The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review
Abstract
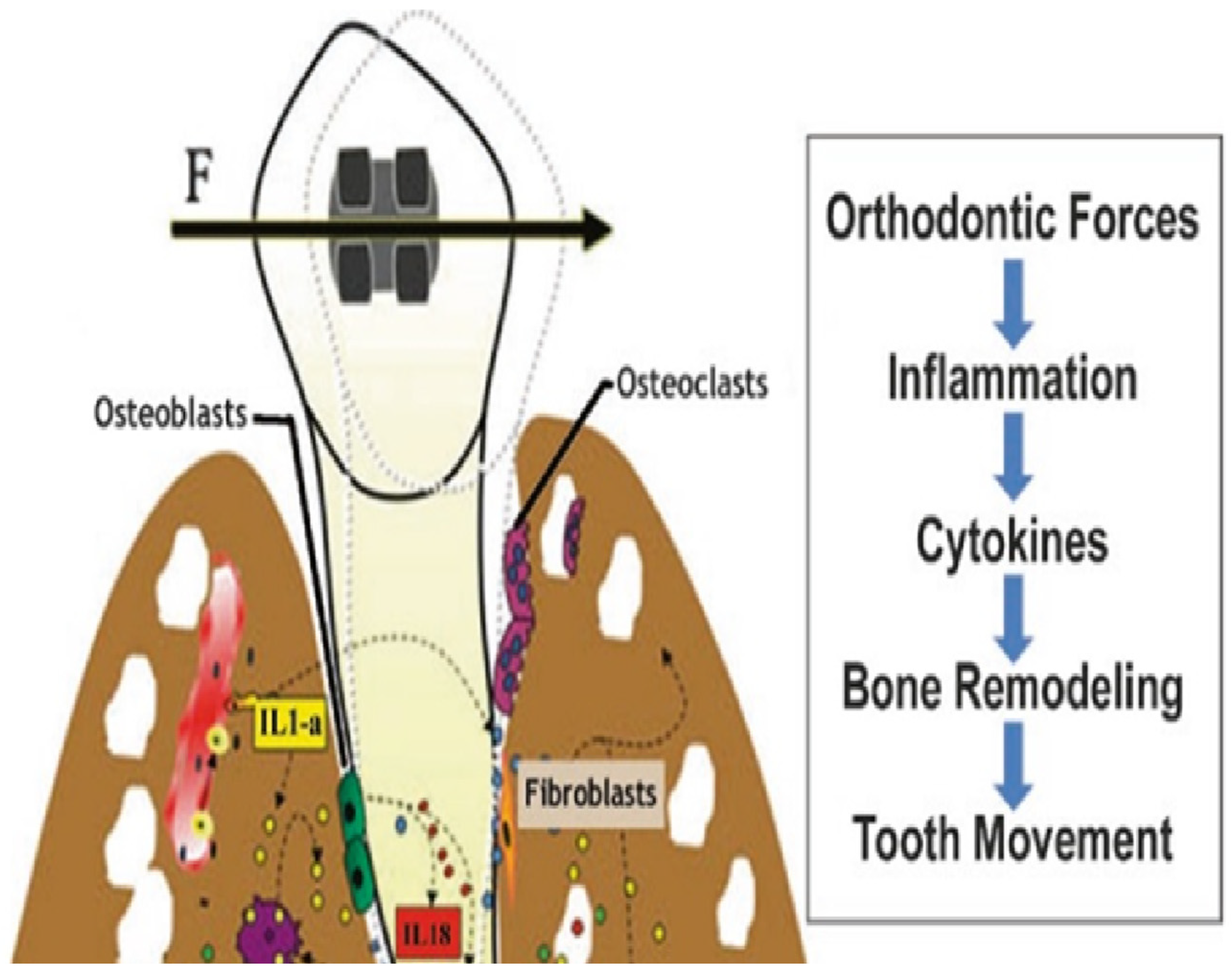
:1. Introduction
2. Methodology
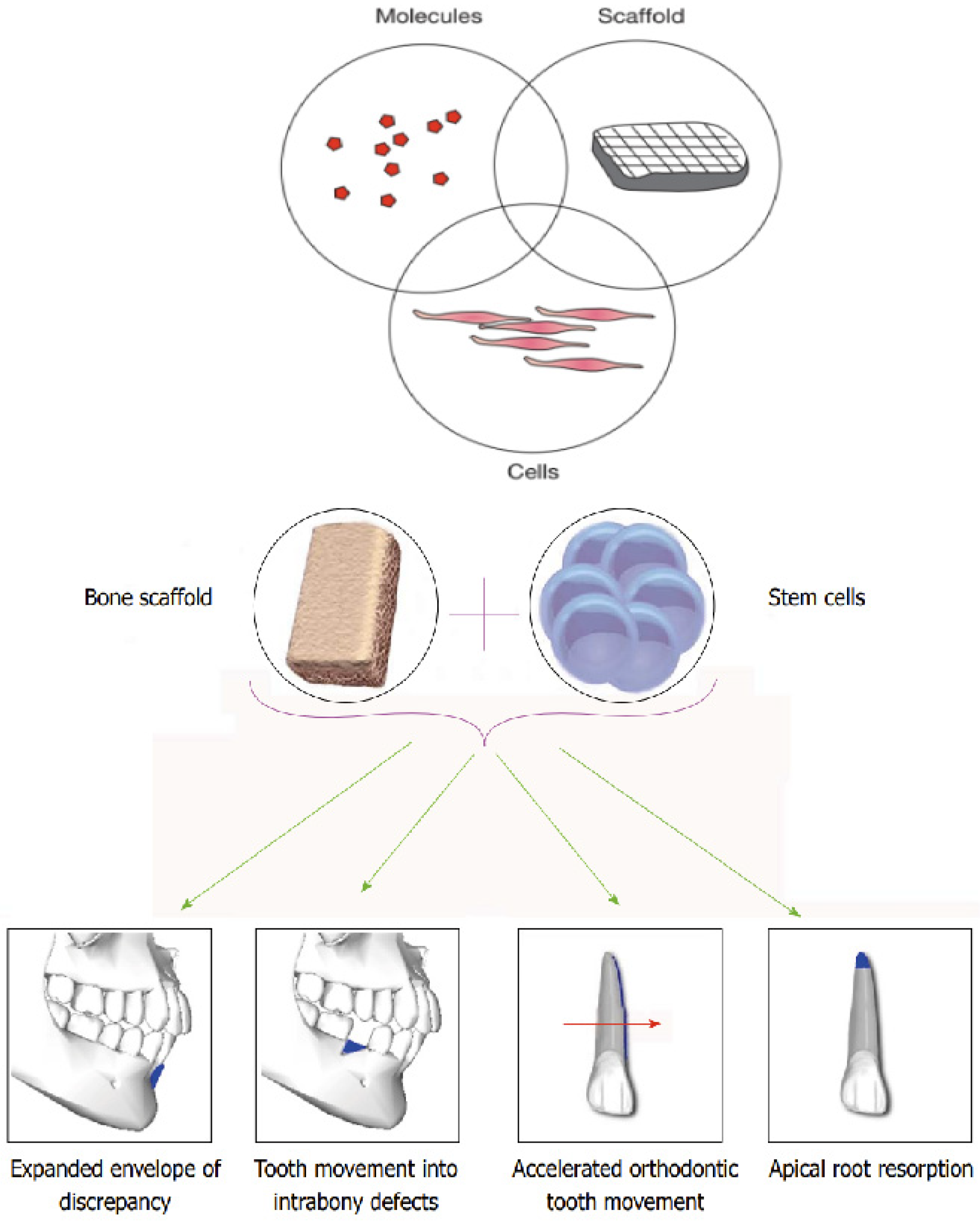
3. Tissue Engineering in Orthodontics
4. Scaffold Systems in Orthodontics
4.1. Transforming Orthodontic Treatment Through Adaptive Technologies
4.2. Advanced Fabrication Techniques
4.2.1. Revolutionizing Scaffold Design with 3D Printing
4.2.2. Functionally Enhanced Scaffold Design
4.2.3. Precision and Customization in Clinical Practice
4.2.4. Integration into Clinical Practice
5. Role of Nanoparticles in Orthodontic Regeneration
5.1. Specific Areas of Application
5.1.1. Brackets and Wires
5.1.2. Adhesives and Cements
5.1.3. Coatings on Appliances
6. Growth Factors in Orthodontic Tissue Engineering: Enhancing Treatment and Recovery
| Growth Factor/Cytokine | Role in Orthodontic Bone Remodeling | Mechanism of Action | Significance in Orthodontics | Refs. |
|---|---|---|---|---|
| Transforming Growth Factor-β (TGF-β) | Influences cell differentiation and proliferation. Modulates bone remodeling. | Stimulates the production of matrix proteins and downregulates matrix degradation, affecting tissue structure. | Essential for the regulation of cellular activities during tooth movement and stabilization of the newly formed bone. | [116,117,118] |
| Vascular Endothelial Growth Factors (VEGFs) | Promotes angiogenesis and tissue regeneration. | Induces endothelial cell proliferation, promotes vessel permeability, and enhances the migration and formation of blood vessels. | Critical for ensuring adequate blood supply for bone healing and regeneration during mechanical stress in orthodontics. | [22,119,120] |
| Platelet-Derived Growth Factors (PDGFs) | Crucial for cell recruitment and proliferation necessary for bone regeneration and repair. | Attracts cells such as fibroblasts, smooth muscle cells, and monocytes to the site of injury, promoting remodeling of the periodontal ligament and alveolar bone. | Supports the movement of teeth and the repair of periodontal tissues, enhancing the response to orthodontic forces. | [118,119,120,121] |
| Fibroblast Growth Factor 2 (FGF2) | Supports proliferation and differentiation of various cells, aiding in angiogenesis and wound healing. | Stimulates endothelial cells, fibroblasts, and other cells, enhancing their proliferation and activities necessary for tissue repair and vascular growth. | Important for rapid tissue repair and regeneration, particularly in the dental pulp during and after orthodontic appliance activation. | [120,121,122] |
| Hepatocyte Growth Factor (HGF) | Supports wound healing and tissue regeneration, responsive to mechanical forces. | Acts on various cell types to promote cellular proliferation, movement, and survival; has anti-fibrotic effects. | Enhance tissue repair and regeneration around moving teeth, minimizing treatment time and improving outcomes. | [115,123,124] |
| Osteoprotegerin (OPG) | Inhibits osteoclast activation, preventing excessive bone resorption. | Functions as a decoy receptor for RANKL, inhibiting its ability to bind RANK on osteoclasts and thus preventing osteoclastogenesis. | Plays a crucial role in maintaining alveolar bone density and preventing unwanted bone loss during tooth movement. | [125,126] |
