Rectal Spacer Placement for Anorectal Reirradiation of De Novo Rectal or Anal Cancer Following Prostate Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
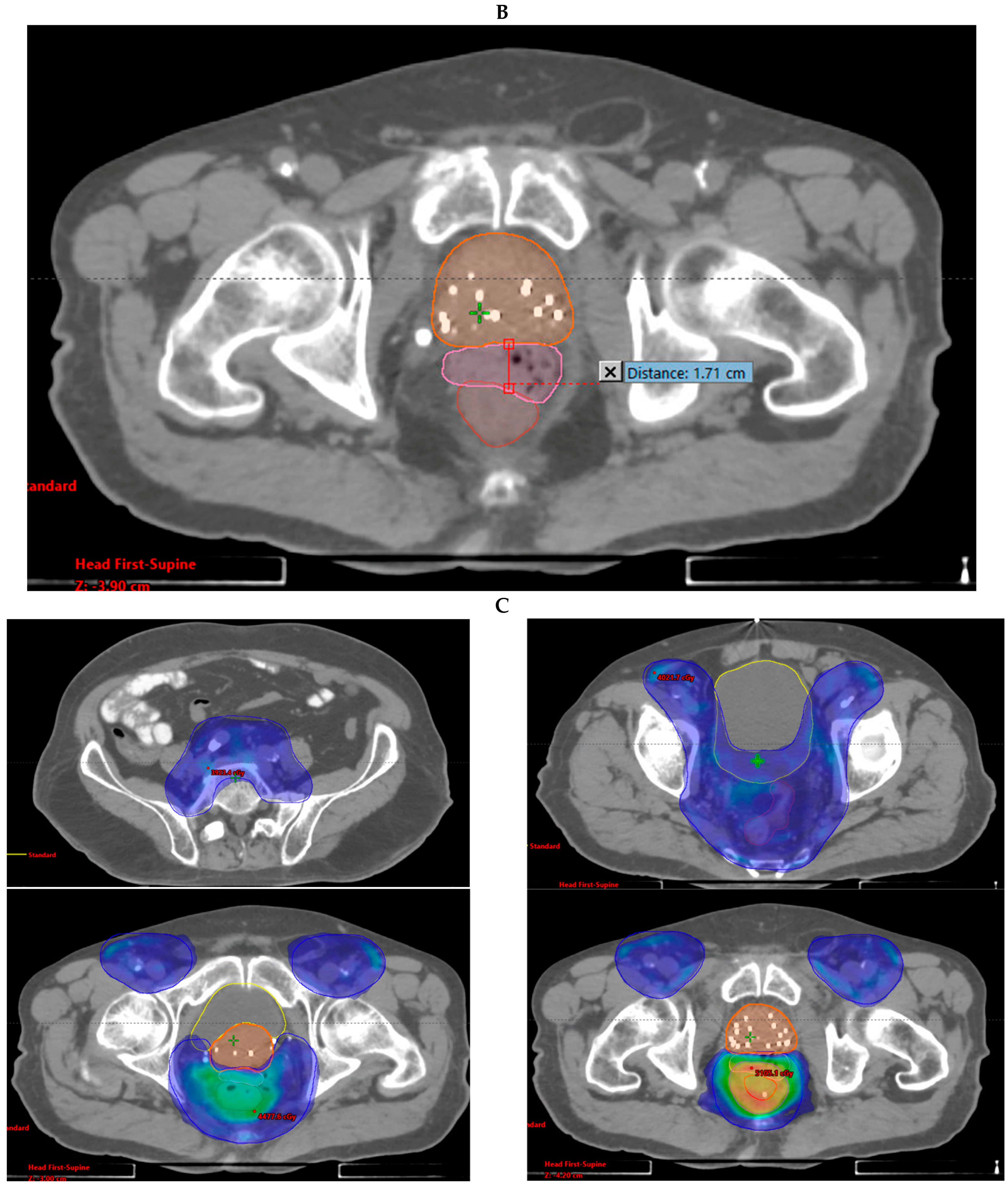
2.1. Rectal Spacer Placement
