Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients
Abstract
:1. Introduction
2. Patients and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Fecher, L.A.; Agarwala, S.S.; Hodi, F.S.; Weber, J.S. Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist 2013, 18, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor response, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.C.; Bakkenist, C.J.; Ferris, R.L.; Clump, D.C. Role of immunotherapy in head and neck cancer. Sem. Radiat. Oncol. 2017, 28, 12–16. [Google Scholar] [CrossRef]
- Larkins, E.; Blumenthal, G.M.; Yuan, W.; He, K.; Sridhara, R.; Subramanian, S.; Zhao, H.; Liu, C.; Yu, J.; Goldberg, K.B.; et al. FDA approval summary: Pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy. Oncologist 2017, 22, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Agrawal, S.; Ning, Y.-M.; Maher, E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA approval summary: Atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist 2019, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Beldelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Chen, Y.; Song, S.Y.; Wang, T.J.; Ji, W.J.; Li, S.W.; Liu, N.; Yan, C.-X. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front. Oncol. 2017, 8, 730. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.H.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef]
- Danlos, F.-X.; Voisin, A.-L.; Dyevre, V.; Michot, J.-M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Khan, A.Y.; Malik, S.U.; Faridi, W.; Fraz, M.A.; Usman, M.; Tariq, M.J.; Durer, S.; Durer, C.; Russ, A.; et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol. Blood Marrow Transpl. 2018, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Hughes, P.; Mann, J.; Zhuangming, L.; Teh, J.J.; Mclean, E.; Edmonds, K.; Lingard, K.; Chauhan, D.; Lynch, J.; et al. An immunotherapy survivor population: Health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support. Care Cancer 2020, 28, 561–570. [Google Scholar] [CrossRef]
- Sundaresan, S.B.A.; Nguyen, K.T.; Nelson, K.C.; Ivan, D.; Patel, A.B. Erythema multiforme major in a patient with metastatic melanoma treated with nivolumab. Dermatol. Online J. 2017, 23, 1–3. [Google Scholar] [CrossRef]
- Rapoport, B.L.; van Eeden, R.; Sibaud, V.; Epstein, J.B.; Klastersky, J.; Aapro, M.; Moodley, D. Supportive care for patients undergoing immunotherapy. Support. Care Cancer 2017, 25, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.; Yueh, H.; Ng, Q.S. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur. J. Cancer 2017, 81, 237–239. [Google Scholar] [CrossRef]
- Salati, M.; Pifferi, M.; Baldessari, C.; Bertolini, F.; Tomasello, C.; Cascinu, S.; Barbieri, F. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann. Oncol. 2018, 29, 283–284. [Google Scholar] [CrossRef]
