Simple Summary
This study investigates the karyotypic structure and microsatellite distribution in three Anatidae species: Amazonetta brasiliensis (Brazilian Teal), Coscoroba coscoroba (Coscoroba Swan), and Dendrocygna viduata (White-faced Whistling Duck). The research aims to understand the role of repetitive DNA sequences in the chromosomal evolution and differentiation of Anseriformes. Despite having a similar macrochromosome morphology, the species show significant variation in diploid numbers (2n = 78 for White-faced Whistling Duck, 2n = 80 for Brazilian Teal, and 2n = 98 for Coscoroba Swan) and in microsatellite sequences distribution. For example, Brazilian Teal and Coscoroba Swan showed extensive accumulation of microsatellites in both their autosomal and sex chromosomes (Z and W), while White-faced Whistling Duck showed few signs of hybridization, without any microsatellites on the sex chromosomes. In view of these differences, the study suggests that transposable elements may be involved in the reorganization of these sequences, contributing to the evolutionary dynamics of chromosome structure. Furthermore, the distinct patterns of microsatellite distribution among the species, particularly between Brazilian Teal and Coscoroba Swan, reflect their closer phylogenetic relationship, compared to White-faced Whistling Duck, which belongs to the more distantly related subfamily Dendrocygninae. The findings of this research provide a better understanding of the genetic diversity and chromosomal evolution within Anseriformes and may serve as a tool for future studies on avian chromosomal evolution.
Abstract
Anseriformes represent a basal order in the phylogeny of neognath birds and are of particular interest in cytogenetic research due to their distinctive chromosomal features. However, aspects of their chromosomal evolution, such as the distribution and organization of microsatellite sequences, remain poorly understood. Given the role of these dynamic repetitive sequences in chromosome organization, differentiation, and evolution, we analyzed microsatellite distribution in three Anatidae species, each representing a different subfamily: Amazonetta brasiliensis-Brazilian Teal (Anatinae), Coscoroba coscoroba-Coscoroba Swan (Anserinae), and Dendrocygna viduata-White-faced Whistling Duck (Dendrocygninae). This is the first karyotypic description for White-faced Whistling Duck (2n = 78) and Brazilian Teal (2n = 80), whereas Coscoroba Swan, previously analyzed, exhibits a notably high diploid number (2n = 98). Despite sharing a similar macrochromosome morphology, the three showed differences in diploid numbers and microsatellite distribution. Extensive microsatellite accumulation was found in both autosomal and sex chromosomes (Z and W) of Brazilian Teal and Coscoroba Swan, while White-faced Whistling Duck displays minimal hybridization signals and an absence of microsatellites on the sex chromosomes. The accumulation of specific microsatellites, such as (CAC)10 and (GAG)10, in centromeric and pericentromeric regions suggests an association with transposable elements, potentially driving chromosomal evolution. Notably, the substantial accumulation of these sequences on the Z and W chromosomes of Brazilian Teal and Coscoroba Swan, but not White-faced Whistling Duck, supports the hypothesis that repetitive sequence expansion occurs in a species-specific manner, contributing to sex chromosome differentiation. These findings highlight microsatellite mapping as a valuable tool for understanding chromosomal evolution and genomic differentiation in Anseriformes.
1. Introduction
Anseriformes (Wagler, 1831) comprise a group of waterfowl with around 178 species, commonly known as ducks, geese, swans, magpie goose, and screamers that are distributed in three families, Anhimidae restricted to South America; Anseranatidae restricted to Oceania; and Anatidae which are globally distributed [1]. This order, together with the Galliformes (chickens, guinea fowl, and pheasants), constitutes the basal clade Galloanserae, which diverged from the Neognathae lineage around 70 to 100 million years ago [2,3].
Whereas the position of Anseriformes in relation to bird phylogeny is corroborated in several studies, the relationships among some species within this order are still discussed, especially considering Anatidae, which is the family with the absolute majority of modern taxonomic and ecological diversity, representing around 98% of the species in the order [3,4,5]. Anatidae encompasses three subfamilies: Anatinae (ducks), Anserinae (swans and geese), and Dendrocygninae (whistling ducks). This last subfamily, which includes the whistling ducks (genus Dendrocygna), has been classified in some studies as an independent family named Dendrocygnidae. However, this taxonomic classification has not been totally accepted, despite the apparent morphological peculiarities and phylogenetic position of the Dendrocygna ducks [3,5].
Although the evolutionary history of Anatidae species remains incompletely understood, recent studies have provided a detailed picture of the diversity, phylogeny, and evolution of Anseriformes [3,6,7,8,9,10]. However, in the field of cytogenetics, research on Anseriformes has largely been limited to karyotypic descriptions using conventional staining. To date, karyotypes have been described for 46 species, approximately 25% of all Anseriformes, while chromosome painting has been applied to only eight species. Despite diploid numbers varying from 2n = 74 to 98, most species exhibit a typical avian karyotype with 78–80 chromosomes, characterized by the presence of microchromosomes [8,11,12].
Understanding the evolutionary diversification of organisms is essential for reconstructing their evolutionary history and identifying factors that influence this process. Anseriformes hold a significant position within the avian phylogeny, sharing close evolutionary relationships with Galliformes, which makes them a key group for investigating genome evolution, sex chromosome differentiation, and speciation in birds [13]. However, despite their relevance, relatively few species have been analyzed in detail. To date, karyotypic analyses using conventional staining have been conducted on only 46 species, while chromosome painting has been applied to just eight species, all within the Anatidae family [14]. Furthermore, in Anseriformes, physical mapping of repetitive sequences on metaphase chromosomes is still limited, with many of these markers presenting an unknown distribution in the order.
Repetitive sequences are abundant components of genomes, and each species has its specific library of repetitive element families, which are organized in chains (transposable elements) or tandem repeats (satellites, minisatellites, microsatellites) [15,16]. Analyses involving the mapping of these DNA sequences have provided important data and valuable insights into the process of karyotypic evolution in different groups of birds [10,17,18,19]. Among the main repetitive elements, microsatellites are the components that are most widely distributed in eukaryotic genomes. These repetitive sequences correspond to short segments of DNA sequences (1–6 base pairs) repeated in tandem and dispersed throughout the genome [20,21]. As these elements evolve very quickly, they are important markers for studies of the genetic variability of populations [22,23]. Studies aimed at analyzing the distribution of microsatellites have contributed to our understanding of the structural and functional organization of chromosomes, the genomic organization and differentiation of macrochromosomes and microchromosomes, as well as playing an important role in the differentiation of sex chromosomes [17,19,23].
Many satellite and microsatellite DNA sequences are organized in large tandem groups, ranging from hundreds to thousands of copies [15,16,20], which makes them readily detectable by fluorescence in situ hybridization (FISH) using fluorescently labeled DNA probes. For this reason, microsatellites are widely recognized as an excellent chromosomal marker for genomic comparison [17,19,23].
Given the importance of repetitive DNA in genomic differentiation and the limited available data on these sequences in Anseriformes, we investigated the karyotypic structure and microsatellite distribution in three Anatidae species: Amazonetta brasiliensis (Brazilian Teal), Coscoroba coscoroba (Coscoroba Swan), and Dendrocygna viduata (White-faced Whistling Duck). Our goal was to explore the role of these sequences in the cytogenetic diversity and chromosomal organization of this family, as well as their potential taxonomic and phylogenetic significance.
2. Materials and Methods
2.1. Cell Culture and Chromosome Preparations
All experiments performed in our study were approved by the Ethics Committee on the Use of Animals of Universidade Federal do Pará (CEUA authorization no. 170/2013). For this study, a representative species of each subfamily of Anatidae was included A. brasiliensis—Brazilian Teal (Anatinae), C. coscoroba—Coscoroba Swan (Anserinae), and D. viduata—White-faced Whistling Duck (Dendrocygninae). Skin biopsies were obtained from one female individual of each species, under the permission from System of Authorization and Information in Biodiversity (SISBIO license no. 33860-3). The cell culture process began with the mechanical dissociation of the biopsies, followed by incubation in collagenase for 1 h, after which they were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal bovine serum, 2% penicillin-streptomycin, and 1% L-glutamine at 37 °C, according to Sasaki et al. [24]. The metaphase chromosomes were obtained using standard protocols, treatment with colcemide (1 h), hypotonic solution (0.075 M KCl, 20 min), and fixation with Carnoy solution (3:1 methanol/acetic acid).
