Implementing Neurosurgery and Cesium-131 Brachytherapy in Veterinary Medicine: A Veterinary Case Study with a Review of Clinical Usage of Cesium-131 for Brain Tumors in Human Patients and Opportunities for Translational Research
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Licensing and Ethical Approval
2.2. Isotope Considerations
2.3. Protocol and Radiation Safety
3. Results
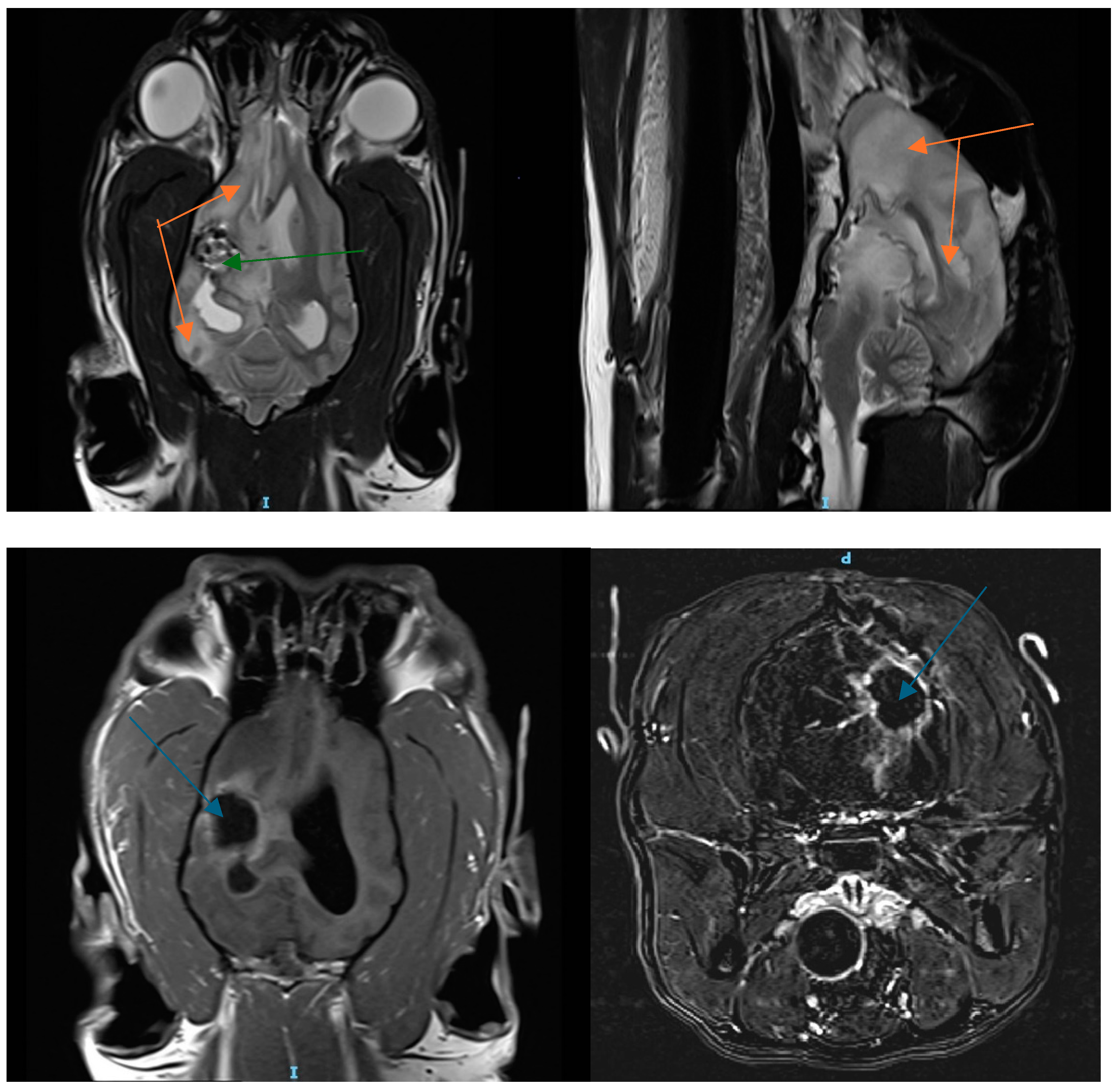
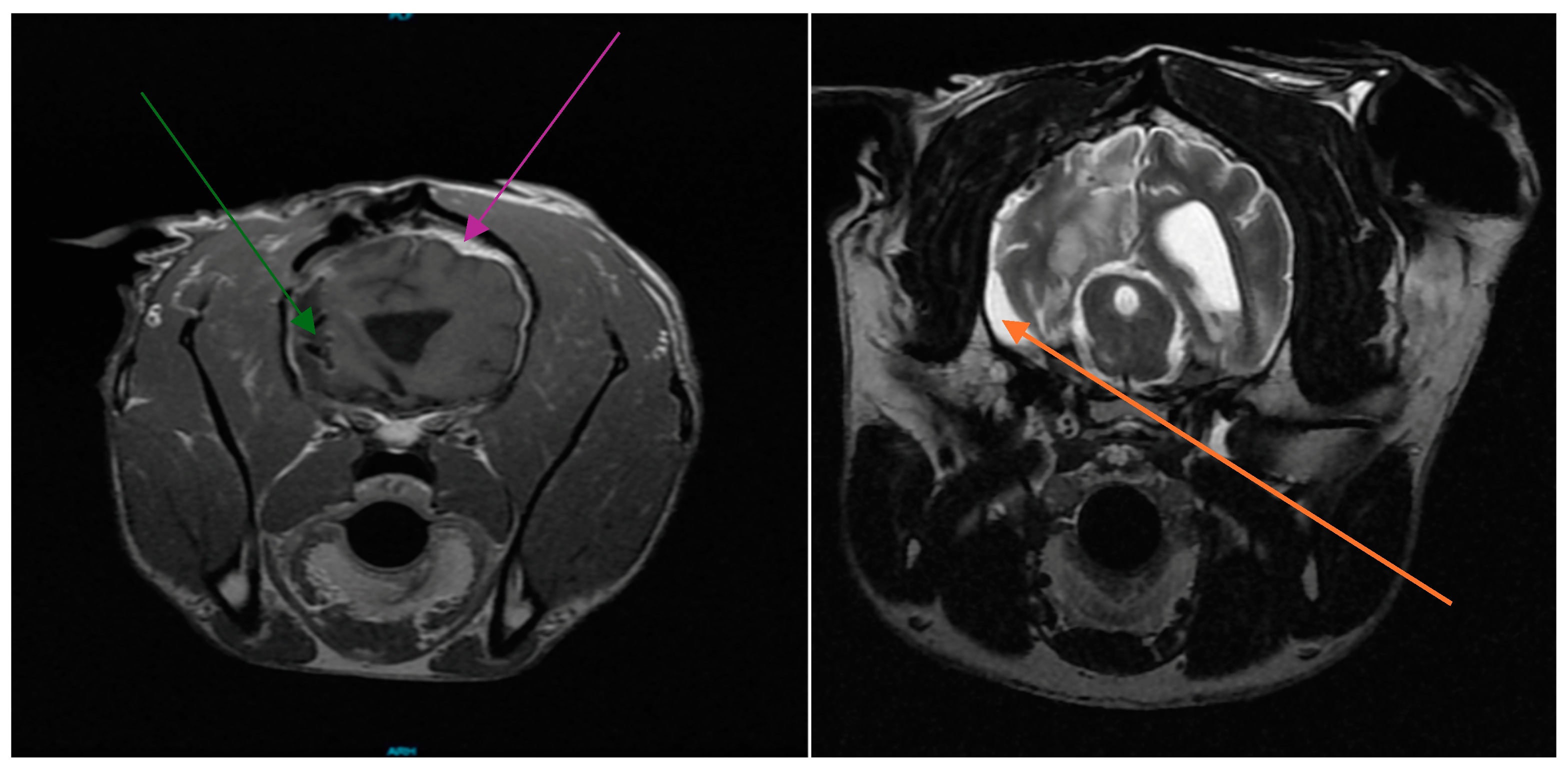
3.1. Patient History
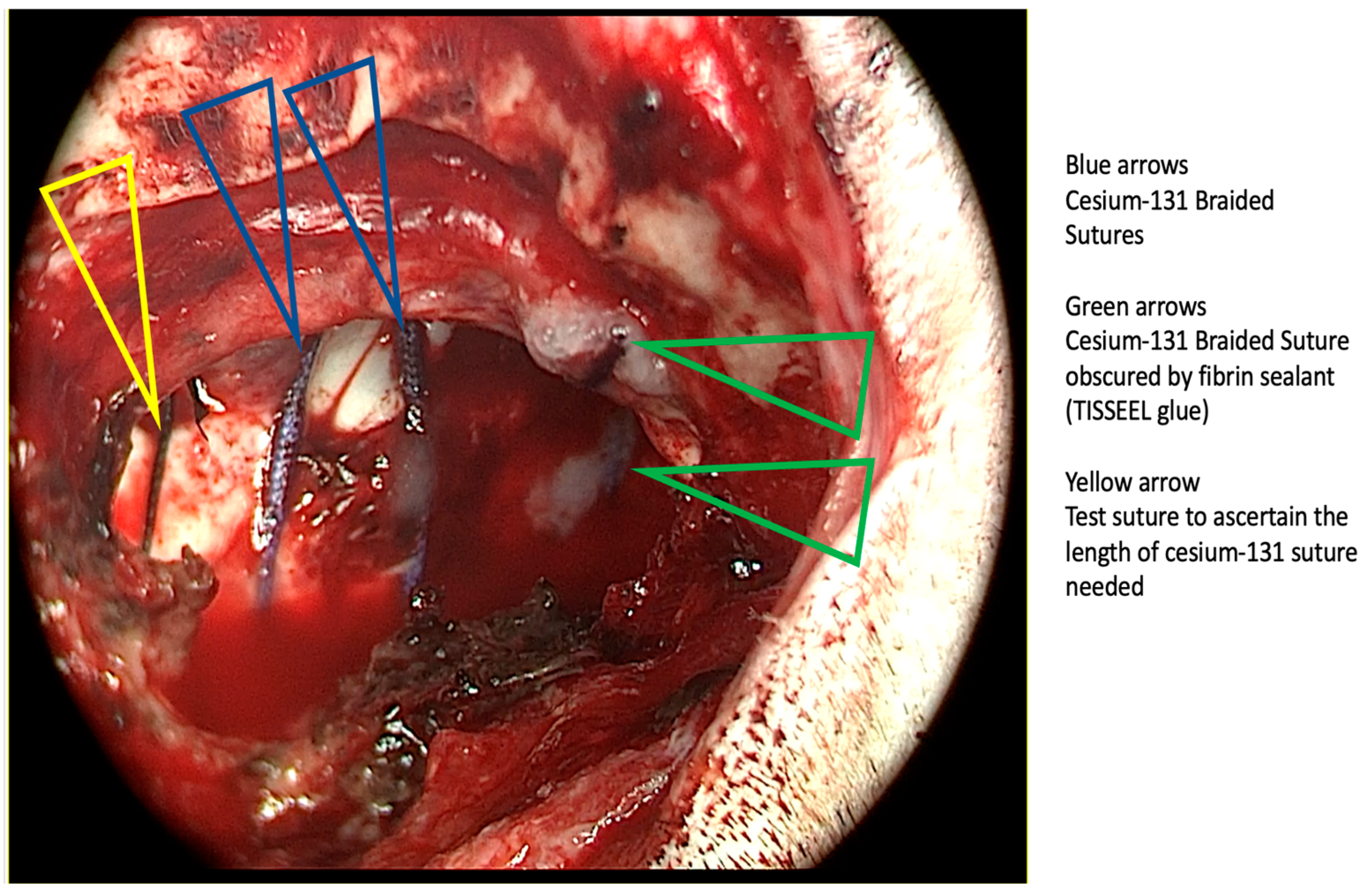
3.2. Cesium Brachytherapy
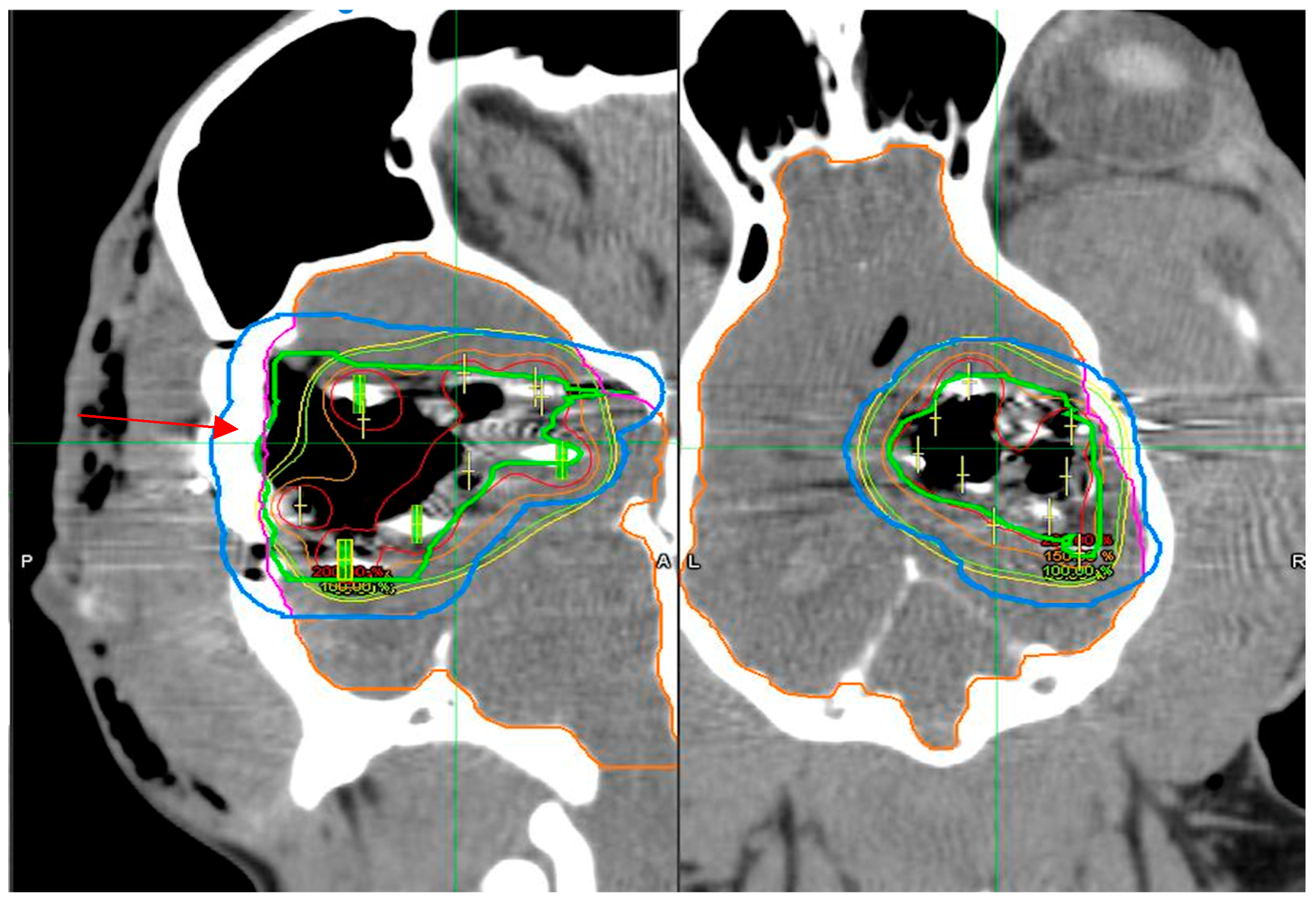
3.3. Dosimetry Verification
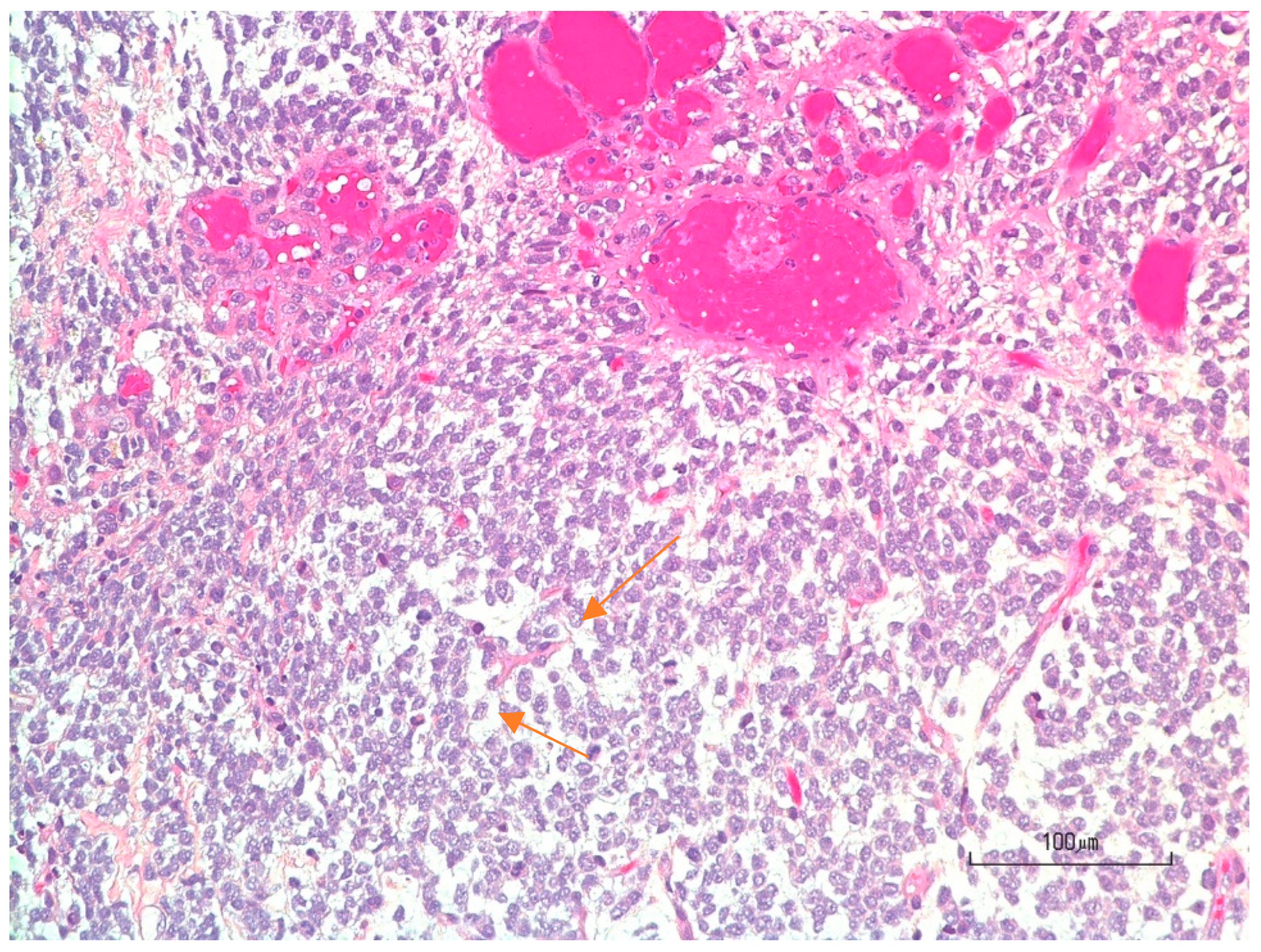
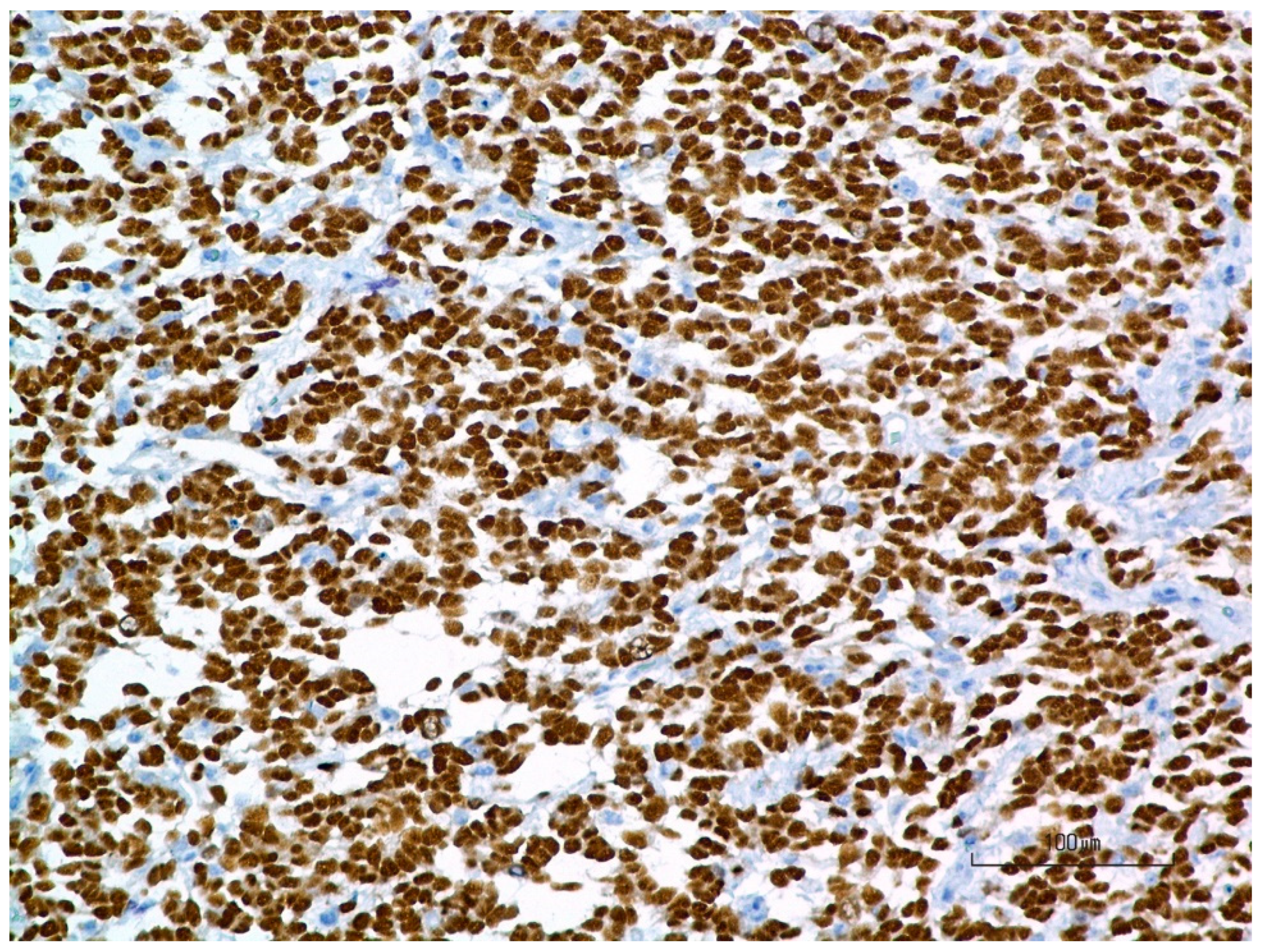
3.4. Pathology
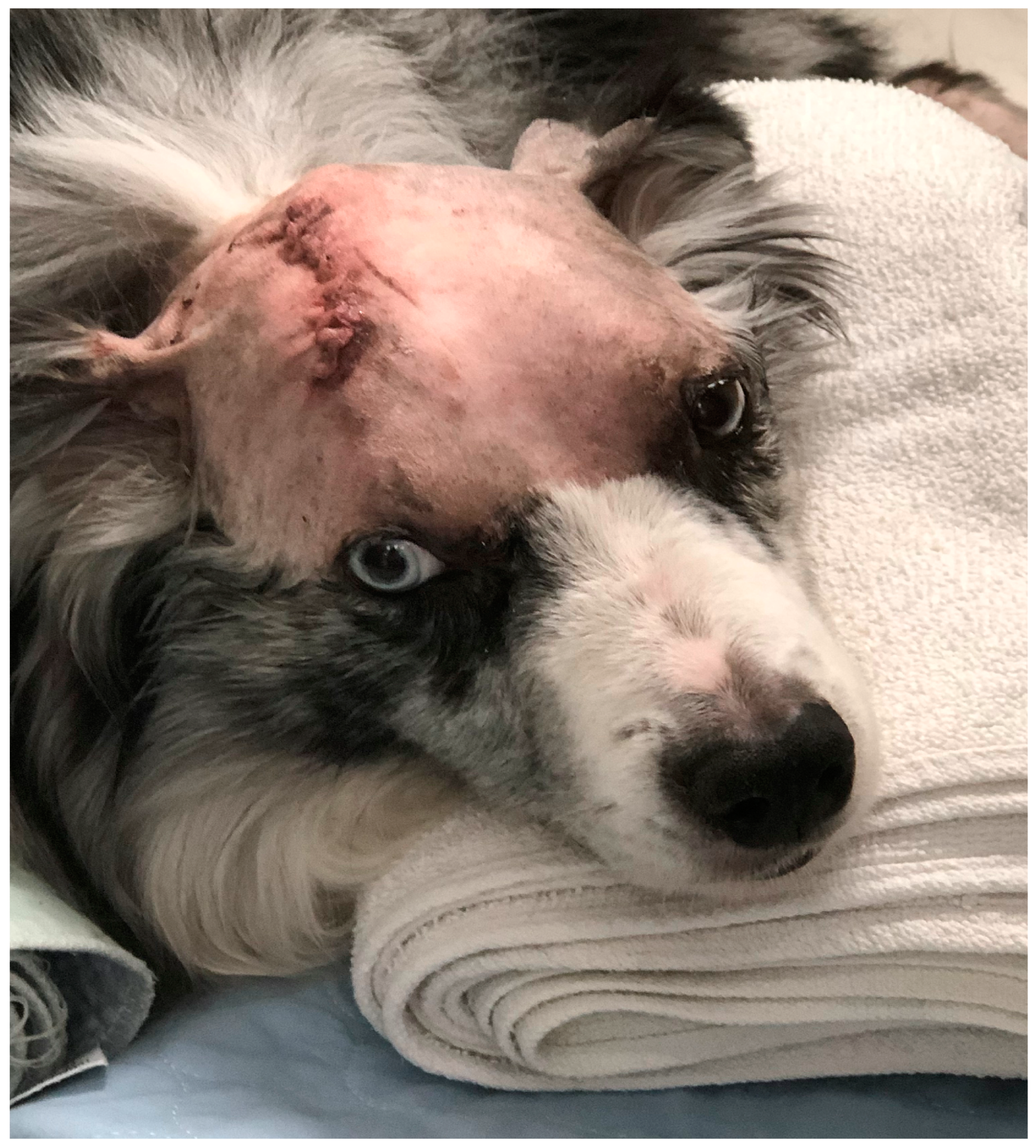
3.5. Patient Follow-Up
4. Discussion
4.1. Future Considerations for Veterinary Brain Brachytherapy with Cesium-131
4.2. The Human Experience in Neuro-Brachytherapy with Cesium-131: What Can We Learn as Veterinarians from Their Experience
4.3. Opportunity for Translational Research (NCI Brain Comparative)
4.4. Advancing Understanding Through Fundamental Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, M.P.; Bagley, R.S.; Harrington, M.L.; Gavin, P.R. Intracranial tumors. Vet. Clin. N. Am. Small Anim. Pract. 1996, 26, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK; estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.T.; Ahmed, A.U.; Yanke, A.B.; Cohen-Gadol, A.A.; Dey, M. Dogs are man’s best friend: In sickness and in Health. Neuro-Oncology 2017, 19, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Platt, S.; Kent, M.; Haley, A. Canine brain tumours: A model for the human disease? Vet. Comp. Oncol. 2017, 15, 252–272. [Google Scholar] [CrossRef]
