New Evidence on the Linkage of Population Trends and Species Traits to Long-Term Niche Changes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
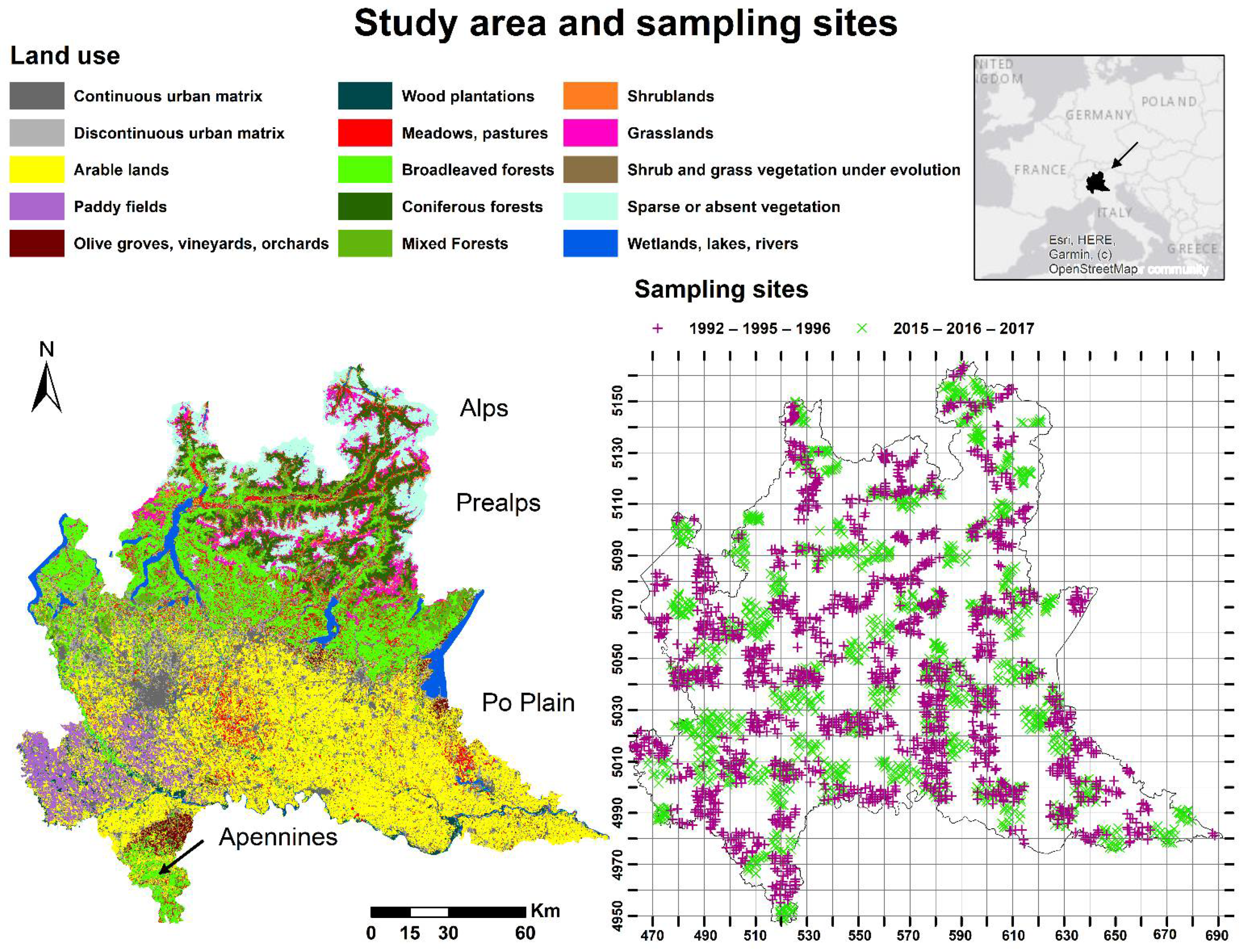
2.1. Study Area and Bird Data
2.2. Habitat and Climatic Variables
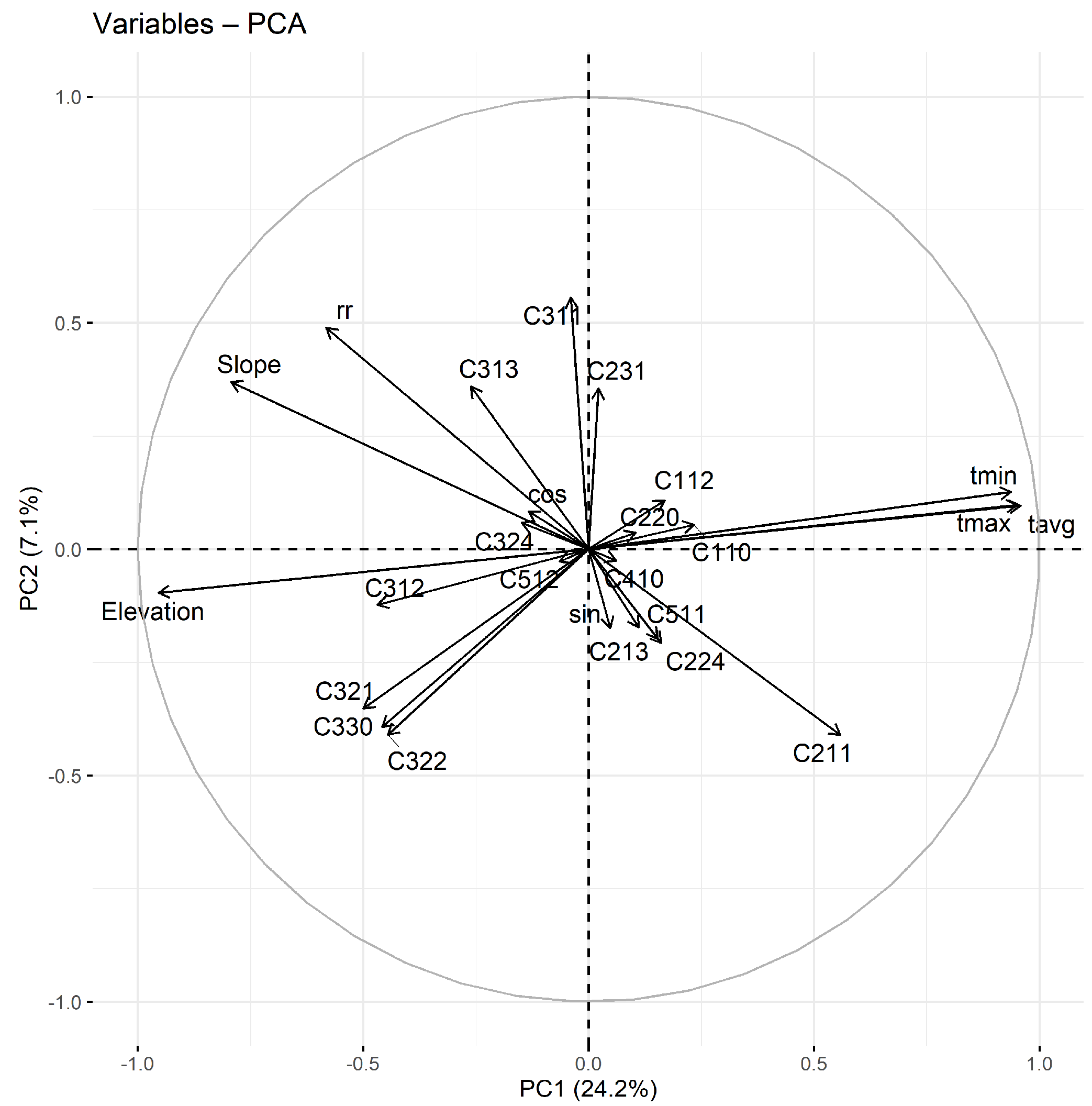
2.3. Niche Quantification and Temporal Changes
2.4. Relationship between Niche Temporal Changes, Population Trends and Species Traits
3. Results
3.1. Niche Quantification and Temporal Changes
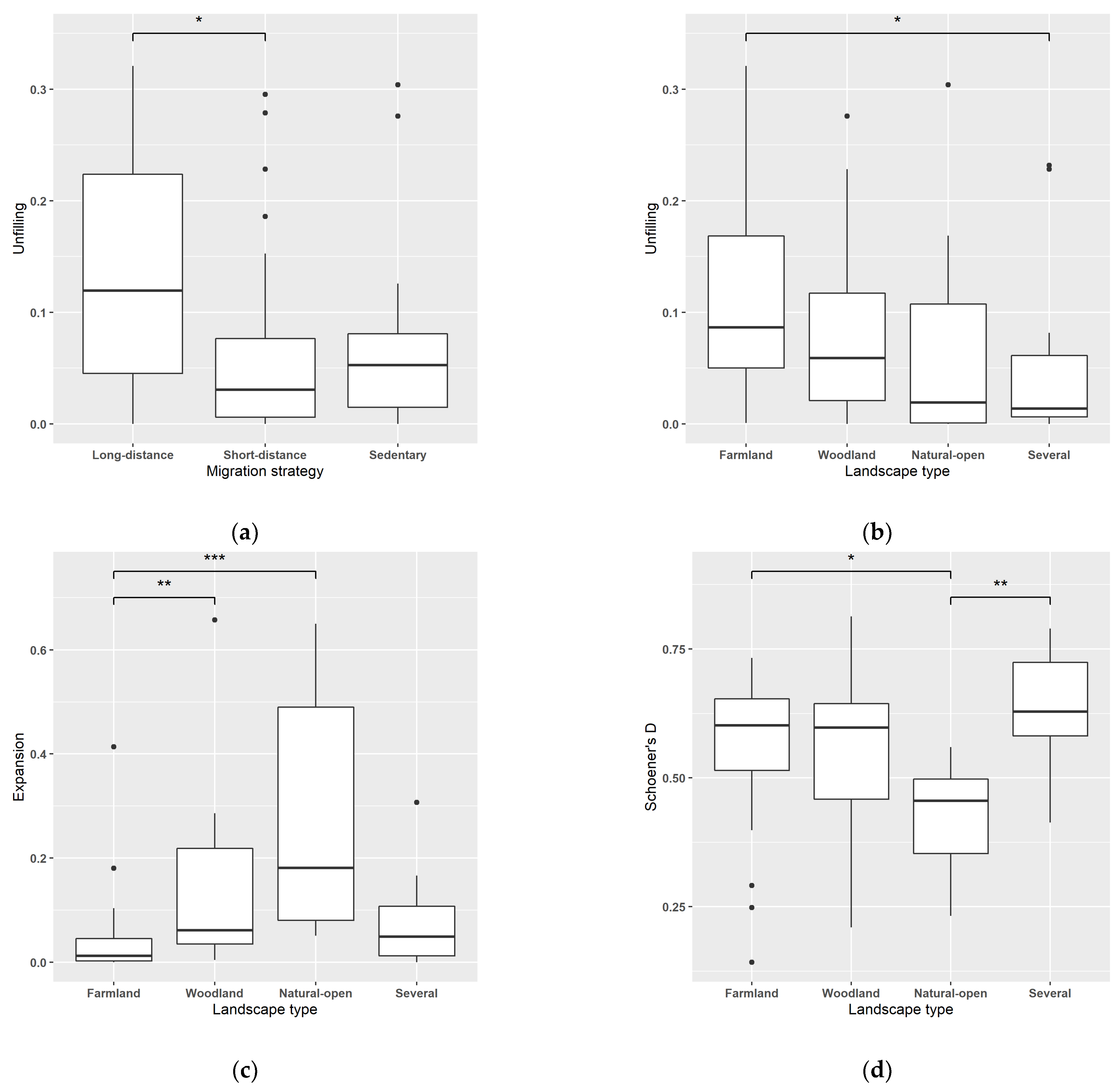
3.2. Relationship between Niche Temporal Changes, Population Trends and Species Traits
4. Discussion
4.1. Niche Quantification and Temporal Changes
4.2. Relationship between Nyche Temporal Changes, Population Trends and Species Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Grinnell, J. Barriers to distribution as regards birds and mammals. Am. Nat. 1914, 48, 248–254. [Google Scholar] [CrossRef]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Soberón, J.; Nakamura, M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef] [Green Version]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strubbe, D.; Broennimann, O.; Chiron, F.; Matthysen, E. Niche conservatism in non-native birds in Europe: Niche unfilling rather than niche expansion. Glob. Ecol. Biogeogr. 2013, 22, 962–970. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including environmental niche information to improve IUCN Red List assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Mothes, C.C.; Stroud, J.T.; Clements, S.L.; Searcy, C.A. Evaluating ecological niche model accuracy in predicting biotic invasions using South Florida’s exotic lizard community. J. Biogeogr. 2019, 46, 432–441. [Google Scholar] [CrossRef]
- Rather, T.A.; Kumar, S.; Khan, J.A. Multi-scale habitat modelling and predicting change in the distribution of tiger and leopard using random forest algorithm. Sci. Rep. 2020, 10, 11473. [Google Scholar] [CrossRef] [PubMed]
- Zink, R.M.; Botero-Cañola, S.; Martinez, H.; Herzberg, K.M. Niche modeling reveals life history shifts in birds at La Brea over the last twenty millennia. PLoS ONE 2020, 15, e0227361. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Hemami, M.-R.; Kaboli, M.; Nazarizadeh, M.; Malekian, M.; Behrooz, R.; Geniez, P.; Alroy, J.; Zimmermann, N.E. The legacy of Eastern Mediterranean mountain uplifts: Rapid disparity of phylogenetic niche conservatism and divergence in mountain vipers. BMC Ecol. Evol. 2021, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Liordos, V.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Valsamidis, E.; Kontsiotis, V.J. Niche analysis and conservation of bird species using urban core areas. Sustainability 2021, 13, 6327. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Broennimann, O.; Ursenbacher, S.; Meyer, A.; Golay, P.; Monney, J.-C.; Schmocker, H.; Guisan, A.; Dubey, S. Influence of climate on the presence of colour polymorphism in two montane reptile species. Biol. Lett. 2014, 10, 20140638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Contreras, I.; Sánchez-González, L.A.; Arizmendi, M.C.; Prieto-Torres, D.A.; Navarro-Sigüenza, A.G. Climatic niche evolution in the Arremon brunneinucha complex (Aves: Passerellidae) in a mesoamerican landscape. Evol. Biol. 2020, 47, 123–132. [Google Scholar] [CrossRef]
- Sillero, N.; Argaña, E.; Matos, C.; Franch, M.; Kaliontzopoulou, A.; Carretero, M.A. Local segregation of realised niches in lizards. ISPRS Int. J. Geo-Inf. 2020, 9, 764. [Google Scholar] [CrossRef]