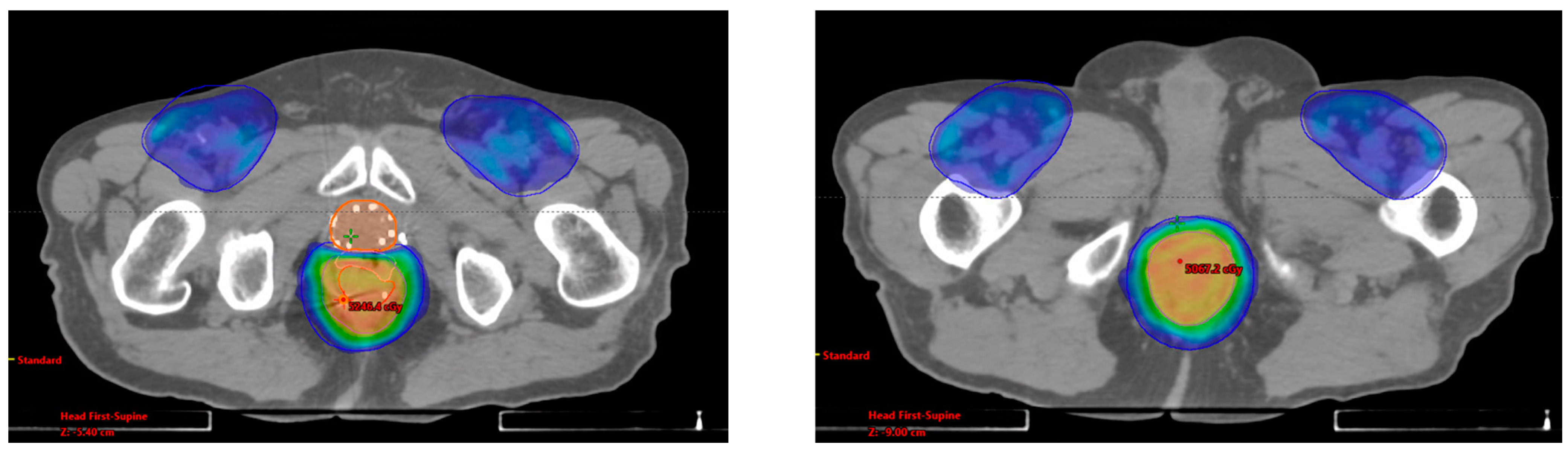
2.2. Radiation Therapy
3. Results
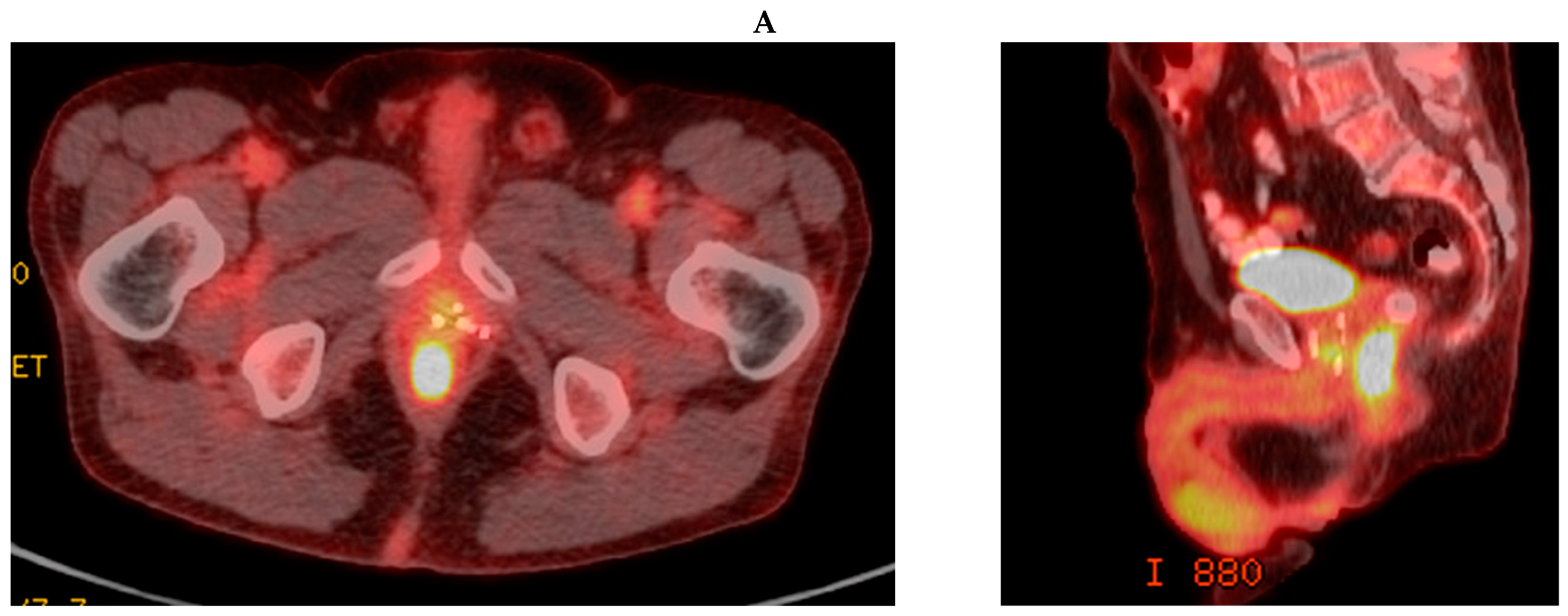
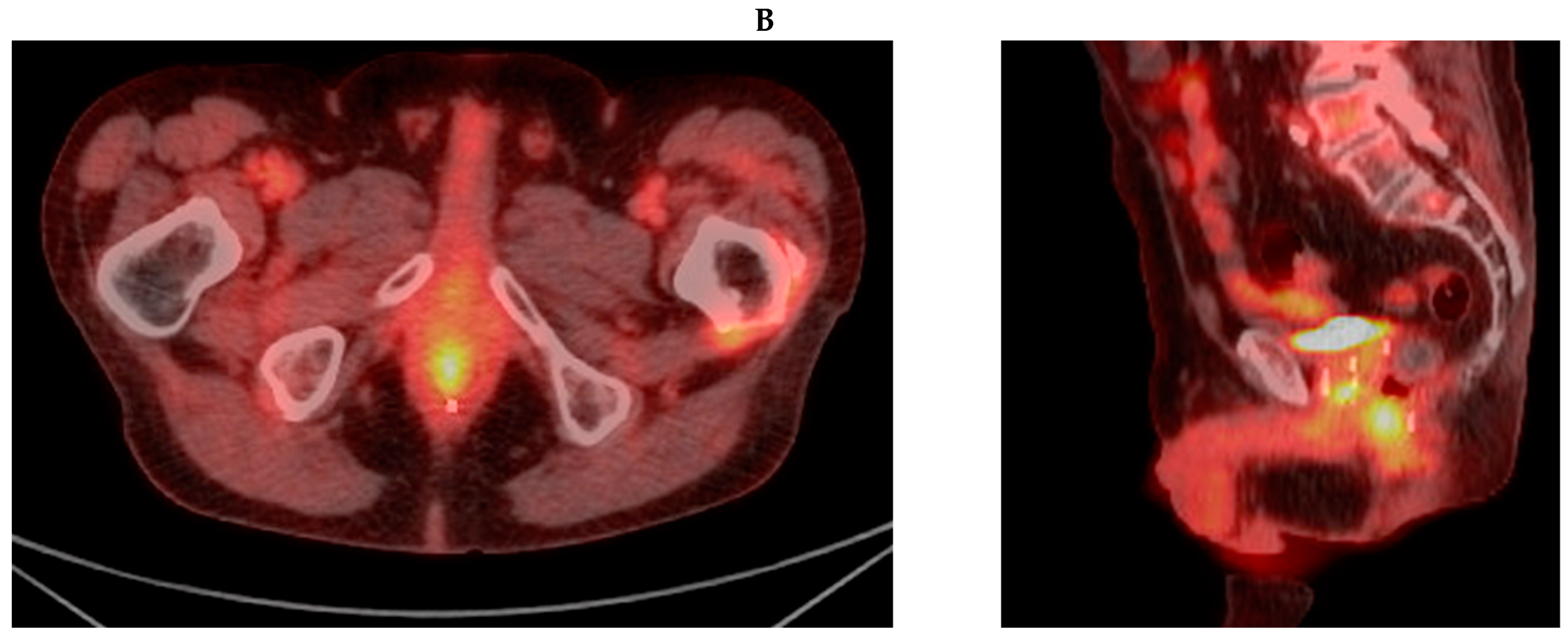
3.1. Case Report 1: Anal Squamous Cell Carcinoma
3.2. Case Report 2: Rectal Adenocarcinoma
3.3. Remaining Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCCN, Rectal Cancer. 2020. Available online: https://www.nccn.org/patients/guidelines/content/PDF/rectal-patient.pdf (accessed on 1 April 2024).