- Hu, H.; Barker, A.; Harcourt-Brown, T.; Jeffery, N. Systematic review of brain tumor treatment in dogs. J. Vet. Intern. Med. 2015, 29, 1456–1463. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Thrall, D.E.; Price, G.S.; Sharp, N.J.; Munana, K.; Page, R.L. Primary Irradiation of Canine Intracranial Masses. Veter-Radiol. Ultrasound 2000, 41, 377–380. [Google Scholar] [CrossRef]
- Targeser, E.; Martin, T.T.; Burkedin, B.; Hart, C.; Leary, D.; Larue, S.; Boss, M.K. Efficacy of stereotactic radiation therapy for the treatment of confirmed presumed canine glioma. Vet. Com. Oncol. 2023, 21, 578–586. [Google Scholar] [CrossRef]
- Walker, M.A. Interstitial implant brachytherapy in small animals. Veter-Clin. North Am. Small Anim. Pract. 1997, 27, 59–71. [Google Scholar] [CrossRef]
- Klueter, S.; Krasteld, D.; Ludewig, E.; Reischauer, A.; Henichke, F.; Pohlmann, S. High-dose-rate brachy-therapy for intranasal tumours in dogs, results of a pilot study. Vet. Comp. Oncol. 2006, 4, 218. [Google Scholar] [CrossRef]
- Groothuis, D.R.; Wright, D.C.; Ostertag, C.B. The effect of 125I interstitial radiotherapy on blood-brain barrier function in normal canine brain. J. Neurosurg. 1987, 67, 895–902. [Google Scholar] [CrossRef]
