Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Fecher, L.A.; Agarwala, S.S.; Hodi, F.S.; Weber, J.S. Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist 2013, 18, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor response, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.C.; Bakkenist, C.J.; Ferris, R.L.; Clump, D.C. Role of immunotherapy in head and neck cancer. Sem. Radiat. Oncol. 2017, 28, 12–16. [Google Scholar] [CrossRef]
- Larkins, E.; Blumenthal, G.M.; Yuan, W.; He, K.; Sridhara, R.; Subramanian, S.; Zhao, H.; Liu, C.; Yu, J.; Goldberg, K.B.; et al. FDA approval summary: Pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist 2017, 22, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Agrawal, S.; Ning, Y.-M.; Maher, E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA approval summary: Atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Beldelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Chen, Y.; Song, S.Y.; Wang, T.J.; Ji, W.J.; Li, S.W.; Liu, N.; Yan, C.-X. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front. Oncol. 2017, 8, 730. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.H.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef]
- Danlos, F.-X.; Voisin, A.-L.; Dyevre, V.; Michot, J.-M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Khan, A.Y.; Malik, S.U.; Faridi, W.; Fraz, M.A.; Usman, M.; Tariq, M.J.; Durer, S.; Durer, C.; Russ, A.; et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol. Blood Marrow Transpl. 2018, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Hughes, P.; Mann, J.; Zhuangming, L.; Teh, J.J.; Mclean, E.; Edmonds, K.; Lingard, K.; Chauhan, D.; Lynch, J.; et al. An immunotherapy survivor population: Health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support. Care Cancer 2020, 28, 561–570. [Google Scholar] [CrossRef]
- Sundaresan, S.B.A.; Nguyen, K.T.; Nelson, K.C.; Ivan, D.; Patel, A.B. Erythema multiforme major in a patient with metastatic melanoma treated with nivolumab. Dermatol. Online J. 2017, 23, 1–3. [Google Scholar] [CrossRef]
- Rapoport, B.L.; van Eeden, R.; Sibaud, V.; Epstein, J.B.; Klastersky, J.; Aapro, M.; Moodley, D. Supportive care for patients undergoing immunotherapy. Support. Care Cancer 2017, 25, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.; Yueh, H.; Ng, Q.S. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur. J. Cancer 2017, 81, 237–239. [Google Scholar] [CrossRef]
- Salati, M.; Pifferi, M.; Baldessari, C.; Bertolini, F.; Tomasello, C.; Cascinu, S.; Barbieri, F. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann. Oncol. 2018, 29, 283–284. [Google Scholar] [CrossRef]