- Escoriza, D.; Pascual, G.; Mestre, L. Climate and habitat niche diversification in a southwest European squamate assemblage. Evol. Ecol. 2021, 35, 761–777. [Google Scholar] [CrossRef]
- Hending, D. Niche-separation and conservation biogeography of Madagascar’s fork-marked lemurs (Cheirogaleidae: Phaner): Evidence of a new cryptic species? Glob. Ecol. Conserv. 2021, 29, e01738. [Google Scholar] [CrossRef]
- Lin, X.; Shih, C.; Hou, Y.; Shu, X.; Zhang, M.; Hu, J.; Jiang, J.; Xie, F. Climatic-niche evolution with key morphological innovations across clades within Scutiger boulengeri (Anura: Megophryidae). Ecol. Evol. 2021, 11, 10353–10368. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jacquemyn, H.; Chen, W.; Janssens, S.B.; He, X.; Yu, S.; Huang, Y. Niche evolution and historical biogeography of lady slipper orchids in North America and Eurasia. J. Biogeogr. 2021, 48, 2727–2741. [Google Scholar] [CrossRef]
- Broennimann, O.; Mráz, P.; Petitpierre, B.; Guisan, A.; Müller-Schärer, H. Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. J. Biogeogr. 2014, 41, 1126–1136. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, X.; Li, X.; Petitpierre, B.; Guisan, A. Residence time, expansion toward the equator in the invaded range and native range size matter to climatic niche shifts in non-native species. Glob. Ecol. Biogeogr. 2014, 23, 1094–1104. [Google Scholar] [CrossRef]
- Jänes, H.; Herkül, K.; Kotta, J. Environmental niche separation between native and non-native benthic invertebrate species: Case study of the northern Baltic Sea. Mar. Environ. Res. 2017, 131, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Louppe, V.; Leroy, B.; Herrel, A.; Veron, G. Current and future climatic regions favourable for a globally introduced wild carnivore, the raccoon Procyon lotor. Sci. Rep. 2019, 9, 9174. [Google Scholar] [CrossRef] [Green Version]
- Changjun, G.; Yanli, T.; Linshan, L.; Bo, W.; Yili, Z.; Haibin, Y.; Xilong, W.; Zhuoga, Y.; Binghua, Z.; Bohao, C. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 2021, 11, 12092–12113. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Olivera, L.; Minghetti, E.; Montemayor, S.I. Ecological niche modeling (ENM) of Leptoglossus clypealis a new potential global invader: Following in the footsteps of Leptoglossus occidentalis? Bull. Entomol. Res. 2021, 111, 289–300. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Liu, X.; Zhang, J. Potential range expansion and niche shift of the invasive Hyphantria cunea between native and invasive countries. Ecol. Entomol. 2021, 46, 910–925. [Google Scholar] [CrossRef]
- Zachariah Atwater, D.; Barney, J.N. Climatic niche shifts in 815 introduced plant species affect their predicted distributions. Glob. Ecol. Biogeogr. 2021, 30, 1671–1684. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Couvet, D.; Jiguet, F. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B Biol. Sci. 2008, 275, 2743–2748. [Google Scholar] [CrossRef] [Green Version]
- Reino, L.; Triviño, M.; Beja, P.; Araújo, M.B.; Figueira, R.; Segurado, P. Modelling landscape constraints on farmland bird species range shifts under climate change. Sci. Total Environ. 2018, 625, 1596–1605. [Google Scholar] [CrossRef]
- Jose, V.S.; Nameer, P.O. The expanding distribution of the Indian Peafowl (Pavo cristatus) as an indicator of changing climate in Kerala, southern India: A modelling study using MaxEnt. Ecol. Indic. 2020, 110, 105930. [Google Scholar] [CrossRef]
