Using Acoustic Data Repositories to Study Vocal Responses to Playback in a Neotropical Songbird
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
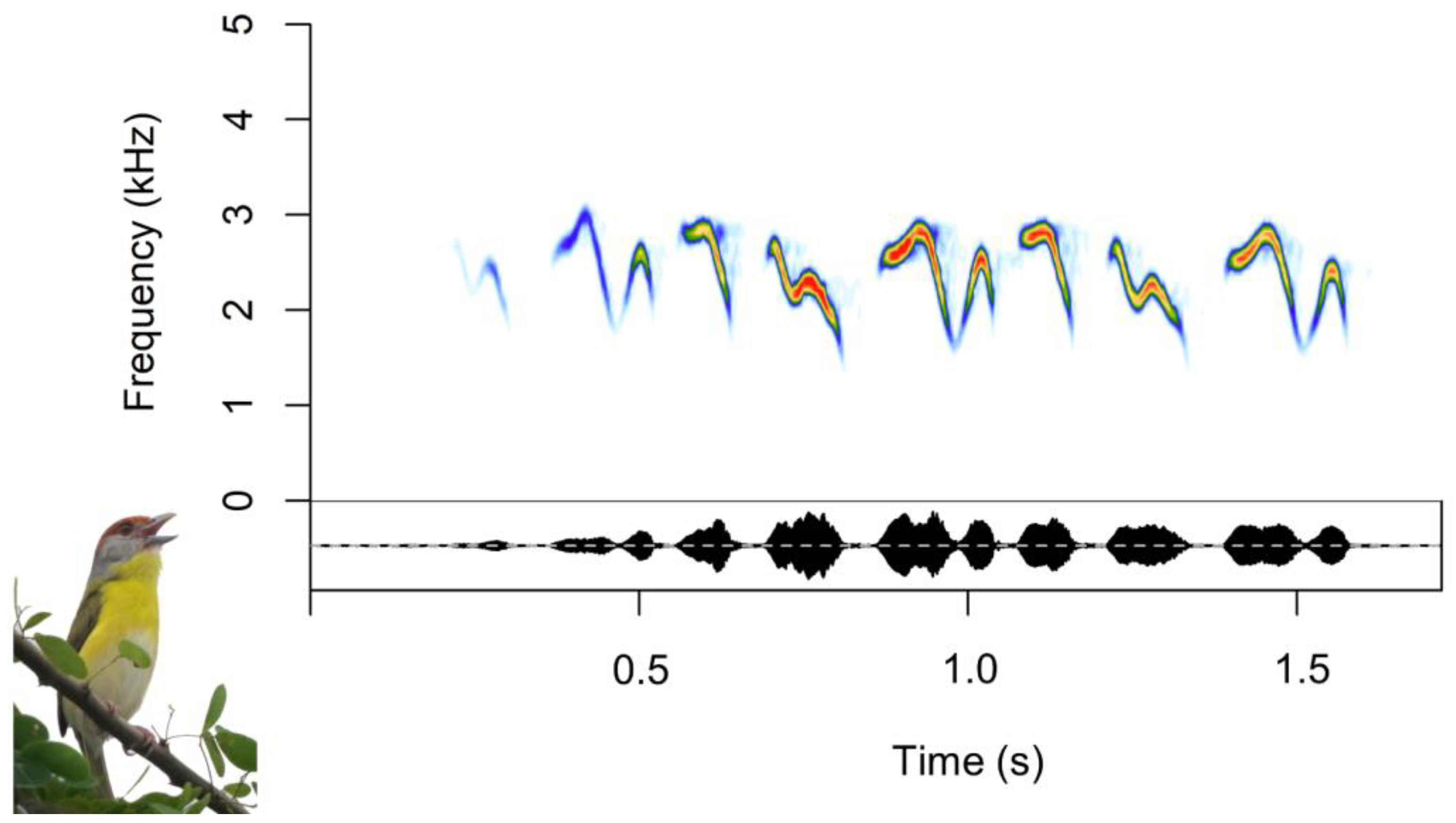
2.1. Study Species
2.2. Song Recordings
2.3. Acoustic Analyses
2.4. Statistical Analyses
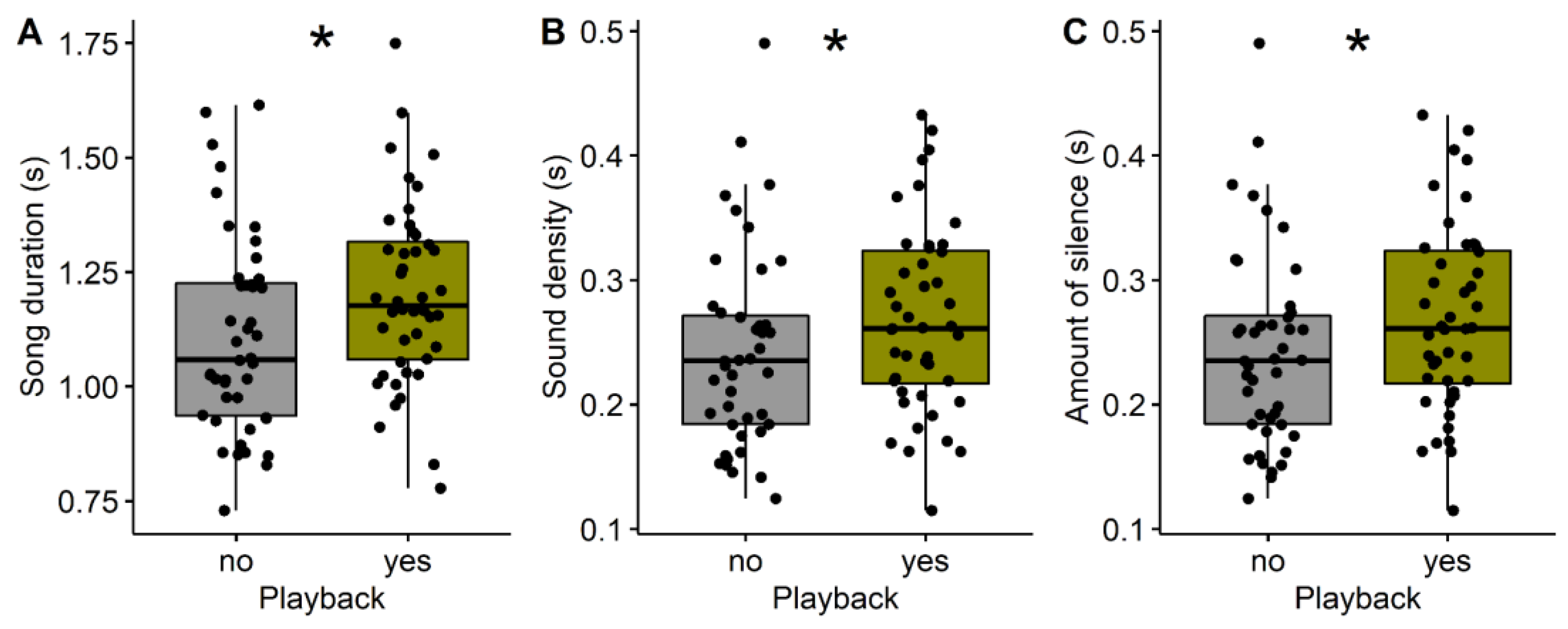
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Searcy, W.A.; Nowicki, S. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems; Princeton University Press: Princeton, NJ, USA, 2005; ISBN 9780691070940. [Google Scholar]
- Wilkins, M.R.; Seddon, N.; Safran, R.J. Evolutionary Divergence in Acoustic Signals: Causes and Consequences. Trends Ecol. Evol. 2013, 28, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication, Second Edition; Sinauer Associates: Sunderland, MA, USA, 2011. [Google Scholar]
- Hogstad, O. It Is Expensive to Be Dominant. Auk 1987, 104, 333–336. [Google Scholar] [CrossRef]
- Bailey, W.J.; Withers, P.C.; Endersby, M.; Gaull, K. The Energetic Costs of Calling the Bushcricket Requena verticalis (Orthoptera: Tettigoniidae: Listroscelidinae). J. Exp. Biol. 1993, 178, 21–37. [Google Scholar] [CrossRef]
- Bretman, A.; Gage, M.J.G.; Chapman, T. Quick-Change Artists: Male Plastic Behavioural Responses to Rivals. Trends Ecol. Evol. 2011, 26, 467–473. [Google Scholar] [CrossRef]
- Kasumovic, M.M. The Multidimensional Consequences of the Juvenile Environment: Towards an Integrative View of the Adult Phenotype. Anim. Behav. 2013, 85, 1049–1059. [Google Scholar] [CrossRef]
- Narango, D.L.; Rodewald, A.D. Urban-Associated Drivers of Song Variation along a Rural-Urban Gradient. Behav. Ecol. 2016, 27, 608–616. [Google Scholar] [CrossRef]
- Gentry, K.E.; Derryberry, E.P.; Danner, R.M.; Danner, J.E.; Luther, D.A. Immediate Signaling Flexibility in Response to Experimental Noise in Urban, but Not Rural, White-crowned Sparrows. Ecosphere 2017, 8, e01916. [Google Scholar] [CrossRef]
- Jablonszky, M.; Canal, D.; Hegyi, G.; Krenhardt, K.; Laczi, M.; Markó, G.; Nagy, G.; Rosivall, B.; Szász, E.; Zsebők, S.; et al. Individual Differences in Song Plasticity in Response to Social Stimuli and Singing Position. Ecol. Evol. 2022, 12, e8883. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, C.K.; Slater, P.J.B. Bird Song: Biological Themes and Variations, 2nd ed.; Cambridge University Press: New York, NY, USA, 2008; ISBN 9780521872423. [Google Scholar]
- Brumm, H.; Slater, P.J.B. Ambient Noise, Motor Fatigue, and Serial Redundancy in Chaffinch Song. Behav. Ecol. Sociobiol. 2006, 60, 475–481. [Google Scholar] [CrossRef]
