Improving the Accuracy of Bone-Scintigraphy Imaging Analysis Using the Skeletal Count Index: A Study Based on Human Trial Data
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Cases
2.2. Equipment and Analysis Software
2.3. Imaging
2.4. Criteria for the Presence or Absence of Bone Metastasis
2.5. Case Grouping and Data Analysis
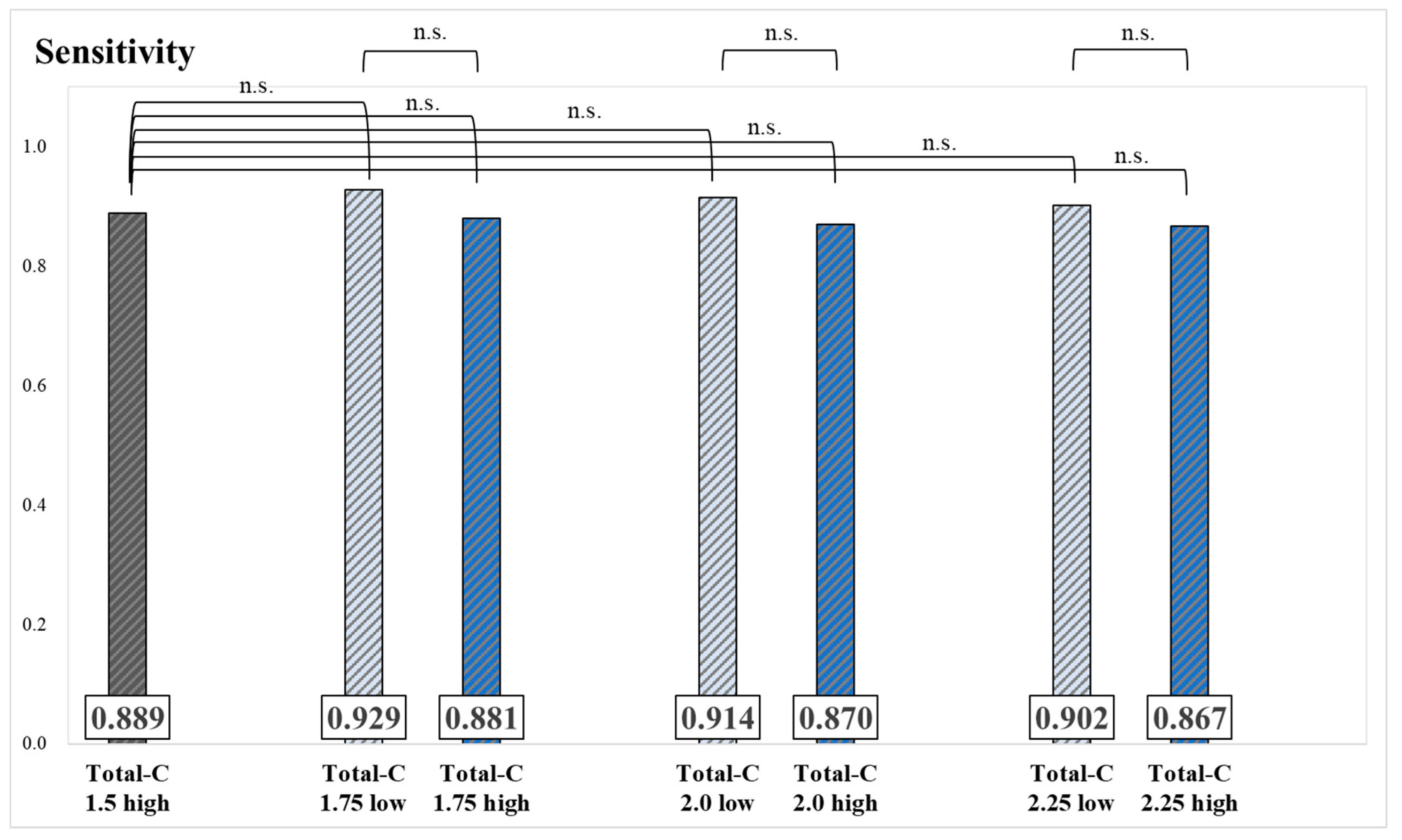
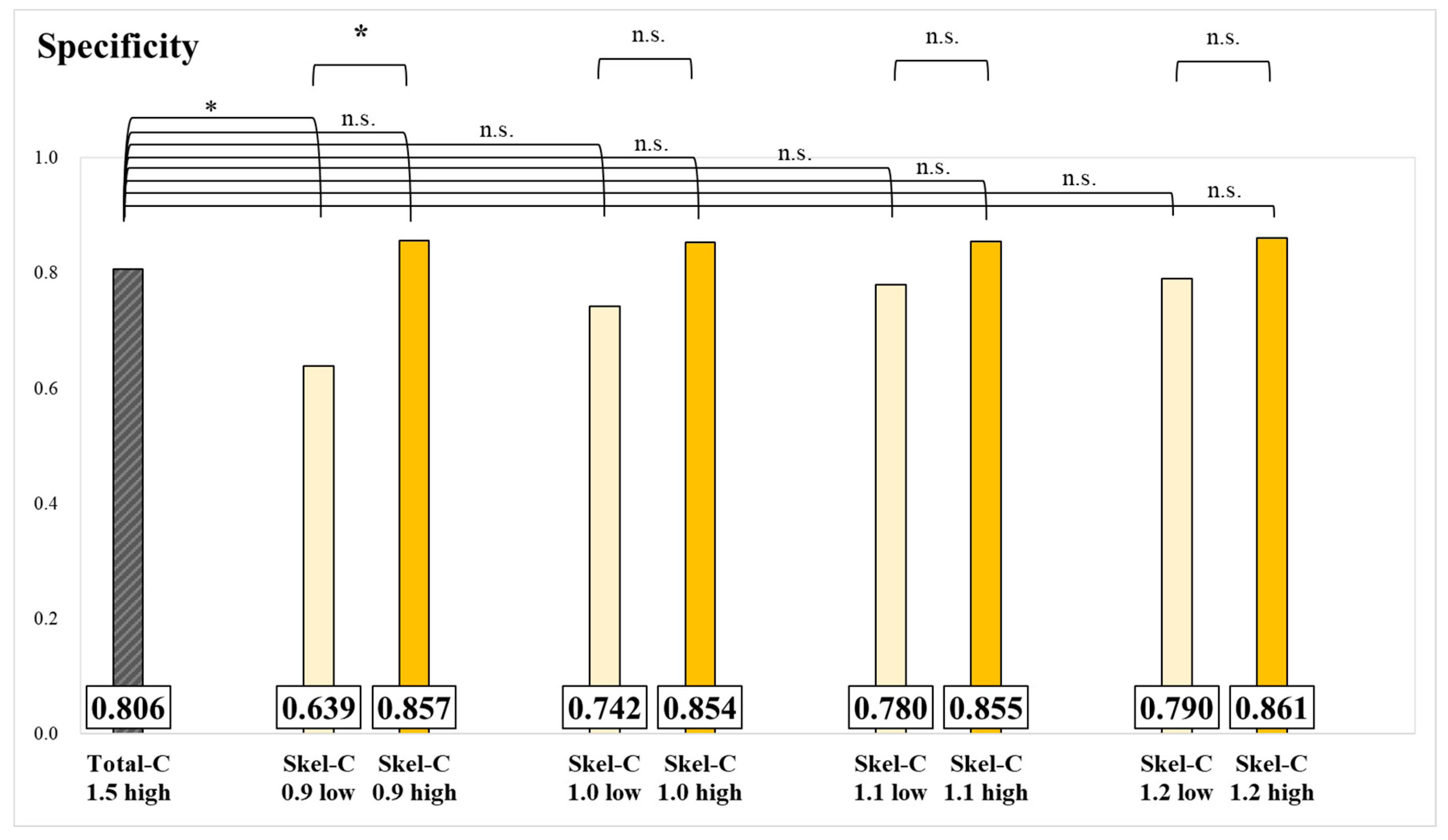
3. Results
4. Discussion
α = 0.9 MC/Lowest Skel-C value observed among the cases (unit: MC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nishiyama, Y.; Kinuya, S.; Kato, T.; Kayano, D.; Sato, S.; Tashiro, M.; Tatsumi, M.; Hashimoto, T.; Baba, S.; Hirata, K.; et al. Nuclear medicine practice in Japan: A report of the eighth nationwide survey in 2017. Ann. Nucl. Med. 2019, 33, 725–732. [Google Scholar] [CrossRef]
- Nakajima, K.; Edenbrandt, L.; Mizokami, A. Bone scan index: A new biomarker of bone metastasis in patients with prostate cancer. Int. J. Urol. 2017, 24, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Makino, T.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Iijima, M.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; Izumi, K.; et al. Initial experience with radium-223 chloride treatment at the Kanazawa University Hospital. Anticancer Res. 2019, 39, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Yonou, H.; Ogawa, Y.; Ochiai, A. Mechanism of osteoblastic bone metastasis of prostate cancer. Clin Calcium. 2006, 16, 557–564. [Google Scholar]