- Bladon, A.J.; Donald, P.F.; Collar, N.J.; Denge, J.; Dadacha, G.; Wondafrash, M.; Green, R.E. Climatic change and extinction risk of two globally threatened Ethiopian endemic bird species. PLoS ONE 2021, 16, e0249633. [Google Scholar] [CrossRef]
- Sierra-Morales, P.; Rojas-Soto, O.; Ríos-Muñoz, C.A.; Ochoa-Ochoa, L.M.; Flores-Rodríguez, P.; Almazán-Núñez, R.C. Climate change projections suggest severe decreases in the geographic ranges of bird species restricted to Mexican humid mountain forests. Glob. Ecol. Conserv. 2021, 30, e01794. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Sánchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Medley, K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Lauzeral, C.; Leprieur, F.; Beauchard, O.; Duron, Q.; Oberdorff, T.; Brosse, S. Identifying climatic niche shifts using coarse-grained occurrence data: A test with non-native freshwater fish. Glob. Ecol. Biogeogr. 2011, 20, 407–414. [Google Scholar] [CrossRef]
- Sutton, W.B.; Barrett, K.; Moody, A.T.; Loftin, C.S.; de Maynadier, P.G.; Nanjappa, P. Predicted changes in climatic niche and climate refugia of conservation priority salamander species in the Northeastern United States. Forests 2015, 6, 1–26. [Google Scholar] [CrossRef]
- Ralston, J.; DeLuca, W.V.; Feldman, R.E.; King, D.I. Realized climate niche breadth varies with population trend and distribution in North American birds. Glob. Ecol. Biogeogr. 2016, 25, 1173–1180. [Google Scholar] [CrossRef]
- Ralston, J.; DeLuca, W.V.; Feldman, R.E.; King, D.I. Population trends influence species ability to track climate change. Glob. Chang. Biol. 2017, 23, 1390–1399. [Google Scholar] [CrossRef]
- Sauer, J.R.; Pardieck, K.L.; Ziolkowski, D.J., Jr.; Smith, A.C.; Hudson, M.-A.R.; Rodriguez, V.; Berlanga, H.; Niven, D.K.; Link, W.A. The first 50 years of the North American breeding bird survey. Condor Ornithol. Appl. 2017, 119, 576–593. [Google Scholar] [CrossRef]
- Hutchinson, G.E. An Introduction to Population Ecology; Yale University Press: New Haven, CT, USA, 1978. [Google Scholar]
- Cheeseman, A.E.; Tanis, B.P.; Finck, E.J. Quantifying temporal variation in dietary niche to reveal drivers of past population declines. Funct. Ecol. 2021, 35, 930–941. [Google Scholar] [CrossRef]
- Sullivan, M.J.P.; Newson, S.E.; Pearce-Higgins, J.W. Using habitat-specific population trends to evaluate the consistency of the effect of species traits on bird population change. Biol. Conserv. 2015, 192, 343–352. [Google Scholar] [CrossRef]
- Rocchia, E.; Luppi, M.; Dondina, O.; Orioli, V.; Bani, L. Can the effect of species ecological traits on birds’ altitudinal changes differ between geographic areas? Acta Oecol. 2018, 92, 26–34. [Google Scholar] [CrossRef]
- Bowler, D.E.; Heldbjerg, H.; Fox, A.D.; de Jong, M.; Böhning-Gaese, K. Long-term declines of European insectivorous bird populations and potential causes. Conserv. Biol. 2019, 33, 1120–1130. [Google Scholar] [CrossRef]
- Kamp, J.; Frank, C.; Trautmann, S.; Busch, M.; Dröschmeister, R.; Flade, M.; Gerlach, B.; Karthäuser, J.; Kunz, F.; Mitschke, A.; et al. Population trends of common breeding birds in Germany 1990–2018. J. Ornithol. 2021, 162, 1–15. [Google Scholar] [CrossRef]
- Tirozzi, P.; Orioli, V.; Dondina, O.; Kataoka, L.; Bani, L. Species traits drive long-term population trends of common breeding birds in northern Italy. Animals 2021, 11, 3426. [Google Scholar] [CrossRef]
- Pigot, A.L.; Sheard, C.; Miller, E.T.; Bregman, T.P.; Freeman, B.G.; Roll, U.; Seddon, N.; Trisos, C.H.; Weeks, B.C.; Tobias, J.A. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat. Ecol. Evol. 2020, 4, 230–239. [Google Scholar] [CrossRef]
