Role of MOB4 in Cell Proliferation and Neurogenesis
Abstract
:1. Introduction
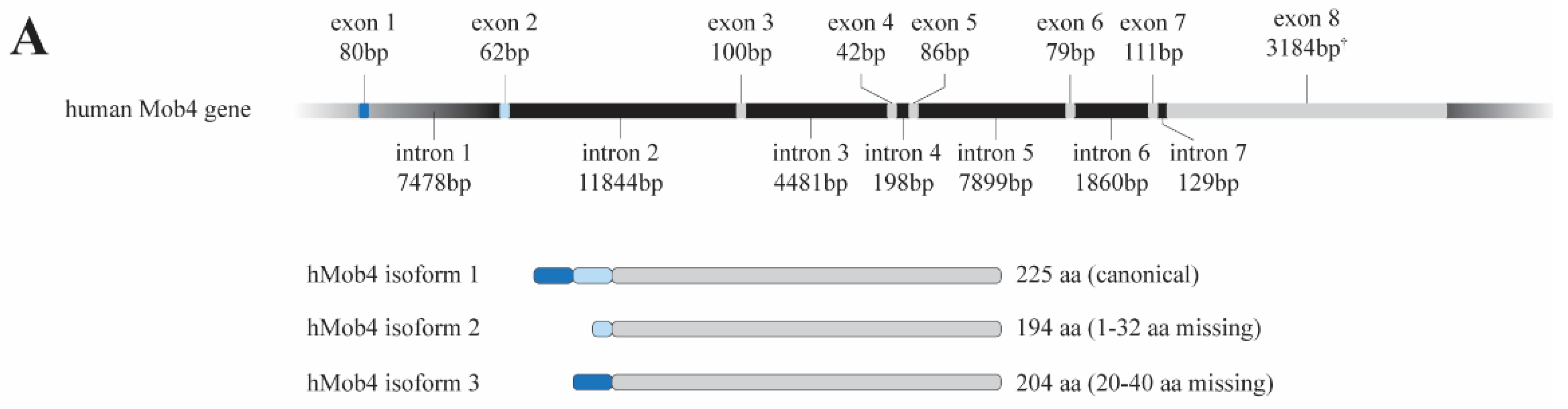
2. MOB4: From the Gene to the Function
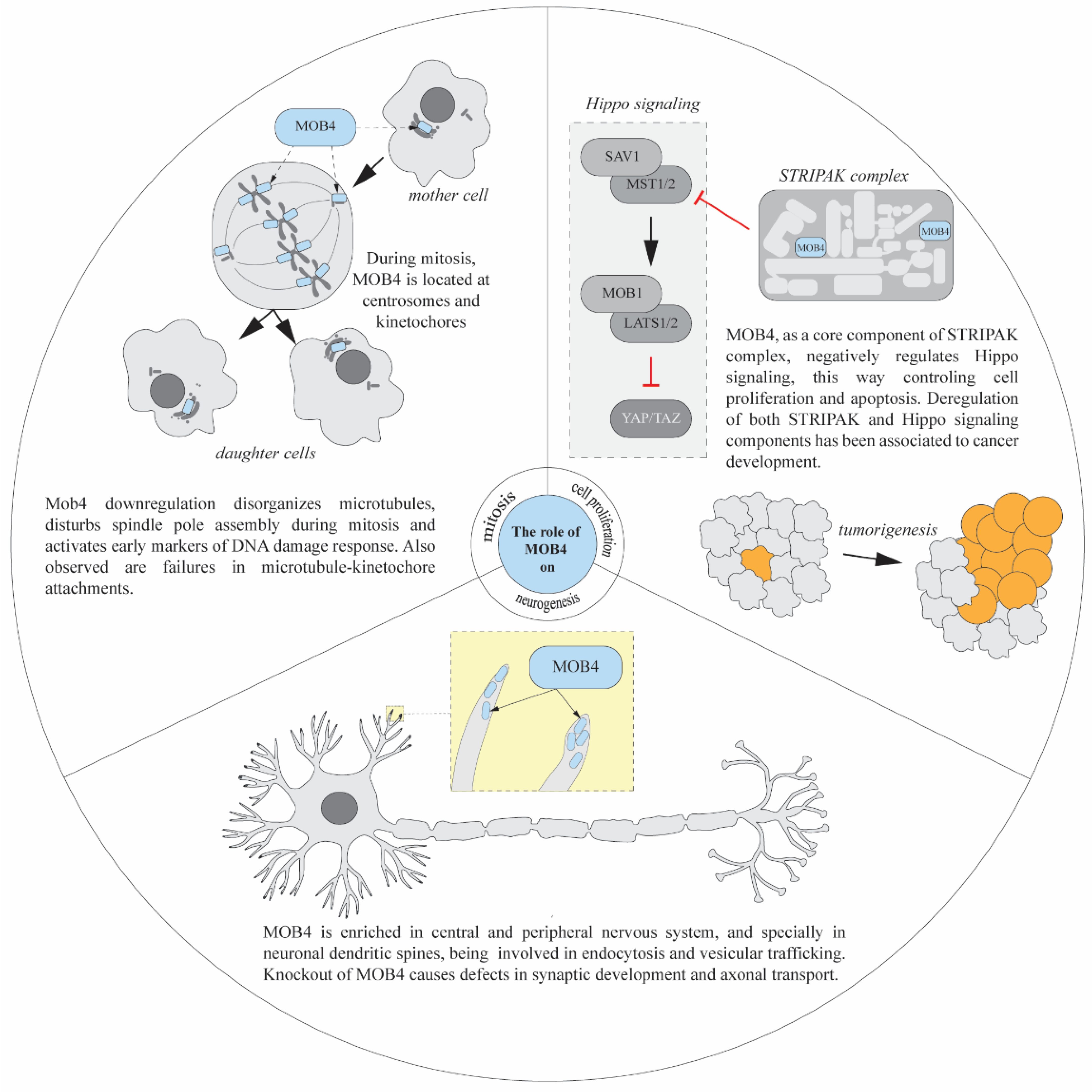
3. Neuronal Functions of Mob4
4. Mob4 and Cytoskeleton
5. Cell Proliferation Function of Mob4
6. Mob4 and Spermatogenesis
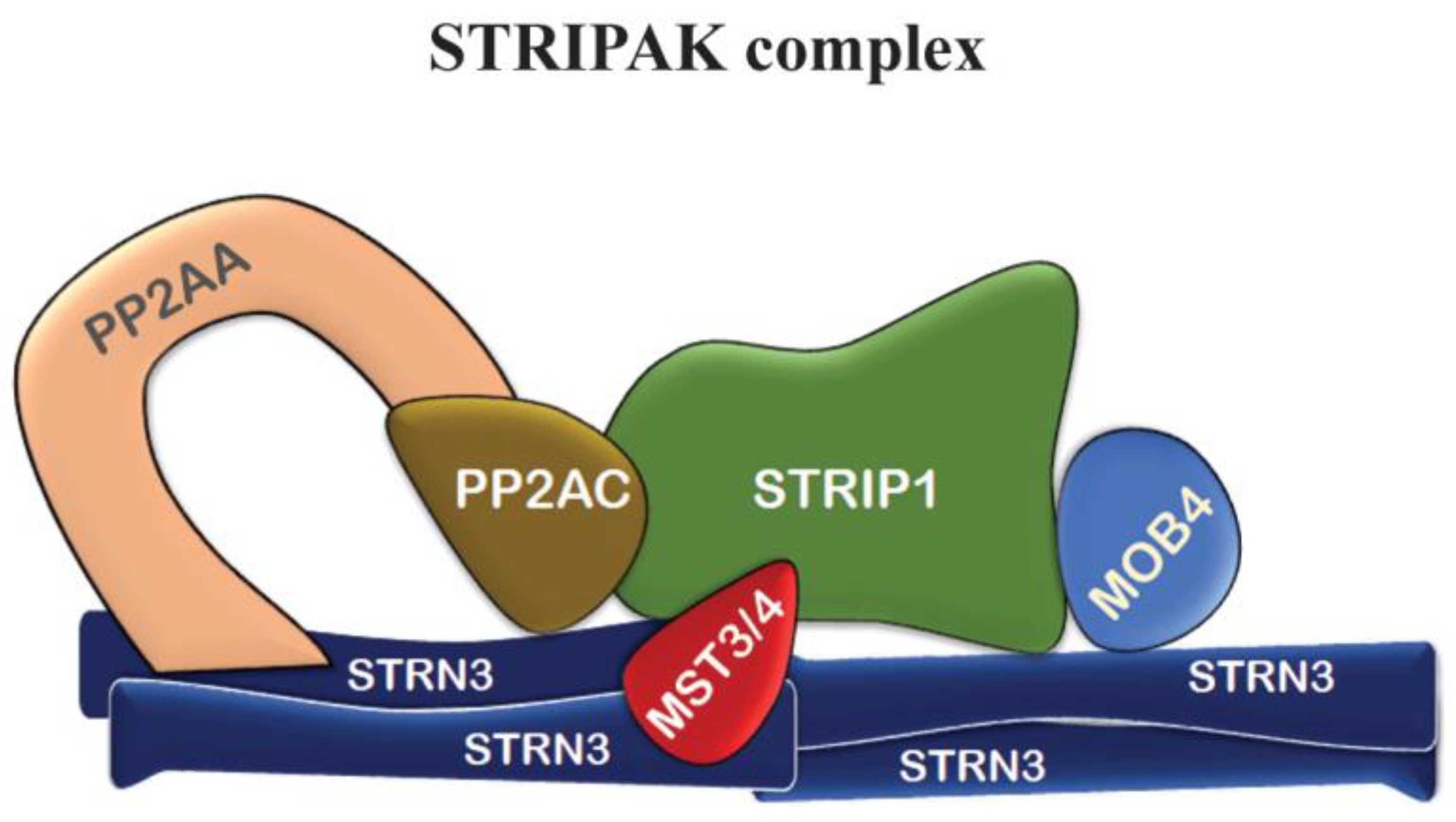
7. Other Mob4 Functions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, I.; Carmona, B.; Nolasco, S.; Santos, D.; Leitão, A.; Soares, H. MOB: Pivotal Conserved Proteins in Cytokinesis, Cell Architecture and Tissue Homeostasis. Biology 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Duhart, J.C.; Raftery, L.A. Mob Family Proteins: Regulatory Partners in Hippo and Hippo-Like Intracellular Signaling Pathways. Front. Cell Dev. Biol. 2020, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.C.; Winey, M. Regulation of Mob1p, an essential budding yeast protein required for completion of mitosis and spindle pole body duplication. Mol. Biol. Cell 1998, 9, 12A. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.C.; Winey, M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol. Biol. Cell 1998, 9, 29–46. [Google Scholar] [CrossRef]
- Ye, X.; Nikolaidis, N.; Nei, M.; Lai, Z. Evolution of the mob Gene Family. Open Cell Signal. J. 2009, 1, 1–11. [Google Scholar] [CrossRef]
- Vitulo, N.; Vezzi, A.; Galla, G.; Citterio, S.; Marino, G.; Ruperti, B.; Zermiani, M.; Albertini, E.; Valle, G.; Barcaccia, G. Characterization and evolution of the cell cycle-associated mob domain-containing proteins in eukaryotes. Evol. Bioinform. 2007, 3, 121–158. [Google Scholar] [CrossRef]
- Lai, Z.C.; Wei, X.; Shimizu, T.; Ramos, E.; Rohrbaugh, M.; Nikolaidis, N.; Ho, L.L.; Li, Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 2005, 120, 675–685. [Google Scholar] [CrossRef]
- Pan, D. Hippo signaling in organ size control. Genes Dev. 2007, 21, 886–897. [Google Scholar] [CrossRef]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef]
- Weiss, E.L.; Kurischko, C.; Zhang, C.; Shokat, K.; Drubin, D.G.; Luca, F.C. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J. Cell Biol. 2002, 158, 885–900. [Google Scholar] [CrossRef]