- Nikolaou, V.; Voudouri, D.; Tsironis, G.; Charpidou, A.; Stamoulis, G.; Triantafyllopoulou, I.; Panoutsopoulou, I.; Xidakis, E.; Bamias, A.; Samantas, E. Cutaneous toxicities of antineoplastic agents: Data from a large cohort of Greek patients. Support. Care Cancer 2019, 27, 4535–4542. [Google Scholar] [CrossRef]
- Psyrri, A.; Harrington, K.; Gillison, M.; Ahn, M.J.; Takahashi, S.; Weiss, J.; Machiels, J.P.; Vasilyev, H.; Karpenko, A. Durvalumab with or without tremelimumab versus the EXTREME regimen, as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann. Oncol. 2023, 34, 263–274. [Google Scholar] [CrossRef]
- Jackson, L.K.; Johnson, D.B.; Sosman, J.A.; Murphy, B.A.; Epstein, J.B. Oral health in oncology: Impact of immunotherapy. Support. Care Cancer 2015, 23, 1–3. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibeaud, V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am. J. Dermatol. 2018, 19, S31–S39. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venerol. 2017, 31, e464–e469. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.A.; Shazib, M.A.; Villa, A.; Alves, F.d.A.; Vacharotayangul, P.; Sonis, S.; Fedele, S.; Treister, N.S. Ιmmune checkpoint inhibitors in cancer therapy: Review of orofacial adverse events and role of the oral healthcare provider. Front. Oral Health 2022, 3, 83. [Google Scholar] [CrossRef]
- Klein, B.A.; Alves, F.A.; Velho, J.D.S.R.; Vachrotayangul, P.; Hanna, G.J.; LeBoeuf, N.R.; Shazib, M.A.; Villa, A.; Woo, S.B.; Sroussi, H.; et al. Oral manifestations of immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Oral Dis. 2021, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Masuzawa, M.; Amoh, Y. Oral lichenoid reaction showing multiple ulcers associated with anti-programmed death cell receptor-1 treatment: A report of two cases and published work review. J. Dermatol. 2018, 45, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, I.; Chehal, H.; Migliorati, C. Severe oral erosive lichenoid reaction to pembrolizumab therapy. Oral Surg. Oral Med. Oral Pathol. 2020, 130, e301–e307. [Google Scholar] [CrossRef]
- Shazib, M.A.; Woo, S.B.; Sroussi, H.; Carvo, I.; Treister, N.; Farag, A.; Schoenfeld, J.; Haddad, R.; LeBoeuf, N.; Villa, A. Oral immune-related adverse events associated with PD-1 inhibitor therapy: A case series. Oral Dis. 2020, 26, 325–333. [Google Scholar] [CrossRef]
- Economopoulou, P.; Nicolatou-Galitis, O.; Kotsantis, I.; Psyrri, A. Nivolumab-related lichen planus of the lip in a patient with head and neck cancer. Oral Oncol. 2020, 104, 104623. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Han, J.J.; Baek, S.K.; Kim, H.J.; Maeng, C.H. Pembrolizumab-induced severe oral mucositis in a patient with squamous cell carcinoma of the lung: A case study. Lung Cancer 2020, 147, 21–25. [Google Scholar] [CrossRef]