- Wiley, R.H.; Richards, D.G. Sound Transmission and Signal Detection. In Acoustic Communication in Birds; Kroodsma, D.E., Miller, E.H., Eds.; Academic Press: New York, USA, 1982; pp. 131–181. [Google Scholar]
- Dabelsteen, T. Public, Private or Anonymous? Facilitating and Countering Eavesdropping. In Animal Communication Networks; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Greene, E.; Meagher, T. Red Squirrels, Tamiasciurus hudsonicus, Produce Predator-Class Specific Alarm Calls. Anim. Behav. 1998, 55, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Oberweger, K.; Goller, F. The Metabolic Cost of Birdsong Production. J. Exp. Biol. 2001, 204, 3379–3388. [Google Scholar] [CrossRef]
- Cuthill, I.C.; Macdonald, W.A. Experimental Manipulation of the Dawn and Dusk Chorus in the Blackbird Turdus merula. Behav. Ecol. Sociobiol. 1990, 26, 209–216. [Google Scholar] [CrossRef]
- Nelson, D.A.; Poesel, A. Song Length Variation Serves Multiple Functions in the White-Crowned Sparrow. Behav. Ecol. Sociobiol. 2011, 65, 1103–1111. [Google Scholar] [CrossRef]
- Gil, D.; Slater, P.J.B.; Graves, J.A. Extra-Pair Paternity and Song Characteristics in the Willow Warbler Phylloscopus trochilus. J. Avian Biol. 2007, 38, 291–297. [Google Scholar] [CrossRef]
- Morton, E.S. On the Occurrence and Significance of Motivation-Structural Rules in Some Bird and Mammal Sounds. Am. Nat. 1977, 111, 855–869. [Google Scholar] [CrossRef]
- Ryan, M.J.; Brenowitz, E.A. The Role of Body Size, Phylogeny, and Ambient Noise in the Evolution of Bird Song. Am. Nat. 1985, 126, 87–100. [Google Scholar] [CrossRef]
- Wallschläger, D. Correlation of Song Frequency and Body Weight in Passerine Birds. Experientia 1980, 36, 412. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Mamede, A.T.; Atwell, J.W.; Mota, P.G.; Ketterson, E.D.; Price, T.D. Song Frequency Does Not Reflect Differences in Body Size among Males in Two Oscine Species. Ethology 2008, 114, 1084–1093. [Google Scholar] [CrossRef]
- Forstmeier, W.; Burger, C.; Temnow, K.; Derégnaucourt, S. The Genetic Basis of Zebra Finch Vocalizations. Evolution 2009, 63, 2114–2130. [Google Scholar] [CrossRef]
- Galeotti, P.; Saino, N.; Sacchi, R.; Møller, A.P. Song Correlates with Social Context, Testosterone and Body Condition in Male Barn Swallows. Anim. Behav. 1997, 53, 687–700. [Google Scholar] [CrossRef]