- NCCN, Anal Cancer. 2020. Available online: https://www.nccn.org/patients/guidelines/content/PDF/anal-patient.pdf (accessed on 1 April 2024).
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Martenson, J.A.; Wieand, H.S.; Krook, J.E.; Macdonald, J.S.; Haller, D.G.; Mayer, R.J.; Gunderson, L.L.; Rich, T.A. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N. Engl. J. Med. 1994, 331, 502–507. [Google Scholar] [CrossRef]
- Wolmark, N.; Wieand, H.S.; Hyams, D.M.; Colangelo, L.; Dimitrov, N.V.; Romond, E.H.; Wexler, M.; Prager, D.; Cruz, A.B., Jr.; Gordon, P.H.; et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J. Natl. Cancer Inst. 2000, 92, 388–396. [Google Scholar] [CrossRef]
- Baxter, N.N.; Tepper, J.E.; Durham, S.B.; Rothenberger, D.A.; Virnig, B.A. Increased risk of rectal cancer after prostate radiation: A population-based study. Gastroenterology 2005, 128, 819–824. [Google Scholar] [CrossRef]
- Boue-Rafle, A.; Briens, A.; Supiot, S.; Blanchard, P.; Baty, M.; Lafond, C.; Masson, I.; Crehange, G.; Cosset, J.M.; Pasquier, D.; et al. Does radiation therapy for prostate cancer increase the risk of second cancers? Cancer Radiother. 2024, 28, 293–307. [Google Scholar] [CrossRef] [PubMed]
- McPartland, C.; Salib, A.; Banks, J.; Mark, J.R.; Lallas, C.D.; Trabulsi, E.J.; Gomella, L.G.; Goldberg, H.; Leiby, B.; Den, R.; et al. Risk of Secondary Malignancies After Pelvic Radiation: A Population-based Analysis. Eur. Urol. Open Sci. 2024, 63, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chrouser, K.; Leibovich, B.; Bergstralh, E.; Zincke, H.; Blute, M. Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. J. Urol. 2005, 174, 107–110; discussion 110–111. [Google Scholar] [CrossRef]
- Ma, J.L.; Hennessey, D.B.; Newell, B.P.; Bolton, D.M.; Lawrentschuk, N. Radiotherapy-related complications presenting to a urology department: A more common problem than previously thought? BJU Int. 2018, 121 (Suppl. 3), 28–32. [Google Scholar] [CrossRef] [PubMed]
- Marguet, C.; Raj, G.V.; Brashears, J.H.; Anscher, M.S.; Ludwig, K.; Mouraviev, V.; Robertson, C.N.; Polascik, T.J. Rectourethral fistula after combination radiotherapy for prostate cancer. Urology 2007, 69, 898–901. [Google Scholar] [CrossRef]
- Stone, N.N.; Stock, R.G. Complications following permanent prostate brachytherapy. Eur. Urol. 2002, 41, 427–433. [Google Scholar] [CrossRef]
- Zoubek, J.; McGuire, E.J.; Noll, F.; DeLancey, J.O. The late occurrence of urinary tract damage in patients successfully treated by radiotherapy for cervical carcinoma. J. Urol. 1989, 141, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Song, D.Y.; Herfarth, K.K.; Uhl, M.; Eble, M.J.; Pinkawa, M.; van Triest, B.; Kalisvaart, R.; Weber, D.C.; Miralbell, R.; Deweese, T.L.; et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: Analysis of dosimetric outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Herfarth, K.; Eble, M.J.; Pinkawa, M.; van Triest, B.; Kalisvaart, R.; Weber, D.C.; Miralbell, R.; Song, D.Y.; DeWeese, T.L. Absorbable hydrogel spacer use in men undergoing prostate cancer radiotherapy: 12 month toxicity and proctoscopy results of a prospective multicenter phase II trial. Radiat. Oncol. 2014, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Folkert, M.R.; Zelefsky, M.J.; Hannan, R.; Desai, N.B.; Lotan, Y.; Laine, A.M.; Kim, D.W.N.; Neufeld, S.H.; Hornberger, B.; Kollmeier, M.A.; et al. A Multi-Institutional Phase 2 Trial of High-Dose SAbR for Prostate Cancer Using Rectal Spacer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 101–109. [Google Scholar] [CrossRef]
- Hatiboglu, G.; Pinkawa, M.; Vallee, J.P.; Hadaschik, B.; Hohenfellner, M. Application technique: Placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012, 110, E647–E652. [Google Scholar] [CrossRef]
- Pinkawa, M.; Corral, N.E.; Caffaro, M.; Piroth, M.D.; Holy, R.; Djukic, V.; Otto, G.; Schoth, F.; Eble, M.J. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother. Oncol. 2011, 100, 436–441. [Google Scholar] [CrossRef]
- Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events (CTCAE)v.5.0 [5x7]. In Cancer Ther Aval Progr 155 (2017). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 1 April 2024).