- Stubbs, J.B.; Frankel, R.H.; Schultz, K.; Crocker, I.; Dillehay, D.; Olson, J.J. Pre-Clinical Evaluation of a Novel Device for Delivering Brachytherapy to the Margins of Resected Brain tumor cavities. J. Neurosurg. 2002, 96, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, A.G.; Yondorf, M.Z.; Peng, L.; Trichter, S.; Nedialova, L.; Sabbas, A.; Khulidzhanov, F.; Pararshar, B.; Nori, D.; Clifford Chao, K.S.; et al. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J. Neurosurg. 2014, 121, 338–348. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Marko, N.F.; Weil, R.J. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J. Clin. Neurosci. 2013, 20, 485–502. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Lazow, S.P.; Taube, S.; Yondorf, M.Z.; Kovanlikaya, I.; Nori, D.; Christos, P.; Boockvar, J.A.; Pannullo, S.; Stieg, P.E.; et al. Surgical Technique and Clinically Relevant Resection Cavity Dynamics Following Implantation of Cesium131(CS-131) Brachytherapy in patients with Brain Metastases. Oper. Neurosurg. 2016, 12, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, A.G.; Yondorf, M.Z.; Parashar, B.; Nori, D.; Chao, K.S.C.; Boockvar, J.A.; Pannullo, S.; Stieg, P.; Schwartz, T.H. The cost-effectiveness of surgical resection and cesium-131 intraoperative brachytherapy versus surgical resection and stereotactic radiosurgery in the treatment of metastatic brain tumors. J. Neuro-Oncol. 2016, 127, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bander, E.D.; Kelly, A.; Ma, X.; Christos, P.J.; Wernicke, A.G.; Stieg, P.E.; Trichter, S.; Knisely, J.P.; Ramakrishna, R.; Schwartz, T.H. Safety and efficacy of Cesium-131 brachytherapy for brain tumors. J. Neuro-Oncol. 2023, 163, 355–365. [Google Scholar] [CrossRef]