- Beheshti, M.; Langsteger, W.; Fogelman, I. Prostate cancer: Role of SPECT and PET in imaging bone metastases. Semin. Nucl. Med. 2009, 39, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Nasibeh, M.; Nazanin, Z.; Sara, H.; Ghasemali, D.; Christian, P.; Mohsen, B. Bone Metastasis in Prostate Cancer: Bone Scan Versus PET Imaging. Semin. Nucl. Med. 2024, 54, 97–118. [Google Scholar]
- Kakehi, Y.; Sugimoto, M.; Taoka, R. Committee for establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition). Int. J. Urol. 2017, 24, 648–666. [Google Scholar] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lv, H.; Hao, X.; Dong, Y.; Dai, H.; Song, Y. Prognostic value of bone scan index as an imaging biomarker in metastatic prostate cancer: A meta-analysis. Oncotarget 2017, 8, 84449–84458. [Google Scholar] [CrossRef]
- Dennis, E.R.; Jia, X.; Mezheritskiy, I.S.; Stephenson, R.D.; Schoder, H.; Fox, J.J.; Heller, G.; Scher, H.I.; Larson, S.M.; Morris, M.J. Bone scan index: A quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J. Clin. Oncol. 2012, 30, 519–524. [Google Scholar] [CrossRef]
- Zhang, X.; Nakajima, K.; Mizokami, A.; Horikoshi, H.; Nishimoto, K.; Hashine, K.; Matsuyama, H.; Takahashi, S.; Wakabayashi, H.; Kinuya, S. Flare phenomenon visualized by 99mTc-bone scintigraphy has prognostic value for patients with metastatic castration-resistant prostate cancer. Ann. Nucl. Med. 2024, 38, 428–440. [Google Scholar] [CrossRef]
- Poulsen, M.H.; Rasmussen, J.; Edenbrandt, L.; Høilund-Carlsen, P.F.; Gerke, O.; Johansen, A.; Lund, L. Bone Scan Index predicts outcome in patients with metastatic hormone-sensitive prostate cancer. BJU Int. 2016, 117, 748–753. [Google Scholar] [CrossRef]
- Sadik, M.; Suurkula, M.; Höglund, P.; Järund, A.; Edenbrandt, L. Quality of planar whole-body bone scan interpretations—A nationwide survey. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Sadik, M.; Hamadeh, I.; Nordblom, P.; Suurkula, M.; Höglund, P.; Ohlsson, M.; Edenbrandt, L. Computer-assisted interpretation of planar whole-body bone scans. J. Nucl. 2008, 49, 1958–1965. [Google Scholar] [CrossRef]
- Horikoshi, H.; Kikuchi, A.; Onoguchi, M.; Sjöstrand, K.; Edenbrandt, L. Computer-aided diagnosis system for bone scintigrams from Japanese patients: Importance of training database. Ann. Nucl. Med. 2012, 26, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Onoguchi, M.; Horikoshi, H.; Sjöstrand, K.; Edenbrandt, L. Automated segmentation of the skeleton in whole-body bone scans: Influence of difference in atlas. Nucl. Med. Commun. 