2.2. Giemsa Staining
Chromosome morphology and diploid numbers (2n) were determined based on the analysis of at least 30 stained metaphases (5% Giemsa in phosphate buffer pH 6.8 for 5 min) from each individual.
2.3. Fluorescence in Situ Hybridization-FISH
Eleven di/trinucleotide microsatellite repeats were used as probes: (CA)15, (GA)15, (TA)15, (GC)15, (CAA)10, (CAC)10, (CAG)10, (CAT)10, (CGG)10, (GAA)10, and (GAG)10, following the procedures adopted by Kubat et al. [25], with modifications as described by Cioffi et al. [26]. All probes used were commercially obtained and labeled directly with Cy3 in the 5′ terminal region during synthesis (Sigma, St. Louis, MO, USA).
The selection of microsatellite probes used in this work was based on the availability of these sequences in our laboratory and on the fact that they have been previously reported in the literature as widely distributed in bird genomes.
2.4. Microscopic Analysis and Image Processing
Karyotypes were organized using GenASIs software, version 7.2.6.19509 (Applied Spectral Imaging, Carlsbad, CA, USA), according to chromosome size and morphology following Guerra [27]. The results of the FISH experiments were registered using a Zeiss Axio ImagerZ.2 epifluorescence microscope, and images were captured and edited with Axio Vision 4.8 software (Zeiss, Jena, Germany).
3. Results
3.1. Conventional Analysis
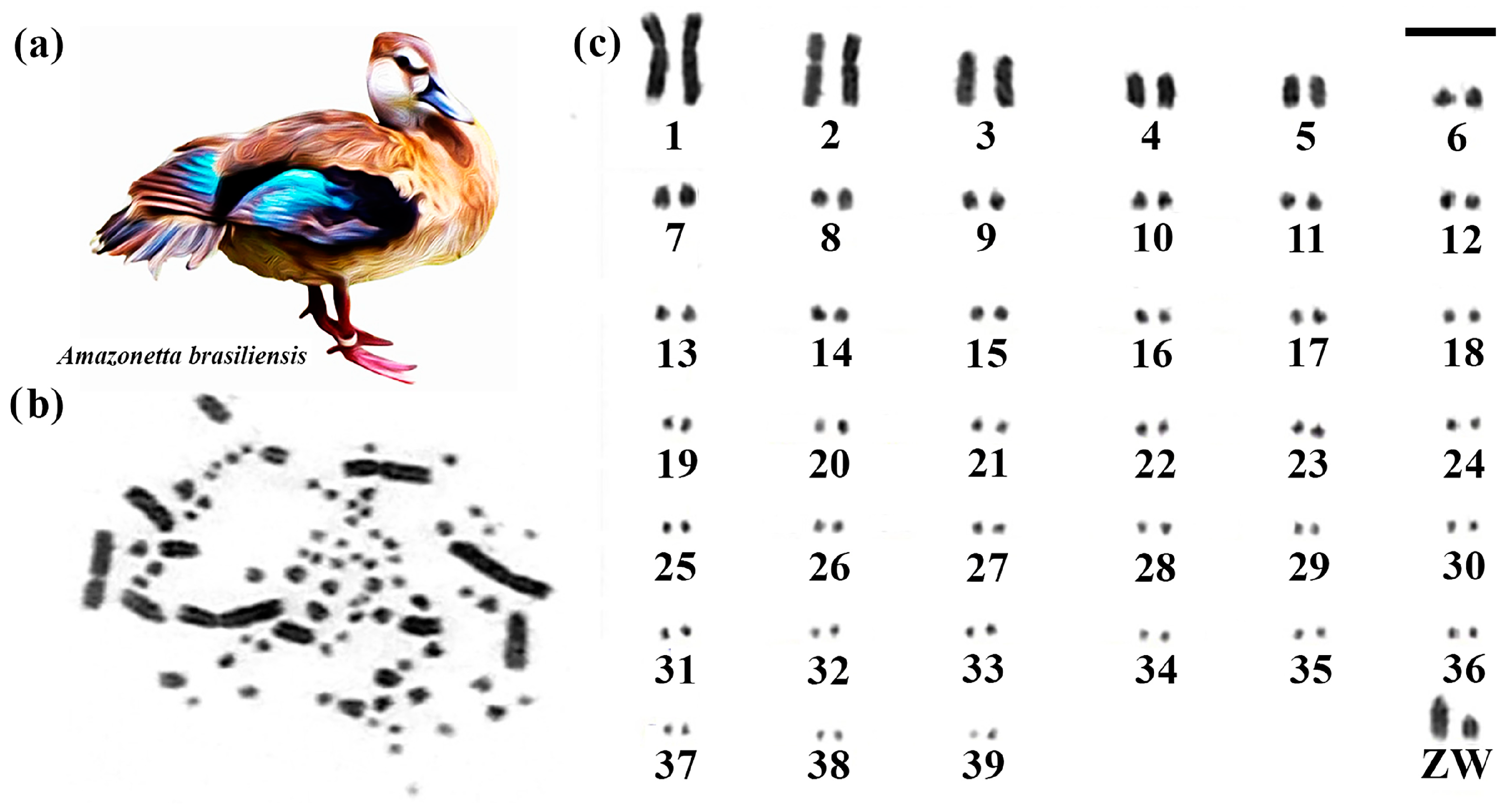
Brazilian Teal had 2n = 80 (Figure 1). Among the macrochromosomes, the first and second pairs are submetacentric, and the others are acrocentric. The Z chromosome is acrocentric, being the fourth largest element, and the W chromosome is acrocentric, with a size close to the eighth pair (Figure 1).
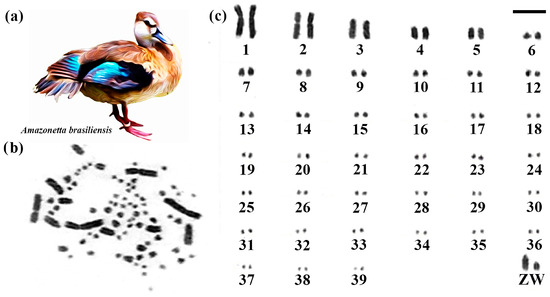
Figure 1.
Karyotype of Brazilian Teal. The letters mean (a) Brazilian Teal species, (b) metaphase, and (c) karyotype in conventional staining (Bar = 5 µm).