- Kamran, S.C.; Zelefsky, M.; Nguyen, P.L.; Lawton, C.A.F. To Radiate or Not to Radiate-The Challenges of Pelvic Reirradiation. Semin. Radiat. Oncol. 2020, 30, 238–241. [Google Scholar] [CrossRef]
- Chung, S.Y.; Koom, W.S.; Keum, K.C.; Chang, J.S.; Shin, S.J.; Ahn, J.B.; Min, B.S.; Lee, K.Y.; Kim, N.K.; Yoon, H.I. Treatment Outcomes of Re-irradiation in Locoregionally Recurrent Rectal Cancer and Clinical Significance of Proper Patient Selection. Front. Oncol. 2019, 9, 529. [Google Scholar] [CrossRef]
- Zilli, T.; Benz, E.; Dipasquale, G.; Rouzaud, M.; Miralbell, R. Reirradiation of Prostate Cancer Local Failures After Previous Curative Radiation Therapy: Long-Term Outcome and Tolerance. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 318–322. [Google Scholar] [CrossRef]
- Chang, D.T.; Koay, E.J.; Herman, J.M.; Hong, T.S.; Das, P. Abdominal and Pelvic Reirradiation for Recurrent Gastrointestinal Cancers. Semin. Radiat. Oncol. 2020, 30, 232–237. [Google Scholar] [CrossRef]
- Das, P.; Delclos, M.E.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.W.; Chang, G.J.; Eng, C.; Bedi, M.; Krishnan, S.; Crane, C.H. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Marks, G.; Marks, J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 2002, 95, 1144–1150. [Google Scholar] [CrossRef]
- Tao, R.; Tsai, C.J.; Jensen, G.; Eng, C.; Kopetz, S.; Overman, M.J.; Skibber, J.M.; Rodriguez-Bigas, M.; Chang, G.J.; You, Y.N.; et al. Hyperfractionated accelerated reirradiation for rectal cancer: An analysis of outcomes and toxicity. Radiother. Oncol. 2017, 122, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Morganti, A.G.; Gambacorta, M.A.; Mohiuddin, M.; Doglietto, G.B.; Coco, C.; De Paoli, A.; Rossi, C.; Di Russo, A.; Valvo, F.; et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Hilal, L.; Wu, A.J.; Reyngold, M.; Cuaron, J.J.; Navilio, J.; Romesser, P.B.; Dreyfuss, A.; Yin, S.; Zhang, Z.; Bai, X.; et al. Radiation therapy for de novo anorectal cancer in patients with a history of prostate radiation therapy. Front. Oncol. 2022, 12, 975519. [Google Scholar] [CrossRef]
- Mahal, B.A.; Ziehr, D.R.; Hyatt, A.S.; Neubauer-Sugar, E.H.; O’Farrell, D.A.; O’Leary, M.P.; Steele, G.S.; Niedermayr, T.R.; Beard, C.J.; Martin, N.E.; et al. Use of a rectal spacer with low-dose-rate brachytherapy for treatment of prostate cancer in previously irradiated patients: Initial experience and short-term results. Brachytherapy 2014, 13, 442–449. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Devlin, P.M.; Beard, C.J.; Orio, P.F., 3rd; O’Leary, M.P.; Wolfsberger, L.D.; O’Farrell, D.A.; Sweeney, C.M.; Hadaschik, B.A. High-dose-rate brachytherapy for prostate cancer in a previously radiated patient with polyethylene glycol hydrogel spacing to reduce rectal dose: Case report and review of the literature. Brachytherapy 2013, 12, 77–83. [Google Scholar] [CrossRef]