| Soluble Receptor Activator of Nuclear Factor Kappa-Β Ligand (sRANKL) | Promotes osteoclast differentiation and activity, influencing bone resorption. | Binds to RANK on osteoclast precursors, promoting their maturation and activity, essential for bone remodeling. | Vital for the controlled resorption of bone necessary to accommodate tooth realignment. | [127,128] |
| Bone Morphogenetic Proteins (BMPs) | Stimulates osteoblast differentiation, crucial for bone formation and healing. | Induces the transformation of stem cells into bone-forming osteoblast cells, also induces the production of other growth factors in the osteoblasts. | Plays a pivotal role in the regeneration of bone defects and enhances the stability of teeth post-orthodontic treatment. | [129,130,131] |
| Insulin-like Growth Factors (IGFs) | Enhances osteoblast proliferation, contributing to bone density and growth. | Mediates growth hormone effects, leading to cell proliferation and inhibition of apoptosis in osteoblastic cells. | Supports rapid remodeling required during tooth movement, ensuring timely adjustment to the desired positions. | [132,133,134] |
| Connective Tissue Growth Factor (CTGF) | Involved in connective tissue development and repair, significant for periodontal ligament adjustments. | Promotes extracellular matrix production in connective tissues, influencing fibroblast proliferation and angiogenesis. | Essential for the repair and regeneration of periodontal ligament and surrounding soft tissue during orthodontic treatment. | [135,136] |
| Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α) | Regulate inflammation and bone resorption processes; influence osteoclastic activity. | Pro-inflammatory cytokines that stimulate osteoclastogenesis and bone resorption while modulating immune response in periodontal tissues. | Their regulation is crucial for balancing bone formation and resorption, affecting the stability and duration of treatment. | [21,137,138] |
7. Future Directions of Role of Artificial Inelligence (AI) and Machine Learning in Personalizing Orthodontic Treatment
7.1. AI in the Design of Tissue-Engineered Products and Nanoparticle-Enhanced Materials
7.2. AI-Driven Personalization: Genetic Profiles and Predictive Modeling
7.3. Multi-Modal Data Integration
7.4. AI-Driven Predictive Models
8. Discussion
8.1. Limitations
8.2. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farhadi, M.; Tashakor, A.; Farjaminejad, R.; Jamilian, A. Treatment of Maxillary Deficiency with Reverse Chin Cup: A Case Report. Eur. J. Dent. Oral Health 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Meeran, N.A. Iatrogenic possibilities of orthodontic treatment and modalities of prevention. J. Orthod. Sci. 2013, 2, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Ganji, N.R.; Hassanvand, A.; Zandi, A.; Rabbanidoost, A.; Safdari, S.; Jouzdani, K.M.; Naseri, M.; Amiri, M. Emerging Innovations: Cutting-Edge Medical and Dental Advances Transforming Healthcare; Nobel Sciences: Pune, India; Available online: https://books.google.com/books/about/Emerging_Innovations_Cutting_Edge_Medica.html?id=icj0EAAAQBAJ (accessed on 17 February 2025).