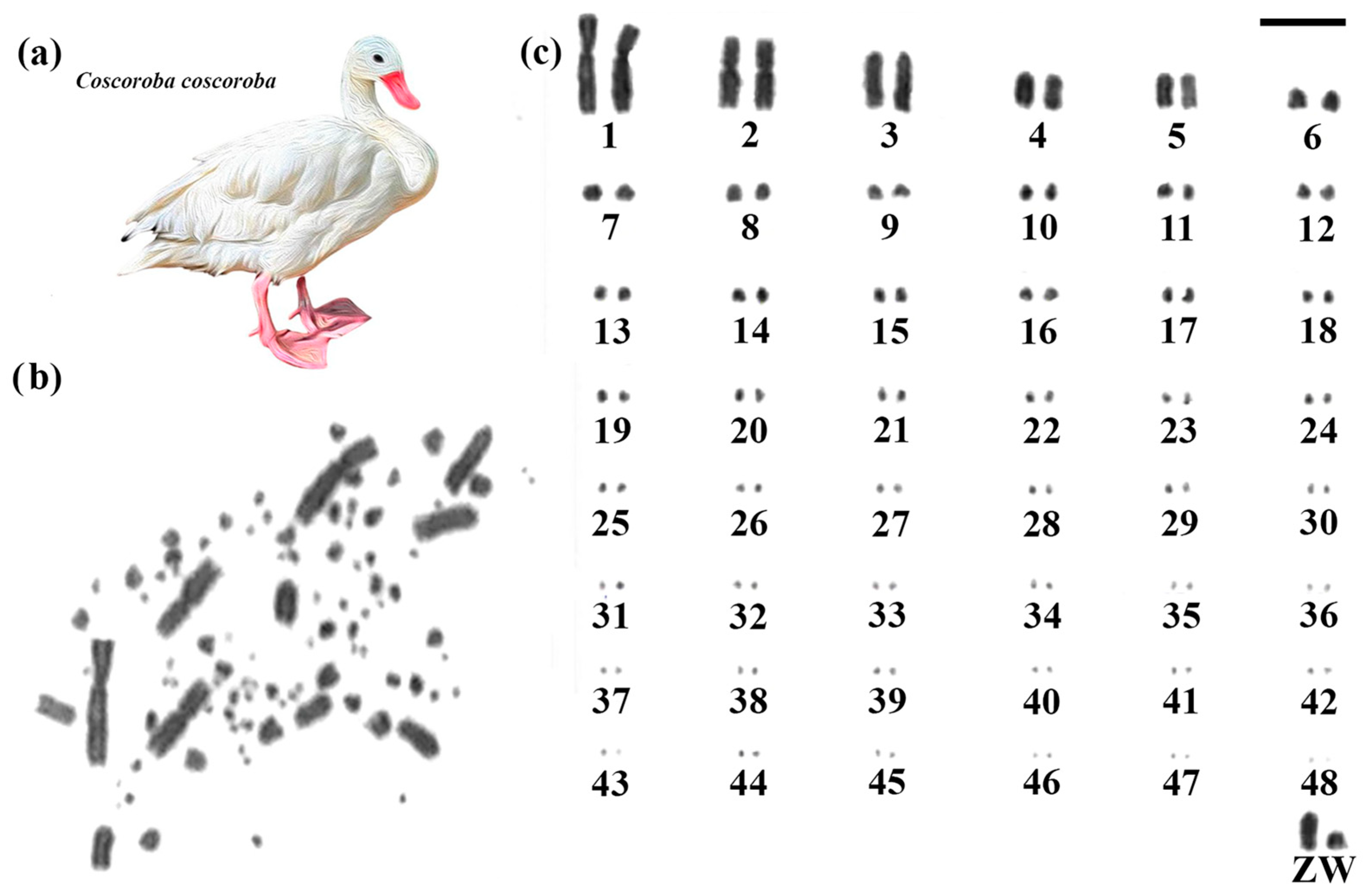
Coscoroba Swan had 2n = 98, and despite the higher diploid number than the other two species, the autosomal macrochromosomes showed a similar morphology, with pair 1 submetacentric and pair 2 metacentric, while the other chromosomes are acrocentric (Figure 2). The sex chromosomes also maintained a similar pattern, with the Z acrocentric and the fourth largest element, while the W has a size close to the sixth pair. A higher number of microchromosomes was observed, which is responsible for the increase in the diploid number (Figure 2).
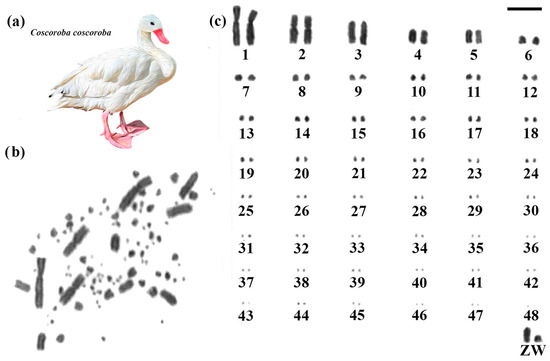
Figure 2.
Karyotype of Coscoroba Swan. The letters mean (a) Coscoroba Swan species, (b) metaphase, and (c) karyotype in conventional staining (Bar = 5 µm).
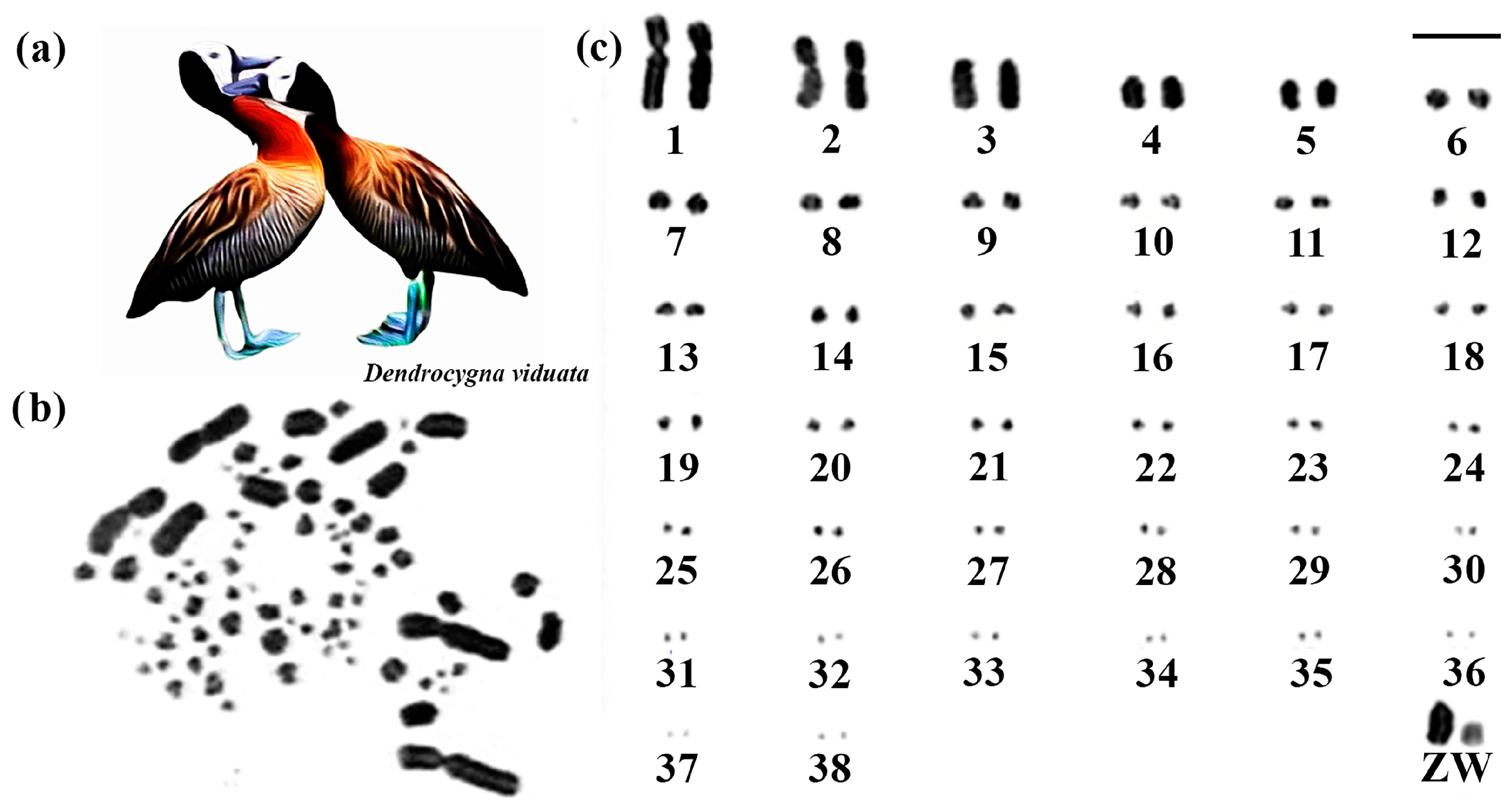
The diploid number of White-faced Whistling Duck species was 2n = 78. Except for submetacentric pair 1 and metacentric pair 2, the other macrochromosomes are acrocentric, including the sex chromosome pair (Figure 3). The Z chromosome corresponds to the fourth largest element, and the W has a size close to the sixth pair (Figure 3).
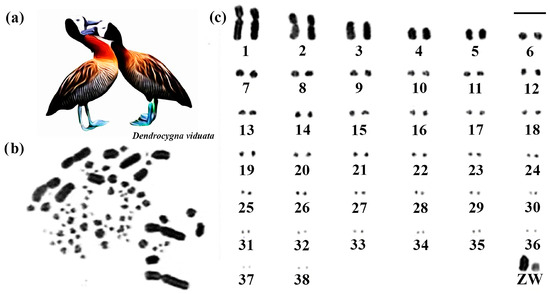
Figure 3.