- Nikolaou, V.; Voudouri, D.; Tsironis, G.; Charpidou, A.; Stamoulis, G.; Triantafyllopoulou, I.; Panoutsopoulou, I.; Xidakis, E.; Bamias, A.; Samantas, E. Cutaneous toxicities of antineoplastic agents: Data from a large cohort of Greek patients. Support. Care Cancer 2019, 27, 4535–4542. [Google Scholar] [CrossRef]
- Psyrri, A.; Harrington, K.; Gillison, M.; Ahn, M.J.; Takahashi, S.; Weiss, J.; Machiels, J.P.; Vasilyev, H.; Karpenko, A. Durvalumab with or without tremelimumab versus the EXTREME regimen, as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann. Oncol. 2023, 34, 263–274. [Google Scholar] [CrossRef]
- Jackson, L.K.; Johnson, D.B.; Sosman, J.A.; Murphy, B.A.; Epstein, J.B. Oral health in oncology: Impact of immunotherapy. Support. Care Cancer 2015, 23, 1–3. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibeaud, V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am. J. Dermatol. 2018, 19, S31–S39. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venerol. 2017, 31, e464–e469. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.A.; Shazib, M.A.; Villa, A.; Alves, F.d.A.; Vacharotayangul, P.; Sonis, S.; Fedele, S.; Treister, N.S. Ιmmune checkpoint inhibitors in cancer therapy: Review of orofacial adverse events and role of the oral healthcare provider. Front. Oral Health 2022, 3, 83. [Google Scholar] [CrossRef]
- Klein, B.A.; Alves, F.A.; Velho, J.D.S.R.; Vachrotayangul, P.; Hanna, G.J.; LeBoeuf, N.R.; Shazib, M.A.; Villa, A.; Woo, S.B.; Sroussi, H.; et al. Oral manifestations of immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Oral Dis. 2021, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Masuzawa, M.; Amoh, Y. Oral lichenoid reaction showing multiple ulcers associated with anti-programmed death cell receptor-1 treatment: A report of two cases and published work review. J. Dermatol. 2018, 45, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, I.; Chehal, H.; Migliorati, C. Severe oral erosive lichenoid reaction to pembrolizumab therapy. Oral Surg. Oral Med. Oral Pathol. 2020, 130, e301–e307. [Google Scholar] [CrossRef]
- Shazib, M.A.; Woo, S.B.; Sroussi, H.; Carvo, I.; Treister, N.; Farag, A.; Schoenfeld, J.; Haddad, R.; LeBoeuf, N.; Villa, A. Oral immune-related adverse events associated with PD-1 inhibitor therapy: A case series. Oral Dis. 2020, 26, 325–333. [Google Scholar] [CrossRef]
- Economopoulou, P.; Nicolatou-Galitis, O.; Kotsantis, I.; Psyrri, A. Nivolumab-related lichen planus of the lip in a patient with head and neck cancer. Oral Oncol. 2020, 104, 104623. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Han, J.J.; Baek, S.K.; Kim, H.J.; Maeng, C.H. Pembrolizumab-induced severe oral mucositis in a patient with squamous cell carcinoma of the lung: A case study. Lung Cancer 2020, 147, 21–25. [Google Scholar] [CrossRef]