- Abid, M.; Jamal, H.; Alsahafi, E.; Dziedzic, A.; Kubina, R. Tissue Engineering Supporting Regenerative Strategies to Enhance Clinical Orthodontics and Dentofacial Orthopaedics: A Scoping, Perspective Review. Biomedicines 2023, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Motyl, S.; Manfredini, D.; Oruba, Z.; Bugajska, J.; Sztefko, K.; Stós, W.; Osiewicz, M.; Loster, B.W.; Lobbezoo, F. Evaluation of interleukin-1 beta and the ratio of interleukin-1 beta to interleukin-1 receptor antagonist in gingival crevicular fluid during orthodontic canine retraction. Dent. Med. Probl. 2021, 58, 47–54. [Google Scholar] [CrossRef]
- Yazarloo, S.; Arab, S.; Mirhashemi, A.H.; Gholamrezayi, E. Systematic review of preventive and treatment measures regarding orthodontically induced white spot lesions. Dent. Med. Probl. 2023, 60, 527–535. [Google Scholar]
- Villaman-Santacruz, H.; Torres-Rosas, R.; Acevedo-Mascarúa, A.E.; Argueta-Figueroa, L. Root resorption factors associated with orthodontic treatment with fixed appliances: A systematic review and meta-analysis. Dent. Med. Probl. 2022, 59, 437–450. [Google Scholar] [CrossRef]
- Soheilifar, S.; Ataei, H.; Mollabashi, V.; Amini, P.; Bakhshaei, A.; Naghdi, N. Extraction versus non-extraction orthodontic treatment: Soft tissue profile changes in borderline class I patients. Dent. Med. Probl. 2020, 57, 275–283. [Google Scholar]
- Tahmasebi, E.; Alam, M.; Yazdanian, M.; Tebyanian, H.; Yazdanian, A.; Seifalian, A.; Mosaddad, S.A. Current biocompatible materials in oral regeneration: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2020, 9, 11731–11755. [Google Scholar] [CrossRef]
- Kaukua, N.; Fried, K.; Mao, J.J. Regenerative Medicine in Orthodontic Therapy. Integr. Clin. Orthod. 2023, 30, 541–564. [Google Scholar]
- Thomas, S.; Grohens, Y.; Ninan, N. (Eds.) Nanotechnology Applications for Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2015. [Google Scholar]
- Rossi, N.J.; Rossi, R.C.; Rossi, N.J.C. Procedures to accelerate orthodontic treatment: Review of techniques and biological bases. Int. J. Dent. Res. 2019, 4, 30–37. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Hasani, M.; Farjaminejad, R.; Foroozandeh, A.; Abdouss, M.; Hasanzadeh, M. The critical role of nano-hydroxyapatites as an advanced scaffold in drug delivery towards efficient bone regeneration: Recent progress and challenges. Carbohydr. Polym. Technol. Appl. 2025, 9, 100692. [Google Scholar]
- Yu, Y.; Li, X. Current Application of Magnetic Materials in the Dental Field. Magnetochemistry 2024, 10, 46. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Kouhi, M.; de Souza Araújo, I.J.; Asa’ad, F.; Zeenat, L.; Bojedla, S.S.R.; Pati, F.; Zolfagharian, A.; Watts, D.C.; Bottino, M.C.; Bodaghi, M. Recent advances in additive manufacturing of patient-specific devices for dental and maxillofacial rehabilitation. Dent. Mater. 2024, 40, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sharpe, P.; Volponi, A.A. Applications of regenerative techniques in adult orthodontics. Front. Dent. Med. 2023, 3, 1100548. [Google Scholar] [CrossRef]
- Palanisamy, S. Exploring the Horizons of Four-Dimensional Printing Technology in Dentistry. Cureus 2024, 16, 4. [Google Scholar] [CrossRef]
- Kaukua, N.; Fried, K.; Mao, J.J. Tissue engineering in orthodontic therapy. In Integrated Cclinical Orthodontics, 1st ed.; Blackwell: Oxford, UK, 2012; pp. 380–391. [Google Scholar]
- Safari, S.; Mahdian, A.; Motamedian, S.R. Applications of stem cells in orthodontics and dentofacial orthopedics: Current trends and future perspectives. World J. Stem Cells 2018, 10, 66. [Google Scholar] [CrossRef]
- Guarino, V.; Pérez, M.A.Á. (Eds.) Current Advances in Oral and Craniofacial Tissue Engineering; CRC Press/Taylor and Francis Group: New York, NY, USA, 2021. [Google Scholar]
- Roberts, W.E.; Huja, S.S. Bone physiology, metabolism, and biomechanics in orthodontic practice. In Orthodontics: Current Principles and Techniques, 6th ed.; Mosby: St Louis, LA, USA, 2016; pp. 99–153. [Google Scholar]
- Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A review on current trends of polymers in orthodontics: BPA-free and smart materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Maroof, M.; Sujithra, R.; Tewari, R. Superelastic and shape memory equi-atomic nickel-titanium (Ni-Ti) alloy in dentistry: A systematic review. Mater. Today Commun. 2022, 33, 104352. [Google Scholar] [CrossRef]
- Gupta, I.; Mangat, P. Bio Smart Dentistry; Dent Publications: Bijnor, India, 2024. [Google Scholar]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; King, M.W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 2011, 58, 423–438. [Google Scholar] [CrossRef]
- Zhang, W.; Yelick, C. Craniofacial tissue engineering. Cold Spring Harb. Perspect. Med. 2018, 8, a025775. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Daniele, R.; Giardino, R.; di Carlo, S.; Piccoli, L.; Cascone, P.; Pompa, G. Prosthetic rehabilitation with partial removable and aesthetic dentures (Valplast) in a patient with recurrent right TMJ ankylosis: A case report. Minerva Stomatol. 2016, 65 (Suppl. S1), 245–246. [Google Scholar]
- Poulton, D.R. Preliminary program of the ninety-ninth annual session, May 14-18, 1999. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 186–215. [Google Scholar] [CrossRef]
- Pathak, K.; Saikia, R.; Das, A.; Das, D.; Islam, M.A.; Pramanik, P.; Parasar, A.; Borthakur, P.P.; Sarmah, P.; Saikia, M.; et al. 3D printing in biomedicine: Advancing personalized care through additive manufacturing. Explor. Med. 2023, 4, 1135–1167. [Google Scholar] [CrossRef]
- Murphy, N.C. In vivo tissue engineering for orthodontists: A modest first step Biological Mechanisms of Tooth Eruption, Resorption and Movement. In Biological Mechanisms of Tooth Eruption, Resorption and Movement; Harvard Society for the Advancement of Orthodontics: Boston, MA, USA, 2006; pp. 385–410. [Google Scholar]
- Gašparovič, M.; Jungová, P.; Tomášik, J.; Mriňáková, B.; Hirjak, D.; Timková, S.; Danišovič, Ľ.; Janek, M.; Bača, Ľ.; Peciar, P.; et al. Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry. Appl. Sci. 2024, 14, 2270. [Google Scholar] [CrossRef]
- Yang, Z.; Han, L.; Guo, Y.; Jia, L.; Yin, C.; Xia, Y. Nanotechnology in Dental Therapy and Oral Tissue Regeneration. In Nanotechnology in Regenerative Medicine and Drug Delivery Therapy; Xu, H., Gu, N., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Cui, H.; You, Y.; Cheng, G.W.; Lan, Z.; Zou, K.L.; Mai, Q.Y.; Han, Y.H.; Chen, H.; Zhao, Y.Y.; Yu, G.T. Advanced materials and technologies for oral diseases. Sci. Technol. Adv. Mater. 2023, 24, 2156257. [Google Scholar] [CrossRef]