Karyotype of White-faced Whistling Duck. The letters mean (a) White-faced Whistling Duck species, (b) metaphase, and (c) karyotype in conventional staining (Bar = 5 µm).
3.2. Microsatellite Mapping
Microsatellite sequences were detected in autosomal and sex chromosomes, as well as in microchromosomes, with variations in hybridization patterns among the species. Of the eleven sequences analyzed, only (TA)15 failed to produce a hybridization signal in any of the species. The microsatellite hybridizations by species are summarized in Table 1.

Table 1.
Summarization of microsatellite hybridization results in the Anseriformes species analyzed.
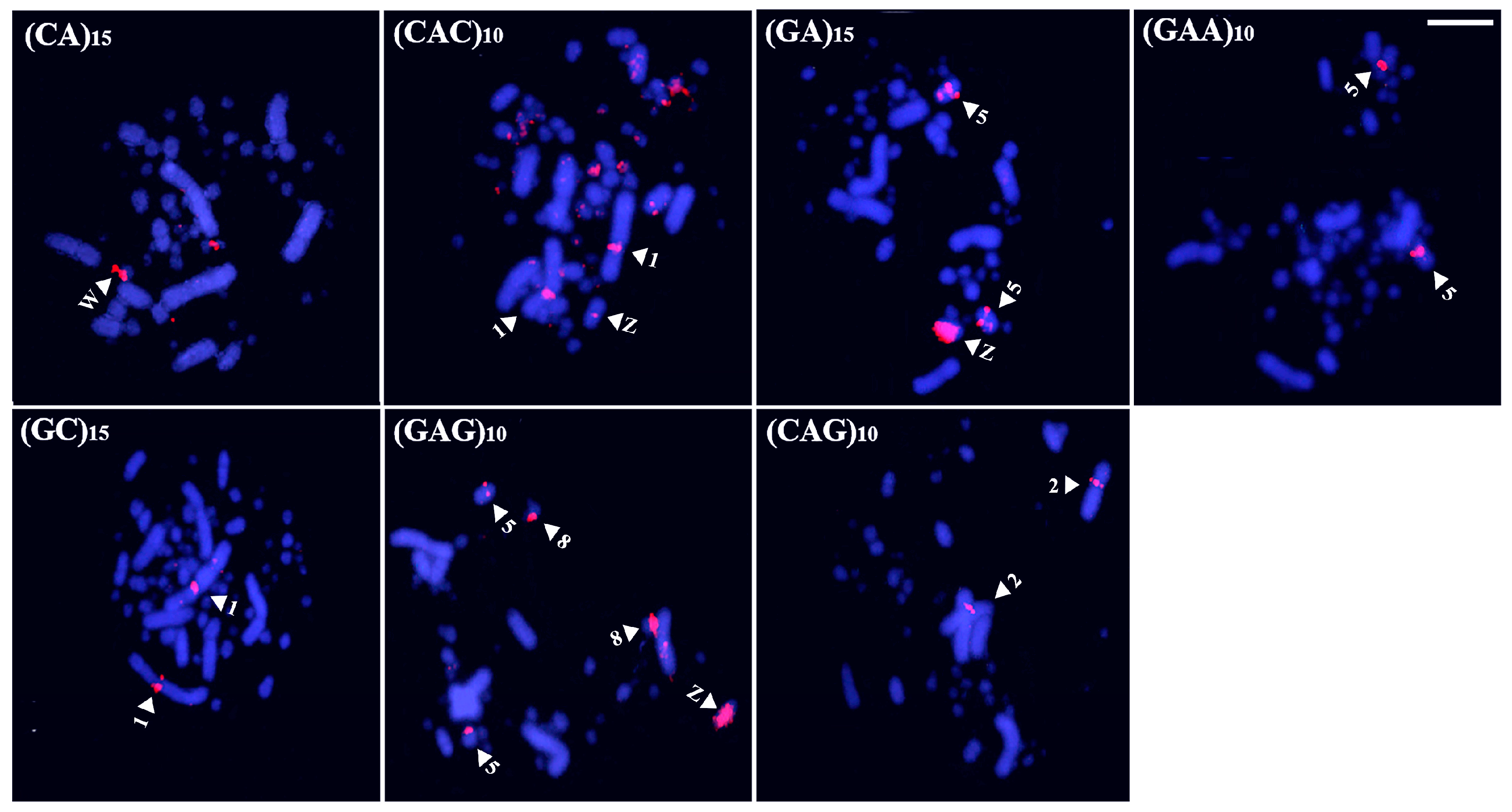
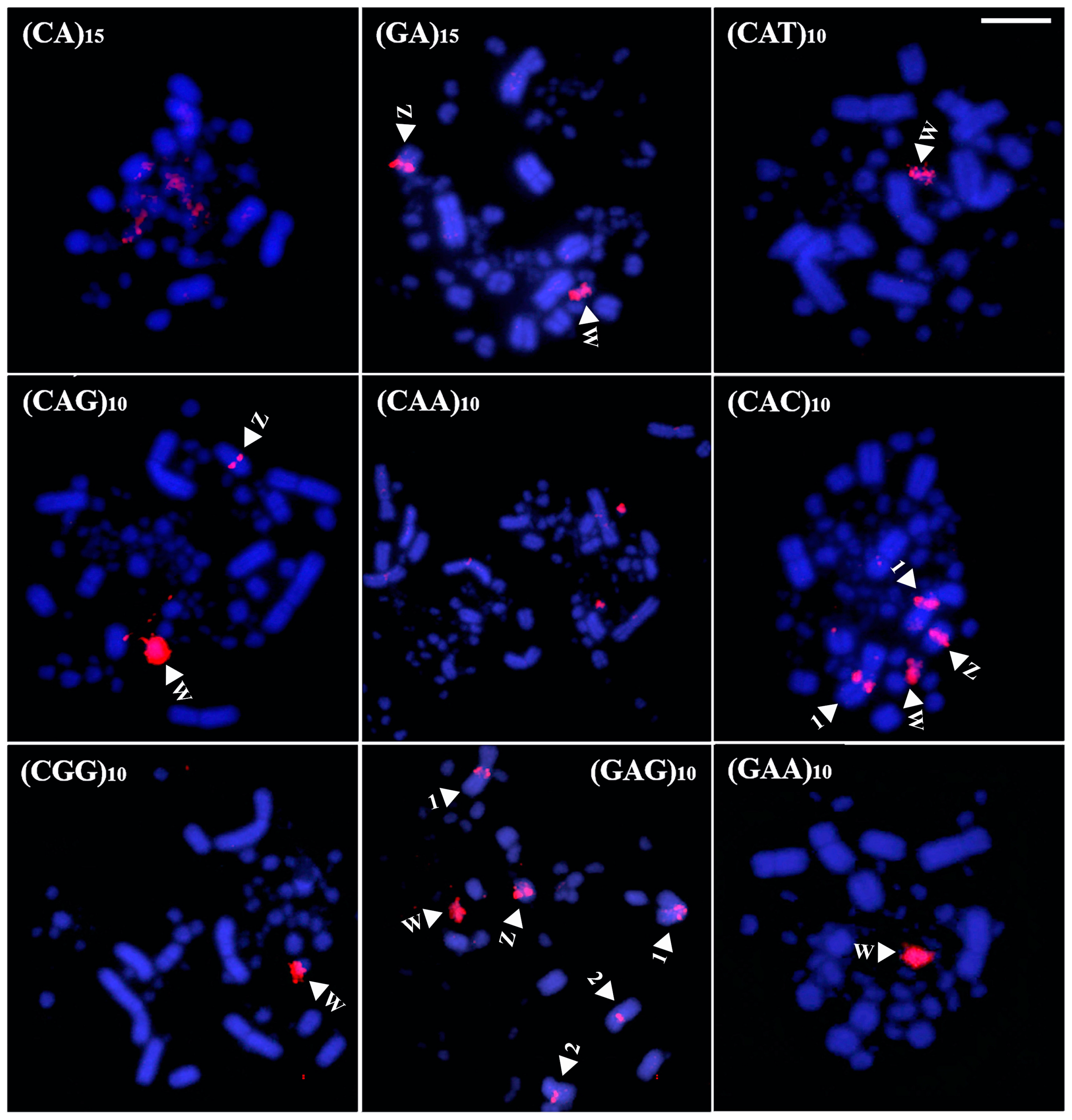
In Brazilian Teal, seven probes produced noticeable hybridization signals (CA)15, (CAC)10, (CAG)10, (GA)15, (GC)15, (GAA)10, and (GAG)10 (Figure 4). Regarding signals on the autosomal macrochromosomes, two were concentrated on the centromere of pair 1, (CAC)10 and (GC)15, and three on the long arm of pair 5, (GA)15, (GAA)10, and (GAG)10. The probes (CAC)10 and (CAG)10 also showed hybridization signals in several microchromosomes and on pair 2, respectively. In the sex chromosomes, three microsatellites were observed in the Z, (GA)15 and (GAG)10, in a more intense way, and (CAC)10, in a more restricted way. In the W, only signals produced by the sequence (CA)15 were detected (Figure 4).