- Sheth, H.; Pragya, R.; Kovale, S.; Deshpande, M.; Mistry, R.; Shreenivas, A.; Limaye, S. Oral mucositis-case series of a rare adverse effect associated with immunotherapy. Support. Care Cancer 2021, 29, 4705–4709. [Google Scholar] [CrossRef]
- Jacob, J.S.; Dutra, B.E.; Garcia-Rodriguez, V.; Panneerselvan, K.; Abraham, A.; Zou, F.; Ma, W.; Grivas, P.; Thompson, J.A.; Altan, M.; et al. Clinical characteristics and outcomes of oral mucositis associated with immune checkpoint inhibitors in patients with cancer. J. Natl. Compr. Cancer Netw. 2021, 12, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, N.; Sonis, S.T.; Villa, A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women’s Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer 2021, 127, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Scordo, M.; Yom, S.K.; Randazzo, J.; Chapman, P.B.; Huryn, J.M.; Estilo, C.L. Osteonecrosis of the jaw a new complication related to ipilimumab. Oral Oncol. 2015, 51, e100–e101. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Kouri, M.; Papadopoulou, E.; Vardas, E.; Galiti, D.; Epstein, J.B.; Elad, S.; Campisi, G.; Tsoukalas, N.; Bektas-Kayhan, K.; et al. Osteonecrosis of the jaw related to non-antiresorptive medications: A systematic review. Support. Care Cancer 2019, 27, 383–394. [Google Scholar] [CrossRef]
- Decaux, J.; Magremanne, M. Medication-related osteonecrosis of the jaw related to epacadostat and pembrolizumab. J. Stomatol. Oral Maxillofac Surg. 2020, 121, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Pundole, X.; Jones, A.L.; Tetzlaff, M.T.; Williams, M.D.; Murphy, W.A., Jr.; Otun, A.; Goepfert, R.P.; Davies, M.A. Osteonecrosis of the jaw induced by treatment with anti-PD-1 immunotherapy: A case report. Immunotherapy 2020, 12, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Perri, F.; Ionna, F.; Ascierto, P.A.; Grimaldi, A.M. New-generation anticancer drugs and medication-related osteonecrosis of the jaw (MRONJ): Late onset 3 years after ipilimumab endovenous administration with a possible role of target therapy. Clin. Case Rep. 2021, 9, 61–66. [Google Scholar] [CrossRef]
- Myoken, Y.; Fujita, Y.; Kawamoto, K.; Toratani, S. Osteonecrosis of the jaw in a metastatic lung cancer patient with bone metastases undergoing pembrolizumab + denosumab combination therapy: Case report and literature review. Oral Oncol. 2020, 111, 104874. [Google Scholar] [CrossRef]
- Bustillos, H.; Indorf, A.; Alwan, L.; Thompson, J.; Jung, L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Support. Care Cancer 2022, 30, 1681–1687. [Google Scholar] [CrossRef]