- Kohler, R.S.; Schmitz, D.; Cornils, H.; Hemmings, B.A.; Hergovich, A. Differential NDR/LATS interactions with the human MOB family reveal a negative role for human MOB2 in the regulation of human NDR kinases. Mol. Cell. Biol. 2010, 30, 4507–4520. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, L.; Xue, G.; Hynx, D.; Wang, Y.; Cron, P.D.; Hundsrucker, C.; Hergovich, A.; Frank, S.; Hemmings, B.A.; et al. hMOB3 modulates MST1 apoptotic signaling and supports tumor growth in glioblastoma multiforme. Cancer Res 2014, 74, 3779–3789. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed]
- Goudreault, M.; D’Ambrosio, L.M.; Kean, M.J.; Mullin, M.J.; Larsen, B.G.; Sanchez, A.; Chaudhry, S.; Chen, G.I.; Sicheri, F.; Nesvizhskii, A.I.; et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteom. 2009, 8, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Glatter, T.; Wepf, A.; Aebersold, R.; Gstaiger, M. An integrated workflow for charting the human interaction proteome: Insights into the PP2A system. Mol. Syst. Biol. 2009, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.S.; Josué, F.; Wepf, A.; Wehr, M.C.; Rinner, O.; Kelly, G.; Tapon, N.; Gstaiger, M. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 2010, 39, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Hwang, J.; Carrier, K.J.; Jones, C.A.; Kern, Q.L.; Moreno, C.S.; Karas, R.H.; Pallas, D.C. Protein phosphatase 2a (PP2A) binds within the oligomerization domain of striatin and regulates the phosphorylation and activation of the mammalian Ste20-Like kinase Mst3. BMC Biochem. 2011, 12, 54. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Gil-Ranedo, J.; Gonzaga, E.; Jaworek, K.J.; Berger, C.; Bossing, T.; Barros, C.S. STRIPAK Members Orchestrate Hippo and Insulin Receptor Signaling to Promote Neural Stem Cell Reactivation. Cell Rep. 2019, 27, 2921–2933.e5. [Google Scholar] [CrossRef]
- Shi, Z.; Jiao, S.; Zhou, Z. STRIPAK complexes in cell signaling and cancer. Oncogene 2016, 35, 4549–4557. [Google Scholar] [CrossRef]
- Baillat, G.; Moqrich, A.; Castets, F.; Baude, A.; Bailly, Y.; Benmerah, A.; Monneron, A. Molecular cloning and characterization of phocein, a protein found from the Golgi complex to dendritic spines. Mol. Biol. Cell 2001, 12, 663–673. [Google Scholar] [CrossRef]