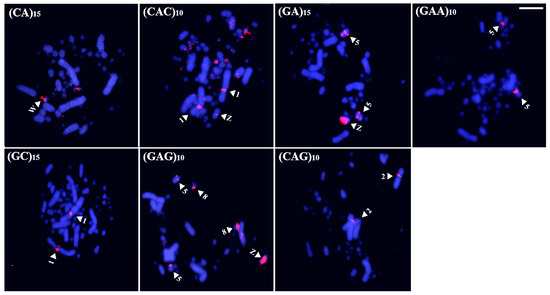
Figure 4.
Distribution of microsatellites in the karyotype of Brazilian Teal (Bar = 5 µm). The arrowheads indicate a specific chromosome.
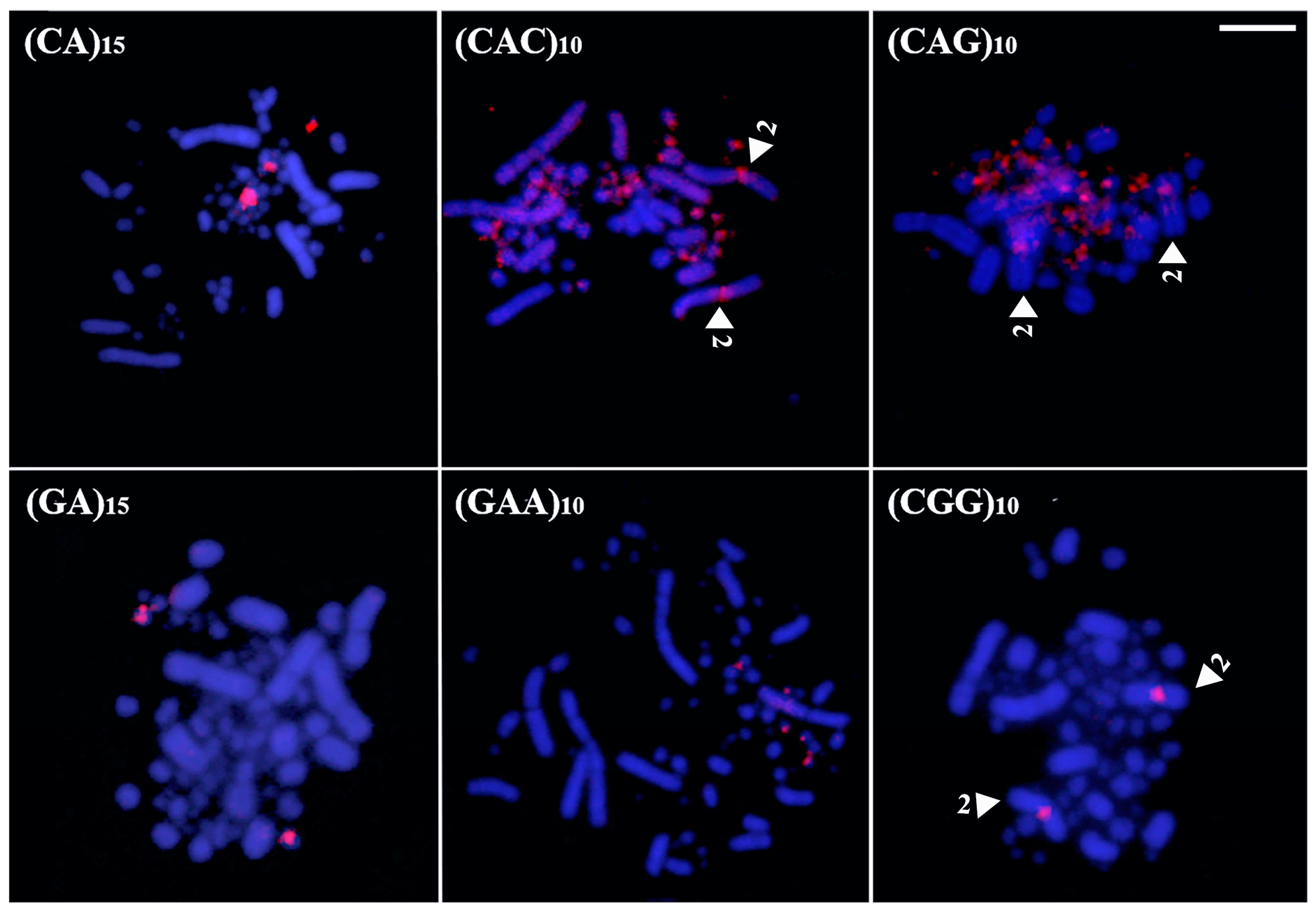
Coscoroba Swan exhibited the highest accumulation of microsatellites in the sex chromosomes. A total of four sequences—(CAC)10, (CAG)10, (GA)15, and (GAG)10—produced signals on both the Z and W chromosomes, while three sequences—(GAA)10, (CAT)10, and (CGG)10—were exclusive to the W chromosome (Figure 5). Additionally, pair 1 displayed (CAC)10 signals in the proximal region of the long arm, whereas (GAG)10 marked the centromeres of pairs 1 and 2. Lastly, (CA)15 and (CAA)10 were restricted to microchromosomes, with the former labeling multiple pairs and the latter marking only one pair (Figure 5).
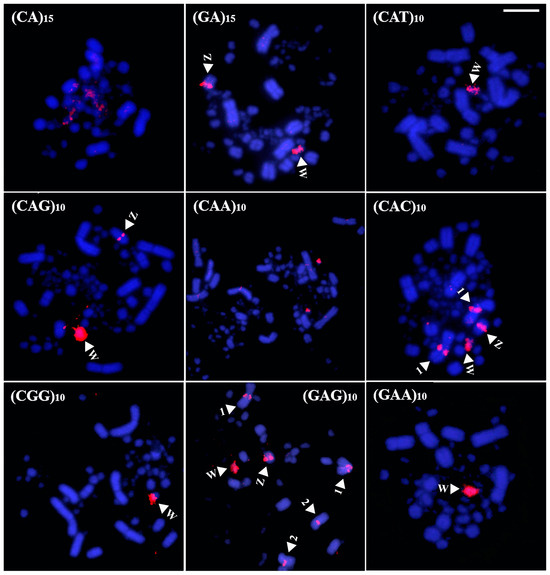
Figure 5.
Distribution of microsatellites in the karyotype of Coscoroba Swan (Bar = 5 µm). The arrowheads indicate a specific chromosome.
In White-faced Whistling Duck, six microsatellite sequences generated hybridization signals: (CA)15, (CAC)10, (CAG)10, (GA)15, (GAA)10, and (CGG)10 (Figure 6). Among the macrochromosomes, only pair 2 was marked, specifically in the pericentromeric region, where three probes—(CAC)10, (CAG)10, and (CGG)10—produced signals (Figure 6). No accumulation was detected on the sex chromosomes, while all other probes hybridized exclusively to microchromosomes.