- Pelegrin, N.; Winemiller, K.O.; Vitt, L.J.; Fitzgerald, D.B.; Pianka, E.R. How do lizard niches conserve, diverge or converge? Further exploration of saurian evolutionary ecology. BMC Ecol. Evol. 2021, 21, 149. [Google Scholar] [CrossRef]
- Shaner, P.-J.L.; Chen, Y.-K.; Hsu, Y.-C. Niche–trait relationships at individual and population level in three co-occurring passerine species. Ecol. Evol. 2021, 11, 7378–7389. [Google Scholar] [CrossRef]
- Zurell, D.; Gallien, L.; Graham, C.H.; Zimmermann, N.E. Do long-distance migratory birds track their niche through seasons? J. Biogeogr. 2018, 45, 1459–1468. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Metzger, B.; Neuling, E.; Austad, M.; Galea, N.; Barbara, N.; Quillfeldt, P. Year-round spatial distribution and migration phenology of a rapidly declining trans-Saharan migrant—Evidence of winter movements and breeding site fidelity in European turtle doves. Behav. Ecol. Sociobiol. 2021, 75, 152. [Google Scholar] [CrossRef]
- ERSAF. Uso del Suolo in Regione Lombardia. I Dati DUSAF, Destinazione d’Uso dei Suoli Agricoli e Forestali. 2018. Available online: https://www.geoportale.regione.lombardia.it/ (accessed on 21 September 2021).
- ERSAF. Uso del Suolo in Regione Lombardia. I Dati DUSAF, Destinazione d’Uso dei Suoli Agricoli e Forestali. 1999. Available online: https://www.geoportale.regione.lombardia.it/ (accessed on 21 September 2021).
- Bani, L.; Massimino, D.; Orioli, V.; Bottoni, L.; Massa, R. Assessment of population trends of common breeding birds in Lombardy, Northern Italy, 1992–2007. Ethol. Ecol. Evol. 2009, 21, 27–44. [Google Scholar] [CrossRef]
- Blondel, J.; Ferry, C.; Frochot, B. Point counts with unlimited distance. Stud. Avian Biol. 1981, 6, 414–420. [Google Scholar]
- Fornasari, L.; Bani, L.; De Carli, E.; Massa, R. Optimum design in monitoring common birds and their habitat. Gibier Faune Sauvag. 1998, 15, 309–322. [Google Scholar]
- Dondina, O.; Orioli, V.; Tirozzi, P.; Bani, L. Long-term dynamic of nestedness in bird assemblages inhabiting fragmented landscapes. Landsc. Ecol. 2022. [Google Scholar] [CrossRef]
- Dondina, O.; Orioli, V.; D’Occhio, P.; Luppi, M.; Bani, L. How does forest species specialization affect the application of the island biogeography theory in fragmented landscapes? J. Biogeogr. 2017, 44, 1041–1052. [Google Scholar] [CrossRef]
- Bani, L.; Luppi, M.; Rocchia, E.; Dondina, O.; Orioli, V. Winners and losers: How the elevational range of breeding birds on Alps has varied over the past four decades due to climate and habitat changes. Ecol. Evol. 2019, 9, 1289–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, R.J.; Langslow, D.R. Estimating numbers of birds by point counts: How long should counts last? Bird Study 1984, 31, 195–202. [Google Scholar] [CrossRef]
- European Climate Assessment & Dataset, ECAD, Version 23.1e. Available online: https://surfobs.climate.copernicus.eu/dataaccess/access_eobs.php#datafiles (accessed on 26 October 2021).
- Lindström, Å.; Green, M.; Paulson, G.; Smith, H.G.; Devictor, V. Rapid changes in bird community composition at multiple temporal and spatial scales in response to recent climate change. Ecography 2013, 36, 313–322. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop; Release 10.7.1; Environmental Systems Research Institute: Redlands, CA, USA, 2019. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 October 2021).