- Hardouin, L.A.; Reby, D.; Bavoux, C.; Burneleau, G.; Bretagnolle, V. Communication of Male Quality in Owl Hoots. Am. Nat. 2007, 169, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Price, J.J.; Earnshaw, S.M.; Webster, M.S. Montezuma Oropendolas Modify a Component of Song Constrained by Body Size during Vocal Contests. Anim. Behav. 2006, 71, 799–807. [Google Scholar] [CrossRef]
- Watson, D.M.; Znidersic, E.; Craig, M.D. Ethical Birding Call Playback and Conservation. Conserv. Biol. 2019, 33, 469–471. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Aycrigg, J.L.; Barry, J.H.; Bonney, R.E.; Bruns, N.; Cooper, C.B.; Damoulas, T.; Dhondt, A.A.; Dietterich, T.; Farnsworth, A.; et al. The eBird Enterprise: An Integrated Approach to Development and Application of Citizen Science. Biol. Conserv. 2014, 169, 31–40. [Google Scholar] [CrossRef]
- Jäckel, D.; Mortega, K.G.; Darwin, S.; Brockmeyer, U.; Sturm, U.; Lasseck, M.; Moczek, N.; Lehmann, G.U.C.; Voigt-Heucke, S.L. Community Engagement and Data Quality: Best Practices and Lessons Learned from a Citizen Science Project on Birdsong. J. Ornithol. 2023, 164, 233–244. [Google Scholar] [CrossRef]
- Searfoss, A.M.; Liu, W.; Creanza, N. Geographically Well-Distributed Citizen Science Data Reveals Range-Wide Variation in the Chipping Sparrow’s Simple Song. Anim. Behav. 2020, 161, 63–76. [Google Scholar] [CrossRef]
- Lewanzik, D.; Straka, T.M.; Lorenz, J.; Marggraf, L.; Voigt-Heucke, S.; Schumann, A.; Brandt, M.; Voigt, C.C. Evaluating the Potential of Urban Areas for Bat Conservation with Citizen Science Data. Environ. Pollut. 2022, 297, 118785. [Google Scholar] [CrossRef]
- Odom, K.J.; Benedict, L. A Call to Document Female Bird Songs: Applications for Diverse Fields. Auk 2018, 135, 314–325. [Google Scholar] [CrossRef]
- Mikula, P.; Valcu, M.; Brumm, H.; Bulla, M.; Forstmeier, W.; Petrusková, T.; Kempenaers, B.; Albrecht, T. A Global Analysis of Song Frequency in Passerines Provides No Support for the Acoustic Adaptation Hypothesis but Suggests a Role for Sexual Selection. Ecol. Lett. 2021, 24, 477–486. [Google Scholar] [CrossRef]
- Kroodsma, D.E.; Byers, B.E.; Goodale, E.; Johnson, S.; Liu, W.-C. Pseudoreplication in Playback Experiments, Revisited a Decade Later. Anim. Behav. 2001, 61, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Chartier, S.L.; Ramsay, S.M.; Otter, K.A. Within-Population Song Evolution in White-Throated Sparrows (Zonotrichia albicollis). Behaviour 2022, 159, 1421–1445. [Google Scholar] [CrossRef]
- Searcy, W.A.; Beecher, M.D. Song as an Aggressive Signal in Songbirds. Anim. Behav. 2009, 78, 1281–1292. [Google Scholar] [CrossRef]
