: A Comparison Study with Metal-Oxide-Semiconductor Field-Effect Transistors. Radiation. 2022; 2(4):338-356. https://doi.org/10.3390/radiation2040026
Chicago/Turabian StyleLopes, Alana, Eric Sabondjian, and Alejandra Rangel Baltazar. 2022. "In Vivo Dosimetry for Superficial High Dose Rate Brachytherapy with Optically Stimulated Luminescence Dosimeters: A Comparison Study with Metal-Oxide-Semiconductor Field-Effect Transistors" Radiation 2, no. 4: 338-356. https://doi.org/10.3390/radiation2040026
APA StyleLopes, A., Sabondjian, E., & Baltazar, A. R. (2022). In Vivo Dosimetry for Superficial High Dose Rate Brachytherapy with Optically Stimulated Luminescence Dosimeters: A Comparison Study with Metal-Oxide-Semiconductor Field-Effect Transistors. Radiation, 2(4), 338-356. https://doi.org/10.3390/radiation2040026