- Villafluerte, K.R.V.; Taba, M., Jr.; Messora, M.; Dos Reis, F.J.C.; Carrara, H.A.; Martinez, C.d.J.H.; Palioto, D.B. Effects of non-surgical periodontal therapy on the cytokine profile in gingival crevicular fluid of breast cancer patients with periodontitis undergoing chemotherapy. Support. Care Cancer 2021, 29, 7505–7513. [Google Scholar] [CrossRef]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Brokstad Herlofson, B.; Ristow, O.; Kofod, T. Workshop of European Task force on medication-related osteonecrosis of the jaw-Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Papadopoulou, L.E.; Vardas, E.; Kouri, M.; Galiti, D.; Galitis, E.; Alexiou, K.E.; Tsiklakis, K.; Ardavanis, A.; Razis, E.; et al. Alveolar bone histological nexcrosis observed prior to extractions in patients, who receive bone-targeting agents. Oral Dis. 2020, 26, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Rückschloß, T.; Moratin, J.; Müller, M.; Kühle, R.; Dominik, H.; Pliz, M.; Shavlokhova, V.; Otto, S.; Hoffman, J.; et al. Wound closure and alveoloplasty after preventive tooth extractions in patients with antiresorptive intake-A randomized pilot study. Oral Dis. 2021, 27, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Aljohani, S.; Fleifel, R.; Ecke, S.; Ristow, O.; Burian, E.; Troeltzsch, M.; Pauke, C.; Ehrenfeld, M. Infection as an important factor in Medication-Related Osteonecrosis of the Jaw (MRONJ). Medicina 2021, 57, 463. [Google Scholar] [CrossRef]






| n | % | ||
|---|---|---|---|
| Gender | 19/5 | 79.2/20.8 | |
| M/F | |||
| Age/years | |||
| Range/median | 49–80/64 | ||
| Mean age/standard deviation | 65.88/9.20 | ||
| Cancer type | |||
| Lung ca | 15 | 62.50 | |
| Renal Cell ca | 4 | 16.66 | |
| Melanoma | 2 | 8.33 | |
| Urothelial ca | 2 | 8.33 | |
| Oral ca | 1 | 4.16 | |
| Immunotherapy | 24 | 100.00 | |
| Nivolumab | 11 | ||
| Pembrolizumab | 10 | ||
| Atezolizumab | 1 | ||
| Ipilimumab switched to Nivolumab | 1 | ||
| Pembrolizumab switched to Ipilimumab | 1 | ||
| Cancer therapy prior to immunotherapy | 20 | 83.33 | |
| CT alone | 14 | 58.33 | |
| CT+Zoledronic acid | 1 | 4.16 | |
| CT+Bevacizumab | 2 | 8.33 | |
| Angiogenesis inhibitors alone | 3 | 12.50 | |
| Sunitinib, n = 1 | |||
| Sunitinib followed by Cabozantinib, n = 1 | |||
| Temsirolimus followed by Sunitinib, n = 1 | |||
| Radiotherapy to Head/Neck | 1 | 4.16 | |
| Therapy concurrent with immunotherapy | 9 | 37.5 | |
| Cytotoxic CT, 1 alone and 3 combined with other drugs | 4 | 16.66 | |
| BTA alone (zol, n = 2, Den = 3) | 5 | 20.83 | |
| BTA in combination (n = 3), as following | 3 | 12.50 | |
| CT+Bevacizumab+Zoledronic acid | 1 | ||
| CT+Zoledronic acid | 1 | ||
| CT+Denosumab | 1 | ||
| Patients with a history of autoimmune disease | 3 | 12.50 | |
| Oral lichen planus & autoimmune biliary cirrhosis | 1 | ||
| Dermal lichen planus | 1 | ||
| Vitiligo | 1 | ||
| n | % | ||
|---|---|---|---|
| Reason for referral | |||