- Fernández-Gómez, R.A.; Morales-Mávil, J.E.; Hernández-Salazar, L.T.; Sosa-López, J.R. Asymmetric Behavioural Responses to Divergent Vocal Signals in Allopatric Neotropical Sparrows. Anim. Behav. 2021, 174, 41–50. [Google Scholar] [CrossRef]
- Dargis, L.; Benedict, L.; Najar, N.A. Female Bird Song Rates Do Not Covary with Population Density in a North American Species. Ethology 2021, 127, 1042–1052. [Google Scholar] [CrossRef]
- Sosa-López, J.R.; Martínez Gómez, J.E.; Mennill, D.J. Divergence in Mating Signals Correlates with Genetic Distance and Behavioural Responses to Playback. J. Evol. Biol. 2016, 29, 306–318. [Google Scholar] [CrossRef]
- Diniz, P.; Duca, C. Anthropogenic Noise, Song, and Territorial Aggression in Southern House Wrens. J. Avian Biol. 2021, 52, 1–14. [Google Scholar] [CrossRef]
- Hyman, J. Seasonal Variation in Response to Neighbors and Strangers by a Territorial Songbird. Ethology 2005, 111, 951–961. [Google Scholar] [CrossRef]
- Tubaro, P.L.; Segura, E.T. Geographic Ecological and Subspecific Variation in the Song of the Rufous-Browed Peppershrike (Cyclarhis gujanensis). Condor 1995, 97, 792–803. [Google Scholar] [CrossRef]
- DuBois, A.L.; Nowicki, S.; Searcy, W.A. Swamp Sparrows Modulate Vocal Performance in an Aggressive Context. Biol. Lett. 2009, 5, 163–165. [Google Scholar] [CrossRef]
- Brewer, D.; Bonan, A.; de Juana, E. Rufous-Browed Peppershrike (Cyclarhis gujanensis). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Tejeda-Cruz, C.; Sutherland, W.J. Bird Responses to Shade Coffee Production. Anim. Conserv. 2004, 7, 169–179. [Google Scholar] [CrossRef]
- Harrison, N.M.; Whitehouse, M.J.; Madureira, L.A.S.P. Observations of the Under-Described Avifauna of the Mostardas Peninsula, Rio Grande Do Sul, Brazil. Check List 2013, 9, 391–399. [Google Scholar] [CrossRef]
- K. Lisa Yang Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software, Version 1.6. [Computer Software]. The Cornell Lab of Ornithology: Ithaca, NY, USA, 2022. Available online: http://www.birds.cornell.edu/raven(accessed on 24 January 2021).
- Zimmerling, J.R.; Ankney, C.D. A Technique That Increases Detectability of Passerine Species during Point Counts. J. Field Ornithol. 2000, 71, 638–649. [Google Scholar] [CrossRef]
- Anikin, A. Soundgen: An Open-Source Tool for Synthesizing Nonverbal Vocalizations. Behav. Res. Methods 2019, 51, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Araya-Salas, M.; Smith-Vidaurre, G. WarbleR: An r Package to Streamline Analysis of Animal Acoustic Signals. Methods Ecol. Evol. 2017, 8, 184–191. [Google Scholar] [CrossRef]
- Diniz, P.; Silva, E.F., Jr.; Webster, M.S.; Macedo, R.H. Duetting Behavior in a Neotropical Ovenbird: Sexual and Seasonal Variation and Adaptive Signaling Functions. J. Avian Biol. 2018, 49, jav-01637. [Google Scholar] [CrossRef]
- Tierney, A.T.; Russo, F.A.; Patel, A.D. The Motor Origins of Human and Avian Song Structure. Proc. Natl. Acad. Sci. USA 2011, 108, 15510–15515. [Google Scholar] [CrossRef]
- Zollinger, S.A.; Podos, J.; Nemeth, E.; Goller, F.; Brumm, H. On the Relationship between, and Measurement of, Amplitude and Frequency in Birdsong. Anim. Behav. 2012, 84, e1–e9. [Google Scholar] [CrossRef]
- Silva, E.F., Jr.; Diniz, P.; Macedo, R.H. Song Varies with Latitude, Climate, and Species Richness in a Neotropical Bird. Behav. Ecol. 2022, 33, 87–100. [Google Scholar] [CrossRef]
- Charif, R.; Strickman, L.; Waack, A. Raven Pro 1.4 User’s Manual. Revision 11; Cornell Lab of Ornithology: Ithaca, NY, USA, 2010. [Google Scholar]
- Palacios, M.G.; Tubaro, P.L. Does Beak Size Affect Acoustic Frequencies in Woodcreepers? Condor 2000, 102, 553–560. [Google Scholar] [CrossRef]
- Perrins, C.M. The timing of birds’ breeding seasons. Ibis 2008, 112, 242–255. [Google Scholar] [CrossRef]
- Hau, M. Timing of Breeding in Variable Environments: Tropical Birds as Model Systems. Horm. Behav. 2001, 40, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-7; 2020. Available online: https://github.com/vegandevs/vegan (accessed on 29 December 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Galeotti, P.; Saino, N.; Perani, E.; Sacchi, R.; Møller, A.R. Age-Related Song Variation in Male Barn Swallows. Ital. J. Zool. 2001, 68, 305–310. [Google Scholar] [CrossRef]
- Linhart, P.; Slabbekoorn, H.; Fuchs, R. The Communicative Significance of Song Frequency and Song Length in Territorial Chiffchaffs. Behav. Ecol. 2012, 23, 1338–1347. [Google Scholar] [CrossRef]
- Osiejuk, T.S.; Jakubowska, A. Song Duration Mediates Responses of Territory Owner in a Songbird Species with a Small Song Repertoire. Acta Ethol. 2017, 20, 137–145. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; van Overveld, T.; Careau, V.; Matthysen, E.; Adriaensen, F.; Slabbekoorn, H. Personality-Dependent Response to Field Playback in Great Tits: Slow Explorers Can Be Strong Responders. Anim. Behav. 2014, 90, 65–71. [Google Scholar] [CrossRef]
- Riebel, K.; Slater, P.J.B. Testing the Flexibility of Song Type Bout Duration in the Chaffinch, Fringilla coelebs. Anim. Behav. 2000, 59, 1135–1142. [Google Scholar] [CrossRef]
- Acero-Murcia, A.C.; Raposo do Amaral, F.; de Barros, F.C.; da Silva Ribeiro, T.; Miyaki, C.Y.; Maldonado-Coelho, M. Ecological and Evolutionary Drivers of Geographic Variation in Songs of a Neotropical Suboscine Bird: The Drab-Breasted Bamboo Tyrant (Hemitriccus diops, Rhynchocyclidae). Ornithology 2021, 138, ukab003. [Google Scholar] [CrossRef]
- Brindley, E.L. Response of European Robins to Playback of Song: Neighbour Recognition and Overlapping. Anim. Behav. 1991, 41, 503–512. [Google Scholar] [CrossRef]
- Nielsen, B.M.B.; Vehrencamp, S.L. Responses of Song Sparrows to Song-Type Matching via Interactive Playback. Behav. Ecol. Sociobiol. 1995, 37, 109–117. [Google Scholar] [CrossRef]
- Ríos-Chelén, A.A.; Garcia, C.M. Responses of a Sub-Oscine Bird during Playback: Effects of Different Song Variants and Breeding Period. Behav. Process. 2007, 74, 319–325. [Google Scholar] [CrossRef]
- Balsby, T.J.S.; Dabelsteen, T. The Meaning of Song Repertoire Size and Song Length to Male Whitethroats Sylvia communis. Behav. Process. 2001, 56, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ritschard, M.; van Oers, K.; Naguib, M.; Brumm, H. Song Amplitude of Rival Males Modulates the Territorial Behaviour of Great Tits During the Fertile Period of Their Mates. Ethology 2012, 118, 197–202. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Beauchamp, G.; Jiang, Z. Flock Size and Human Disturbance Affect Vigilance of Endangered Red-Crowned Cranes (Grus japonensis). Biol. Conserv. 2011, 144, 101–105. [Google Scholar] [CrossRef]
- Podos, J.; Warren, P.S. The Evolution of Geographic Variation in Birdsong. Adv. Study Behav. 2007, 37, 403–458. [Google Scholar] [CrossRef]
- Wright, T.F.; Dahlin, C.R. Vocal Dialects in Parrots: Patterns and Processes of Cultural Evolution. Emu-Austral Ornithol. 2018, 118, 50–66. [Google Scholar] [CrossRef]
- Schubert, S.C.; Manica, L.T.; Guaraldo, A.D.C. Revealing the Potential of a Huge Citizen-Science Platform to Study Bird Migration. Emu-Austral Ornithol. 2019, 119, 364–373. [Google Scholar] [CrossRef]
- Zulian, V.; Miller, D.A.W.; Ferraz, G. Integrating Citizen-Science and Planned-Survey Data Improves Species Distribution Estimates. Divers. Distrib. 2021, 27, 2498–2509. [Google Scholar] [CrossRef]
- Lima, A.M.X.; Roper, J.J. The Use of Playbacks Can Influence Encounters with Birds: An Experiment. Rev. Bras. Ornitol. 2009, 17, 37–40. [Google Scholar]