- Kida, D.; Zakrzewska, A.; Zborowski, J.; Szulc, M.; Karolewicz, B. Polymer-based carriers in dental local healing—Review and future challenges. Materials 2021, 14, 3948. [Google Scholar] [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N. Effects of drugs and systemic factors on orthodontic treatment. Quintessence Int. 2001, 32, 5. [Google Scholar]
- Farjaminejad, S.; Farjaminejad, R.; Garcia-Godoy, F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. J. Funct. Biomater. 2024, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Sidhu, M.S.; Grover, S.; Prabhakar, M. “A Century of Orthodontic Progress”–Innovations in Orthodontics. Orthod. J. Nepal 2021, 11, 65–71. [Google Scholar] [CrossRef]
- Konda, P.; Rafiq, A.M. 3D printing: Changing the landscape of orthodontics. Technology 2024, 7, 9. [Google Scholar]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Farjaminejad, R.; Farjaminejad, S.; Nucci, L.; d’Apuzzo, F.; Grassia, V.; Majidi, K.; Jamilian, A. 3D Printing Approach in Maxillofacial Surgery in Iran: An Evaluation Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework. Appl. Sci. 2024, 14, 3075. [Google Scholar] [CrossRef]
- Omigbodun, F.T.; Oladapo, B.I.; Osa-uwagboe, N. Exploring the frontier of Polylactic Acid/Hydroxyapatite composites in bone regeneration and their revolutionary biomedical applications–A review. J. Reinf. Plast. Compos. 2024, 1–21. [Google Scholar] [CrossRef]
- Nesic, D.; Schaefer, B.M.; Sun, Y.; Saulacic, N.; Sailer, I. 3D printing approach in dentistry: The future for personalized oral soft tissue regeneration. J. Clin. Med. 2020, 9, 2238. [Google Scholar] [CrossRef]
- Taneva, E.; Kusnoto, B.; Evans, C.A. 3D scanning, imaging, and printing in orthodontics. Issues Contemp. Orthod. 2015, 148, 862–867. [Google Scholar]
- Abueed, O.; Gharaibeh, B.; Alalawin, A.; Mahfouf, M.; Alsoussi, A.; Albashabsheh, N. The development of a radial based integrated network for the modelling of 3D fused deposition. Rapid Prototyp. J. 2022, 29, 408–421. [Google Scholar]
- Ryan, K.R.; Down, M.; Banks, C.E. Future of additive manufacturing: Overview of 4D and 3D printed smart and advanced materials and their applications. Chem. Eng. J. 2021, 403, 126162. [Google Scholar] [CrossRef]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on bone substitutes through 3D bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Atwal, N.; Bhatnagar, D. Evaluating and Comparing Flexure Strength of Dental Models Printed Using Fused Deposition Modelling, Digital Light Processing, and Stereolithography Apparatus Printers. Cureus 2024, 16, 16. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-printing: Transferring art from the laboratories to the clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Canzi, P.; Marconi, S.; Montasser, M.A.; Bressani, D.; Gandini, P.; Sfondrini, M.F. Properties of CAD/CAM 3D printing dental materials and their clinical applications in orthodontics: Where are we now? Appl. Sci. 2022, 12, 551. [Google Scholar] [CrossRef]
- Vyas, C. Development of a Multi-Material and Multi-Scale 3d Bioprinted Scaffold for Osteochondral Tissue Engineering; The University of Manchester: Manchester, UK, 2019. [Google Scholar]
- Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Wang, Y.; Peng, C.; Wu, D. Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration. Mater. Today Bio 2022, 16, 100409. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Pouliezou, I.; Gravia, A.P.; Vasoglou, M. Digital Model in Orthodontics: Is It Really Necessary for Every Treatment Procedure? A Scoping Review. Oral 2024, 4, 243–262. [Google Scholar] [CrossRef]
- Tsoukala, E.; Lyros, I.; Tsolakis, A.I.; Maroulakos, M.; Tsolakis, I.A. Direct 3d-printed orthodontic retainers. a systematic review. Children 2023, 10, 676. [Google Scholar] [CrossRef]
- Sehrawat, S.; Kumar, A.; Prabhakar, M.; Nindra, J. The expanding domains of 3D printing pertaining to the speciality of orthodontics. Mater. Today Proc. 2022, 50, 1611–1618. [Google Scholar] [CrossRef]
- Zhang, L.; Morsi, Y.; Wang, Y.; Li, Y.; Ramakrishna, S. Review scaffold design and stem cells for tooth regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 14–26. [Google Scholar] [CrossRef]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 16. [Google Scholar]
- Soraya, L.; Najoua, L.; Houda, L.; Malika, S.I. Latest Intelligent and Sustainable Materials for Dental Application. In Handbook of Intelligent and Sustainable Smart Dentistry; Taylor and Francis: Abingdon, UK, 2024; pp. 87–129. [Google Scholar]
- Batra, P. Applications of nanoparticles in orthodontics. Dent. Appl. Nanotechnol. 2018, 81–105. Available online: https://link.springer.com/chapter/10.1007/978-3-319-97634-1_5 (accessed on 17 February 2025).
- Farjaminejad, S.; Shojaei, S.; Goodarzi, V.; Khonakdar, H.A.; Abdouss, M. Tuning properties of bio-rubbers and its nanocomposites with addition of succinic acid and ɛ-caprolactone monomers to poly (glycerol sebacic acid) as main platform for application in tissue engineering. Eur. Polym. J. 2021, 159, 110711. [Google Scholar] [CrossRef]
- Al Othman, Z.A.; Alam, M.M.; Naushad, M.; Inamuddin, I.; Khan, M.F. Inorganic nanoparticles and nanomaterials based on titanium (Ti): Applications in medicine. In Materials Science Forum; Trans Tech Publications Ltd.: Bach, Switzerland, 2013; Volume 754, pp. 21–87. [Google Scholar]
- Zhang, R.; Han, B.; Liu, X. Functional surface coatings on orthodontic appliances: Reviews of friction reduction, antibacterial properties, and corrosion resistance. Int. J. Mol. Sci. 2023, 24, 6919. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, M.; Movahedi Fazel, S.; Rangrazi, A. Incorporation of chitosan nanoparticles into a cold-cure orthodontic acrylic resin: Effects on mechanical properties. Biomimetics 2021, 6, 7. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials application in orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Gil, F.J.; Espinar-Escalona, E.; Clusellas, N.; Fernandez-Bozal, J.; Artes-Ribas, M.; Puigdollers, A. New bactericide orthodonthic archwire: NiTi with silver nanoparticles. Metals 2020, 10, 702. [Google Scholar] [CrossRef]
- Indumathi, P.; Singh, D.; Sharma, V.K.; Shukla, N.K.; Chaturvedi, T. The effect of various nanoparticle coating on the frictional resistance at orthodontic wire and bracket interface: A systematic review. J. Orthod. Sci. 2022, 11, 7. [Google Scholar]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial properties of nanoparticles in dental restorative materials. A systematic review and meta-analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Qin, D.; Wei, F.; Xiaobing, L. Application of antibacterial nanoparticles in orthodontic materials. Nanotechnol. Rev. 2022, 11, 2433–2450. [Google Scholar] [CrossRef]