| Oral pain | 18 | 75.00 | |
| Dental/mandible pain | 5 | 20.83 | |
| Burning/itching | 7 | 29.16 | |
| Xerostomia | 5 | 20.83 | |
| Gingival bleeding | 3 | 12.50 | |
| Swelling | 3 | 12.50 | |
| Taste problems | 3 | 12.50 | |
| Dental extraction follow-up | 1 | 4.16 | |
| Oral mucosal lesions | |||
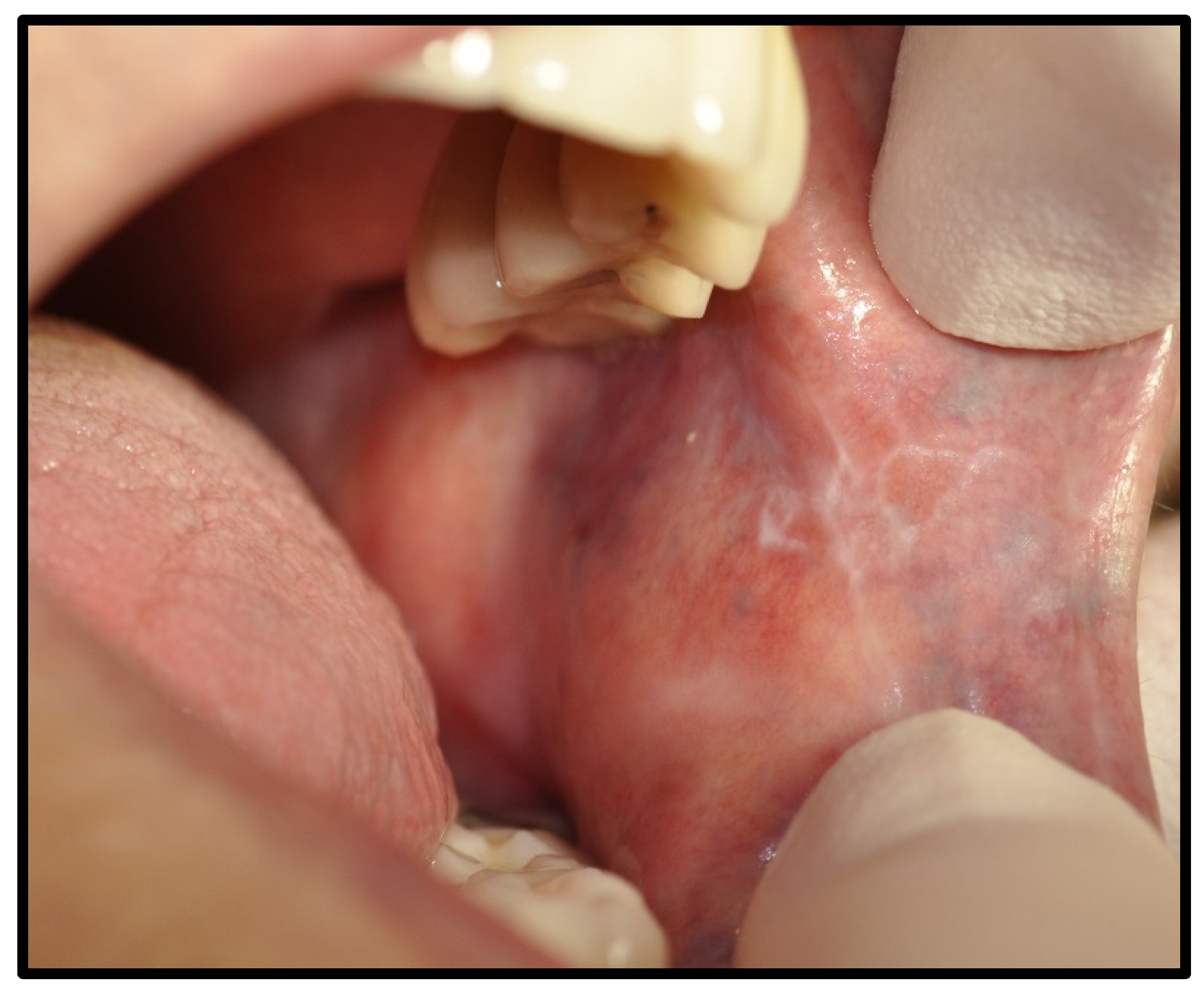
| Immune Related | 15 | 62.50 | |
| OLP/lichenoid reaction (3 were exacerbations of previous autoimmune disease) | 12 | 50.00 | |
| Benign mucous membrane pemphigoid | 3 | 12.50 | |
| Management | Costicosteroid mouthwash, with good response | ||
| Infections | Candidiasis, pseudomembranous (3), & erythematous (1), managed with oral fluconazole | 4 | 16.66 |
| Herpes simplex, lip ulcers, managed with oral acyclovir & topical cream on lip | 2 | 8.33 | |
| Osteonecrosis of the mandible, exposed type | 6 | 25.00 | |
| Medications with known ONJ risk, prior or concurrent with Immunotherapy | |||
| Prior bevacizumab | 1 | ||
| Concurrent zoledronic | 1 | ||
| Prior sunitininb, followed by cabozantinib, concurrent denosumab | 1 | ||
| Concurrent bevacizumab & zoledronic acid | 1 | ||
| Prior & concurrent zoledronic acid | 1 | ||
| Concurrent denosumab | 1 | ||
| Management | Conservative, antibiotics | 6 | |
| Patients with more than one lesion at presentation | 2 | 8.33 | |
| Lichenoid reaction+candidiasis+herpes | 1 | ||
| MRONJ + Candidiasis | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolatou-Galitis, O.; Psyrri, A.; Tsoukalas, N.; Galitis, E.; Linardou, H.; Galiti, D.; Athansiadis, I.; Kalapanida, D.; Razis, E.; Katirtzoglou, N.; et al. Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral 2023, 3, 123-133. https://doi.org/10.3390/oral3010011
Nicolatou-Galitis O, Psyrri A, Tsoukalas N, Galitis E, Linardou H, Galiti D, Athansiadis I, Kalapanida D, Razis E, Katirtzoglou N, et al. Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral. 2023; 3(1):123-133. https://doi.org/10.3390/oral3010011
Chicago/Turabian StyleNicolatou-Galitis, Ourania, Amanda Psyrri, Nikolaos Tsoukalas, Evangelos Galitis, Helena Linardou, Dimitra Galiti, Ilias Athansiadis, Despoina Kalapanida, Evangelia Razis, Nikolaos Katirtzoglou, and et al. 2023. "Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients" Oral 3, no. 1: 123-133. https://doi.org/10.3390/oral3010011
APA StyleNicolatou-Galitis, O., Psyrri, A., Tsoukalas, N., Galitis, E., Linardou, H., Galiti, D., Athansiadis, I., Kalapanida, D., Razis, E., Katirtzoglou, N., Kentepozidis, N., Kosmidis, P., Stavridi, F., Kyrodimos, E., Daliani, D., Tsironis, G., Mountzios, G., Karageorgopoulou, S., Gouveris, P., & Syrigos, K. (2023). Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral, 3(1), 123-133. https://doi.org/10.3390/oral3010011