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Peterson, A.T.; Campbell, L.P.; Soberón, J.; Ji, L.; Escobar, L.E. NicheA: Creating virtual species and ecological niches in multivariate environmental scenarios. Ecography 2016, 39, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Nunes, L.A.; Pearson, R.G. A null biogeographical test for assessing ecological niche evolution. J. Biogeogr. 2017, 44, 1331–1343. [Google Scholar] [CrossRef]
- Brown, J.L.; Carnaval, A.C. A tale of two niches: Methods, concepts, and evolution. Front. Biogeogr. 2019, 11, e44158. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broennimann, O.; Di Cola, V.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods. R Package Version 3.1. 2020. Available online: https://CRAN.R-project.org/package=ecospat (accessed on 10 October 2021).
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Kendall, M.G. A new measure of rank correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Koma, Z.; Grootes, M.W.; Meijer, C.W.; Nattino, F.; Seijmonsbergen, A.C.; Sierdsema, H.; Foppen, R.; Kissling, W.D. Niche separation of wetland birds revealed from airborne laser scanning. Ecography 2021, 44, 907–918. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, Q. Tolerance niche expansion and potential distribution prediction during Asian openbill bird range expansion. Ecol. Evol. 2021, 11, 5562–5574. [Google Scholar] [CrossRef]
- Kendall, M.G. The treatment of ties in rank problems. Biometrika 1945, 33, 239–251. [Google Scholar] [CrossRef]
- Storchová, L.; Hořák, D. Life-history characteristics of European birds. Glob. Ecol. Biogeogr. 2018, 27, 400–406. [Google Scholar] [CrossRef]
- Jiguet, F.; Gadot, A.-S.; Julliard, R.; Newson, S.E.; Couvet, D. Climate envelope, life history traits and the resilience of birds facing global change. Glob. Chang. Biol. 2007, 13, 1672–1684. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Nestedness in fragmented landscapes: A case study on birds, arboreal marsupials and lizards. J. Biogeogr. 2005, 32, 1737–1750. [Google Scholar] [CrossRef]
- Li, C.; Zhao, B.; Wang, Y. Nestedness of waterbird assemblages in the subsidence wetlands recently created by underground coal mining. Curr. Zool. 2018, 65, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julliard, R.; Clavel, J.; Devictor, V.; Jiguet, F.; Couvet, D. Spatial segregation of specialists and generalists in bird communities. Ecol. Lett. 2006, 9, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Pearman, P.B.; Lavergne, S.; Roquet, C.; Wüest, R.; Zimmermann, N.E.; Thuiller, W. Phylogenetic patterns of climatic, habitat and trophic niches in a European avian assemblage. Glob. Ecol. Biogeogr. 2014, 23, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef] [Green Version]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 28 November 2021).
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Gill, F.; Donsker, D.; Rasmussen, P. (Eds.) IOC World Bird List (v12.1); International Ornithologists Union: Baton Rouge, LA, USA, 2022. [Google Scholar] [CrossRef]
- Broennimann, O.; Guisan, A. Predicting current and future biological invasions: Both native and invaded ranges matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.J.; Beltrán, J.F.; Tejedo, M.; Nicieza, A.G.; Llusia, D.; Márquez, R.; Aragón, P. Niche models at inter- and intraspecific levels reveal hierarchical niche differentiation in midwife toads. Sci. Rep. 2020, 10, 10942. [Google Scholar] [CrossRef]
- Cuervo, P.F.; Flores, F.S.; Venzal, J.M.; Nava, S. Niche divergence among closely related taxa provides insight on evolutionary patterns of ticks. J. Biogeogr. 2021, 48, 2865–2876. [Google Scholar] [CrossRef]
- Peterson, A.T. Projected climate change effects on Rocky Mountain and Great Plains birds: Generalities of biodiversity consequences. Glob. Change Biol. 2003, 9, 647–655. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. WIREs Clim. Change 2014, 5, 317–335. [Google Scholar] [CrossRef] [Green Version]
- Tingley, M.W.; Monahan, W.B.; Beissinger, S.R.; Moritz, C. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 19637–19643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayleur, C.; Caplat, P.; Massimino, D.; Johnston, A.; Jonzén, N.; Smith, H.G.; Lindström, A. Swedish birds are tracking temperature but not rainfall: Evidence from a decade of abundance changes. Glob. Ecol. Biogeogr. 2015, 24, 859–872. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Tracking of climatic niche boundaries under recent climate change. J. Anim. Ecol. 2012, 81, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D.E.; Negro, M.; Caprio, E.; Rolando, A. Assessing the sensitivity of alpine birds to potential future changes in habitat and climate to inform management strategies. Biol. Conserv. 2013, 167, 127–135. [Google Scholar] [CrossRef]
- Pearce-Higgins, J.W.; Beale, C.M.; Oliver, T.H.; August, T.A.; Carroll, M.; Massimino, D.; Ockendon, N.; Savage, J.; Wheatley, C.J.; Ausden, M.A.; et al. A national-scale assessment of climate change impacts on species: Assessing the balance of risks and opportunities for multiple taxa. Biol. Conserv. 2017, 213, 124–134. [Google Scholar] [CrossRef]
- Scridel, D.; Brambilla, M.; Martin, K.; Lehikoinen, A.; Iemma, A.; Matteo, A.; Jähnig, S.; Caprio, E.; Bogliani, G.; Pedrini, P.; et al. A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. IBIS 2018, 160, 489–515. [Google Scholar] [CrossRef] [Green Version]
- Lehikoinen, A.; Brotons, L.; Calladine, J.; Campedelli, T.; Escandell, V.; Flousek, J.; Grueneberg, C.; Haas, F.; Harris, S.; Herrando, S.; et al. Declining population trends of European mountain birds. Glob. Chang. Biol. 2019, 25, 577–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambilla, M.; Gustin, M.; Cento, M.; Ilahiane, L.; Celada, C. Habitat, climate, topography and management differently affect occurrence in declining avian species: Implications for conservation in changing environments. Sci. Total Environ. 2020, 742, 140663. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; Piccardi, B.; Celona, F.; Guglini, M.; Galani, G.; Ravanelli, G.; Ciampitti, M.; Bazzoli, M.; Craveri, L.; Ballardini, P.; et al. Rapporto Sullo Stato delle Foreste in Lombardia 2017; Piccardi, B., Celona, F., Eds.; ERSAF: Milan, Italy, 2017; pp. 8–11. Available online: https://www.ersaf.lombardia.it/it/servizi-alle-filiere/foreste-legno/rapporto-stato-delle-foreste (accessed on 28 January 2022).