- Brézulier, D.; Chaigneau, L.; Jeanne, S.; Lebullenger, R. The challenge of 3D bioprinting of composite natural polymers PLA/bioglass: Trends and benefits in cleft palate surgery. Biomedicines 2021, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ferreira, J.M. 5—Bioresorbable composites for bone repair. In Biomedical Composites: Materials, Manufacturing and Engineering; De Gruyter: Boston, MA, USA; Berlin, Germany, 2014; pp. 69–88. [Google Scholar]
- Shah, R.; Sinanan, A.C.M.; Knowles, J.C.; Hunt, N.; Lewis, M. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials 2005, 26, 1497–1505. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamaguchi, M.; Tanimoto, Y.; Yao-Umezawa, E.; Kasai, K. Biological evaluation of a prototype material made of polyglycolic acid and hydroxyapatite. J. Hard Tissue Biol. 2015, 24, 375–384. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, Q.; Xu, L.; Hu, M.; Xu, J.; Wang, Y.; Song, Y. Preparation and performance evaluation of a novel orthodontic adhesive incorporating composite dimethylaminohexadecyl methacrylate—Polycaprolactone fibers. PLoS ONE 2024, 19, e0304143. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Q.; Xu, X.; Du, Y.; Xu, J.; Song, Y.; Wang, Y. Development and Characterization of Novel Orthodontic Adhesive Containing PCL–Gelatin–AgNPs Fibers. J. Funct. Biomater. 2022, 13, 303. [Google Scholar] [CrossRef]
- Kamran, M.A.; Alnazeh, A.A.; Hameed, M.S.; Yassin, S.M.; Mannakandath, M.L.; Alshahrani, I. Formulation and clinical performance of nanosilver loaded poly-l-glycolic acid modified orthodontic adhesive for orthodontic bonding. J. Mol. Struct. 2022, 1249, 131490. [Google Scholar] [CrossRef]
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear bond strength, adhesive remnant index, and anti-biofilm effects of a photoexcited modified orthodontic adhesive containing curcumin doped poly lactic-co-glycolic acid nanoparticles: An ex-vivo biofilm model of S. mutans on the enamel slab bonded brackets. Photodiagnosis Photodyn. Ther. 2020, 30, 101674. [Google Scholar]
- Sodagar, A.; Akhavan, A.; Hashemi, E.; Arab, S.; Pourhajibagher, M.; Sodagar, K.; Kharrazifard, M.J.; Bahador, A. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog. Orthod. 2016, 17, 1–7. [Google Scholar] [CrossRef]
- Mohammed-Salih, H.S.; Ghazi, A.; Mahmood, R.I.; Al-Qazzaz, H.H.; Supian, F.L.; Al-Obaidi, J.R.; Jabir, M. Enhancing orthodontic treatment control with fish scale-derived hydroxyapatite nanoparticles: Insights from an animal model study. Saudi Dent. J. 2024, 36, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Nishioka-Sakamoto, K.; Hotokezaka, H.; Hotokezaka, Y.; Nashiro, Y.; Funaki, M.; Ohba, S.; Yoshida, N. Fixation of an orthodontic anchor screw using beta-tricalcium phosphate in a screw-loosening model in rats. Angle Orthod. 2023, 93, 341–347. [Google Scholar] [CrossRef]
- Niwa, K.; Ogawa, K.; Miyazawa, K.; Aoki, T.; Kawai, T.; Goto, S. Application of α-tricalcium phosphate coatings on titanium subperiosteal orthodontic implants reduces the time for absolute anchorage: A study using rabbit femora. Dent. Mater. J. 2009, 28, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Weijs, W.L.J.; Siebers, T.J.H.; Kuijpers-Jagtman, A.M.; Bergé, S.J.; Meijer, G.J.; Borstlap, W.A. Early secondary closure of alveolar clefts with mandibular symphyseal bone grafts and β-tri calcium phosphate (β-TCP). Int. J. Oral Maxillofac. Surg. 2010, 39, 424–429. [Google Scholar] [CrossRef]
- El Shazley, N.; Hamdy, A.; El-Eneen, H.A.; El Backly, R.M.; Saad, M.M.; Essam, W.; Moussa, H.; El Tantawi, M.; Jain, H.; Marei, M.K. Bioglass in alveolar bone regeneration in orthodontic patients: Randomized controlled clinical trial. JDR Clin. Transl. Res. 2016, 1, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Domagała, I.; Przystupa, K.; Firlej, M.; Pieniak, D.; Niewczas, A.; Biedziak, B. Bending behaviour of polymeric materials used on biomechanics orthodontic appliances. Materials 2020, 13, 5579. [Google Scholar] [CrossRef]
- Schickert, S.D.L. Polymer-Ceramic Nancomposites for Bone Regeneration. PhD Thesis, Universidade Catolica Portuguesa, Lisboa, Portugal, 2014. [Google Scholar]
- Cacciafesta, V.; Sfondrini, M.F.; Norcini, A.; Macchi, A. Fiber-reinforced composites in lingual orthodontics. J. Clin. Orthod. 2005, 39, 710. [Google Scholar]
- Khan, A.A.; Zafar, M.S.; Fareed, M.A.; AlMufareh, N.A.; Alshehri, F.; AlSunbul, H.; Lassila, L.; Garoushi, S.; Vallittu, K. Fiber-reinforced composites in dentistry–An insight into adhesion aspects of the material and the restored tooth construct. Dent. Mater. 2023, 39, 141–151. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B. Recent advances in alginates as material for biomedical applications. Alginates 2019, 25–88. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780429023439-2/recent-advances-alginates-material-biomedical-applications-milan-milivojevic-ivana-pajic-lijakovic-branko-bugarski (accessed on 17 February 2025).
- Atia, G.A.; Shalaby, H.K.; Roomi, A.B.; Ghobashy, M.M.; Attia, H.A.; Mohamed, S.Z.; Abdeen, A.; Abdo, M.; Fericean, L.; Bănățean Dunea, I.; et al. Macro, micro, and nano-inspired bioactive polymeric biomaterials in therapeutic, and regenerative orofacial applications. Drug Design Dev. Ther. 2023, 17, 2985–3021. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Akkurt, M.D.; Amasyali, M.; Ozcan, S.; Yagci, A.; Basak, F.; Sagdic, D. Does a chitosan-containing dentifrice prevent demineralization around orthodontic brackets? Angle Orthod. 2011, 81, 319–325. [Google Scholar] [CrossRef]
- Ly, N.T.K.; Shin, H.; Gupta, K.C.; Kang, I.K.; Yu, W. Bioactive antibacterial modification of orthodontic microimplants using chitosan biopolymer. Macromol. Res. 2019, 27, 504–510. [Google Scholar] [CrossRef]
- Juárez, E.N. Biological effects of chitosan in Dentistry. Mex. J. Med. Res. ICSA 2023, 11, 36–40. [Google Scholar] [CrossRef]
- Tallarico, M.; Park, C.J.; Lumbau, A.I.; Annucci, M.; Baldoni, E.; Koshovari, A.; Meloni, S.M. Customized 3D-printed titanium mesh developed to regenerate a complex bone defect in the aesthetic zone: A case report approached with a fully digital workflow. Materials 2020, 13, 3874. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Kareem, S.A.; Ozah, B.N.; Alshahrani, H.A.; Ajibuwa, O.A. Application of finite element analysis for optimizing selection and design of Ti-based biometallic alloys for fractures and tissues rehabilitation: A review. J. Mater. Res. Technol. 2022, 19, 121–139. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, M.; Lee, D.; Han, S.; Moon, J.H.; Lim, H.N.; Kwon, I.K. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic applications of antimicrobial silver-based biomaterials in dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- He, L.; Zhang, W.; Liu, J.; Pan, Y.; Li, S.; Xie, Y. Applications of nanotechnology in orthodontics: A comprehensive review of tooth movement, antibacterial properties, friction reduction, and corrosion resistance. BioMed. Eng. OnLine 2024, 23, 72. [Google Scholar] [CrossRef]
