MALDI MS-Based Investigations for SARS-CoV-2 Detection
Abstract
1. Introduction
2. MALDI-TOF and MALDI-FT-ICR Mass Spectrometry: A Brief Presentation
3. MALDI-MS for Pathogen Detection: A General Overview
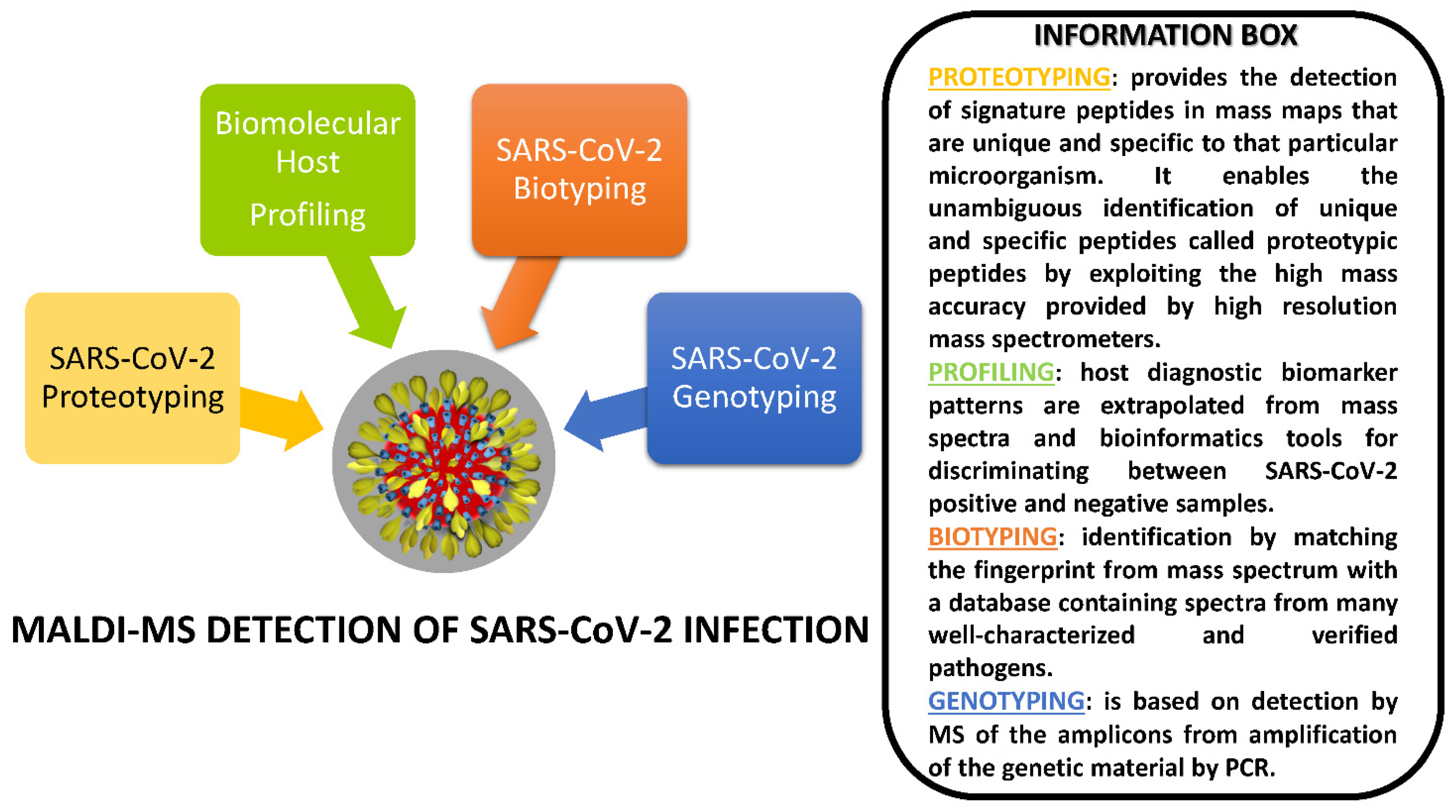
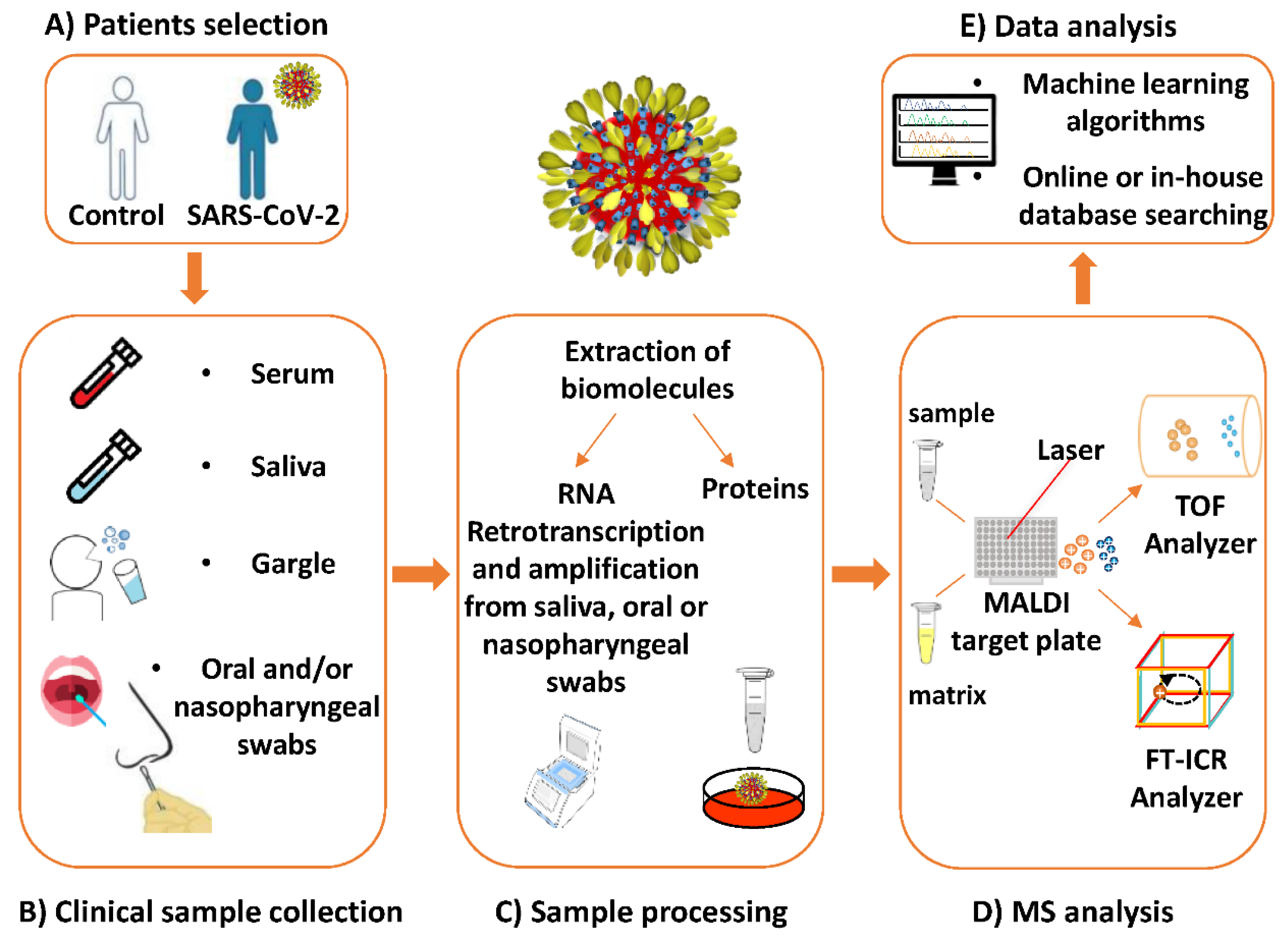
4. MALDI-MS Investigations Targeting SARS-CoV-2
4.1. Proteotyping Approach for SARS-CoV-2 Detection by MALDI-MS
4.2. Biotyping Approach for SARS-CoV-2 Detection by MALDI-MS
4.3. Genotyping Approach for SARS-CoV-2 Detection by MALDI-MS
4.4. Biomolecular Host Profiling Approach for SARS-CoV-2 Detection by MALDI-MS
5. Discussion
5.1. Comparison of MALDI-MS vs. RT-PCR Techniques for SARS-CoV-2 Detection: Advantages and Limitations
5.2. Pre-Analytical and Analytical Issues for Molecular Detection of SARS-CoV-2
5.3. Detection of SARS-CoV-2 in Different Types of Clinical Specimens
5.4. Asymptomatic Infection and the Spread of New SARS-CoV-2 Variants
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Update (Live): 92,895,303 Cases and 1,989,457 Deaths from COVID-19 Virus Pandemic—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 21 October 2021).
- Peiris, J.S.; Yuen, K.Y.; Osterhaus, A.D.; Stöhr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef]
- Muller, C.P. Do asymptomatic carriers of SARS-COV-2 transmit the virus? Lancet Reg. Health Eur. 2021, 4, 100082. [Google Scholar] [CrossRef]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef] [PubMed]
- Atripaldi, L.; Sale, S.; Capone, M.; Montesarchio, V.; Parrella, R.; Botti, G.; Ascierto, P.A.; Madonna, G. Could asymptomatic carriers spread the SARS-CoV-2 infection? Experience from the Italian second wave. J. Transl. Med. 2021, 19, 93. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Mirzapour, P.; Dadras, O.; Pashaei, Z.; Karimi, A.; MohsseniPour, M.; Soleymanzadeh, M.; Barzegary, A.; Afsahi, A.M.; Vahedi, F.; et al. Characterization of SARS-CoV-2 diferent variants and related morbidity and mortality: A systematic review. Eur. J. Med. Res. 2021, 26, 51. [Google Scholar] [CrossRef]
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef]
- D’Cruz, R.J.; Currier, A.W.; Sampson, V.B. Laboratory Testing Methods for Novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). Front. Cell Dev. Biol. 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Ciapponi, A.; Sued, O.; Martinez-García, L.; Rutjes, A.W.; Low, N.; et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE 2020, 15, e0242958. [Google Scholar] [CrossRef]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert. Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Rosebrock, A.P. DNA Cross-Reactivity of the CDC-Specified SARS-CoV-2 Specimen Control Leads to Potential for False Negatives and Underreporting of Viral Infection. Clin. Chem. 2020, 67, 435–437. [Google Scholar] [CrossRef]
- Kanji, J.N.; Zelyas, N.; MacDonald, C.; Pabbaraju, K.; Khan, M.N.; Prasad, A.; Hu, J.; Diggle, M.; Berenger, B.M.; Tipples, G. False negative rate of COVID-19 PCR testing: A discordant testing analysis. Virol. J. 2021, 18, 1–6. [Google Scholar] [CrossRef]
- Huggett, J.F.; Benes, V.; Bustin, S.A.; Garson, J.A.; Harris, K.; Kammel, M.; Kubista, M.; McHugh, T.D.; Moran-Gilad, J.; Nolan, T.; et al. Cautionary Note on Contamination of Reagents Used for Molecular Detection of SARS-CoV-2. Clin. Chem. 2020, 66, 1369–1372. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, Y.; Lu, R.; Dong, Y.; Zhang, C. Rapid antigen detection alone may not be sufficient for early diagnosis and/or mass screening of COVID-19. J. Med. Virol. 2021, 93, 6462–6464. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512–e00520. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, R.; Preianò, M.; Fregola, A.; Pelaia, C.; Montalcini, T.; Savino, R. Mapping the SARS-CoV-2-Host Protein-Protein Interactome by Affinity Purification Mass Spectrometry and Proximity-Dependent Biotin Labeling: A Rational and Straightforward Route to Discover Host-Directed Anti-SARS-CoV-2 Therapeutics. Int. J. Mol. Sci. 2021, 22, 532. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S. Empowering Clinical Diagnostics with Mass Spectrometry. ACS Omega 2020, 5, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.W.S.; Sugumar, V.; Ren, A.H.; Kulasingam, V. Emerging role of clinical mass spectrometry in pathology. J. Clin. Pathol. 2020, 73, 61–69. [Google Scholar] [CrossRef]