- Paniccia, C.; Di Febbraro, M.; Frate, L.; Sallustio, L.; Santopuoli, G.; Altea, T.; Posillico, M.; Marchetti, M.; Loy, A. Effect of imperfect detection on the estimation of niche overlap between two forest dormice. IForest 2018, 11, 482–490. [Google Scholar] [CrossRef]
- Newson, S.E.; Massimino, D.; Johnston, A.; Baillie, S.R.; Pearce-Higgins, J.W. Should we account for detectability in population trends? Bird Study 2013, 60, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Pulliam, H.R. Sources, sinks, and population regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation dynamics. Nature 1998, 396, 41–49. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Siriwardena, G.M. The effects of agricultural intensification on Skylarks (Alauda arvensis): Evidence from monitoring studies in Great Britain. Environ. Rev. 2000, 8, 95–113. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Vickery, J.A.; Gough, S. Spatial and temporal distribution of breeding Skylarks Alauda arvensis in relation to crop type in periods of population increase and decrease. Ardea 2000, 88, 61–73. [Google Scholar]
- Eraud, C.; Marie Boutin, J. Density and productivity of breeding Skylarks Alauda arvensis in relation to crop type on agricultural lands in Western France. Bird Study 2002, 49, 287–296. [Google Scholar] [CrossRef]
- Koleček, J.; Reif, J.; Weidinger, K. The abundance of a farmland specialist bird, the skylark, in three European regions with contrasting agricultural management. Agric. Ecosyst. Environ. 2015, 212, 30–37. [Google Scholar] [CrossRef]
- Schurr, F.M.; Pagel, J.; Cabral, J.S.; Groeneveld, J.; Bykova, O.; O’Hara, R.B.; Hartig, F.; Kissling, W.D.; Linder, H.P.; Midgley, G.F.; et al. How to understand species’ niches and range dynamics: A demographic research agenda for biogeography. J. Biogeogr. 2012, 39, 2146–2162. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Birds, body size and the threat of extinction. Philos. Trans. R. Soc. B Biol. Sci. 1995, 347, 205–212. [Google Scholar] [CrossRef]
- Henle, K.; Davies, K.; Kleyer, M.; Margules, C.; Settele, J. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Wang, Y.; Si, X.; Bennett, P.M.; Chen, C.; Zeng, D.; Zhao, Y.; Wu, Y.; Ding, P. Ecological correlates of extinction risk in Chinese birds. Ecography 2018, 41, 782–794. [Google Scholar] [CrossRef]
- Dardanelli, S.; Bellis, L.M. Nestedness structure of bird assemblages in a fragmented forest in central argentina: The role of selective extinction and colonization processes. Anim. Biodivers. Conserv. 2021, 44, 17–29. [Google Scholar] [CrossRef]
- Dondina, O.; Orioli, V.; Colli, L.; Luppi, M.; Bani, L. Ecological network design from occurrence data by simulating species perception of the landscape. Landsc. Ecol. 2018, 33, 275–287. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Saino, N.; Ambrosini, R.; Rubolini, D.; von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O.; Lehikoinen, A.; Lehikoinen, E.; Rainio, K.; et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B Biol. Sci. 2011, 278, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.M.; Laughlin, A.J.; Hall, R.J. The response of migratory populations to phenological change: A migratory flow network modelling approach. J. Anim. Ecol. 2016, 85, 648–659. [Google Scholar] [CrossRef]
- Newton, I. The Migration Ecology of Birds, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Jonzén, N.; Lindén, A.; Ergon, T.; Knudsen, E.; Vik, J.O.; Rubolini, D.; Piacentini, D.; Brinch, C.; Spina, F.; Karlsson, L.; et al. Rapid advance of spring arrival dates in long-distance migratory birds. Science 2006, 312, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Newson, S.E.; Moran, N.J.; Musgrove, A.J.; Pearce-Higgins, J.W.; Gillings, S.; Atkinson, P.W.; Miller, R.; Grantham, M.J.; Baillie, S.R. Long-term changes in the migration phenology of UK breeding birds detected by large-scale citizen science recording schemes. IBIS 2016, 158, 481–495. [Google Scholar] [CrossRef]