- Shoorgashti, R.; Shahrzad, H.; Nowroozi, S.; Ghadamgahi, B.; Mehrara, R.; Oroojalian, F. Evaluation of the antibacterial and cytotoxic activities of Ag/ZnO nanoparticles loaded polycaprolactone/chitosan composites for dental applications. Nanomed. J. 2023, 10, 68. [Google Scholar]
- Sreenivasalu, K.; Dora, C.; Swami, R.; Jasthi, V.C.; Shiroorkar, N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K. Nanomaterials in dentistry: Current applications and future scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Musuc, A.M.; Santos, A.C.; Paszkiewicz, S.; Idumah, C.I.; Singh, S.; Varma, R.S.; Berrada, M. Nano-hydroxyapatite (nHAp) scaffolds for bone regeneration: Preparation, characterization and biological applications. J. Drug Deliv. Sci. Technol. 2024, 18, 105601. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Yfanti, Z.; Christopoulos, P.; Donta, C.; Damaskos, S.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.M.; Tosios, K.I.; Tsiklakis, K. Imaging of nano-hydroxyapatite/chitosan scaffolds using a cone beam computed tomography device on rat calvarial defects with histological verification. Clin. Oral Investig. 2020, 24, 437–446. [Google Scholar] [CrossRef]
- Aguiar, R.C.D.O.; Nunes, L.; Batista, E.S.; Viana, M.M.; Rodrigues, M.C.; Bueno-Silva, B.; Roscoe, M.G. Experimental composite containing silicon dioxide-coated silver nanoparticles for orthodontic bonding: Antimicrobial activity and shear bond strength. Dent. Press J. Orthod. 2022, 27, e222116. [Google Scholar] [CrossRef] [PubMed]
- Shadlou, S.; Ahmadi-Moghadam, B.; Taheri, F. Nano-Enhanced Adhesives. Prog. Adhes. Adhes. 2015, 31, 357–396. [Google Scholar]
- Wang, Y.L.; Liu, Z.J. Unique Features of Nanomaterials and their Combination Support Applications in Orthodontics. Chin. J. Dent. Res. 2023, 26, 143–152. [Google Scholar]
- Gracco, A.; Siviero, L.; Dandrea, M.; Crivellin, G. Use of nanotechnology for the superlubrication of orthodontic wires. In Nanobiomaterials in Dentistry; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 241–267. [Google Scholar]
- Ravi, J.; Eshwar, A. Tissue Engineering in Orthodontics—A Review. J. Adv. Med. Dent. Sci. Res. 2020, 8, 92–97. [Google Scholar]
- Mundy, G.R. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin. Orthop. Relat. Res. 1996, 324, 24–27. [Google Scholar] [CrossRef]
- Komath, M.; Varma, H.K.; John, A.; Krishnan, V.; Simon, D.; Ramanathan, M.; Bhuvaneshwar, G.S. Designing Bioactive Scaffolds for Dental Tissue Engineering. In Regenerative Medicine: Laboratory to Clinic; Springer: Singapore, 2017; pp. 423–447. [Google Scholar]
- Radermacher, C.; Craveiro, R.B.; Jahnen-Dechent, W.; Beier, J.; Bülow, A.; Wolf, M.; Neuss, S. Impact of compression forces on different mesenchymal stem cell types regarding orthodontic indication. Stem Cells Transl. Med. 2024, 13, szae057. [Google Scholar] [CrossRef]
- Derringer, K.A.; Linden, R.W.A. Vascular endothelial growth factor, fibroblast growth factor 2, platelet derived growth factor and transforming growth factor beta released in human dental pulp following orthodontic force. Arch. Oral Biol. 2004, 49, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jianguo, L.; Qi, S.; Mu, S.; Jiangtao, Z.; Xiaoyan, G.; Juxiang, P. Synergistic effect of platelet-derived growth factor-BB and transforming growth factor-β 1 on expression of integrin β 3 in periodontal membrane of rat orthodontic tooth. West China J. Stomatol. 2014, 32, 4. [Google Scholar]
- Pawinru, A.S.; Achmad, H.; Wahyuni, S.; Erwansyah, E.; Narmada, I.B.; Samad, R.; Bukhari, A.; Habar, E.H. Effect of ethanol intake on bone remodeling process during orthodontic treatment in male wistar rats. J. Dentomaxillofacial Sci. 2024, 9, 95–99. [Google Scholar]
- Wei, F.; Yang, S.; Xu, H.; Guo, Q.; Li, Q.; Hu, L.; Liu, D.; Wang, C. Expression and function of hypoxia inducible factor-1α and vascular endothelial growth factor in pulp tissue of teeth under orthodontic movement. Mediat. Inflamm. 2015, 2015, 215761. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Wang, J.; Yu, W.; Zhang, K.; Zhang, N.; Cao, L.; Jiang, X.; Bai, Y. Biocompatible reduced graphene oxide stimulated BMSCs induce acceleration of bone remodeling and orthodontic tooth movement through promotion on osteoclastogenesis and angiogenesis. Bioact. Mater. 2022, 15, 409–425. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z.E. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469–e1. [Google Scholar] [CrossRef]
- Sonmez, A.B.; Castelnuovo, J. Applications of basic fibroblastic growth factor (FGF-2, bFGF) in dentistry. Dent. Traumatol. 2014, 30, 107–111. [Google Scholar] [CrossRef]
- Barton, E.R.; Crowder, C. Growth factor targets for orthodontic treatments. Semin. Orthod. 2010, 16, 128–134. [Google Scholar] [CrossRef]
- Altun, S. Characterization of microRNA-101,-124,-143,-145,-223 in GCF During Orthodontic Treatment. Master’s Thesis, University of Illinois Chicago, Chicago, IL, USA, 2020. [Google Scholar]
- Hamman, N.R. Modulation of RANKL and Osteoprotegerin in Adolescents Using Orthodontic Forces; The University of Tennessee Health Science Center: Memphis, TN, USA, 2010. [Google Scholar]
- Dunn, M.D.; Park, C.H.; Kostenuik, J.; Kapila, S.; Giannobile, W.V. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone 2007, 41, 446–455. [Google Scholar] [CrossRef]
- Pandey, T. The RANK/RANKL/OPG System: A Genetic Jigsaw in Bone Remodelling. J. Orofac. Health Sci. 2016, 7, 14–17. [Google Scholar] [CrossRef]
- Elango, J.; Bao, B.; Wu, W. The hidden secrets of soluble RANKL in bone biology. Cytokine 2021, 144, 155559. [Google Scholar] [CrossRef] [PubMed]
- Branch, V. Levels of Rankl and Opg in Gingival Crevicular Fluid During Orthodontic Tooth Movement. Master’s Thesis, The Tamil Nadu Dr. M.G.R. Medical University, Chennai, India, 2011. [Google Scholar]
- Chandra, R.V.; Rachala, M.R.; Madhavi, K.; Kambalyal, P.; Reddy, A.A.; Ali, M.H. Periodontally accelerated osteogenic orthodontics combined with recombinant human bone morphogenetic protein-2: An outcome assessment. J. Indian Soc. Periodontol. 2019, 23, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Wang, L.; Han, G.; Cheng, Y. Mesenchymal stem cell-derived exosomes: A potential cell-free therapy for orthodontic tooth stability management. Stem Cell Res. Ther. 2024, 15, 342. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Z.; He, S.; Zhou, C.; Wang, H.; Yin, X.; Zou, S.; Duan, P. Intermittent parathyroid hormone improves orthodontic retention via insulin-like growth factor-1. Oral Dis. 2021, 27, 290–300. [Google Scholar] [CrossRef]
- Zeichner-David, M. Genetic influences on orthodontic tooth movement. Biol. Mech. Tooth Mov. 2021, 169–188. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119608912.ch12 (accessed on 17 February 2025).