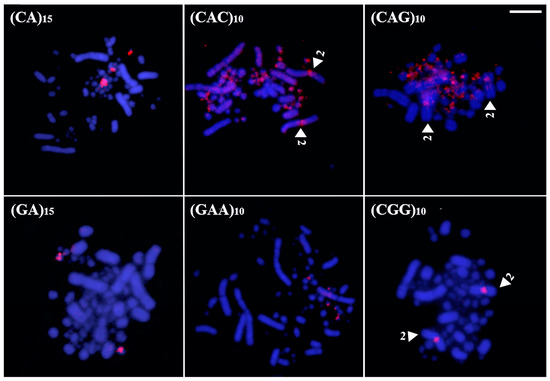
Figure 6.
Distribution of microsatellites in the karyotype of White-faced Whistling Duck (Bar = 5 µm). The arrowheads indicate a specific chromosome.
4. Discussion
Birds of the order Anseriformes are a very interesting group for cytogenetic research, especially for studies involving the distribution of repetitive sequences, due to their basal position in the phylogeny of neognath birds and their great chromosomal variation [3,8,12].
Similar to most species within the Galloanserae clade, the Anatidae whose karyotypes are described here for the first time, Brazilian Teal (2n = 80) and White-faced Whistling Duck (2n = 78), exhibit conserved karyotypes. Their chromosome numbers are close to 2n = 80, and their macrochromosome morphology is similar, a characteristic commonly observed among Anseriformes [12,14]. On the other hand, the karyotype of the Coscoroba Swan, with 2n = 98 chromosomes, represents an exception within the group. Even though the macrochromosomes are morphologically similar to the other species analyzed, the significantly higher number of microchromosomes suggests the occurrence of chromosomal rearrangements, such as fissions or amplifications involving exclusively these elements, a hypothesis already suggested by Rodrigues et al. [12] in their study using Gallus gallus chromosome-specific probes in Coscoroba Swan. The similarity among the macrochromosomes, even in the face of this great variation in diploid number, instigates a comparative evaluation of the distribution of microsatellite sequences in these species, since mapping microsatellites in the karyotype of birds provides detailed insights into the genetic structure, genomic variability, and morphological differentiation of the sex chromosomes [28].
Microsatellite accumulations were identified in both euchromatic and heterochromatic regions, with a preferential accumulation in the latter, supporting the hypothesis that repetitive DNA is predominantly found in condensed and transcriptionally inactive regions of the genome [20]. While microsatellites are more commonly associated with non-coding DNA, their presence in coding regions suggests a potential influence on protein structure and function [29,30].
Despite their phylogenetic proximity and morphological similarity, the distribution patterns and number of microsatellite clusters varied among the analyzed species. The Brazilian Teal and Coscoroba Swan exhibited more similar microsatellite distribution profiles compared to White-faced Whistling Duck. Notably, hybridization patterns differed in pairs 1 and 2 among these species. For instance, the (CAC)10 probe marked the centromere of pair 1 in Brazilian Teal and the interstitial region of the long arm of pair 1 in Coscoroba Swan, but it did not produce signals on this chromosome in White-faced Whistling Duck. Instead, it marked the centromere of pair 2 in this species. The most parsimonious explanation for this variation suggests that this sequence may be part of a centromeric transposon in anatids. Based on the phylogenetic proximity among the species analyzed, we propose that the original position of this transposon would be in pair 1 in Brazilian Teal and Coscoroba Swan, which belong to related subfamilies, and subsequently, in Coscoroba Swan, it would have been relocated to the interstitial region of the same pair. In White-faced Whistling Duck, a species belonging to a more external subfamily, the transposon moved to the centromeric region of pair 2.
Transposable elements show a wide genomic distribution; however, they usually present a disproportionate abundance in the centromeric and/or pericentromeric regions in a wide range of phylogenetically unrelated species [31]. Although there is no direct evidence confirming the association of the (CAC)10 microsatellite with transposable elements, the presence of this microsatellite sequence also on the Z chromosome of Brazilian Teal and on the Z and W of Coscoroba Swan may indicate that this sequence is associated with or is part of a centromeric transposon. However, in the sex chromosomes, this sequence would have expanded, since it is not restricted to the centromeric region of these chromosomes.
The presence of microsatellites associated with transposable elements can play a role in the plasticity of the genome, potentially influencing the adaptation of species by affecting the regulation of genes and chromosomal stability, thus playing a fundamental role in the evolution of organisms [32].
The transposon hypothesis could also explain the presence of microsatellites (GAG)10, which appears in pairs 1 and 2 of Coscoroba Swan and in pair 2 of Brazilian Teal. Also, considering the autosomal macrochromosomes, three sequences were observed in pair 5 of Brazilian Teal: (GAG)10, (GAA)10, and (GA)15. The similarity in the nucleotide composition of these sequences may explain the location of their signals in the same region of this chromosome pair.
In addition, the distribution of microsatellites in White-faced Whistling Duck differs significantly from that observed in the other two species, Brazilian Teal and Coscoroba Swan. The similarity in repetitive sequence distribution between Brazilian Teal and Coscoroba Swan likely reflects their classification within two closely related subfamilies (Anatinae and Anserinae), which are more distant from the Dendrocygninae subfamily to which White-faced Whistling Duck belongs. Some authors even elevate Dendrocygninae to a separate family [3,33]. Therefore, the microsatellite mapping performed in this study may reveal valuable evolutionary and phylogenetic information.
Regarding the bird sex chromosomes, we highlighted the occurrence of a higher density of repeats on both the Z and W sex chromosomes of Brazilian Teal and Coscoroba Swan. Distinct microsatellite hybridization patterns have been described on these chromosomes, despite the small amount of these DNA classes in their genome. In general, microsatellites accumulate mainly on the W chromosome, as observed in Columbiformes, Cuculiformes, Gruiformes, Passeriformes, Psittaciformes, and Trogoniformes. While a few orders show more slight signs on the Z chromosome, such as Passeriformes, Psittaciformes, and Trogoniformes, except for Piciformes, which shows a large accumulation of microsatellites on this sex chromosome [17,23,34,35,36].
In addition, it is important to note that some microsatellites, such as (CAC)10, (CAG)10, and (GAG)10, which showed signs on both sex chromosomes of Coscoroba Swan and Brazilian Teal, show a more general predilection for the sex chromosomes of the most diverse groups of birds (Cuculiformes, Passeriformes, Psittaciformes, and Piciformes) [23,34,35,36]. Specifically in relation to the Galloanserae clade, the study by Matsubara et al. [36] mapped the distribution of microsatellites in the Gallus gallus species (Galliformes) and revealed a predominance of the (GA)15 and (GAG)10 sequences on the W chromosome, with a lower frequency on the Z and autosomes of the species. This same pattern was observed for Brazilian Teal and Coscoroba Swan, reinforcing the hypothesis that microsatellites are not randomly distributed in eukaryotic genomes and that closely related species, such as Galliformes and Anseriformes, tend to share both the same microsatellite sequences and their chromosomal locations.
Conversely, none of the microsatellite sequences hybridized to the sex chromosomes of White-faced Whistling Duck. In addition, most repeats on sex chromosomes are species-specific and highly variable, even among closely related taxa [37,38,39], which in turn would explain the differences in the amount of hybridized microsatellites in Coscoroba Swan and Brazilian Teal. Although both the Z and W chromosomes normally show an accumulation of repetitive sequences [40], the strong marking observed in Brazilian Teal is unusual, suggesting rapid amplification and molecular differentiation that occurred repeatedly after divergence from a common ancestor.
These differences in the accumulation of repetitive sequences in the sex chromosomes of these species may be linked to speciation mechanisms, as they can lead to reproductive isolation through genomic incompatibilities [41,42]. In addition, from an ecological perspective, variations in chromosome organization may be correlated with species-specific adaptations to different environments, influencing characteristics such as reproductive success, behavior, or resistance to environmental stressors [43].