- Mayor, S.J.; Guralnick, R.P.; Tingley, M.W.; Otegui, J.; Withey, J.C.; Elmendorf, S.C.; Andrew, M.E.; Leyk, S.; Pearse, I.S.; Schneider, D.C. Increasing phenological asynchrony between spring green-up and arrival of migratory birds. Sci. Rep. 2017, 7, 1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, M.D.; Smith, K.W.; Evans, K.L.; Leech, D.; Pearce-Higgins, J.W.; Branston, C.J.; Briggs, K.; Clark, J.R.; du Feu, C.R.; Lewthwaite, K.; et al. Tritrophic phenological match–mismatch in space and time. Nat. Ecol. Evol. 2018, 2, 970–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangerter, A.B.; Heiser, E.R.; Carlisle, J.D.; Miller, R.A. Local weather explains annual variation in northern goshawk reproduction in the Northern Great Basin, USA. J. Raptor Res. 2021, 55, 471–484. [Google Scholar] [CrossRef]
- Wittwer, T.; O’Hara, R.B.; Caplat, P.; Hickler, T.; Smith, H.G. Long-term population dynamics of a migrant bird suggests interaction of climate change and competition with resident species. Oikos 2015, 124, 1151–1159. [Google Scholar] [CrossRef]
- Møller, A.P.; Rubolini, D.; Lehikoinen, E. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl. Acad. Sci. USA 2008, 105, 16195–16200. [Google Scholar] [CrossRef] [Green Version]
- Howard, C.; Stephens, P.A.; Pearce-Higgins, J.W.; Gregory, R.D.; Butchart, S.H.M.; Willis, S.G. Disentangling the relative roles of climate and land cover change in driving the long-term population trends of European migratory birds. Divers. Distrib. 2020, 26, 1442–1455. [Google Scholar] [CrossRef]
- Newton, I. The recent declines of farmland bird populations in Britain: An appraisal of causal factors and conservation actions. IBIS 2004, 146, 579–600. [Google Scholar] [CrossRef]
- Elton, C. Animal Ecology; Sedgwick and Jackson: London, UK, 1927. [Google Scholar]

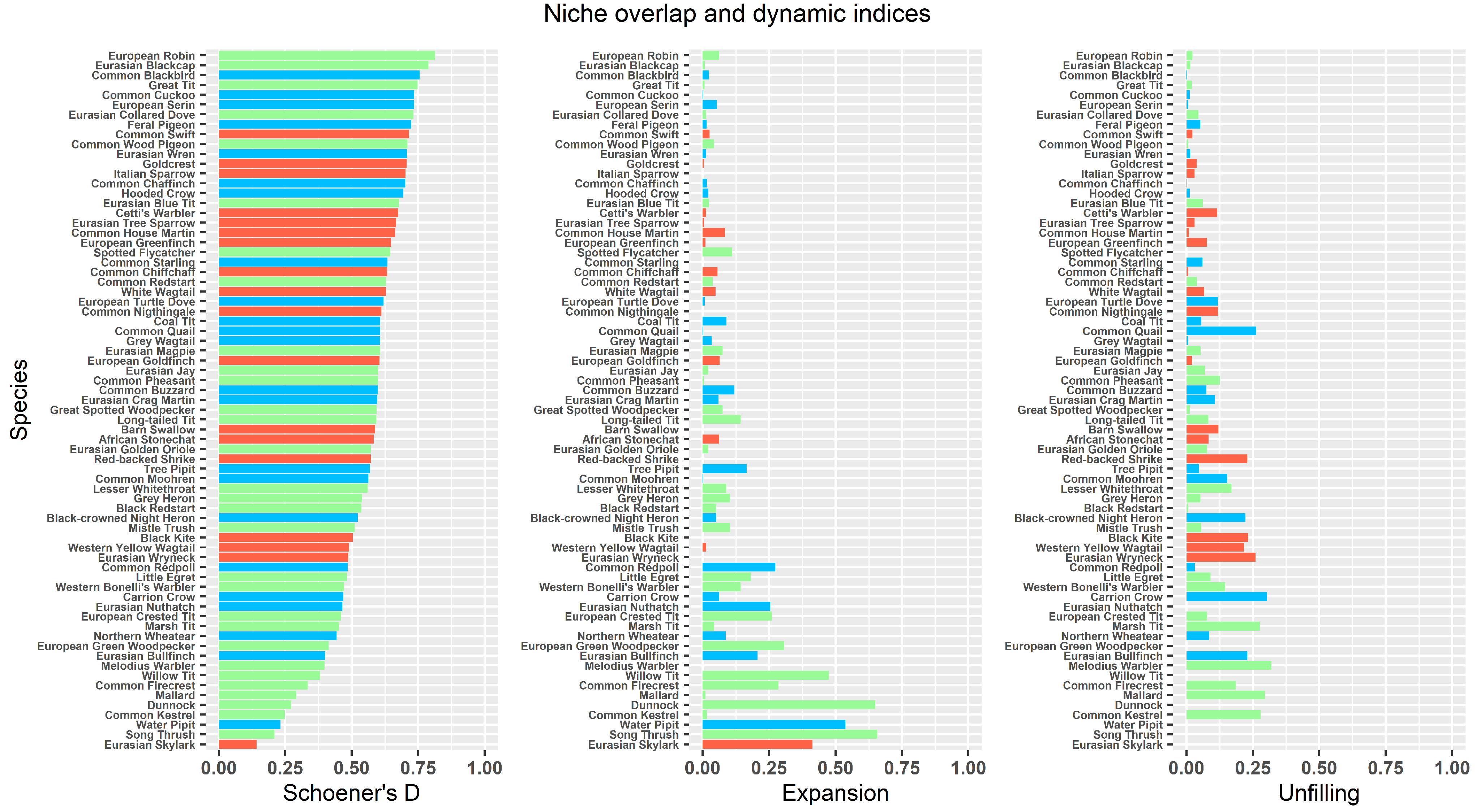


| Species | Schoener’s D | E (D) | E (C) | S (D) | S (C) |
|---|---|---|---|---|---|
| Black-crowned Night Heron (Nycticorax nycticorax) | 0.52 | 0.007 | 0.994 | 0.968 | 0.026 |
| Little Egret (Egretta garzetta) | 0.48 | 0.023 | 0.977 | 0.947 | 0.038 |
| Grey Heron (Ardea cinerea) | 0.54 | 0.003 | 1.000 | 0.950 | 0.035 |
| Mallard (Anas platyrhynchos) | 0.29 | 0.004 | 0.996 | 0.812 | 0.211 |
| Black Kite (Milvus migrans) | 0.50 | 0.172 | 0.840 | 0.965 | 0.042 |
| Common Buzzard (Buteo buteo) | 0.60 | 0.297 | 0.690 | 0.990 | 0.011 |
| Common Kestrel (Falcon tinnunculus) | 0.25 | 0.034 | 0.970 | 0.762 | 0.229 |
| Common Quail (Coturnix coturnix) | 0.61 | 0.399 | 0.627 | 0.995 | 0.012 |
| Common Pheasant (Phasianus colchicus) | 0.60 | 0.036 | 0.979 | 0.968 | 0.018 |
| Common Moohren (Gallinula chloropus) | 0.56 | 0.010 | 0.995 | 0.968 | 0.032 |
| Feral Pigeon (Columba livia domestica) | 0.72 | 0.033 | 0.966 | 0.993 | 0.010 |
| Common Wood Pigeon (Columba palumbus) | 0.71 | 0.435 | 0.591 | 0.998 | 0.009 |
| Eurasian Collared Dove(Streptopelia decaocto) | 0.73 | 0.119 | 0.864 | 0.991 | 0.008 |
| European Turtle Dove (Streptopelia turtur) | 0.62 | 0.033 | 0.984 | 0.962 | 0.028 |
| Common Cuckoo (Cuculus canorus) | 0.74 | 0.999 | <0.001 | 1.000 | 0.002 |
| Common Swift (Apus apus) | 0.71 | 0.933 | 0.061 | 0.995 | 0.010 |
| Eurasian Wryneck (Jinx torquilla) | 0.49 | 0.709 | 0.295 | 0.968 | 0.048 |
| European Green Woodpecker (Picus viridis) | 0.41 | 0.054 | 0.956 | 0.915 | 0.114 |
| Great Spotted Woodpecker (Dendrocopos major) | 0.59 | 0.444 | 0.589 | 0.961 | 0.032 |
| Eurasian Skylark (Alauda arvensis) | 0.14 | <0.001 | 1.000 | 0.836 | 0.179 |
| Eurasian Crag Martin (Ptyonoprogne rupestris) | 0.60 | 0.531 | 0.487 | 0.942 | 0.033 |
| Barn Swallow (Hirundo rustica) | 0.59 | <0.001 | 1.000 | 0.971 | 0.021 |
| Common House Martin (Delichon urbicum) | 0.66 | 0.454 | 0.535 | 0.996 | 0.018 |
| Tree Pipit (Anthus trivialis) | 0.57 | 0.842 | 0.168 | 0.976 | 0.015 |
| Water Pipit (Anthus spinoletta) | 0.23 | 0.181 | 0.821 | 0.905 | 0.101 |
| Western Yellow Wagtail (Motacilla flava) | 0.49 | <0.001 | 1.000 | 0.959 | 0.036 |
| Grey Wagtail (Motacilla cinerea) | 0.61 | 0.752 | 0.259 | 0.987 | 0.008 |
| White Wagtail (Motacilla alba) | 0.63 | 0.440 | 0.564 | 0.997 | 0.006 |
| Eurasian Wren (Troglodytes troglodytes) | 0.71 | 0.993 | 0.003 | 0.999 | 0.002 |
| Dunnock (Prunella modularis) | 0.27 | 0.029 | 0.982 | 0.884 | 0.101 |