- Yan, L.; Sun, S.; Qu, L. Insulin-like growth factor-1 promotes the proliferation and odontoblastic differentiation of human dental pulp cells under high glucose conditions. Int. J. Mol. Med. 2017, 40, 1253–1260. [Google Scholar] [CrossRef]
- Uzel, M.I.; Kantarci, A.; Hong, H.H.; Uygur, C.; Sheff, M.C.; Firatli, E.; Trackman, C. Connective tissue growth factor in drug-induced gingival overgrowth. J. Periodontol. 2001, 72, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Kaya, F.A.; Hamamci, N.; Basaran, G.; Dogru, M.; Yildirim, T.T. TNF-α, IL-1β AND IL-8 levels in tooth early levelling movement orthodontic treatment. J. Int. Dent. Med. Res. 2010, 3, 3. [Google Scholar]
- Aristizábal, J.F.; Ríos, H.; Rey, D.; Álvarez, M.A.; Parra Patiño, B.; Ortiz, M. Interleukin 1-beta (IL-1β) polymorphism and orthodontics: A systematic review. Rev. Fac. Odontol. Univ. Antioq. 2019, 31, 147–161. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamaguchi, M.; Shimizu, M.; Yamada, K.; Kasai, K. TNF-α aggravates the progression of orthodontically-induced inflammatory root resorption in the presence of RANKL. J. Hard Tissue Biol. 2014, 23, 155–162. [Google Scholar] [CrossRef]
- Suwardi, A.; Wang, F.; Xue, K.; Han, M.Y.; Teo, P.; Wang, P.; Wang, S.; Liu, Y.; Ye, E.; Li, Z.; et al. Machine learning-driven biomaterials evolution. Adv. Mater. 2022, 34, 2102703. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, N.R.; Bokade, S. Aids of Machine Learning for Additively Manufactured Bone Scaffold. Emerg. Technol. Healthc. Internet Things Deep Learn. Models 2021, 359–380. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119792345.ch15 (accessed on 17 February 2025).
- MacKay, B.S. Labelling, Modelling, and Predicting Cell Biocompatibility Using Deep Neural Networks. PhD Thesis, University of Southampton, Southampton, UK, 2021. [Google Scholar]
- Khong, J.; Wang, P.; Gan, T.R.; Ng, J.; Anh, T.T.L.; Blasiak, A.; Kee, T.; Ho, D. The role of artificial intelligence in scaling nanomedicine toward broad clinical impact. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 385–407. [Google Scholar]
- Mech, D.J.; Murugappan, S.; Buddhiraju, H.S.; Eranki, A.; Rengan, A.K.; Rizvi, M.S. AI on DDS for regenerative medicine. In Artificial Intelligence in Tissue and Organ Regeneration; Academic Press: New York, NY, USA, 2023; pp. 133–153. [Google Scholar]
- Chen, Y.W.; Stanley, K.; Att, W. Artificial intelligence in dentistry: Current applications and future perspectives. Quintessence Int. 2020, 51, 248–257. [Google Scholar]
- Asiri, S.N. Can Artificial Intelligence Predict Growth and Treatment Outcomes Among Orthodontic Patients. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2021. [Google Scholar]
- Elnagar, M.H.; Venugopalan, S.R.; Allareddy, V. Applications of Artificial Intelligence and Big Data Analytics in Orthodontics. In Orthodontics-E-Book; Elsevier: Amsterdam, The Netherlands, 2022; Volume 176. [Google Scholar]
- Sharma, M.S.; Jadhav, V.; Bhuskute, K.; Reche, A. Artificial Intelligence: An Innovative Approach in Orthodontics: A Narrative Review. J. Clin. Diagn. Res. 2023, 17, 12. [Google Scholar] [CrossRef]
- Gargouri, M. Artificial Intelligence and Orthodontic: Achievements, Expectations and Challenges. PhD Thesis, Instituto Universitário Egas Moniz (IUEM), Almeida, Portugal, 2024. [Google Scholar]
- Sun, M.L.; Liu, Y.; Liu, G.; Cui, D.; Heidari, A.A.; Jia, W.Y.; Ji, X.; Chen, H.; Luo, Y. Application of machine learning to stomatology: A comprehensive review. IEEE Access 2020, 8, 184360–184374. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-Ehaideb, A.; Vishwanathaiah, S.; Maganur, C.; Patil, S.; Naik, S.; Baeshen, H.A.; Sarode, S.S. Scope and performance of artificial intelligence technology in orthodontic diagnosis, treatment planning, and clinical decision-making-a systematic review. J. Dent. Sci. 2021, 16, 482–492. [Google Scholar] [CrossRef]
- Surendran, A.; Daigavane, P.; Shrivastav, S.; Kamble, R.; Sanchla, A.D.; Bharti, L.; Shinde, M. The Future of Orthodontics: Deep Learning Technologies. Cureus 2024, 16, 6. [Google Scholar] [CrossRef]
- Francisco, I.; Ribeiro, M.; Marques, F.; Travassos, R.; Nunes, C.; Pereira, F.; Caramelo, F.; Paula, A.B.; Vale, F. Application of three-dimensional digital technology in orthodontics: The state of the art. Biomimetics 2022, 7, 23. [Google Scholar] [CrossRef]
- Aljabaa, A.; McDonald, F.; Newton, J.T. A systematic review of randomized controlled trials of interventions to improve adherence among orthodontic patients aged 12 to 18. Angle Orthod. 2015, 85, 305–313. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, S.; Zhao, Z.; Wu, Z. Machine learning in orthodontics: Challenges and perspectives. Adv. Clin. Exp. Med. 2021, 30, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Farrag, G.; Flügge, T.; Timm, L.H. Predicting Outcome in Clear Aligner Treatment: A Machine Learning Analysis. J. Clin. Med. 2024, 13, 3672. [Google Scholar] [CrossRef]
- Panayi, N.C.; Efstathiou, S.; Christopoulou, I.; Kotantoula, G.; Tsolakis, I.A. Digital orthodontics: Present and future. AJO-DO Clin. Companion 2024, 4, 14–25. [Google Scholar] [CrossRef]
- Caruso, S.; Caruso, S.; Pellegrino, M.; Skafi, R.; Nota, A.; Tecco, S. A knowledge-based algorithm for automatic monitoring of orthodontic treatment: The dental monitoring system. Two cases. Sensors 2021, 21, 1856. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Shivalkar, S.; Pal, A.; Verma, M.L.; Sahoo, A.K.; Roy, D.N. Nanotechnology for enhanced bioactivity of bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–466. [Google Scholar]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef]
- Seifi, M.; Eskandarloo, F.; Amdjadi, P.; Farmany, A. Investigation of mechanical properties, remineralization, antibacterial effect, and cellular toxicity of composite orthodontic adhesive combined with silver-containing nanostructured bioactive glass. BMC Oral Health 2024, 24, 650. [Google Scholar] [CrossRef]
- Gandedkar, N.H.; Dalci, O.; Darendeliler, M.A. February. Accelerated orthodontics (AO): The past, present and the future. In Seminars in Orthodontics; WB Saunders: Philadelphia, PA, USA, 2024. [Google Scholar]
- Eliades, T.; Brantley, W.A. The inappropriateness of conventional orthodontic bond strength assessment protocols. Eur. J. Orthod. 2000, 22, 13–23. [Google Scholar] [CrossRef]
- Squires, T.; Michelogiannakis, D.; Rossouw, E.; Javed, F. An evidence-based review of the scope and potential ethical concerns of teleorthodontics. J. Dent. Educ. 2021, 85, 92–100. [Google Scholar] [CrossRef]