- Jeong, B.C.; Bae, S.J.; Ni, L.; Zhang, X.; Bai, X.C.; Luo, X. Cryo-EM structure of the Hippo signaling integrator human STRIPAK. Nat. Struct. Mol. Biol. 2021, 28, 290–299. [Google Scholar] [CrossRef]
- Moreno, C.S.; Lane, W.S.; Pallas, D.C. A mammalian homolog of yeast MOB1 is both a member and a putative substrate of striatin family-protein phosphatase 2A complexes. J. Biol. Chem. 2001, 276, 24253–24260. [Google Scholar] [CrossRef]
- Haeberlé, A.M.; Castets, F.; Bombarde, G.; Baillat, G.; Bailly, Y. Immunogold localization of MOB4 in dendritic spines. J. Comp. Neurol. 2006, 495, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Bailly, Y.J.R.; Castets, F. Phocein: A potential actor in vesicular trafficking at Purkinje cell dendritic spines. Cerebellum 2007, 6, 344–352. [Google Scholar] [CrossRef]
- Schulte, J.; Sepp, K.J.; Jorquera, R.A.; Wu, C.; Song, Y.; Hong, P.; Littleton, J.T. DMob4/Phocein regulates synapse formation, axonal transport, and microtubule organization. J. Neurosci. 2010, 30, 5189–5203. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Mimoso, J.M.; Palma, S.L.; Gonçalves, C.; Silvestre, D.; Campinho, M.; Tavares, Á.A. Mob4 is required for neurodevelopment in zebrafish. microPubl. Biol. 2023. [Google Scholar] [CrossRef]
- Hwang, J.; Pallas, D.C. STRIPAK complexes: Structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 2014, 47, 118–148. [Google Scholar] [CrossRef]
- Li, D.; Musante, V.; Zhou, W.; Picciotto, M.R.; Nairn, A.C. Striatin-1 is a B subunit of protein phosphatase PP2A that regulates dendritic arborization and spine development in striatal neurons. J. Biol. Chem. 2018, 293, 11179–11194. [Google Scholar] [CrossRef]
- Trammell, M.A.; Mahoney, N.M.; Agard, D.A.; Vale, R.D. Mob4 plays a role in spindle focusing in Drosophila S2 cells. J. Cell Sci. 2008, 121, 1284–1292. [Google Scholar] [CrossRef]
- Berger, J.; Berger, S.; Currie, P.D. Mob4-dependent STRIPAK involves the chaperonin TRiC to coordinate myofibril and microtubule network growth. PLoS Genet. 2022, 18, e1010287. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.B.; Farr, G.W.; Miklos, D.; Horwich, A.L.; Sternlicht, M.L.; Sternlicht, H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 1992, 358, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Berger, S.; Li, M.; Jacoby, A.S.; Arner, A.; Bavi, N.; Stewart, A.G.; Currie, P.D. In Vivo Function of the Chaperonin TRiC in α-Actin Folding during Sarcomere Assembly. Cell Rep. 2018, 22, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ghozlan, H.; Cox, A.; Nierenberg, D.; King, S.; Khaled, A.R. The TRiCky Business of Protein Folding in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 906530. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, K.; Sato, M.; Sato, K.; Nakajima-Shimada, J.; Harada, A.; Sato, K.; Geisler, F.; Gerhardus, H.; Carberry, K.; Davis, W.; et al. Caenorhabditis elegans chaperonin CCT/TRiC is required for actin and tubulin biogenesis and microvillus formation in intestinal epithelial cells. Mol. Biol. Cell 2014, 25, 3095–3104. [Google Scholar] [CrossRef]
- Khabirova, E.; Moloney, A.; Marciniak, S.J.; Williams, J.; Lomas, D.A.; Oliver, S.G.; Favrin, G.; Sattelle, D.B.; Crowther, D.C. The TRiC/CCT chaperone is implicated in Alzheimer’s disease based on patient GWAS and an RNAi screen in Abeta-expressing Caenorhabditis elegans. PLoS ONE 2014, 9, e102985. [Google Scholar] [CrossRef] [PubMed]
- Schad, E.G.; Petersen, C.P. STRIPAK Limits Stem Cell Differentiation of a WNT Signaling Center to Control Planarian Axis Scaling. Curr. Biol. 2020, 30, 254–263.e2. [Google Scholar] [CrossRef]
- Wagner, D.E.; Wang, I.E.; Reddien, P.W. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 2011, 332, 811–816. [Google Scholar] [CrossRef]
- Reddien, P.W. The Cellular and Molecular Basis for Planarian Regeneration. Cell 2018, 175, 327–345. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Gurley, K.A.; Rink, J.C.; Sanchez Alvarado, A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 2008, 319, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Reddien, P.W. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 2008, 319, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Sileo, P.; Simonin, C.; Melnyk, P.; Chartier-Harlin, M.C.; Cotelle, P. Crosstalk between the Hippo Pathway and the Wnt Pathway in Huntington’s Disease and Other Neurodegenerative Disorders. Cells 2022, 11, 3631. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Gonçalves, J.; Florindo, C.; Tavares, A.A.; Soares, H. Mob1: Defining cell polarity for proper cell division. J. Cell Sci. 2012, 125, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Perdigão, J.; Fesquet, D.; Schiebel, E.; Pines, J.; Tavares, A.A. Human Mob1 proteins are required for cytokinesis by controlling microtubule stability. J. Cell Sci. 2012, 125, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Piroli, M.E.; Blanchette, J.O.; Jabbarzadeh, E. Polarity as a physiological modulator of cell function. Front. Biosci. 2019, 24, 451–462. [Google Scholar] [CrossRef]
- Bernhards, Y.; Pöggeler, S. The phocein homologue SmMOB3 is essential for vegetative cell fusion and sexual development in the filamentous ascomycete Sordaria macrospora. Curr. Genet. 2011, 57, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Iwasa, H.; Kuroyanagi, H.; Hata, Y. Loss of Caenorhabditis elegans homologue of human MOB4 compromises life span, health life span and thermotolerance. Genes Cells 2021, 26, 798–806. [Google Scholar] [CrossRef]
- Iwasa, H.; Maimaiti, S.; Kuroyanagi, H.; Kawano, S.; Inami, K.; Timalsina, S.; Ikeda, M.; Nakagawa, K.; Hata, Y. Yes-associated protein homolog, YAP-1, is involved in the thermotolerance and aging in the nematode Caenorhabditis elegans. Exp. Cell Res. 2013, 319, 931–945. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, H.; Shi, Z.; Li, Y.; Zhang, X.; Gao, Z.; Zhou, L.; Ma, J.; Xu, Q.; Guan, J.; et al. The MST4-MOB4 complex disrupts the MST1–MOB1 complex in the Hippo–YAP pathway and plays a pro-oncogenic role in pancreatic cancer. J. Biol. Chem. 2018, 293, 14455–14469. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kück, U.; Radchenko, D.; Teichert, I. STRIPAK, a highly conserved signaling complex, controls multiple eukaryotic cellular and developmental processes and is linked with human diseases. Biol. Chem. 2019, 400, 1005–1022. [Google Scholar] [CrossRef]
- Chiu, I.M. Growth factor genes as oncogenes. Mol. Chem. Neuropathol. 1989, 10, 37–52. [Google Scholar] [CrossRef]
- Miller, M.S.; Miller, L.D. RAS Mutations and Oncogenesis: Not all RAS Mutations are Created Equally. Front. Genet. 2012, 2, 100. [Google Scholar] [CrossRef] [PubMed]
- Morrish, F.; Neretti, N.; Sedivy, J.M.; Hockenbery, D.M. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle 2008, 7, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, D.W. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene 2006, 25, 5233–5243. [Google Scholar] [CrossRef]