- Ferreira, C.; Sterling, D.; Reynolds, M.; Dusenbery, K.; Chen, C.; Alaei, P. First clinical implementation of Gammatile permanent brain implants after FDA clearance. Brachytherapy 2021, 20, 673–685. [Google Scholar] [CrossRef]
- Choi, M.; Martin, B. Gammatile Surgically Targeted Radiation Therapy Improving Access Care Without Compromising Treatment Outcomes; Clinical White Paper; Gammatile Company: Richland, WA, USA, 2020. [Google Scholar]
- Nakaji, P.; Smith, K.; Youssef, E.; Thomas, T.; Pinnaduwage, D.; Rogers, L.; Wallstrom, G.; Brachman, D. Resection and Surgically Targeted Radiation Therapy for the treatment of larger recurrent or Newly diag-nosed Brain Metastasis: Results from a prospective trial. Cureus 2020, 12, e11570. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; Aleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post operative radiosurgery versus observation for completely resected brain metastasis; a single center, randomized, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Ling, D.C.; Vargo, J.A.; Wegner, R.E.; Flickinger, J.C.; Burton, S.A.; Engh, J.; Amankulor, N.; Ozhasoglu, C.; Quinn, A.E.; Heron, D.E. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases; Clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neuroseurgery 2015, 76, 150–157. [Google Scholar] [CrossRef]
- Brown, P.D.; Balkman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107c/CEC.3) a multicentre, randomized controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Brachman, D.G.; Youssef, E.; Dardis, C.J.; Sanai, N.; Zabramski, J.; Smith, K.A.; Little, A.S.; Shetter, A.G.; McBride, H.L.; Sorensen, S.; et al. Resection and Permanent intracranial brachytherapy using modular, biocompatible cesium 131 implants results in 20 recurrent previously irradiated meningiomas. J. Neurosurg. 2019, 131, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszynski, A.P.; Ohri, N.; Andrews, D.W.; Evans, J.J.; Dicker, A.P.; Werner, M. Wasik reirradiation of re-current meningioma. J. Clin. Neurosci. 2012, 19, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Gessler, D.G.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Ozutemiz, C.; Genturk, M.; et al. GammatiIe TM brachytherapy in the treat-ment of recurrent glioblastomas. Neuro-Oncol. Adv. 2022, 4, vdab185. [Google Scholar] [CrossRef]
- Budnick, H.C.; Richardson, A.M.; Shiue, K.; Watson, G.; Ng, S.K.; Le, Y.; Shah, M.V. Gamma Tile for Gliomas: A Single-Center case series. Cureus 2021, 13, e19390. [Google Scholar] [CrossRef]
- Nakaiji, P.; Brachman, D.; Smith, P.K.; Thomas, T.; Daris, C.; Pinnaduwage, D.; Wallsrrom, G.; Rogers, C.L.; Youssef, E.F. Resection and surgically targeted radiation therapy for locally recurrent GBM. J. Clin. Oncol. 2021, 39, 2054. [Google Scholar] [CrossRef]
- Vanhaezebrouck, I.F.; Scarpelli, M.L. Companion Animal as the key to success translating radiation. Cancers 2023, 15, 3377. [Google Scholar] [CrossRef]
- Parel, R.B.; Baniel, C.C.; Sriramaneni, R.N.; Bradley, K.; Markovina, S.; Morris, Z.S. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination; A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2018, 17, 995–1003. [Google Scholar] [CrossRef]
- Lukas, L.; Zhang, H.; Cheng, K. A Epstein Immune Priming with spatially fractionated Radiation therapy. Curr. Oncol. Rep. 2023, 12, 1483–1496. [Google Scholar] [CrossRef]
- Cho, Y.B.; Yoon, N.; Suh, J.H.; Scott, J.G. Radio-immune response modeling for spatially fractionated radiotherapy. Phys. Med. Biol. 2023, 68, 165010. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic therapy for treating Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Ito, H.; Masuoka, J.; Abe, T. Boron Neutron Capture Therapy and Photodynamic Therapy for High-grade Meningiomas. Cancers 2020, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Van Solinge, T.S.; Nieland, L.; Chiocca, E.A.; Broekman, M.L. Advances in local therapy for glioblastoma—Taking the fight to the tumour. Nat. Rev. Neurol. 2022, 18, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Herpai, D.; Quigley, M.; Cecere, T.E.; Robertson, J.L.; D’Agostino, R.B.; Hinckley, J.; Tatter, S.B.; Dickinson, P.J.; Debinski, W. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro Oncol. 2021, 23, 422–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenbluth, K.H.; Martin, A.J.; Mittermeyer, S.; Eschermann, J.; Dickinson, P.J.; Bankiewicz, K.S. Rapid inverse planning for pressure-driven drug infusions in the brain. PLoS ONE 2013, 8, e56397. [Google Scholar] [CrossRef] [PubMed Central]
- Monjazeb, A.M.; Kent, M.S.; Grossenbacher, S.K.; Mall, A.E.; Zamora, C.; Mirsoian, A.; Chen, M.; Kol, A.; Shiao, S.L.; Reddy, A.; et al. Blocking Indolamine-2-3 dioxygenase rebound immune suppression boosts antitumor effects of radioimmunotherapy in murine models and spontaneous canine malignancies. Clin. Cancer Res. 2016, 22, 4328–4340. [Google Scholar] [CrossRef]
- Boss, M.K.; Watts, R.; Harrison, L.G.; Hopkins, S.; Chow, L.; Trageser, E.; Eaton, C.; Larue, S.M.; Regan, D.; Dewhirst, M.W.; et al. Immunologic effects of stereotactic radiation therapy in dogs with spontaneous tumors and the impact of intratumora OX40/TLR agonist immunotherapy. Int. J. Mol. sci. 2022, 23, 826. [Google Scholar] [CrossRef]
- Litak, J.; Grochowski, C.; Litak, J.; OsuschowskaI, I.; Gisk, K.; Radzikowska, E.; Kmaieniak, P.; Rolinski, J. TLR4 signaling bs Immune checkpoints, miRNAs molecules, cancer stem cells and wingless signaling interplay in glioblastoma multiforme-future perspectives. Int. J. Mol. Sci. 2020, 21, 3114. [Google Scholar] [CrossRef]




| Radionuclide Type | Provider | Emitted Energy (Kev) | Decay Properties—Half-Life |
|---|---|---|---|
| Cesium-131(CS-131) | GammaTile Therapy/GT Medical Technologies https://gtmedtech.com (accessed on 25 September 2024) | 30.4 | 9.7 days |
| Palladium-107 (Pd-103) Theraseed™ | BD-850 W Rios Salado Pkwy, Tempe, AZ 85281 USA https://www.bd.com (accessed on 25 September 2024) | 20.7 | 17.0 days |
| Iodine-125 (I-125) Isoseed ® I-125 | Eckert & Ziegler https://Medical.ezag.com (accessed on 25 September 2024) Robert-Rossle-Strasse 10 13125 Berlin, Germany | 28.4 | 59.4 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhaezebrouck, I.F.; Bentley, R.T.; Georgiades, A.; Arnold, S.; Young, J.A.; Claus, N.; Danaher, L.; Klutzke, J.B.; Scarpelli, M.L. Implementing Neurosurgery and Cesium-131 Brachytherapy in Veterinary Medicine: A Veterinary Case Study with a Review of Clinical Usage of Cesium-131 for Brain Tumors in Human Patients and Opportunities for Translational Research. Radiation 2025, 5, 13. https://doi.org/10.3390/radiation5020013
Vanhaezebrouck IF, Bentley RT, Georgiades A, Arnold S, Young JA, Claus N, Danaher L, Klutzke JB, Scarpelli ML. Implementing Neurosurgery and Cesium-131 Brachytherapy in Veterinary Medicine: A Veterinary Case Study with a Review of Clinical Usage of Cesium-131 for Brain Tumors in Human Patients and Opportunities for Translational Research. Radiation. 2025; 5(2):13. https://doi.org/10.3390/radiation5020013
Chicago/Turabian StyleVanhaezebrouck, Isabelle F., R. Timothy Bentley, Alex Georgiades, Susan Arnold, Joshua A. Young, Nathan Claus, Laura Danaher, Joshua B. Klutzke, and Matthew L. Scarpelli. 2025. "Implementing Neurosurgery and Cesium-131 Brachytherapy in Veterinary Medicine: A Veterinary Case Study with a Review of Clinical Usage of Cesium-131 for Brain Tumors in Human Patients and Opportunities for Translational Research" Radiation 5, no. 2: 13. https://doi.org/10.3390/radiation5020013
APA StyleVanhaezebrouck, I. F., Bentley, R. T., Georgiades, A., Arnold, S., Young, J. A., Claus, N., Danaher, L., Klutzke, J. B., & Scarpelli, M. L. (2025). Implementing Neurosurgery and Cesium-131 Brachytherapy in Veterinary Medicine: A Veterinary Case Study with a Review of Clinical Usage of Cesium-131 for Brain Tumors in Human Patients and Opportunities for Translational Research. Radiation, 5(2), 13. https://doi.org/10.3390/radiation5020013