| Primary Disease | Prostate RT | Anorectal RT | Initial Response | Current Status | In-Field POD (mo) | Out-of-Field POD (mo) | OS from RT End |
|---|---|---|---|---|---|---|---|
| Anal | 2006: -LDR | 2018: -Anal canal + LNs 36–48 Gy/24 fx QD | -POD; liver -CR; local | NED | No POD | 2 mo; liver treated with SBRT | 48 mo |
| Rectal | 2013: -EBRT 81 Gy/45 fx | 2019: -Rectum + LNs 45 Gy/30 fx BID | CR | NED | No POD | No POD | 36 mo |
| Rectal | 1998: -Brachy 2 | 2020: -Rectum + LNs 36 Gy/24 fx QD -Rectum CD 39 Gy/26 fx QD | CR | NED | 14 mo; treated with LAR | 6 and 9 mo; liver and lungs treated with IR ablation | 24 mo |
| Rectal | 2010: -EBRT | 2020: -Rectum + LNs 43.5 Gy/29 fx QD | POD; rectum | Died of disease | 5 mo; treated with chemo | No POD | 11 mo |
| Anal | 2018: -LDR 2,3 | 2020: -Anal canal + LNs 4 43–50 Gy/25 fx QD | POD; anus | Died of prostate/anal disease | 3 mo; treated with diverting ostomy + Pembro | No POD | 10 mo |
| Disease | GTV V100% | PTV V100% | Mean Rectal Dose (cGy) | Mean Bladder Dose (cGy) | Max Distance of Spacer (cm) |
|---|---|---|---|---|---|
| Anal | 100% | 96% | 4024.7 | 2730.1 | 1.71 |
| Rectal | 62% 1 | 73% 2 | 4462.5 | 3957.6 | 1.10 |
| Rectal | 100% | 100% | 3920.6 | 1840.9 | 1.76 |
| Rectal | 56% 1 | 62% 2 | 4091.6 (29/30 fx delivered) | 1592.7 (29/30 fx delivered) | 0.72 |
| Anal | 85% 1 | 81% 2 | 4574.3 | 2046.0 | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreyfuss, A.D.; Navilio, J.P.; Kim, N.; Shim, A.; Romesser, P.B.; Reyngold, M.; Zelefsky, M.J.; Crane, C.H.; Hajj, C. Rectal Spacer Placement for Anorectal Reirradiation of De Novo Rectal or Anal Cancer Following Prostate Radiation Therapy. Radiation 2024, 4, 242-252. https://doi.org/10.3390/radiation4030019
Dreyfuss AD, Navilio JP, Kim N, Shim A, Romesser PB, Reyngold M, Zelefsky MJ, Crane CH, Hajj C. Rectal Spacer Placement for Anorectal Reirradiation of De Novo Rectal or Anal Cancer Following Prostate Radiation Therapy. Radiation. 2024; 4(3):242-252. https://doi.org/10.3390/radiation4030019
Chicago/Turabian StyleDreyfuss, Alexandra D., John P. Navilio, Neal Kim, Andy Shim, Paul B. Romesser, Marsha Reyngold, Michael J. Zelefsky, Christopher H. Crane, and Carla Hajj. 2024. "Rectal Spacer Placement for Anorectal Reirradiation of De Novo Rectal or Anal Cancer Following Prostate Radiation Therapy" Radiation 4, no. 3: 242-252. https://doi.org/10.3390/radiation4030019
APA StyleDreyfuss, A. D., Navilio, J. P., Kim, N., Shim, A., Romesser, P. B., Reyngold, M., Zelefsky, M. J., Crane, C. H., & Hajj, C. (2024). Rectal Spacer Placement for Anorectal Reirradiation of De Novo Rectal or Anal Cancer Following Prostate Radiation Therapy. Radiation, 4(3), 242-252. https://doi.org/10.3390/radiation4030019