- He, H.; Cao, J.; Wang, D.; Gu, B.; Guo, H.; Liu, H. Gene-modified stem cells combined with rapid prototyping techniques: A novel strategy for periodontal regeneration. Stem Cell Rev. Rep. 2010, 6, 137–141. [Google Scholar] [CrossRef]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z. 4D printing in biomedical engineering: Advancements, challenges, and future directions. J. Funct. Biomater. 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, P.; Xue, S.; Sharma, B.; Sanchez-Martin, M.; Wang, F.; Beaty, K.A.; Dehan, E.; Parikh, B. Translating cancer genomics into precision medicine with artificial intelligence: Applications, challenges and future perspectives. Hum. Genet. 2019, 138, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Oberoi, S.; Solem, R.C.; Kapila, S. Moving towards precision orthodontics: An evolving paradigm shift in the planning and delivery of customized orthodontic therapy. Orthod. Craniofacial Res. 2017, 20, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, N.; Kazimierczak, W.; Serafin, Z.; Nowicki, P.; Nożewski, J.; Janiszewska-Olszowska, J. AI in orthodontics: Revolutionizing diagnostics and treatment planning—A comprehensive review. J. Clin. Med. 2024, 13, 344. [Google Scholar] [CrossRef]
- Wang, X.; Mu, M.; Yan, J.; Han, B.; Ye, R.; Guo, G. 3D printing materials and 3D printed surgical devices in oral and maxillofacial surgery: Design, workflow and effectiveness. Regen. Biomater. 2024, 11, rbae066. [Google Scholar] [CrossRef]
- Ariano, A.; Posa, F.; Storlino, G.; Mori, G. Molecules inducing dental stem cells differentiation and bone regeneration: State of the art. Int. J. Mol. Sci. 2023, 24, 9897. [Google Scholar] [CrossRef]
- Shaikh, K.; Bekal, S.V.; Marei, H.F.A.; Elsayed, W.S.M.; Surdilovic, D.; Jawad, L.A. Artificial Intelligence in Dentistry; Springer Nature: New York, NY, USA, 2022. [Google Scholar]


| No. | Scaffold Type | Material Type | Role in Orthodontics and Orthognathic Surgery | Importance in Orthodontic and Orthognathic Treatment | References |
|---|---|---|---|---|---|
| 1 | Polylactic Acid (PLA) | Polymer | Used for gradual tissue integration and scaffold stability in bone regeneration. | Ideal for controlled resorption in guided bone regeneration in orthognathic surgery | [11,74,75] |
| 2 | Polyglycolic Acid (PGA) | Polymer | Facilitates rapid cell integration and healing in post-surgical applications. | Beneficial for quick scaffold degradation in orthognathic surgery | [76,77] |
| 3 | Polycaprolactone (PCL) | Polymer | Serves as a framework for long-term tissue engineering in orthognathic surgeries. | Provides extended support for complex craniofacial reconstructions. | [78,79] |
| 4 | Poly(lactic-co-glycolic acid) (PLGA) | Polymer | Offers tailored degradation for scaffolds in alignment and bone defect corrections. | Customizable for patient-specific needs in orthodontics and orthognathic surgery. | [80,81] |
| 5 | Hydroxyapatite (HA) | Ceramic | Supports bone osseointegration and density improvement in surgical areas. | Essential for bone grafting and enhancing osteoconductivity in surgical repairs. | [82,83] |
| 6 | Tricalcium Phosphate (TCP) | Ceramic | Used in alveolar ridge augmentation and cleft palate repairs. | Promotes bone regeneration critical for orthodontic anchor points and device stability. | [84,85,86] |
| 7 | Bioactive Glass | Ceramic | Enhances bone bonding and supports soft tissue healing around implants. | Useful in complex craniofacial reconstructions and as a filler in bone defects. | [87,88] |
| 8 | Polymer–Ceramic Composites | Composite | Combines strength and bioactivity for load-bearing applications in orthodontics. | Provides durable and biocompatible options for long-term craniofacial scaffolding. | [89,90] |
| 9 | Fiber-Reinforced Composites | Composite | Utilized for reinforcing bone grafts and orthodontic appliances needing high strength. | Offers robust mechanical support where significant bite forces are involved. | [91,92] |
| 10 | Alginate | Natural | Used for impression making and as a carrier for bioactive molecules. | Important for creating precise dental molds and delivering therapeutic agents. | [93,94] |
| 11 | Chitosan | Natural | Facilitates hemostasis and has antimicrobial properties for wound healing. | Supports post-surgical recovery and is used in drug delivery systems in orthodontics. | [95,96,97] |
| 12 | Titanium Mesh | Metal | Provides rigid support for bone grafts in extensive reconstructive surgery. | Used for structural support in segmental osteotomies and large defect bridging. | [98,99] |
| 13 | Silver Nanoparticles (Ag NPs) | Metal Nanoparticle | Used in coatings on brackets and archwires to prevent microbial colonization and reduce dental plaque. | Provides broad-spectrum antimicrobial properties essential for reducing the risk of infection and improving oral hygiene during orthodontic treatment. | [100,101,102] |
| 14 | Zinc Oxide Nanoparticles (ZnO NPs) | Metal Oxide Nanoparticle | Incorporated into dental cements and adhesives to enhance antimicrobial properties against oral pathogens. | Helps in preventing decay and infection at critical application sites, improving the longevity and success of orthodontic treatments. | [103,104] |
| 15 | Titanium Dioxide Nanoparticles (TiO2 NPs) | Metal Oxide Nanoparticle | Utilized in composite materials for fixed appliances to improve antimicrobial activity and mechanical properties. | Enhances the durability and infection resistance of orthodontic appliances under varied oral conditions, beneficial for long-term treatment stability. | [64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] |
| 16 | Hydroxyapatite Nanoparticles (nHAP) | Ceramic Nanoparticle | Applied in surface treatments of implants to enhance bone integration and regeneration. | Supports rapid osseointegration due to its similarity to natural bone mineral components, crucial for the stability of orthodontic implants. | [106,107] |
| 17 | Silica Nanoparticles (SiO2 NPs) | Ceramic Nanoparticle | Used to improve the physical properties of orthodontic acrylics and composites, making devices more durable and effective. | Enhances the mechanical strength and clarity of orthodontic appliances, important for maintaining appliance integrity and aesthetics during treatment. | [108,109] |
| 18 | Carbon Nanotubes | Carbon-based Nanoparticle | Reinforces orthodontic polymers to increase durability and reduce the risk of fractures in orthodontic appliances. | Provides structural integrity to polymers, significantly enhancing their mechanical properties and resistance to the mechanical forces during orthodontic treatment. | [110,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farjaminejad, R.; Farjaminejad, S.; Hasani, M.; Garcia-Godoy, F.; Sayahpour, B.; Marya, A.; Jamilian, A. The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral 2025, 5, 21. https://doi.org/10.3390/oral5010021
Farjaminejad R, Farjaminejad S, Hasani M, Garcia-Godoy F, Sayahpour B, Marya A, Jamilian A. The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral. 2025; 5(1):21. https://doi.org/10.3390/oral5010021
Chicago/Turabian StyleFarjaminejad, Rosana, Samira Farjaminejad, Melika Hasani, Franklin Garcia-Godoy, Babak Sayahpour, Anand Marya, and Abdolreza Jamilian. 2025. "The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review" Oral 5, no. 1: 21. https://doi.org/10.3390/oral5010021
APA StyleFarjaminejad, R., Farjaminejad, S., Hasani, M., Garcia-Godoy, F., Sayahpour, B., Marya, A., & Jamilian, A. (2025). The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral, 5(1), 21. https://doi.org/10.3390/oral5010021