Finally, some limitations of this study must be discussed, such as the small sample size, as only one individual per species was analyzed. This restricts the ability to generalize the findings to the broader populations of Brazilian Teal, Coscoroba Swan, and White-faced Whistling Duck, as intraspecific variation in microsatellite distribution cannot be assessed. The exclusive use of female individuals should not introduce biases, particularly in the analysis of sex chromosomes, since females represent the heterogametic sex and possess both sex chromosomes. The methodology employed, such as the use of specific microsatellite probes, may not capture the full diversity of repetitive elements present in these genomes, and the hybridization signals could also be influenced by chromatin condensation states, potentially affecting the interpretation of microsatellite distribution.
Although microsatellites are well known for their high polymorphism at the population level, particularly in the number of repeat units, several studies have demonstrated that their chromosomal distribution is sufficiently conserved to make them reliable markers in comparative cytogenetics [44,45]. While variation in the number of microsatellite copies within and among species can influence signal intensity, it rarely alters the chromosomal location of these sequences. Nevertheless, microsatellite markers may have limitations in detecting subtle chromosomal rearrangements or low-copy-number sites. Thus, interpretations of interspecific differences based on microsatellite mapping should account for these potential constraints, and the use of complementary approaches may enhance the robustness of future studies. In this way, future studies should incorporate larger sample sizes, including both male and female individuals, and complementary genomic approaches, such as next-generation sequencing, to provide a more comprehensive understanding of the role of microsatellites in the evolutionary dynamics of the Anseriformes karyotype.
5. Conclusions
Our results provide valuable insights into the distribution patterns of microsatellites and their potential use as phylogenetic markers. We observed a more similar distribution profile between Brazilian Teal and Coscoroba Swan, whereas White-faced Whistling Duck, which is phylogenetically more distant, exhibited a distinct pattern. Additionally, we propose that the presence of microsatellites on different chromosome pairs may be linked to the activity of transposable elements in reorganizing these sequences among species, highlighting their possible role in the structural and evolutionary dynamics of chromosomes.
Sex chromosomes exhibited high variation in microsatellite accumulation, once again revealing a similar pattern in Brazilian Teal and Coscoroba Swan, with strong hybridization signals on both the Z and W chromosomes. In contrast, White-faced Whistling Duck showed no hybridization signals on these chromosomes. This pattern reinforces the hypothesis that the amplification of repetitive sequences occurs in a species-specific manner, with significant implications for the evolution of sex chromosomes.
Author Contributions
Conceptualization, P.S.B.C., R.P.C.d.S. and E.H.C.d.O.; methodology, P.S.B.C., R.P.C.d.S. and E.H.C.d.O.; validation, E.H.C.d.O.; formal analysis, P.S.B.C., A.J.B.G., B.S.R. and R.P.C.d.S.; investigation, B.S.R. and R.P.C.d.S.; resources, E.H.C.d.O.; writing—original draft preparation, P.S.B.C., B.S.R. and R.P.C.d.S.; writing—review and editing, A.J.B.G., R.P.C.d.S. and E.H.C.d.O.; visualization, R.P.C.d.S.; supervision, E.H.C.d.O.; funding acquisition, E.H.C.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) through a research project E.H.C.d.O. (307382/2019-2).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on the Use of Animals of Universidade Federal do Pará (CEUA authorization no. 170/2013).
Data Availability Statement
All data from this study are available in the manuscript. In addition, other information can be obtained on request from the corresponding author.
Acknowledgments
Authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Instituto Evandro Chagas for technical support. Finally, we would also like to thank Parque Mangal das Garças (Belém, PA) for kindly providing the biological samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gill, F., 3rd; Donsker, D.; Rasmussen, P. IOC World Bird List (v14.2). Available online: https://www.worldbirdnames.org/new/ (accessed on 5 January 2025).
- Van Tuinen, M., 3rd; Hedges, S.B. Calibration of avian molecular clocks. Mol. Biol. Evol. 2001, 18, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, T.; Hu, C.; Sun, L.; Ding, H.; Wang, H.; Zhang, C.; Jin, H.; Chang, Q.; Kan, X.; et al. Rapid and recent diversification patterns in Anseriformes birds: Inferred from molecular phylogeny and diversification analyses. PLoS ONE 2017, 12, e0184529. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, F.; McLaughlin, J.F.; Cheek, R.G.; McCracken, K.G.; Glenn, T.C.; Winker, K. Population genomics indicate three different modes of divergence and speciation with gene flow in the green-winged teal duck complex. Mol. Phylogenet. Evol. 2023, 182, 107733. [Google Scholar] [CrossRef]
- Zelenkov, N.V. The oldest Asian duck (Anseriformes: Romainvilla) and the origin of Anatidae. Dokl. Biol. Sci. 2018, 483, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Musser, G.; Clarke, J.A. A new Paleogene fossil and a new dataset for waterfowl (Aves: Anseriformes) clarify phylogeny, ecological evolution, and avian evolution at the K-Pg Boundary. PLoS ONE 2024, 19, e0278737. [Google Scholar] [CrossRef]
- Houde, P.; Dickson, M.; Camarena, D. Basal Anseriformes from the Early Paleogene of North America and Europe. Diversity 2023, 15, 233. [Google Scholar] [CrossRef]
- Beklemisheva, V.R.; Tishakova, K.V.; Romanenko, S.A.; Andreushkova, D.A.; Yudkin, V.A.; Interesova, E.A.; Yang, F.; Ferguson-Smith, M.A.; Graphodatsky, A.S.; Proskuryakova, A. Detailed cytogenetic analysis of three duck species (the northern pintail, mallard, and common goldeneye) and karyotype evolution in the family Anatidae (Anseriformes, Aves). Vavilovskii Zhurnal Genet. Sel. 2024, 28, 759–769. [Google Scholar] [CrossRef]
- Abu-Almaaty, A.H.; Hassan, M.K.; El-Bakary, N.E.; Ahmed, S.H. Chromosomal evolution and molecular genetic analysis of four species of genus Anas (Aves: Anatidae). Genetika 2019, 51, 103–119. [Google Scholar] [CrossRef]
- Uno, Y.; Nishida, C.; Hata, A.; Ishishita, S.; Matsuda, Y. Molecular cytogenetic characterization of repetitive sequences comprising centromeric heterochromatin in three Anseriformes species. PLoS ONE 2019, 14, e0214028. [Google Scholar] [CrossRef]
- Islam, F.B.; Uno, Y.; Nunome, M.; Nishimura, O.; Tarui, H.; Agata, K.; Matsuda, Y. Comparison of the chromosome structures between the chicken and three anserid species, the domestic duck (Anas platyrhynchos), Muscovy duck (Cairina moschata), and Chinese goose (Anser cygnoides), and the delineation of their karyotype evolution by comparative chromosome mapping. J. Poult. Sci. 2014, 51, 1–13. [Google Scholar]
- Rodrigues, B.S.; Assis, M.D.F.L.; O’Brien, P.C.; Ferguson-Smith, M.A.; Oliveira, E.H. Chromosomal studies on Coscoroba coscoroba (Aves: Anseriformes) reinforce the Coscoroba–Cereopsis clade. Biol. J. Linn. Soc. 2014, 111, 274–279. [Google Scholar] [CrossRef]
- Ebied, A.M.; Hassan, H.A.; Almaaty, A.H.A.; Yaseen, A.E. Karyotypic Characterization of Ten Species of Birds. Cytologia 2005, 70, 181–194. [Google Scholar] [CrossRef]
- Degrandi, T.M.; Barcellos, S.A.; Costa, A.L.; Garnero, A.D.V.; Hass, I.; Gunski, R.J. Introducing the Bird Chromosome Database: An Overview of Cytogenetic Studies in Birds. Cytogenet. Genome Res. 2020, 160, 199–205. [Google Scholar] [CrossRef]
- López-Flores, I.; Garrido-Ramos, M.A. The repetitive DNA content of eukaryotic genomes. Genome Dyn. 2012, 7, 1–28. [Google Scholar] [PubMed]
- Paço, A.; Freitas, R.; Vieira-da-Silva, A. Conversion of DNA sequences: From a transposable element to a tandem repeat or to a gene. Genes 2019, 10, 1014. [Google Scholar] [CrossRef]
- Kretschmer, R.; de Oliveira, T.D.; de Oliveira Furo, I.; Oliveira Silva, F.A.; Gunski, R.J.; del Valle Garnero, A.; Cioffi, M.B.; de Oliveira, E.H.C.; de Freitas, T.R.O. Repetitive DNAs and shrink genomes: A chromosomal analysis in nine Columbidae species (Aves, Columbiformes). Genet. Mol. Biol. 2018, 41, 98–106. [Google Scholar] [CrossRef]
- Furo, I.D.O.; Kretschmer, R.; O’Brien, P.C.; Pereira, J.C.; Garnero, A.D.V.; Gunski, R.J.; O’Connor, R.; Griffin, D.K.; Gomes, A.J.B.; Ferguson-Smith, M.A.; et al. Chromosomal evolution in the phylogenetic context: A remarkable karyotype reorganization in neotropical parrot Myiopsitta monachus (Psittacidae). Front. Genet. 2020, 11, 721. [Google Scholar] [CrossRef]
- Gunski, R.J.; Kretschmer, R.; Santos de Souza, M.; de Oliveira Furo, I.; Barcellos, S.A.; Costa, A.L.; Cioffi, M.B.; de Oliveira, E.H.C.; del Valle Garnero, A. Evolution of bird sex chromosomes narrated by repetitive sequences: Unusual W chromosome enlargement in Gallinula melanops (Aves: Gruiformes: Rallidae). Cytogenet. Genome Res. 2019, 158, 152–159. [Google Scholar] [CrossRef]
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef]
- Martins, C. Chromosomes and repetitive DNAs: A contribution to the knowledge of fish genome. In Fish Cytogenetics; CRC Press: Boca Raton, FL, USA, 2007; Volume 421, p. 452. [Google Scholar]
- Gemayel, R.; Vinces, M.D.; Legendre, M.; Verstrepen, K.J. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 2010, 44, 445–477. [Google Scholar] [CrossRef]
- de Oliveira, T.D.; Kretschmer, R.; Bertocchi, N.A.; Degrandi, T.M.; de Oliveira, E.H.C.; Cioffi, M.D.B.; Garnero, A.V.; Gunski, R. Genomic organization of repetitive DNA in woodpeckers (Aves, Piciformes): Implications for karyotype and ZW sex chromosome differentiation. PLoS ONE 2017, 12, e0169987. [Google Scholar] [CrossRef]
- Sasaki, M.; Ikeuchi, T.; Makino, S. A feather pulp culture technique for avian chromosomes, with notes on the chromosomes of the peafowl and the ostrich. Experientia 1968, 24, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovsky, E. Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Martins, C.; Centofante, L.; Jacobina, U.; Bertollo, L.A.C. Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: Mapping of three classes of repetitive DNAs. Cytogenet. Genome Res. 2009, 125, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.S. Reviewing the chromosome nomenclature of Levan et al. Braz. J. Genet. 1986, 9, 741–743. [Google Scholar]
- Huang, J.; Li, W.; Jian, Z.; Yue, B.; Yan, Y. Genome-wide distribution and organization of microsatellites in six species of birds. Biochem. Syst. Ecol. 2016, 67, 95–102. [Google Scholar] [CrossRef]
- Kretschmer, R.; Ferguson-Smith, M.A.; De Oliveira, E.H.C. Karyotype evolution in birds: From conventional staining to chromosome painting. Genes 2018, 9, 181. [Google Scholar] [CrossRef]
- de Sousa, R.P.C.; de Oliveira Furo, I.; Silva-Oliveira, G.C.; de Sousa-Felix, R.C.; Bessa-Brito, C.D.; Mello, R.C.; Sampaio, I.; Artoni, R.F.; Vallinoto, M. Comparative cytogenetics of microsatellite distribution in two tetra fishes Astyanax bimaculatus (Linnaeus, 1758) and Psalidodon scabripinnis (Jenyns, 1842). PeerJ 2024, 12, e16924. [Google Scholar] [CrossRef]
- Wong, L.H.; Hoo, K.H.A. Evolutionary dynamics of transposable elements at the centromere. Trend Genet. 2004, 20, 611–616. [Google Scholar] [CrossRef]
- Chak, S.T.; Harris, S.E.; Hultgren, K.M.; Jeffery, N.W.; Rubenstein, D.R. Eusociality in snapping shrimps is associated with larger genomes and an accumulation of transposable elements. Proc. Natl. Acad. Sci. USA 2021, 118, e2025051118. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, L.; Zhang, L.; Luo, Z.; Xu, W. The complete mitochondrial genome of Bean goose (Anser fabalis) and implications for Anseriformes taxonomy. PLoS ONE 2013, 8, e63334. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Furo, I.; Kretschmer, R.; dos Santos, M.S.; de Lima Carvalho, C.A.; Gunski, R.J.; O’Brien, P.; Ferguson-Smith, M.A.; Cioffi, M.B.; de Oliveira, E.H. Chromosomal mapping of repetitive DNAs in Myiopsitta monachus and Amazona aestiva (Psittaciformes, Psittacidae) with emphasis on the sex chromosomes. Cytogenet. Genome Res. 2017, 151, 151–160. [Google Scholar] [CrossRef]
- Barcellos, S.; Kretschmer, R.; de Souza, M.S.; Costa, A.L.; Degrandi, T.M.; dos Santos, M.S.; de Oliveira, E.H.C.; Cioffi, M.B.; Gunski, R.J.; Garnero, A.D. Karyotype evolution and distinct evolutionary history of the W chromosomes in swallows (Aves, Passeriformes). Cytogenet. Genome Res. 2019, 158, 98–105. [Google Scholar] [CrossRef]
- Saraiva, D.M.; de Souza, M.S.; Tura, V.; de Rosso, V.O.; Zefa, E.; Garnero, A.D.V.; Gunski, R.J.; Sassi, F.M.Z.; Cioffi, M.B.; Kretschmer, R. Comparative Cytogenetics in Tyrannidae (Aves, Passeriformes): High Genetic Diversity despite Conserved Karyotype Organization. Cytogenet. Genome Res. 2024, 164, 43–51. [Google Scholar] [CrossRef]
- Matsubara, K.; O’Meally, D.; Azad, B.; Georges, A.; Sarre, S.D.; Graves, J.A.M.; Matsuda, Y.; Ezaz, T. Amplification of microsatellite repeat motifs is associated with the evolutionary differentiation and heterochromatinization of sex chromosomes in Sauropsida. Chromosoma 2016, 125, 111–123. [Google Scholar] [CrossRef]
- Vítková, M.; Fuková, I.; Kubíčková, S.; Marec, F. Molecular divergence of the W chromosomes in Pyralid moths (Lepidoptera). Chromosome Res. 2007, 15, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.F.; Skaletsky, H.; Pyntikova, T.; Graves, T.A.; Van Daalen, S.K.; Minx, P.J.; Fulton, R.S.; McGrath, S.D.; Locke, D.P.; Friedman, C.; et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature 2010, 463, 536–539. [Google Scholar] [CrossRef] [PubMed]
- O’meally, D.; Patel, H.R.; Stiglec, R.; Sarre, S.D.; Georges, A.; Marshall Graves, J.A.; Ezaz, T. Non-homologous sex chromosomes of birds and snakes share repetitive sequences. Chromosome Res. 2010, 18, 787–800. [Google Scholar] [CrossRef]
- Hooper, D.M.; Price, T.D. Chromosomal inversion differences correlate with range overlap in passerine birds. Nat. Ecol. Evol. 2017, 1, 1526–1534. [Google Scholar] [CrossRef]
- Ellegren, H. Evolutionary stasis: The stable chromosomes of birds. Trends Ecol. Evol. 2010, 25, 283–291. [Google Scholar] [CrossRef]
- Campagna, L.; Toews, D.P.L. The genomics of adaptation in birds. Curr. Biol. 2022, 32, R1173–R1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Barkley, N.A.; Jenkins, T.M. Microsatellite Markers in Plants and Insects. Genes Genomes Genom. 2009, 3, 54–67. [Google Scholar]
- Santos, J.; Serra, L.; Solé, E.; Pascual, M. FISH mapping of microsatellite loci from Drosophila subobscura and its comparison to related species. Chromosome Res. 2010, 18, 213–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).