| European Robin (Erithacus rubecula) | 0.81 | 1.000 | <0.001 | 1.000 | <0.001 |
| Common Nigthingale (Luscinia megarhynchos) | 0.61 | <0.001 | 1.000 | 0.974 | 0.031 |
| Black Redstart(Phoenicurus ochruros) | 0.54 | 0.514 | 0.506 | 0.968 | 0.027 |
| Common Redstart (Phoenicurus phoenicurus) | 0.63 | 0.063 | 0.934 | 0.987 | 0.012 |
| African Stonechat(Saxicola torquatus) | 0.58 | 0.483 | 0.524 | 0.957 | 0.028 |
| Northern Wheatear (Oenanthe oenanthe) | 0.44 | 0.782 | 0.213 | 0.886 | 0.112 |
| Common Blackbird (Turdus merula) | 0.76 | 0.998 | 0.002 | 0.997 | 0.002 |
| Song Thrush (Turdus philomelos) | 0.21 | 0.049 | 0.946 | 0.908 | 0.105 |
| Mistle Trush (Turdus viscivorus) | 0.51 | 0.926 | 0.074 | 0.911 | 0.082 |
| Cetti’s Warbler (Cettia cetti) | 0.68 | 0.812 | 0.200 | 0.988 | 0.012 |
| Melodius Warbler (Hippolais polyglotta) | 0.40 | 0.046 | 0.942 | 0.913 | 0.083 |
| Lesser Whitethroat (Curruca curruca) | 0.56 | 0.974 | 0.025 | 0.970 | 0.029 |
| Eurasian Blackcap (Sylvia atricapilla) | 0.79 | 1.000 | <0.001 | 1.000 | <0.001 |
| Western Bonelli’s Warbler (Phylloscopus bonelli) | 0.47 | 0.253 | 0.754 | 0.881 | 0.118 |
| Common Chiffchaff (Phylloscopus collybita) | 0.63 | 0.410 | 0.595 | 0.988 | 0.019 |
| Goldcrest (Regulus regulus) | 0.71 | 0.979 | 0.028 | 0.998 | 0.007 |
| Common Firecrest (Regulus ignicapilla) | 0.33 | 0.190 | 0.833 | 0.832 | 0.175 |
| Spotted Flycatcher (Muscicapa striata) | 0.65 | 0.102 | 0.892 | 0.988 | 0.014 |
| Long-tailed Tit (Aegithalos caudatus) | 0.59 | 0.058 | 0.956 | 0.982 | 0.013 |
| Marsh Tit (Poecile palustris) | 0.45 | 0.009 | 0.994 | 0.955 | 0.041 |
| Willow Tit (Poecile montanus) | 0.38 | 0.207 | 0.738 | 0.963 | 0.057 |
| European Crested Tit (Lophophanes cristatus) | 0.46 | 0.526 | 0.499 | 0.873 | 0.168 |
| Coal Tit (Periparus ater) | 0.61 | 0.231 | 0.784 | 0.945 | 0.046 |
| Eurasian Blue Tit (Cyanistes caeruleus) | 0.68 | 0.092 | 0.890 | 0.995 | 0.004 |
| Great Tit (Parus major) | 0.75 | 0.415 | 0.536 | 0.995 | 0.003 |
| Eurasian Nuthatch (Sitta europea) | 0.46 | 0.099 | 0.909 | 0.938 | 0.048 |
| Eurasian Golden Oriole (Oriolus oriolus) | 0.57 | 0.055 | 0.937 | 0.970 | 0.018 |
| Red-backed Shrike (Lanius collurio) | 0.57 | 0.063 | 0.939 | 0.993 | 0.014 |
| Eurasian Jay (Garrulus glandarius) | 0.60 | 0.036 | 0.968 | 0.943 | 0.050 |
| Eurasian Magpie(Pica pica) | 0.61 | 0.003 | 0.999 | 0.974 | 0.018 |
| Carrion Crow (Corvus corone) | 0.47 | 0.582 | 0.425 | 0.958 | 0.047 |
| Hooded Crow (Corvus cornix) | 0.69 | 0.402 | 0.644 | 0.994 | 0.010 |
| Common Starling (Sturnus vulgaris) | 0.63 | <0.001 | 1.000 | 0.993 | 0.020 |
| Italian Sparrow (Passer italiae) | 0.70 | 0.061 | 0.926 | 0.992 | 0.009 |
| Eurasian Tree Sparrow (Passer montanus) | 0.67 | 0.002 | 1.000 | 0.988 | 0.009 |
| Common Chaffinch (Fringilla coelebs) | 0.70 | 0.931 | 0.069 | 0.999 | 0.002 |
| European Serin (Serinus serinus) | 0.73 | 0.313 | 0.691 | 0.991 | 0.010 |
| European Greenfinch (Chloris chloris) | 0.65 | 0.104 | 0.893 | 0.994 | 0.007 |
| European Goldfinch (Carduelis carduelis) | 0.60 | 0.004 | 0.996 | 0.992 | 0.016 |
| Common Redpoll (Acanthis flammea) | 0.48 | 0.613 | 0.374 | 0.979 | 0.021 |
| Eurasian Bullfinch (Pyrrhula pyrrhula) | 0.40 | 0.035 | 0.961 | 0.943 | 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirozzi, P.; Orioli, V.; Dondina, O.; Bani, L. New Evidence on the Linkage of Population Trends and Species Traits to Long-Term Niche Changes. Birds 2022, 3, 149-171. https://doi.org/10.3390/birds3010011
Tirozzi P, Orioli V, Dondina O, Bani L. New Evidence on the Linkage of Population Trends and Species Traits to Long-Term Niche Changes. Birds. 2022; 3(1):149-171. https://doi.org/10.3390/birds3010011
Chicago/Turabian StyleTirozzi, Pietro, Valerio Orioli, Olivia Dondina, and Luciano Bani. 2022. "New Evidence on the Linkage of Population Trends and Species Traits to Long-Term Niche Changes" Birds 3, no. 1: 149-171. https://doi.org/10.3390/birds3010011
APA StyleTirozzi, P., Orioli, V., Dondina, O., & Bani, L. (2022). New Evidence on the Linkage of Population Trends and Species Traits to Long-Term Niche Changes. Birds, 3(1), 149-171. https://doi.org/10.3390/birds3010011