- Aggarwal, S.; Acharjee, A.; Mukherjee, A.; Baker, M.S.; Srivastava, S. Role of Multiomics Data to Understand Host–Pathogen Interactions in COVID-19 Pathogenesis. J. Proteome Res. 2021, 20, 1107–1132. [Google Scholar] [CrossRef]
- Trauger, S.A.; Junker, T.; Siuzdak, G. Investigating viral proteins and intact viruses with mass spectrometry. In Modern Mass Spectrometry, 1st ed.; Schalley, C.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 225, pp. 265–282. [Google Scholar]
- Downard, K.M.; Morrissey, B.; Schwahn, A.B. Mass spectrometry analysis of the influenza virus. Mass Spectrom. Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Ganova-Raeva, L.M.; Khudyakov, Y.E. Application of mass spectrometry to molecular diagnostics of viral infections. Expert Rev. Mol. Diagn. 2013, 13, 377–388. [Google Scholar] [CrossRef]
- Milewska, A.; Ner-Kluza, J.; Dabrowska, A.; Bodzon-Kulakowska, A.; Pyrc, K.; Suder, P. Mass spectrometry in virological sciences. Mass Spectrom. Rev. 2020, 39, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Majchrzykiewicz-Koehorst, J.A.; Heikens, E.; Trip, H.; Hulst, A.G.; de Jong, A.L.; Viveen, M.C.; Sedee, N.J.; van der Plas, J.; Coenjaerts, F.E.; Paauw, A. Rapid and generic identification of influenza A and other respiratory viruses with mass spectrometry. J. Virol. Methods 2015, 213, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.W.; Gerhardt, G.; Robitaille, L.; Plante, P.L.; Boivin, G.; Corbeil, J.; Moseley, M.A. Targeted Proteomics of Human Metapneumovirus in Clinical Samples and Viral Cultures. Anal. Chem. 2015, 87, 10247–10254. [Google Scholar] [CrossRef]
- Santana, W.I.; Williams, T.L.; Winne, E.K.; Pirkle, J.L.; Barr, J.R. Quantification of viral proteins of the avian H7 subtype of influenza virus: An isotope dilution mass spectrometry method applicable for producing more rapid vaccines in the case of an influenza pandemic. Anal. Chem. 2014, 86, 4088–4095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, Z.-C.; Hu, B. Mass Spectrometry-Based Human Breath Analysis: Towards COVID-19 Diagnosis and Research. J. Anal. Test. 2021, 5, 287–297. [Google Scholar] [CrossRef]
- Griffin, J.H.; Downard, K.M. Mass spectrometry analytical responses to the SARS-CoV2 coronavirus in review. Trends Analyt. Chem. 2021, 142, 116328. [Google Scholar] [CrossRef]
- Mahmud, I.; Garrett, T.J. Mass Spectrometry Techniques in Emerging Pathogens Studies: COVID-19 Perspectives. J. Am. Soc. Mass Spectrom. 2020, 31, 2013–2024. [Google Scholar] [CrossRef]
- Rais, Y.; Fu, Z.; Drabovich, A.P. Mass spectrometry-based proteomics in basic and translational research of SARS-CoV-2 coronavirus and its emerging mutants. Clin. Proteom. 2021, 18, 19. [Google Scholar] [CrossRef]
- De Silva, I.W.; Nayek, S.; Singh, V.; Reddy, J.; Granger, J.K.; Verbeck, G.F. Paper Spray Mass Spectrometry Utilizing Teslin® Substrate for Rapid Detection of Lipid Metabolite Changes during COVID-19 Infection. Analyst 2020, 145, 5725–5732. [Google Scholar] [CrossRef]
- Singh, P.; Chakraborty, R.; Marwal, R.; Radhakrishan, V.S.; Bhaskar, A.K.; Vashisht, H.; Dhar, M.S.; Pradhan, S.; Ranjan, G.; Imran, M. A Rapid and Sensitive Method to Detect SARS-CoV-2 virus using Targeted-Mass Spectrometry. J. Proteins Proteom. 2020, 11, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Lebkuchen, A.; Okai, G.G.; Schuch, R.A.; Viana, L.G.; Olive, A.N.; dos Santos Lazari, C.; Fraga, A.M.; Granato, C.F.H.; Pintão, M.C.T.; et al. Establishing a mass spectrometry-based system for rapid detection of SARS-CoV-2 in large clinical sample cohorts. Nat. Commun. 2020, 11, 6201. [Google Scholar] [CrossRef] [PubMed]
- Kipping, M.; Tänzler, D.; Sinz, A. A rapid and reliable liquid chromatography/mass spectrometry method for SARS-CoV-2 analysis from gargle solutions and saliva. Anal. Bioanal. Chem. 2021, 413, 6503–6511. [Google Scholar] [CrossRef]
- Greco, V.; Piras, C.; Pieroni, L.; Ronci, M.; Putignani, L.; Roncada, P.; Urbani, A. Applications of MALDI-TOF mass spectrometry in clinical proteomics. Expert Rev. Proteom. 2018, 15, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.P.; Blakney, G.T.; Hendrickson, C.L.; Ellis, S.R.; Heeren, R.M.A.; Smith, D.F. Ultra-High Mass Resolving Power, Mass Accuracy, and Dynamic Range MALDI Mass Spectrometry Imaging by 21-T FT-ICR MS. Anal. Chem. 2020, 92, 3133–3142. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Emerging Technologies for the Clinical Microbiology Laboratory. Clin. Microbiol. Rev. 2014, 27, 783–822. [Google Scholar] [CrossRef]
- Karas, M.; Bachmann, D.; Bahr, U.; Hillenkamp, F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Process. 1987, 78, 53–68. [Google Scholar] [CrossRef]
- Whitehouse, C.M.; Dreyer, R.N.; Yamashita, M.; Fenn, J.B. Electrospray interface for liquid chromatographs and mass spectrometers. Anal. Chem. 1985, 57, 675–679. [Google Scholar] [CrossRef]
- Hillenkamp, F.; Peter-Katalinic, J. The MALDI Process and Method. In MALDI MS: A Practical Guide to Instrumentation, Methods and Application, 1st ed.; Hillenkam, F., Karas, M., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2007; pp. 1–28. [Google Scholar]
- Baker, T.C.; Han, J.; Borchers, C.H. Recent advancements in matrix-assisted laser desorption/ionization mass spectrometry imaging. Curr. Opin. Biotechnol. 2017, 43, 62–69. [Google Scholar] [CrossRef]
- Ivanova, M.; Dyadyk, O.; Ivanov, D.; Clerici, F.; Smith, A.; Magni, F. Matrix-assisted laser desorption/ionization mass spectrometry imaging to uncover protein alterations associated with the progression of IgA nephropathy. Virchows Arch. 2020, 476, 903–914. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Wang, X.; Liu, L.; Gong, J.; Zhai, Z.; He, L.; Meng, F.; Xiao, D. A multisite SNP genotyping and macrolide susceptibility gene method for Mycoplasma pneumoniae based on MALDI-TOF MS. iScience 2021, 24, 102447. [Google Scholar] [CrossRef]
- Chinello, C.; Cazzaniga, M.; De Sio, G.; Smith, A.J.; Gianazza, E.; Grasso, A.; Rocco, F.; Signorini, S.; Grasso, M.; Bosari, S.; et al. Urinary signatures of Renal Cell Carcinoma investigated by peptidomic approaches. PLoS ONE 2014, 9, e106684. [Google Scholar] [CrossRef]
- Terracciano, R.; Preianò, M.; Maggisano, G.; Pelaia, C.; Savino, R. Hexagonal Mesoporous Silica as a Rapid, Efficient and Versatile Tool for MALDI-TOF MS Sample Preparation in Clinical Peptidomics Analysis: A Pilot Study. Molecules 2019, 24, 2311. [Google Scholar] [CrossRef]
- Terracciano, R.; Preianò, M.; Palladino, G.P.; Carpagnano, G.E.; Foschino Barbaro, M.P.; Pelaia, G.; Savino, R.; Maselli, R. Peptidome profiling of induced sputum by mesoporous silica beads and MALDI-TOF MS for non-invasive biomarker discovery of chronic inflammatory lung diseases. Proteomics 2011, 11, 3402–3414. [Google Scholar] [CrossRef] [PubMed]
- Corigliano, A.; Preianò, M.; Terracciano, R.; Savino, R.; De Gori, M.; Galasso, O.; Gasparini, G. C3f is a potential tool for the staging of osteoarthritis. J. Biol. Regul. Homeost. Agents 2017, 31, 29–35. [Google Scholar] [PubMed]
- Preianò, M.; Maggisano, G.; Murfuni, M.; Villella, C.; Colica, C.; Fregola, A.; Pelaia, C.; Lombardo, N.; Pelaia, G.; Savino, R.; et al. Rapid Detection and Identification of Antimicrobial Peptide Fingerprints of Nasal Fluid by Mesoporous Silica Particles and MALDI-TOF/TOF Mass Spectrometry: From the Analytical Approach to the Diagnostic Applicability in Precision Medicine. Int. J. Mol. Sci. 2018, 19, 4005. [Google Scholar] [CrossRef]
- Lombardo, N.; Preianò, M.; Maggisano, G.; Murfuni, M.S.; Messina, L.; Pelaia, G.; Savino, R.; Terracciano, R. A rapid differential display analysis of nasal swab fingerprints to distinguish allergic from non-allergic rhinitis subjects by mesoporous silica particles and MALDI-TOF mass spectrometry. Proteomics 2017, 17, 1600215. [Google Scholar] [CrossRef]
- Preianò, M.; Maggisano, G.; Murfuni, M.S.; Villella, C.; Pelaia, C.; Montalcini, T.; Lombardo, N.; Pelaia, G.; Savino, R.; Terracciano, R. An Analytical Method for Assessing Optimal Storage Conditions of Gingival Crevicular Fluid and Disclosing a Peptide Biomarker Signature of Gingivitis by MALDI-TOF MS. Proteom. Clin. Appl. 2018, 12, e1800005. [Google Scholar] [CrossRef]
- Angeletti, S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 2017, 138, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Arcangeletti, M.-C.; Rodighiero, I.; Buttrini, M.; Gorrini, C.; Motta, F.; Germini, D.; Medici, M.-C.; Chezzi, C.; De conto, F. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry applied to virus identification. Sci. Rep. 2014, 4, 6803. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F. Application of maldi-tof mass spectrometry in clinical virology: A review. Open Virol. J. 2013, 7, 84–90. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Advances in identification of clinical yeast isolates by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.C.; Gabriel, F.; Riat, A.; Cassagne, C.; Bourgeois, N.; Huguenin, A.; Chauvin, P.; De Geyter, D.; Bexkens, M.; Rubio, E.; et al. Optimization of MALDI-ToF mass spectrometry for yeast identification: A multicenter study. Med. Mycol. 2020, 58, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ahmed, A.; Islam, A.; Kim, S. Developments in FT-ICR MS instrumentation, ionization techniques, and data interpretation methods for petroleomics. Mass Spectrom. Rev. 2015, 34, 248–263. [Google Scholar] [CrossRef]
- Shaw, J.B.; Lin, T.Y.; Leach, F.E., 3rd; Tolmachev, A.V.; Tolić, N.; Robinson, E.W.; Koppenaal, D.W.; Paša-Tolić, L. 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer Greatly Expands Mass Spectrometry Toolbox. J. Am. Soc. Mass Spectrom. 2016, 27, 1929–1936. [Google Scholar] [CrossRef]
- Sun, X.; Wu, P.; Zhao, C.; Zheng, F.; Hu, C.; Lu, X.; Xu, G. Protein profiling analysis based on matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry and its application in typing Streptomyces isolates. Talanta 2020, 208, 120439. [Google Scholar] [CrossRef]
- Dilillo, M.; Ait-Belkacem, R.; Esteve, C.; Pellegrini, D.; Nicolardi, S.; Costa, M.; Vannini, E.; de Graaf, E.L.; Caleo, M.; McDonnell, L.A. Ultra-High Mass Resolution MALDI Imaging Mass Spectrometry of Proteins and Metabolites in a Mouse Model of Glioblastoma. Sci. Rep. 2017, 7, 603. [Google Scholar] [CrossRef]
- Piga, I.; Heijs, B.; Nicolardi, S.; Giusti, L.; Marselli, L.; Marchetti, P.; Mazzoni, M.R.; Lucacchini, A.; McDonnell, L.A. Ultra-high resolution MALDI-FTICR-MSI analysis of intact proteins in mouse and human pancreas tissue. Int. J. Mass Spectrom. 2017, 437, 10–16. [Google Scholar] [CrossRef]
- Patel, R. MALDI-TOF-MS for the diagnosis of infectious diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’hom, G.; Greub, G. Application of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2021, 36, 380–407. [Google Scholar] [CrossRef]
- Welker, M.; van Belkum, A.; Girard, V.; Charrier, J.P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef]
- Sjöholm, M.I.L.; Dillner, J.; Carlson, J. Multiplex detection of human herpesviruses from archival specimens by using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2008, 46, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Hsu, W.; Wang, C.-H.; Chen, Y.-J.; Fang, J.-M. Rapid and specific influenza virus detection by functionalized magnetic nanoparticles and mass spectrometry. J. Nanobiotechnol 2011, 9, 52. [Google Scholar] [CrossRef]
- Piao, J.; Jiang, J.; Xu, B.; Wang, X.; Guan, Y.; Wu, W.; Liu, L.; Zhang, Y.; Huang, X.; Wang, P.; et al. Simultaneous detection and identification of enteric viruses by PCR-mass assay. PLoS ONE 2012, 7, e42251. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yang, F.; Xiong, Z.; Guo, J.; Du, J.; Hu, Y.; Jin, Q. Sensitive and rapid detection of viruses associated with hand foot and mouth disease using multiplexed MALDI-TOF analysis. J. Clin. Virol. 2013, 56, 170–174. [Google Scholar] [CrossRef]
- Iles, R.K.; Zmuidinaite, R.; Iles, J.K.; Carnell, G.; Sampson, A.; Heeney, J.L. Development of a Clinical MALDI-ToF Mass Spectrometry Assay for SARS-CoV-2: Rational Design and Multi-Disciplinary Team Work. Diagnostics 2020, 10, 746. [Google Scholar] [CrossRef]
- Chivte, P.; LaCasse, Z.; Seethi, V.D.R.; Bharti, P.; Bland, J.; Kadkol, S.S.; Gaillard, E.R. MALDI-ToF Protein Profiling as Potential Rapid Diagnostic Platform for COVID-19. J. Mass Spectrom. Adv. Clin. Lab. 2021, 21, 31–41. [Google Scholar]
- Wandernoth, P.; Kriegsmann, K.; Groh-Mohanu, C.; Daeumer, M.; Gohl, P.; Harzer, O.; Kriegsmann, M.; Kriegsmann, J. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Mass Spectrometry. Viruses 2020, 12, 849. [Google Scholar] [CrossRef]
- Rybicka, M.; Miłosz, E.; Bielawski, K.P. Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection. Viruses 2021, 13, 730. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Banu, R.; Shrestha, P.; Pate, A.; Chen, F.; Cao, L.; Fabre, S.; Tan, J.; Lopez, H.; Chiu, N.; et al. RT-PCR/MALDI-TOF mass spectrometry-based detection of SARS-CoV-2 in saliva specimens. J. Med. Virol. 2021, 93, 5481–5486. [Google Scholar] [CrossRef]
- Dollman, N.L.; Griffin, J.H.; Downard, K.M. Detection, Mapping, and Proteotyping of SARS-CoV-2 Coronavirus with High Resolution Mass Spectrometry. ACS Infect. Dis. 2020, 6, 3269–3276. [Google Scholar] [CrossRef]
- Nachtigall, F.M.; Pereira, A.; Trofymchuk, O.S.; Santos, L.S. Detection of SARS-CoV-2 in nasal swabs using MALDI-MS. Nat. Biotechnol. 2020, 38, 1168–1173. [Google Scholar] [CrossRef]
- Tran, N.K.; Howard, T.; Walsh, R.; Pepper, J.; Loegering, J.; Phinney, B.; Salemi, M.R.; Rashidi, H.H. Novel application of automated machine learning with MALDI-TOF-MS for rapid high-throughput screening of COVID-19: A proof of concept. Sci. Rep. 2021, 11, 8219. [Google Scholar] [CrossRef] [PubMed]
- Deulofeu, M.; García-Cuesta, E.; Peña-Méndez, E.M.; Conde, J.E.; Jiménez-Romero, O.; Verdú, E.; Serrando, M.T.; Salvadó, V.; Boadas-Vaello, P. Detection of SARS-CoV-2 Infection in Human Nasopharyngeal Samples by Combining MALDI-TOF MS and Artificial Intelligence. Front. Med. (Lausanne) 2021, 8, 661358. [Google Scholar] [CrossRef]
- Rocca, M.F.; Zintgraff, J.C.; Dattero, M.E.; Santos, L.S.; Ledesma, M.; Vay, C.; Prieto, M.; Benedetti, E.; Avaro, M.; Russo, M.; et al. A combined approach of MALDI-TOF mass spectrometry and multivariate analysis as a potential tool for the detection of SARS-CoV-2 virus in nasopharyngeal swabs. J. Virol. Methods 2020, 286, 113991. [Google Scholar] [CrossRef]
- Yan, L.; Yi, J.; Huang, C.; Zhang, J.; Fu, S.; Li, Z.; Lyu, Q.; Xu, Y.; Wang, K.; Yang, H. Rapid Detection of COVID-19 Using MALDI-TOF-Based Serum Peptidome Profiling. Anal. Chem. 2021, 93, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Chenau, J.; Fenaille, F.; Caro, V.; Haustant, M.; Diancourt, L.; Klee, S.R.; Junot, C.; Ezan, E.; Goossens, P.L.; Becher, F. Identification and validation of specific markers of Bacillus anthracis spores by proteomics and genomics approaches. Mol. Cell. Proteom. 2014, 13, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Parker, C.H.; Croley, T.R.; McFarland, M.A. Identification of Salmonella Taxon-Specific Peptide Markers to the Serovar Level by Mass Spectrometry. Anal. Chem. 2019, 91, 4388–4395. [Google Scholar] [CrossRef]
- Karlsson, R.; Gonzales-Siles, L.; Gomila, M.; Busquets, A.; Salvà-Serra, F.; Jaén-Luchoro, D.; Jakobsson, H.E.; Karlsson, A.; Boulund, F.; Kristiansson, E.; et al. Proteotyping bacteria: Characterization, differentiation and identification of pneumococcus and other species within the Mitis Group of the genus Streptococcus by tandem mass spectrometry proteomics. PLoS ONE 2018, 13, e0208804. [Google Scholar] [CrossRef]
- Gekenidis, M.-T.; Studer, P.; Wuthrich, S.; Brunisholz, R.; Drissner, D. Beyond the Matrix-Assisted Laser Desorption Ionization (MALDI) Biotyping Workflow: In Search of Microorganism-Specific Tryptic Peptides Enabling Discrimination of Subspecies. Appl. Environ. Microbiol. 2014, 80, 4234–4241. [Google Scholar] [CrossRef]
- Downard, K.M. Proteotyping for the Rapid Identification of Pandemic Influenza Virus and other Biopathogens. Chem. Soc. Rev. 2013, 42, 8584–8595. [Google Scholar] [CrossRef]
- Nguyen, A.P.; Downard, K.M. Proteotyping of the Parainfluenza Virus with High Resolution Mass Spectrometry. Anal. Chem. 2013, 85, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Downard, K.M. Subtyping of Hepatitis C Virus with High Resolution Mass Spectrometry. Clin. Mass Spectrom. 2017, 4−5, 19–24. [Google Scholar] [CrossRef]
- Yi, X.; Li, J.; Yu, S.; Zhang, A.; Xu, J.; Yi, J.; Zou, J.; Nie, X.; Huang, J.; Wang, J. A new PCR-based mass spectrometry system for high-risk HPV, part I: Methods. Am. J. Clin. Pathol. 2011, 136, 913–919. [Google Scholar] [CrossRef] [PubMed]
- von Wintzingerode, F.; Böcker, S.; Schlötelburg, C.; Chiu, N.H.L.; Storm, N.; Jurinke, C.; Cantor, C.R.; Göbel, U.B.; van den Boom, D. Base-specific fragmentation of amplified 16S rRNA genes analyzed by mass spectrometry: A tool for rapid bacterial identification. Proc. Natl. Acad. Sci. USA 2002, 99, 7039–7044. [Google Scholar] [CrossRef]
- Lefmann, M.; Honisch, C.; Böcker, S.; Storm, N.; von Wintzingerode, F.; Schlötelburg, C.; Moter, A.; van den Boom, D.; Göbel, U.B. Novel mass spectrometry-based tool for genotypic identification of mycobacteria. J. Clin. Microbiol. 2004, 42, 339–346. [Google Scholar] [CrossRef]
- MassARRAY® SARS-CoV-2 Panel-Agena Bioscience. Available online: https://agenabio.com/wp-content/uploads/2020/04/GEN0027-02-CoV2-EUA-Product-Sheet-WEB.pdf (accessed on 5 October 2021).
- Bittremieux, W.; Adams, C.; Laukens, K.; Dorrestein, P.C.; Bandeira, N. Open Science Resources for the Mass Spectrometry-Based Analysis of SARS-CoV-2. J. Proteome Res. 2021, 20, 1464–1475. [Google Scholar] [CrossRef]
- SoRelle, J.A.; Patel, K.; Filkins, L.; Park, J.Y. Mass Spectrometry for COVID-19. Clin. Chem. 2020, 66, 1367–1368. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Tran, N.K.; Albahra, S.; Pham, T.N.; Holmes IV, J.H.; Greenhalgh, D.; Palmieri, T.L.; Wajda, J.; Rashidi, H.H. Novel application of an automated-machine learning development tool for predicting burn sepsis: A proof of concept. Sci. Rep. 2020, 10, 12354. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Rao, R.; Wilson, R.S.; Punyamurtula, U.; Salvato, F.; Petersen, M.; Ahmed, M.K.; Abid, M.R.; Verburgt, J.C.; Kihara, D.; et al. Mass spectrometry-based proteomic platforms for better understanding of SARS-CoV-2 induced pathogenesis and potential diagnostic approaches. Proteomics 2021, 21, e2000279. [Google Scholar] [CrossRef]
- Haas, P.; Muralidharan, M.; Krogan, N.J.; Kaake, R.M.; Hüttenhain, R. Proteomic Approaches to Study SARS-CoV-2 Biology and COVID-19 Pathology. J. Proteome Res. 2021, 20, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Barzelighi, H.M.; Daraei, B. The Review of SARS-CoV-2: Recent Perspective and Advances in Detection. Infect. Epidemiol. Microbiol. 2020, 6, 229–249. [Google Scholar]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDITOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Rodriguez, C.L.; García-Riestra, C. Application and Perspectives of MALDI–TOF Mass Spectrometry in Clinical Microbiology Laboratories. Microorganisms 2021, 9, 1539. [Google Scholar] [CrossRef]
- Grossegesse, M.; Hartkopf, F.; Nitsche, A.; Schaade, L.; Doellinger, J.; Muth, T. Perspective on Proteomics for Virus Detection in Clinical Samples. J. Proteome Res. 2020, 19, 4380–4388. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Simundic, A.M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Woloshin, S.; Patel, N.; Kesselheim, A.S. False Negative Tests for SARS-CoV-2 Infection-Challenges and Implications. N. Engl. J. Med. 2020, 383, e38. [Google Scholar] [CrossRef]
- Green, D.A.; Zucker, J.; Westblade, L.F.; Whittier, S.; Rennert, H.; Velu, P.; Craney, A.; Cushing, M.; Liu, D.; Sobieszczyk, M.E.; et al. Clinical Performance of SARS-CoV-2 Molecular Tests. J. Clin. Microbiol. 2020, 58, e00995-20. [Google Scholar] [CrossRef] [PubMed]
- Keaney, D.; Whelan, S.; Finn, K.; Lucey, B. Misdiagnosis of SARS-CoV-2: A Critical Review of the Influence of Sampling and Clinical Detection Methods. Med. Sci. 2021, 9, 36. [Google Scholar] [CrossRef]
- Preianò, M.; Falcone, D.; Maggisano, G.; Montalcini, T.; Navarra, M.; Paduano, S.; Savino, R.; Terracciano, R. Assessment of pre-analytical and analytical variables affecting peptidome profiling of gingival crevicular fluid by MALDI-TOF mass spectrometry. Clin. Chim. Acta 2014, 437, 120–128. [Google Scholar] [CrossRef]
- Preianò, M.; Maggisano, G.; Lombardo, N.; Montalcini, T.; Paduano, S.; Pelaia, G.; Savino, R.; Terracciano, R. Influence of storage conditions on MALDI-TOF MS profiling of gingival crevicular fluid: Implications on the role of S100A8 and S100A9 for clinical and proteomic based diagnostic investigations. Proteomics 2016, 16, 1033–1045. [Google Scholar] [CrossRef]
- Manconi, B.; Castagnola, M.; Cabras, T.; Olianas, A.; Vitali, A.; Desiderio, C.; Sanna, M.T.; Messana, I. The intriguing heterogeneity of human salivary proline-rich proteins: Short title: Salivary proline-rich protein species. J. Proteom. 2016, 134, 47–56. [Google Scholar] [CrossRef]
- Esser, D.; Alvarez-Llamas, G.; de Vries, M.P.; Weening, D.; Vonk, R.J.; Roelofsen, H. Sample Stability and Protein Composition of Saliva: Implications for Its Use as a Diagnostic Fluid. Biomark. Insights 2008, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Loefelholz, M.J.; Tang, Y.W. Laboratory diagnosis of emerging human coronavirus infections—the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, 81, e45–e50. [Google Scholar] [CrossRef]
- Azzi, L.; Maurino, V.; Baj, A.; Dani, M.; d’Aiuto, A.; Fasano, M.; Lualdi, M.; Sessa, F.; Alberio, T. Diagnostic Salivary Tests for SARS-CoV-2. J. Dent. Res. 2021, 100, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Chowdhry, A.; Kharbanda, O.P.; Popli, D.B.; Gautam, K.; Saini, V. Exploring salivary diagnostics in COVID-19: A scoping review and research suggestions. BDJ Open 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Mathuria, J.P.; Yadav, R.; Rajkumar. Laboratory diagnosis of SARS-CoV-2-A review of current methods. J. Infect. Public Health 2020, 13, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Griffin, S. Covid-19: Lateral flow tests are better at identifying people with symptoms, finds Cochrane review. BMJ 2021, 372, n823. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Ge, C.; Ding, Y.; Zhang, T.; Cao, S.; Meng, L.; Lu, S. Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection. J. Pharm. Anal. 2021, 11, 257–264. [Google Scholar] [CrossRef]
- Mann, C.; Griffin, J.H.; Downard, K.M. Detection and evolution of SARS-CoV-2 coronavirus variants of concern with mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 7241–7249. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A. COVID-19 Genomics UK (COG-UK) Consortium SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell 2021, 184, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Adikari, T.N.; Ferguson, J.M.; Hammond, J.M.; Stevanovski, I.; Beukers, A.G.; Naing, Z.; Yeang, M.; Verich, A.; Gamaarachchiet, H.; et al. Analytical validity of nanopore sequencing for rapid SARS-CoV-2 genome analysis. Nat. Commun. 2020, 11, 6272. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.M.; Banu, R.; Gonzalez-Reiche, A.S.; van de Guchte, A.; Khan, Z.; Shrestha, P.; Cao, L.; Chen, F.; Shi, H.; Hanna, A.; et al. Robust clinical detection of SARS-CoV-2 variants by RT-PCR/MALDI-TOF multi-target approach. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.09.09.21263348v1.full (accessed on 21 October 2021).
- Zhao, F.; Zhang, J.; Wang, X.; Hou, X.; Qin, T.; Meng, F.; Xu, X.; Li, T.; Zhou, H.; Kan, B.; et al. A novel strategy for the detection of SARS-CoV-2 variants based on multiplex PCR-MALDI-TOF MS. medRxiv 2021, 17, e0126721. [Google Scholar]
- National Library of Medicine (U.S.). Exploration of Feasability of Blood and Saliva Proteomic Analysis through Harvesting by Silica Matrix (NanoDx-CoV-19) (NanoDxCoV19). Identifier: NCT04597216. October 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04597216 (accessed on 21 October 2021).
- National Library of Medicine (U.S.). SARS-CoV2 (COVID-19) Diagnosis in Human Saliva by MALDI-TOF MS Profiling (CoviDiagMS). Identifier: NCT0446063. March 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04460638 (accessed on 21 October 2021).

| Specimen | Sample Size | MS Instrumentation | Identification Method | Molecular Target | Use of Database or Algorithms for Identification | Time | Diagnostic Performances | References |
|---|---|---|---|---|---|---|---|---|
| Nasopharyngeal samples | Not specified | MALDI-FT-ICR | Proteotyping | Viral proteins:
| Online database searching | Similar to RT-PCR time frame (few minutes for mass spectra acquisition) | Only analytical performances | Dollman et al. [74] |
| Saliva or gargle samples | 35 samples | MALDI-TOF MS | Biotyping and Biomolecular Host Profiling | Host proteins; viral proteins:
| Output data processed with appropriate software (not specified) | 45 min for sample preparation, 3 min per sample for MALDI-TOF analysis, a few seconds for data results analysis | Sensitivity of ~100% (5) | Iles et al. [69] |
| Gargle samples |
| MALDI-TOF MS | Biotyping and Biomolecular Host Profiling | Host proteins; viral proteins:
| Online database searching | Not mentioned |
| Chivte et al. [70] |
| Oral or nasopharyngeal samples |
| MALDI-TOF MS | Genotyping | Viral genes:
| Not specified | 8 h for the entire process | Not mentioned | Wandernoth et al. [71] |
| Oral or nasopharyngeal samples | 168 suspected COVID-19 samples | MALDI-TOF MS | Genotyping | Viral genes:
| Online database searching | 8 h for the entire process | Not mentioned | Rybicka et al. [72] |
| Saliva samples |
| MALDI-TOF MS | Genotyping | Viral genes:
| Not specified | Not mentioned |
| Hernandez et al. [73] |
| Nasopharyngeal samples |
| MALDI-TOF MS | Biomolecular Host Profiling | Host proteins | Machine learning algorithms | Not mentioned |
| Nachtigall et al. [75] |
| Nasal samples |
| MALDI-TOF MS | Biomolecular Host Profiling | Host proteins | Machine learning algorithms | Total turnaround time < 1 h |
| Tran et al. [76] |
| Nasopharyngeal samples | 237 samples | MALDI-TOF MS | Biomolecular Host Profiling | Host proteins | Machine learning algorithms | Turnaround time < 2 h |
| Deulofeu et al. [77] |
| Nasopharyngeal samples | 311 samples | MALDI-TOF MS | Biomolecular Host Profiling | Host proteins | In-house database searching and machine learning algorithms | Not mentioned |
| Rocca et al. [78] |
| Serum samples |
| MALDI-TOF MS | Biomolecular Host Profiling | Host proteins | Machine learning algorithms | Less than 1 min per sample for MALDI-TOF analysis |
| Yan et al. [79] |
| Specimen | Test Category | LOD (1) | Analysis Time | Estimated Cost per Sample | Key Points | References |
|---|---|---|---|---|---|---|
| Respiratory tract specimens | RT-PCR | <10 copies/reactions (103–104 copies) (2) | 4–6 h | USD 10–15 | Current gold standard for COVID-19 diagnosis. | Kevadiya et al. [93] |
| Nasopharyngeal samples | MALDI-FT-ICR | 105 copies | Similar to RT-PCR time frame | USD 100 | LOD could be improved in automated selected ion monitoring (SIM) strategy (reaching 103–104 copies). | Dollman et al. [74] |
| Saliva or gargle samples | MALDI-TOF MS | ~10–102 copies | ~50 min | Less than USD 1 | Specificity at 10–102 copies was reached only for the S1 protein peak. | Iles et al. [69] |
| Gargle samples | MALDI-TOF MS | ~30 copies | Not mentioned | Not mentioned | MS protocol was sensitive and comparable with RT-PCR forlow viral loads. | Chivte et al. [70] |
| Oral or nasopharyngeal samples | RT-PCR/MALDI-TOF MS | ~10 copies | 8 h | Not mentioned | Time-to-results was faster for RT-PCR, while hands-on time was comparable between RT-PCR and MS assay techniques. | Wandernoth et al. [71] |
| Oral or nasopharyngeal samples | RT-PCR/MALDI-TOF MS | ~10 copies | 8 h | ~ EUR 10 | The MS assay was able to detect SARS-CoV-2 in low viral load specimens. | Rybicka et al. [72] |
| Saliva samples | RT-PCR/MALDI-TOF MS | ~103 copies | Not mentioned | Not mentioned | The LOD of 103 copies was obtained for the N2 target. | Hernandez et al. [73] |
| Specimen | Sample Size | Patient Classification | Protein/Peptide Identity | m/z | Expression (Downregulated ↓, Upregulated ↑) Against Control | Bioinformatic Tool | Sensitivity/ Specificity of ML Diagnostic | References |
|---|---|---|---|---|---|---|---|---|
| Gargle | 60 samples |
| Immunoglobulin heavy chain or amylase | 55,500–59,000 | ↑ |
| ML (3) not applied. | Chivte et al. [70] |
| Immunoglobulin heavy chain doubly charged | 27,900–29,400 | ↑ | ||||||
| Not identified | ~112,000 | ↑ | ||||||
| Nasopharyngeal | 362 samples |
| Not identified | 3358 | ↓ |
|
| Nachtigall et al. [75] |
| 3095 | ↓ | |||||||
| 4532 | ↑ | |||||||
| 3337 | ↓ | |||||||
| 3152 | ↑ | |||||||
| 10,444 | ↓ | |||||||
| 7612 | ↓ | |||||||
| Nasal | 199 samples |
| Not identified | Not specified | Not specified |
|
| Tran et al. [76] |
| Nasopharyngeal | 237 samples | Not mentioned | Not identified | Not specified | Not specified |
|
| Deulofeu et al. [77] |
| Nasopharyngeal | 311 samples | Not mentioned | Not identified | 3372 | ↓ |
|
| Rocca et al. [78] |
| 3442 | ↓ | |||||||
| 3465 | ↓ | |||||||
| 3488 | ↓ | |||||||
| 6347 | ↓ | |||||||
| 10,836 | ↓ | |||||||
| Serum | 298 samples |
| Not identified | 6357 | ↓ |
|
| Yan et al. [79] |
| 6654 | ↓ | |||||||
| 6639 | ↓ | |||||||
| 28,232 | ↓ | |||||||
| Platelet basic protein | 13,886 | ↓ | ||||||
| Platelet factor 4 variant | 7614 | ↑ | ||||||
| Hemoglobin subunit alpha | 15,123 | ↑ | ||||||
| Hemoglobin subunit beta | 15,867 | ↑ | ||||||
| WD repeat-containing protein | 28,091 | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preianò, M.; Correnti, S.; Pelaia, C.; Savino, R.; Terracciano, R. MALDI MS-Based Investigations for SARS-CoV-2 Detection. BioChem 2021, 1, 250-278. https://doi.org/10.3390/biochem1030018
Preianò M, Correnti S, Pelaia C, Savino R, Terracciano R. MALDI MS-Based Investigations for SARS-CoV-2 Detection. BioChem. 2021; 1(3):250-278. https://doi.org/10.3390/biochem1030018
Chicago/Turabian StylePreianò, Mariaimmacolata, Serena Correnti, Corrado Pelaia, Rocco Savino, and Rosa Terracciano. 2021. "MALDI MS-Based Investigations for SARS-CoV-2 Detection" BioChem 1, no. 3: 250-278. https://doi.org/10.3390/biochem1030018
APA StylePreianò, M., Correnti, S., Pelaia, C., Savino, R., & Terracciano, R. (2021). MALDI MS-Based Investigations for SARS-CoV-2 Detection. BioChem, 1(3), 250-278. https://doi.org/10.3390/biochem1030018