- Smith, A.L.; Robin, T.P.; Ford, H.L. Molecular pathways: Targeting the TGF-β pathway for cancer therapy. Clin. Cancer Res. 2012, 18, 4514–4521. [Google Scholar] [CrossRef]
- Savage, K.I.; Harkin, D.P. BRCA1, a ‘complex’ protein involved in the maintenance of genomic stability. FEBS J. 2015, 282, 630–646. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Nishio, M.; Hamada, K.; Kawahara, K.; Sasaki, M.; Noguchi, F.; Chiba, S.; Mizuno, K.; Suzuki, S.O.; Dong, Y.; Tokuda, M.; et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J. Clin. Investig. 2012, 122, 4505–4518. [Google Scholar] [CrossRef]
- St John, M.A.; Tao, W.; Fei, X.; Fukumoto, R.; Carcangiu, M.L.; Brownstein, D.G.; Parlow, A.F.; McGrath, J.; Xu, T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat. Genet. 1999, 21, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef]
- Lee, K.P.; Lee, J.H.; Kim, T.S.; Kim, T.H.; Park, H.D.; Byun, J.S.; Kim, M.C.; Jeong, W.I.; Calvisi, D.F.; Kim, J.M.; et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 8248–8253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Yao, G.; Hu, L.; Yan, Y.; Liu, J.; Shi, J.; Chang, Y.; Zhang, Y.; Liang, D.; Shen, D.; et al. MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling. Cell Death Dis. 2020, 11, 230. [Google Scholar] [CrossRef]
- Santos, I.B.; Wainman, A.; Garrido-Maraver, J.; Pires, V.; Riparbelli, M.G.; Kovács, L.; Callaini, G.; Glover, D.M.; Tavares, Á.A. Mob4 is essential for spermatogenesis in Drosophila melanogaster. Genetics 2023, 224, iyad104. [Google Scholar] [CrossRef]
- Frost, A.; Elgort, M.G.; Brandman, O.; Ives, C.; Collins, S.R.; Miller-Vedam, L.; Weibezahn, J.; Hein, M.Y.; Poser, I.; Mann, M.; et al. Functional repurposing revealed by comparing S. pombe and S. cerevisiae genetic interactions. Cell 2012, 149, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, R.D.; Soni, D.V.; Wollman, R.; Hahn, A.T.; Yee, M.C.; Guan, A.; Hesley, J.A.; Miller, S.C.; Cromwell, E.F.; Solow-Cordero, D.E.; et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol. Cell 2009, 35, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lee, T.Y.; