Integumentary Colour Allocation in the Stork Family (Ciconiidae) Reveals Short-Range Visual Cues for Species Recognition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Species and Phenotypic Variables
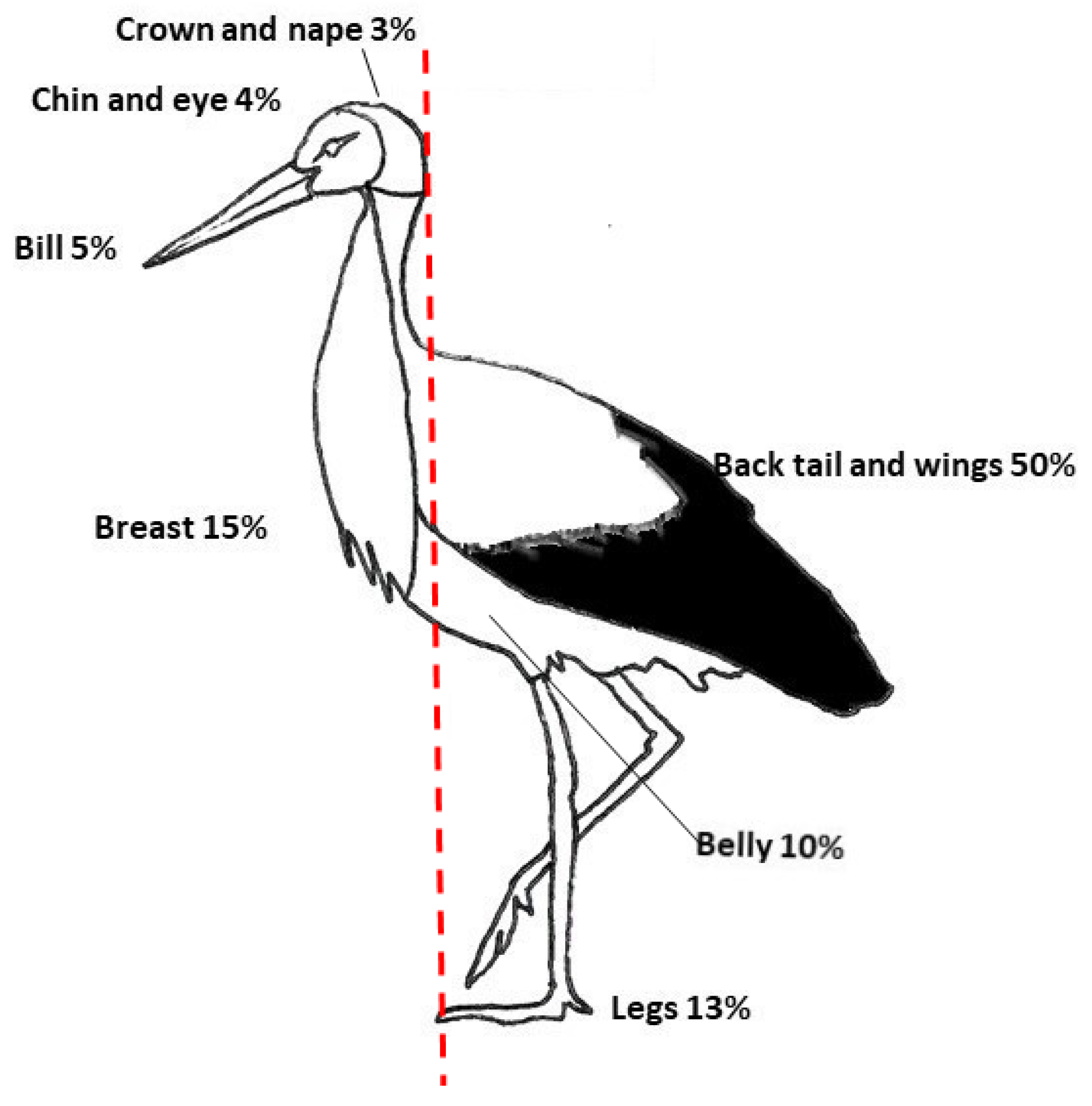
2.2. Phylogeny Data
2.3. Conservatism Measurements
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Hoyo, J. (Ed.) All the Birds of the World; Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Hancock, J.; Kushlan, J.A.; Kahl, M.P. Storks, Ibises and Spoonbills of the World; A&C Black: London, UK, 2010. [Google Scholar]
- Prum, R.O.; Quinn, T.; Torres, R.H. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 2006, 209, 748–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galván, I.; Negro, J.J.; Rodríguez, A.; Carrascal, L.M. On showy dwarfs and sober giants: Body size as a constraint for the evolution of bird plumage colouration. Acta Ornithol. 2013, 48, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Oliphant, L.W.; Hudon, J.; Bagnara, J.T. Pigment cell refugia in homeotherms—the unique evolutionary position of the iris. Pigment Cell Res. 1992, 5, 367–371. [Google Scholar] [CrossRef]
- Negro, J.J.; Blázquez, M.C.; Galván, I. Intraspecific eye colour variability in birds and mammals: A recent evolutionary event exclusive to humans and domestic animals. Front. Zool. 2017, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Negro, J.J.; Sarasola, J.H.; Fariñas, F.; Zorrilla, I. Function and occurrence of facial flushing in birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 78–84. [Google Scholar] [CrossRef]
- Toral, G.M.; Figuerola, J.; Negro, J.J. Multiple ways to become red: Pigment identification in red feathers using spectrometry. Comp. Biochem. Physiol. Part B 2008, 150, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E. Sexiness, individual condition, and species identity: The information signaled by ornaments and assessed by choosing females. Evolut. Biol. 2015, 42, 251–259. [Google Scholar] [CrossRef]
- Galván, I.; García-Campa, J.; Negro, J.J. Complex plumage patterns can be produced only with the contribution of melanins. Physiol. Biochem. Zool. 2017, 90, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Surmacki, A.; Minias, P.; Kudelska, K. Occurrence and function of melanin-based grey colouration in Western Palaearctic songbirds (Aves: Passeriformes). IBIS 2020. [Google Scholar] [CrossRef]
- Burtt, E.H. An analysis of physical, physiological, and optical aspects of avian colouration with emphasis on wood-warblers. Ornithol. Monogr. 1986, 38, 1–126. [Google Scholar] [CrossRef]
- Kazimirski, P.P.; Kaczmarski, M.; Zagalska-Neubauer, M.M.; Żołnierowicz, K.M.; Tobółka, M. Absence of sex differences in digit ratio in nestlings of the White Stork Ciconia ciconia, a monomorphic bird species. Bird Study 2019, 66, 503–509. [Google Scholar] [CrossRef]
- Fiske, P.; Rintamäki, P.T.; Karvonen, E. Mating success in lekking males: A meta-analysis. Behav. Ecol. 1998, 9, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Ishtiaq, F.; Javed, S.; Coulter, M.C.; Rahmani, A.R. Resource partitioning in three sympatric species of storks in Keoladeo National Park, India. Waterbirds 2010, 33, 41–49. [Google Scholar] [CrossRef]
- Slikas, B. Phylogeny of the Avian Family Ciconiidae (Storks) Based on Cytochrome b Sequences and DNA–DNA Hybridization Distances. Mol. Phylogen. Evol. 1997, 8, 275–300. [Google Scholar] [CrossRef] [PubMed]
- McNaught, M.K.; Owens, I.P. Interspecific variation in plumage colour among birds: Species recognition or light environment? J. Evol. Biol. 2002, 15, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Uy, J.A.C.; Moyle, R.G.; Filardi, C.E.; Cheviron, Z.A. Difference in plumage colour used in species recognition between incipient species is linked to a single amino acid substitution in the melanocortin-1 receptor. Am. Nat. 2009, 174, 244–254. [Google Scholar] [CrossRef]
- Wallace, A.R. Darwinism: An Exposition of the Theory of Natural Selection with Some of Its Applications; Macmillan & Co.: London, UK; New York, NY, USA, 1889. [Google Scholar]
- Dale, J. Intraspecific Variation in Colouration. In Bird Colouration: Function and Evolution; Hill, G.E., McGraw, K.J., Eds.; Harvard University Press: Cambridge, MA, USA, 2006; Volume 2, pp. 597–602. [Google Scholar]
- Martin, P.R.; Montgomerie, R.; Lougheed, S.C. Colour patterns of closely related bird species are more divergent at intermediate levels of breeding-range sympatry. Am. Nat. 2015, 185, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Leighton, G.R.; Hugo, P.S.; Roulin, A.; Amar, A. Just Google it: Assessing the use of Google Images to describe geographical variation in visible traits of organisms. Methods Ecol. Evol. 2016, 7, 1060–1070. [Google Scholar] [CrossRef]
- Bergeron, Z.T.; Fuller, R.C. Using human vision to detect variation in avian colouration: How bad is it? Am. Nat. 2018, 191, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Negro, J.J.; Figueroa-Luque, E.; Galván, I. Melanin-based sexual dichromatism in the Western Palearctic avifauna implies darker males and lighter females. J. Avian Biol. 2018, e01657. [Google Scholar] [CrossRef]
- Negro, J.J.; Grande, J.M.; Tella, J.L.; Garrido, J.; Hornero, D.; Donázar, J.A.; Sanchez-Zapata, J.A.; Benítez, J.R.; Barcell, M. An unusual source of essential carotenoids. Nature 2002, 416, 807–808. [Google Scholar] [CrossRef]
- Yezerinac, S.M.; Weatherhead, P.J. Plumage colouration, differential attraction of vectors and haematozoa infections in birds. J. Anim. Ecol. 1995, 64, 528–537. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Münkemüller, T.; Lavergne, S.; Bzeznik, B.; Dray, S.; Jombart, T.; Schiffers, K.; Thuiller, W. How to measure and test phylogenetic signal. Methods Ecol. Evol. 2012, 3, 743–756. [Google Scholar] [CrossRef]
- Pennell, M.; Eastman, J.; Slater, G.; Brown, J.; Uyeda, J.; Fitzjohn, R.; Alfaro, M.; Harmon, L. Geiger v2.0: An expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 2014, 30, 2216–2218. [Google Scholar] [CrossRef]
- Eastman, J.M.; Alfaro, M.E.; Joyce, P.; Hipp, A.L.; Harmon, L.J. A novel comparative method for identifying shifts in the rate of character evolution on trees. Evol. Int. J. Org. Evol. 2011, 65, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Green, P.J. Reversible jump Markov chain Monte Carlo computation and Bayesian model determination. Biometrika 1995, 82, 711–732. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; Parker, G.A. The evolution of bird colouration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1979, 287, 63–130. [Google Scholar]
- Durrer, H. Colouration. In Biology of the Integument; Springer: Berlin/Heidelberg, Germany, 1986; pp. 239–247. [Google Scholar]
- Stoddard, M.C.; Prum, R.O. How colourful are birds? Evolution of the avian plumage colour gamut. Behav. Ecol. 2011, 22, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Sumner, P.; Mollon, J.D. Colours of primate pelage and skin: Objective assessment of conspicuousness. Am. J. Primatol. 2003, 59, 67–91. [Google Scholar] [CrossRef] [PubMed]
- Negro, J.J.; Doña, J.; Blázquez, M.C.; Rodríguez, A.; Herbert-Read, J.E.; Brooke, M.d.L. Contrasting stripes are a widespread feature of group living in birds, mammals and fishes. Proc. R. Soc. B 2020, 287, 20202021. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Systematics and the Origin of Species; Columbia University Press: New York, NY, USA, 1942. [Google Scholar]
- Uy, J.A.C.; Moyle, R.G.; Filardi, C.E. Plumage and song differences mediate species recognition between incipient flycatcher species of the Solomon Islands. Evol. Int. J. Org. Evol. 2009, 63, 153–164. [Google Scholar] [CrossRef]
- Lerner, H.R.; Meyer, M.; James, H.F.; Hofreiter, M.; Fleischer, R.C. Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian honeycreepers. Curr. Biol. 2011, 21, 1838–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, N.; Botero, C.A.; Tobias, J.A.; Dunn, P.O.; MacGregor, H.E.; Rubenstein, D.R.; Uy, J.A.; Weir, J.T.; Whittingham, L.A.; Safran, R.J. Sexual selection accelerates signal evolution during speciation in birds. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131065. [Google Scholar] [CrossRef] [Green Version]
- Allen, W.; Stevens, M.; Higham, J. Character displacement of Cercopithecini primate visual signals. Nat. Commun. 2014, 5, 4266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, N. Ecological adaptation and species recognition drive vocal evolution in neotropical suboscine birds. Evolution 2005, 59, 200–215. [Google Scholar] [CrossRef]
- Kaefer, I.L.; Tsuji-Nishikido, B.M.; Mota, E.P.; Farias, I.P.; Lima, A.P. The early stages of speciation in Amazonian forest frogs: Phenotypic conservatism despite strong genetic structure. Evol. Biol. 2013, 40, 228–245. [Google Scholar] [CrossRef]
- Bonser, R.H. The mechanical properties of feather keratin. J. Zool. 1996, 239, 477–484. [Google Scholar] [CrossRef]
- Bonser, R.H. Melanin and the abrasion resistance of feathers. Condor 1995, 97, 590–591. [Google Scholar]
- Mendelson, T.C.; Shaw, K.L. The (mis) concept of species recognition. Trends Ecol. Evol. 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Lowe, W.H.; Muhlfeld, C.C.; Allendorf, F.W. Spatial sorting promotes the spread of maladaptive hybridization. Trends Ecol. Evol. 2015, 30, 456–462. [Google Scholar] [CrossRef]
- Terborgh, J. Mixed flocks and polyspecific associations: Costs and benefits of mixed groups to birds and monkeys. Am. J. Primatol. 1990, 21, 87–100. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.J.; Beltrán, J.F.; Tejedo, M.; Nicieza, A.G.; Llusia, D.; Márquez, R.; Aragón, P. Niche models at inter-and intraspecific levels reveal hierarchical niche differentiation in midwife toads. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Wagner, G.P.; Schwenk, K. Evolutionarily stable configurations: Functional integration and the evolution of phenotypic stability. In Evolutionary Biology; Springer: Boston, MA, USA, 2000; pp. 155–217. [Google Scholar]
- Lyu, N.; Lloyd, H.; Sun, Y.H. Delayed plumage maturation in birds and the significance of condition-dependent parental care. Behav. Ecol. Sociobiol. 2015, 69, 1003–1010. [Google Scholar] [CrossRef]
- Nagy, M.; Ákos, Z.; Biro, D.; Vicsek, T. Hierarchical group dynamics in pigeon flocks. Nature 2010, 464, 890–893. [Google Scholar] [CrossRef]


| Area. | Log-Likelihood | AIC | AICc |
|---|---|---|---|
| Bill | −40.571838 | 85.143677 | 85.943677 |
| Legs | −16.961067 | 35.922135 | 66.753341 |
| Facial | −32.251670 | 66.503341 | 66.753341 |
| Iris | −24.953299 | 51.906597 | 52.156597 |
| Ventral | −4.838572 | 11.677143 | 11.927143 |
| Dorsum | −16.448905 | 34.897810 | 35.147810 |
| Remiges | −15.936578 | 33.873156 | 34.123156 |
| Tail | −19.509732 | 41.019464 | 41.269464 |
| Neck | −32.956012 | −32.956012 | 68.162024 |
| Head | −29.908035 | −29.908035 | 62.066070 |
| Degree of Conservatism | Body Regions | % of Body Surface |
|---|---|---|
| Low | Bill, facial skin, neck | 12% |
| Medium | Iris, head | 8% |
| High | Remiges, tail, dorsum, legs | 63% |
| Very high | ventral | 17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, E.J.; Negro, J.J. Integumentary Colour Allocation in the Stork Family (Ciconiidae) Reveals Short-Range Visual Cues for Species Recognition. Birds 2021, 2, 138-146. https://doi.org/10.3390/birds2010010
Rodríguez-Rodríguez EJ, Negro JJ. Integumentary Colour Allocation in the Stork Family (Ciconiidae) Reveals Short-Range Visual Cues for Species Recognition. Birds. 2021; 2(1):138-146. https://doi.org/10.3390/birds2010010
Chicago/Turabian StyleRodríguez-Rodríguez, Eduardo J., and Juan J. Negro. 2021. "Integumentary Colour Allocation in the Stork Family (Ciconiidae) Reveals Short-Range Visual Cues for Species Recognition" Birds 2, no. 1: 138-146. https://doi.org/10.3390/birds2010010
APA StyleRodríguez-Rodríguez, E. J., & Negro, J. J. (2021). Integumentary Colour Allocation in the Stork Family (Ciconiidae) Reveals Short-Range Visual Cues for Species Recognition. Birds, 2(1), 138-146. https://doi.org/10.3390/birds2010010