- Mentesana, L.; Adreani, N.M. Acute Aggressive Behavior Perturbates the Oxidative Status of a Wild Bird Independently of Testosterone and Progesterone. Horm. Behav. 2021, 128, 104913. [Google Scholar] [CrossRef]
- Harris, J.B.C.; Haskell, D.G. Simulated Birdwatchers’ Playback Affects the Behavior of Two Tropical Birds. PLoS ONE 2013, 8, e77902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landys, M.M.; Goymann, W.; Schwabl, I.; Trapschuh, M.; Slagsvold, T. Impact of Season and Social Challenge on Testosterone and Corticosterone Levels in a Year-Round Territorial Bird. Horm. Behav. 2010, 58, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, M.; Grade, A.M.; Warren, P.S. Is “Pishing” Tantamount to Mobbing? Black-Capped Chickadees Respond Similarly to Human Pishing and Conspecific Mobbing Calls in Rural and Suburban Forests. Northeast. Nat. 2019, 26, 580. [Google Scholar] [CrossRef]

| Spontaneous Songs | Post-Playback Songs | tdf | p | |
|---|---|---|---|---|
| Rate (songs/min) | 11.83 ± 3.73 | 11.48 ± 2.10 | 0.3518 | 0.73 |
| Duration (s) | 1.11 ± 0.22 | 1.20 ± 0.20 | –2.2043 | 0.03 |
| Median frequency (Hz) | 2513 ± 177 | 2478 ± 208 | 0.9843 | 0.33 * |
| Minimum frequency (Hz) | 1352 ± 141 | 1363 ± 203 | –0.1134 | 0.91 * |
| Maximum frequency (Hz) | 2703 ± 249 | 2701 ± 295 | 0.0843 | 0.93 * |
| Bandwidth (Hz) | 1336 ± 219 | 1366 ± 234 | –0.6834 | 0.50 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, P.O.; Guimarães, L.C.; Oliveira, R.R.; Almeida, F.; Diniz, P. Using Acoustic Data Repositories to Study Vocal Responses to Playback in a Neotropical Songbird. Birds 2023, 4, 61-72. https://doi.org/10.3390/birds4010005
Guimarães PO, Guimarães LC, Oliveira RR, Almeida F, Diniz P. Using Acoustic Data Repositories to Study Vocal Responses to Playback in a Neotropical Songbird. Birds. 2023; 4(1):61-72. https://doi.org/10.3390/birds4010005
Chicago/Turabian StyleGuimarães, Pietra Oliveira, Letícia Campos Guimarães, Renato Rodrigues Oliveira, Fernando Almeida, and Pedro Diniz. 2023. "Using Acoustic Data Repositories to Study Vocal Responses to Playback in a Neotropical Songbird" Birds 4, no. 1: 61-72. https://doi.org/10.3390/birds4010005
APA StyleGuimarães, P. O., Guimarães, L. C., Oliveira, R. R., Almeida, F., & Diniz, P. (2023). Using Acoustic Data Repositories to Study Vocal Responses to Playback in a Neotropical Songbird. Birds, 4(1), 61-72. https://doi.org/10.3390/birds4010005