- Sheth, H.; Pragya, R.; Kovale, S.; Deshpande, M.; Mistry, R.; Shreenivas, A.; Limaye, S. Oral mucositis-case series of a rare adverse effect associated with immunotherapy. Support. Care Cancer 2021, 29, 4705–4709. [Google Scholar] [CrossRef]
- Jacob, J.S.; Dutra, B.E.; Garcia-Rodriguez, V.; Panneerselvan, K.; Abraham, A.; Zou, F.; Ma, W.; Grivas, P.; Thompson, J.A.; Altan, M.; et al. Clinical characteristics and outcomes of oral mucositis associated with immune checkpoint inhibitors in patients with cancer. J. Natl. Compr. Cancer Netw. 2021, 12, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, N.; Sonis, S.T.; Villa, A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women’s Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer 2021, 127, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Scordo, M.; Yom, S.K.; Randazzo, J.; Chapman, P.B.; Huryn, J.M.; Estilo, C.L. Osteonecrosis of the jaw a new complication related to ipilimumab. Oral Oncol. 2015, 51, e100–e101. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Kouri, M.; Papadopoulou, E.; Vardas, E.; Galiti, D.; Epstein, J.B.; Elad, S.; Campisi, G.; Tsoukalas, N.; Bektas-Kayhan, K.; et al. Osteonecrosis of the jaw related to non-antiresorptive medications: A systematic review. Support. Care Cancer 2019, 27, 383–394. [Google Scholar] [CrossRef]
- Decaux, J.; Magremanne, M. Medication-related osteonecrosis of the jaw related to epacadostat and pembrolizumab. J. Stomatol. Oral Maxillofac Surg. 2020, 121, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Pundole, X.; Jones, A.L.; Tetzlaff, M.T.; Williams, M.D.; Murphy, W.A., Jr.; Otun, A.; Goepfert, R.P.; Davies, M.A. Osteonecrosis of the jaw induced by treatment with anti-PD-1 immunotherapy: A case report. Immunotherapy 2020, 12, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Perri, F.; Ionna, F.; Ascierto, P.A.; Grimaldi, A.M. New-generation anticancer drugs and medication-related osteonecrosis of the jaw (MRONJ): Late onset 3 years after ipilimumab endovenous administration with a possible role of target therapy. Clin. Case Rep. 2021, 9, 61–66. [Google Scholar] [CrossRef]
- Myoken, Y.; Fujita, Y.; Kawamoto, K.; Toratani, S. Osteonecrosis of the jaw in a metastatic lung cancer patient with bone metastases undergoing pembrolizumab + denosumab combination therapy: Case report and literature review. Oral Oncol. 2020, 111, 104874. [Google Scholar] [CrossRef]
- Bustillos, H.; Indorf, A.; Alwan, L.; Thompson, J.; Jung, L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Support. Care Cancer 2022, 30, 1681–1687. [Google Scholar] [CrossRef]
- Villafluerte, K.R.V.; Taba, M., Jr.; Messora, M.; Dos Reis, F.J.C.; Carrara, H.A.; Martinez, C.d.J.H.; Palioto, D.B. Effects of non-surgical periodontal therapy on the cytokine profile in gingival crevicular fluid of breast cancer patients with periodontitis undergoing chemotherapy. Support. Care Cancer 2021, 29, 7505–7513. [Google Scholar] [CrossRef]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Brokstad Herlofson, B.; Ristow, O.; Kofod, T. Workshop of European Task force on medication-related osteonecrosis of the jaw-Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Papadopoulou, L.E.; Vardas, E.; Kouri, M.; Galiti, D.; Galitis, E.; Alexiou, K.E.; Tsiklakis, K.; Ardavanis, A.; Razis, E.; et al. Alveolar bone histological nexcrosis observed prior to extractions in patients, who receive bone-targeting agents. Oral Dis. 2020, 26, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Rückschloß, T.; Moratin, J.; Müller, M.; Kühle, R.; Dominik, H.; Pliz, M.; Shavlokhova, V.; Otto, S.; Hoffman, J.; et al. Wound closure and alveoloplasty after preventive tooth extractions in patients with antiresorptive intake-A randomized pilot study. Oral Dis. 2021, 27, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Aljohani, S.; Fleifel, R.; Ecke, S.; Ristow, O.; Burian, E.; Troeltzsch, M.; Pauke, C.; Ehrenfeld, M. Infection as an important factor in Medication-Related Osteonecrosis of the Jaw (MRONJ). Medicina 2021, 57, 463. [Google Scholar] [CrossRef]






| n | % | ||
|---|---|---|---|
| Gender | 19/5 | 79.2/20.8 | |
| M/F | |||
| Age/years | |||
| Range/median | 49–80/64 | ||
| Mean age/standard deviation | 65.88/9.20 | ||
| Cancer type | |||
| Lung ca | 15 | 62.50 | |
| Renal Cell ca | 4 | 16.66 | |
| Melanoma | 2 | 8.33 | |
| Urothelial ca | 2 | 8.33 | |
| Oral ca | 1 | 4.16 | |
| Immunotherapy | 24 | 100.00 | |
| Nivolumab | 11 | ||
| Pembrolizumab | 10 | ||
| Atezolizumab | 1 | ||
| Ipilimumab switched to Nivolumab | 1 | ||
| Pembrolizumab switched to Ipilimumab | 1 | ||
| Cancer therapy prior to immunotherapy | 20 | 83.33 | |
| CT alone | 14 | 58.33 | |
| CT+Zoledronic acid | 1 | 4.16 | |
| CT+Bevacizumab | 2 | 8.33 | |
| Angiogenesis inhibitors alone | 3 | 12.50 | |
| Sunitinib, n = 1 | |||
| Sunitinib followed by Cabozantinib, n = 1 | |||
| Temsirolimus followed by Sunitinib, n = 1 | |||
| Radiotherapy to Head/Neck | 1 | 4.16 | |
| Therapy concurrent with immunotherapy | 9 | 37.5 | |
| Cytotoxic CT, 1 alone and 3 combined with other drugs | 4 | 16.66 | |
| BTA alone (zol, n = 2, Den = 3) | 5 | 20.83 | |
| BTA in combination (n = 3), as following | 3 | 12.50 | |
| CT+Bevacizumab+Zoledronic acid | 1 | ||
| CT+Zoledronic acid | 1 | ||
| CT+Denosumab | 1 | ||
| Patients with a history of autoimmune disease | 3 | 12.50 | |
| Oral lichen planus & autoimmune biliary cirrhosis | 1 | ||
| Dermal lichen planus | 1 | ||
| Vitiligo | 1 | ||
| n | % | ||
|---|---|---|---|
| Reason for referral | |||
| Oral pain | 18 | 75.00 | |
| Dental/mandible pain | 5 | 20.83 | |
| Burning/itching | 7 | 29.16 | |
| Xerostomia | 5 | 20.83 | |
| Gingival bleeding | 3 | 12.50 | |
| Swelling | 3 | 12.50 | |
| Taste problems | 3 | 12.50 | |
| Dental extraction follow-up | 1 | 4.16 | |
| Oral mucosal lesions | |||
| Immune Related | 15 | 62.50 | |
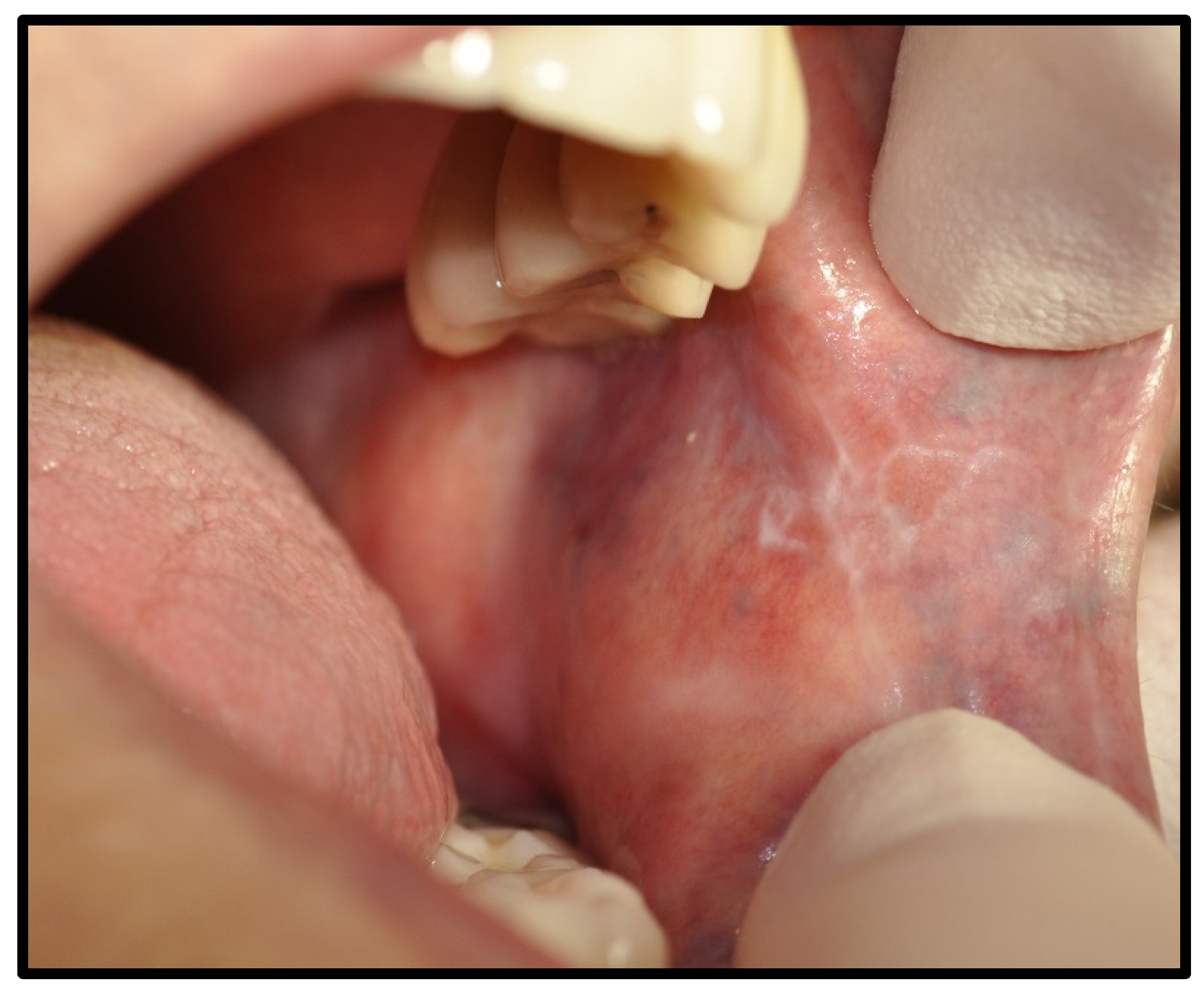
| OLP/lichenoid reaction (3 were exacerbations of previous autoimmune disease) | 12 | 50.00 | |
| Benign mucous membrane pemphigoid | 3 | 12.50 | |
| Management | Costicosteroid mouthwash, with good response | ||
| Infections | Candidiasis, pseudomembranous (3), & erythematous (1), managed with oral fluconazole | 4 | 16.66 |
| Herpes simplex, lip ulcers, managed with oral acyclovir & topical cream on lip | 2 | 8.33 | |
| Osteonecrosis of the mandible, exposed type | 6 | 25.00 | |
| Medications with known ONJ risk, prior or concurrent with Immunotherapy | |||
| Prior bevacizumab | 1 | ||
| Concurrent zoledronic | 1 | ||
| Prior sunitininb, followed by cabozantinib, concurrent denosumab | 1 | ||
| Concurrent bevacizumab & zoledronic acid | 1 | ||
| Prior & concurrent zoledronic acid | 1 | ||
| Concurrent denosumab | 1 | ||
| Management | Conservative, antibiotics | 6 | |
| Patients with more than one lesion at presentation | 2 | 8.33 | |
| Lichenoid reaction+candidiasis+herpes | 1 | ||
| MRONJ + Candidiasis | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolatou-Galitis, O.; Psyrri, A.; Tsoukalas, N.; Galitis, E.; Linardou, H.; Galiti, D.; Athansiadis, I.; Kalapanida, D.; Razis, E.; Katirtzoglou, N.; et al. Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral 2023, 3, 123-133. https://doi.org/10.3390/oral3010011
Nicolatou-Galitis O, Psyrri A, Tsoukalas N, Galitis E, Linardou H, Galiti D, Athansiadis I, Kalapanida D, Razis E, Katirtzoglou N, et al. Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral. 2023; 3(1):123-133. https://doi.org/10.3390/oral3010011
Chicago/Turabian StyleNicolatou-Galitis, Ourania, Amanda Psyrri, Nikolaos Tsoukalas, Evangelos Galitis, Helena Linardou, Dimitra Galiti, Ilias Athansiadis, Despoina Kalapanida, Evangelia Razis, Nikolaos Katirtzoglou, and et al. 2023. "Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients" Oral 3, no. 1: 123-133. https://doi.org/10.3390/oral3010011
APA StyleNicolatou-Galitis, O., Psyrri, A., Tsoukalas, N., Galitis, E., Linardou, H., Galiti, D., Athansiadis, I., Kalapanida, D., Razis, E., Katirtzoglou, N., Kentepozidis, N., Kosmidis, P., Stavridi, F., Kyrodimos, E., Daliani, D., Tsironis, G., Mountzios, G., Karageorgopoulou, S., Gouveris, P., & Syrigos, K. (2023). Oral Toxicities in Cancer Patients, Who Receive Immunotherapy: A Case Series of 24 Patients. Oral, 3(1), 123-133. https://doi.org/10.3390/oral3010011