2012, 33, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Nakajima, Y.; Horikoshi, H.; Ueno, M.; Wakabayashi, H.; Shiga, T.; Yoshimura, M.; Ohtake, E.; Sugawara, Y.; Matsuyama, H.; et al. Enhanced diagnostic accuracy for quantitative bone scan using an artificial neural network system: A Japanese multi-center database project. EJNMMI Res. 2013, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Sadik, M.; Suurkula, M.; Höglund, P.; Järund, A.; Edenbrandt, L. Improved classifications of planar whole-body bone scans using a computer-assisted diagnosis system: A multicenter, multiple-reader, multiple-case study. J. Nucl. Med. 2009, 50, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Miyaji, N.; Murata, T.; Motegi, K.; Miwa, K.; Koyama, M.; Terauchi, T.; Wagatsuma, K.; Kawakami, K.; Richter, J. Evaluation of a revised version of computer-assisted diagnosis system, BONENAVI version 2.1.7, for bone scintigraphy in cancer patients. Ann. Nucl. Med. 2015, 29, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Motegi, K.; Koyama, M.; Terauchi, T.; Yuasa, T.; Yonese, J. Diagnostic performance of a computer-assisted diagnosis system for bone scintigraphy of newly developed skeletal metastasis in prostate cancer patients: Search for low-sensitivity subgroups. Ann. Nucl. Med. 2017, 31, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Matsushita, S.; Nakajima, Y.; Yamaguchi, K.; Okuda, I.; Kojima, Y.; Tsugawa, K. Comparison of diagnostic precision for bone metastasis of primary breast cancer between BONENAVI version 1 and BONENAVI version 2. Nucl. Med. Commun. 2019, 40, 1148–1153. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Morris, M.J.; Kaboteh, R.; Reza, M.; Trägårdh, E.; Matsunaga, N.; Edenbrandt, L.; Bjartell, A.; Larson, S.M.; Minarik, D. A Preanalytic Validation Study of Automated Bone Scan Index: Effect on Accuracy and Reproducibility Due to the Procedural Variabilities in Bone Scan Image Acquisition. J. Nucl. Med. 2016, 57, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kaneko, G.; Takahashi, S.; Matsuyama, H.; Shiina, H.; Ichikawa, T.; Horikoshi, H.; Hashine, K.; Sugiyama, Y.; Miyao, T.; et al. Role of bone scan index in the prognosis and effects of therapy on prostate cancer with bone metastasis: Study design and rationale for the multicenter Prostatic Cancer Registry of Standard Hormonal and Chemotherapy Using Bone Scan Index (PROSTAT-BSI) study. Int. J. Urol. 2018, 25, 492–499. [Google Scholar]
- Mitsui, Y.; Shiina, H.; Yamamoto, Y.; Haramoto, M.; Arichi, N.; Yasumoto, H.; Kitagaki, H.; Igawa, M. Prediction of survival benefit using an automated bone scan index in patients with castration-resistant prostate cancer. BJU. Int. 2012, 110, E628–E634. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- James, J.; Teo, M.; Ramachandran, V.; Law, M.; Ip, E.; Cheng, M. Looking for Metastasis in Early Breast Cancer: Does Bone Scan Help? A Retrospective Review. Clin Breast Cancer. 2021, 21, e18–e21. [Google Scholar] [CrossRef] [PubMed]
- Tada, K.; Kumamaru, H.; Miyata, H.; Asaga, S.; Iijima, K.; Ogo, E.; Kadoya, T.; Kubo, M.; Kojima, Y.; Tanakura, K.; et al. Characteristics of female breast cancer in japan: Annual report of the National Clinical Database in 2018. Breast Cancer. 2023, 30, 157–166. [Google Scholar] [CrossRef]
- R Core Team. _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/ (accessed on 1 December 2024).
- Koizumi, M.; Wagatsuma, K.; Miyaji, N.; Murata, T.; Miwa, K.; Takiguchi, T.; Makino, T.; Koyama, M. Evaluation of a computer-assisted diagnosis system, BONENAVI version 2, for bone scintigraphy in cancer patients in a routine clinical setting. Ann. Nucl. Med. 2015, 29, 138–148. [Google Scholar] [CrossRef]
- Shintawati, R.; Achmad, A.; Higuchi, T.; Shimada, H.; Hirasawa, H.; Arisaka, Y.; Takahashi, A.; Nakajima, T.; Tsushima, Y. Evaluation of bone scan index change over time on automated calculation in bone scintigraphy. Ann. Nucl. Med. 2015, 29, 911–920. [Google Scholar] [CrossRef]
- Kaboteh, R.; Minarik, D.; Reza, M.; Sadik, M.; Trägårdh, E. Evaluation of changes in Bone Scan Index at different acquisition time-points in bone scintigraphy. Clin. Physiol. Funct. Imaging 2018, 38, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Senda, K.; Itoh, S. Evaluation of diffusely high uptake by the calvaria in bone scintigraphy. Ann. Nucl. Med. 1987, 1, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Koizumi, M.; Fukai, S.; Miyaji, N.; Motegi, K.; Nakazawa, S.; Takiguchi, T. Evaluation of bone metastatic burden by bone SPECT/CT in metastatic prostate cancer patients: Defining threshold value for total bone uptake and assessment in radium-223 treated patients. Ann. Nucl. Med. 2018, 32, 105–113. [Google Scholar] [CrossRef] [PubMed]
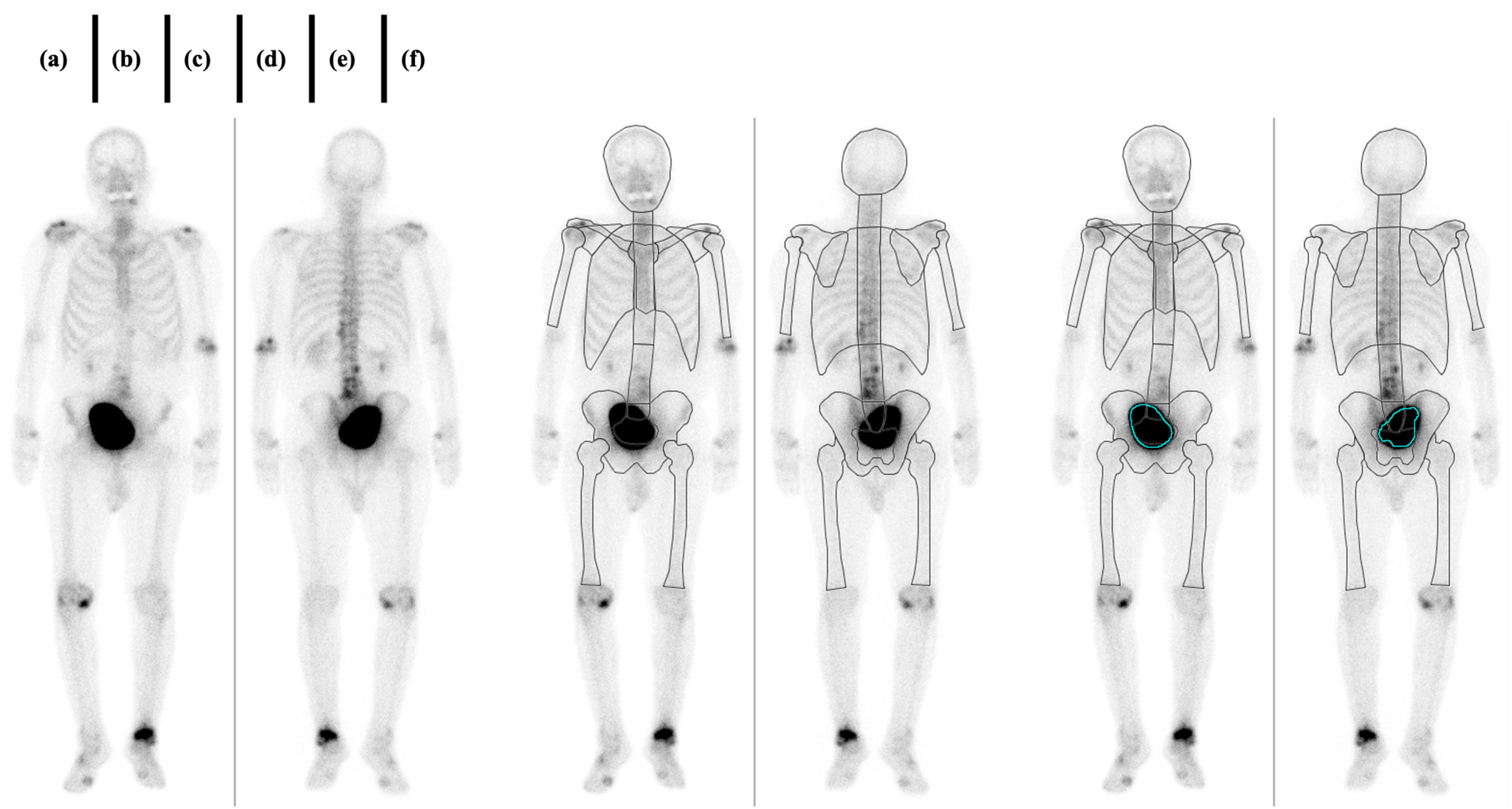

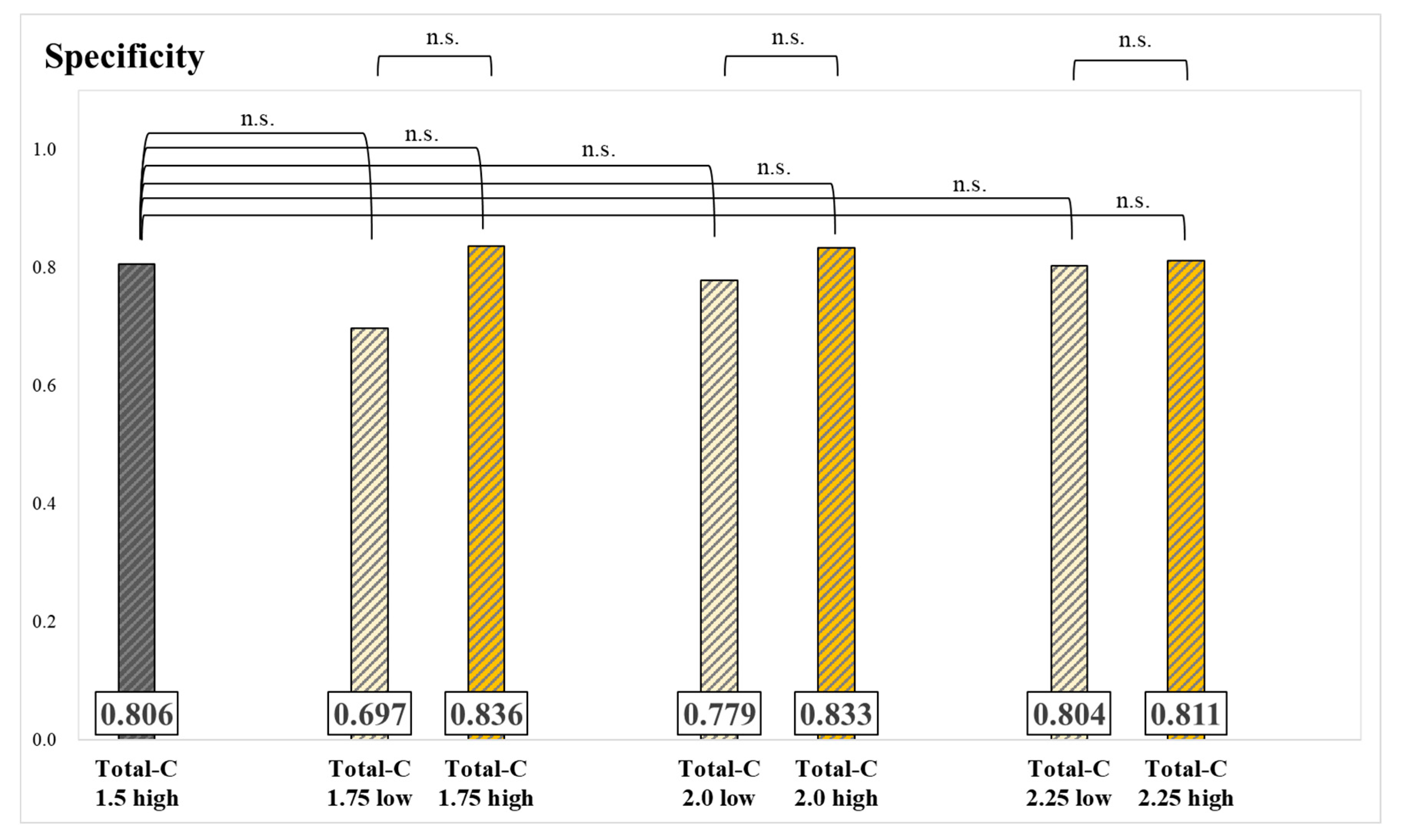




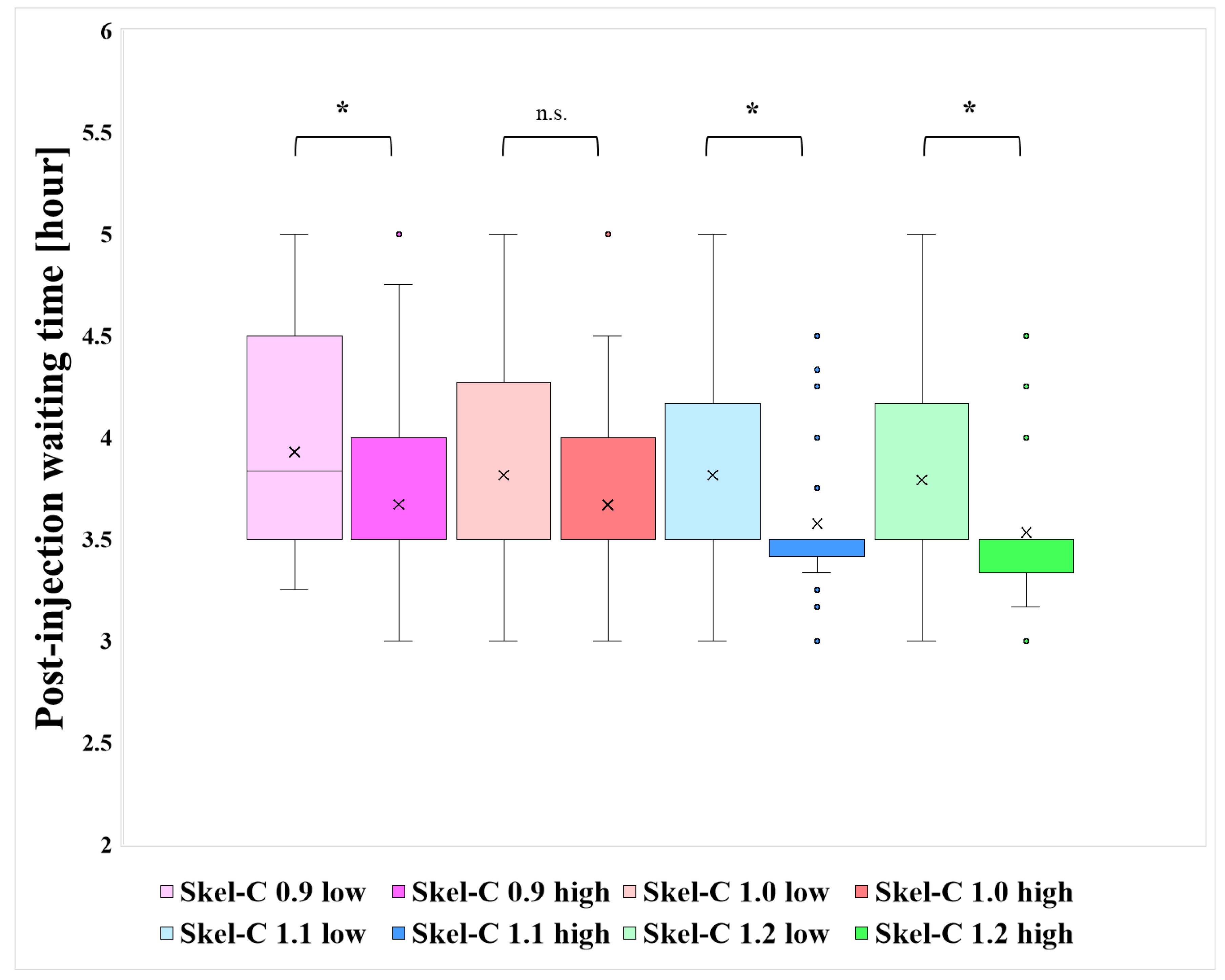
| N | Bone Meta (+) | Bone Meta (−) | Injected Dose [MBq] | Post-Injection Waiting Time [Hour] | |
|---|---|---|---|---|---|
| Skel-C 0.9 low | 42 | 6 | 36 | 755 | 3.93 |
| Skel-C 1.0 low | 87 | 21 | 66 | 757 | 3.83 |
| Skel-C 1.1 low | 137 | 37 | 100 | 752 | 3.84 |
| Skel-C 1.2 low | 167 | 48 | 119 | 753 | 3.82 |
| Skel-C 0.9 high | 194 | 75 | 119 | 751 | 3.73 |
| Skel-C 1.0 high | 149 | 60 | 89 | 749 | 3.72 |
| Skel-C 1.1 high | 99 | 44 | 55 | 751 | 3.66 |
| Skel-C 1.2 high | 69 | 33 | 36 | 747 | 3.63 |
| Total-C 1.75 low | 47 | 14 | 33 | 757 | 3.88 |
| Total-C 2.0 low | 112 | 35 | 77 | 758 | 3.72 |
| Total-C 2.25 low | 153 | 51 | 102 | 758 | 3.83 |
| Total-C 1.75 high | 189 | 67 | 122 | 758 | 3.68 |
| Total-C 2.0 high | 124 | 46 | 78 | 758 | 3.81 |
| Total-C 2.25 high | 83 | 30 | 53 | 758 | 3.63 |
| Total-C 1.5 high | 236 | 81 | 155 | 752 | 3.76 |
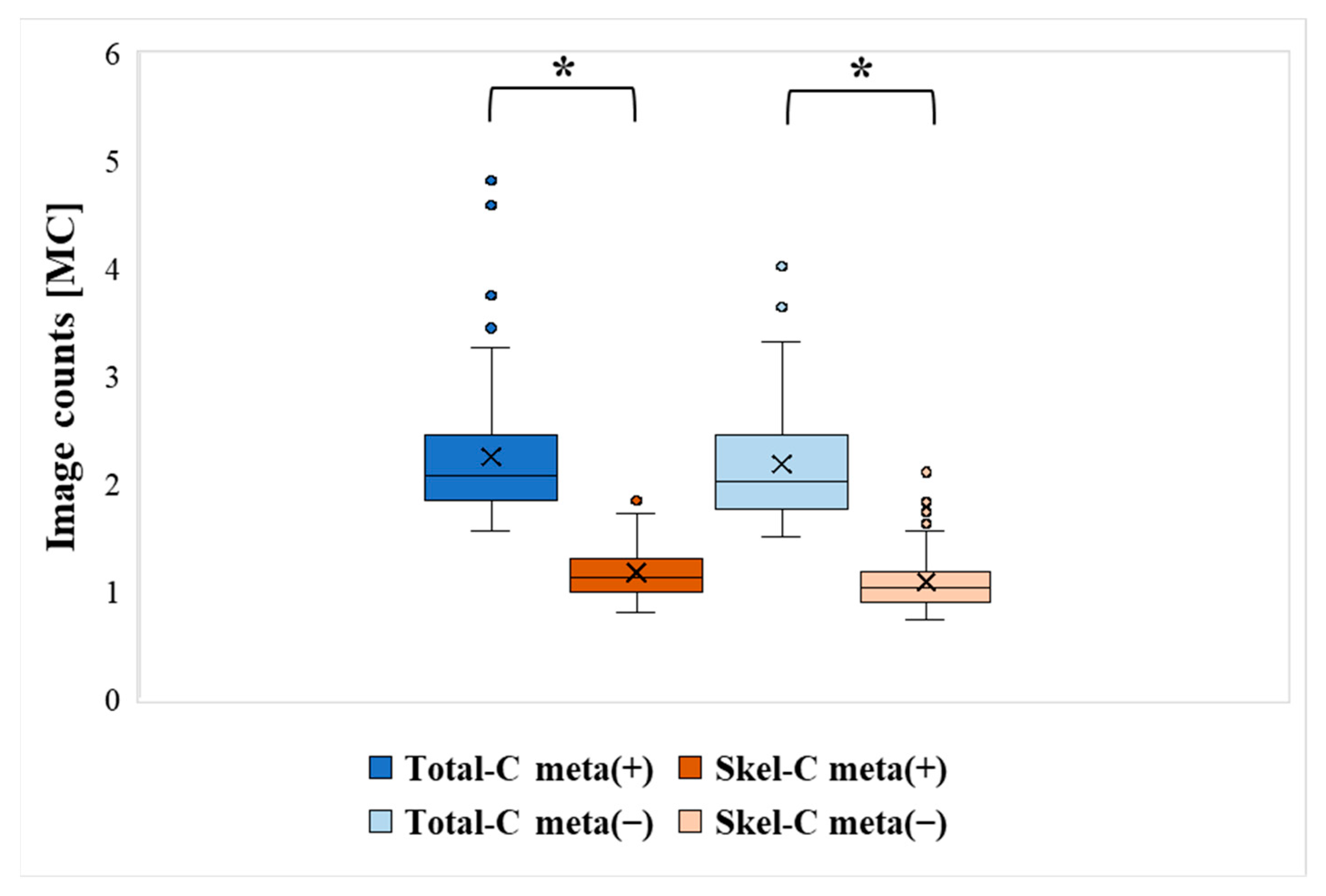
| Bone Meta (+) | Bone Meta (−) | |||
|---|---|---|---|---|
| Total-C | Skel-C | Total-C | Skel-C | |
| Mean [MC] | 2.25 | 1.18 | 2.18 | 1.09 |
| Median [MC] | 2.07 | 1.13 | 2.02 | 1.03 |
| Min [MC] | 1.56 | 0.81 | 1.51 | 0.73 |
| Max [MC] | 4.82 | 1.85 | 4.11 | 2.14 |
| SD | 0.63 | 0.23 | 0.54 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miki, R.; Tsuchitani, T.; Takahashi, Y.; Kitajima, K.; Takahashi, Y. Improving the Accuracy of Bone-Scintigraphy Imaging Analysis Using the Skeletal Count Index: A Study Based on Human Trial Data. Radiation 2025, 5, 5. https://doi.org/10.3390/radiation5010005
Miki R, Tsuchitani T, Takahashi Y, Kitajima K, Takahashi Y. Improving the Accuracy of Bone-Scintigraphy Imaging Analysis Using the Skeletal Count Index: A Study Based on Human Trial Data. Radiation. 2025; 5(1):5. https://doi.org/10.3390/radiation5010005
Chicago/Turabian StyleMiki, Ryosuke, Tatsuya Tsuchitani, Yoshiyuki Takahashi, Kazuhiro Kitajima, and Yasuyuki Takahashi. 2025. "Improving the Accuracy of Bone-Scintigraphy Imaging Analysis Using the Skeletal Count Index: A Study Based on Human Trial Data" Radiation 5, no. 1: 5. https://doi.org/10.3390/radiation5010005
APA StyleMiki, R., Tsuchitani, T., Takahashi, Y., Kitajima, K., & Takahashi, Y. (2025). Improving the Accuracy of Bone-Scintigraphy Imaging Analysis Using the Skeletal Count Index: A Study Based on Human Trial Data. Radiation, 5(1), 5. https://doi.org/10.3390/radiation5010005






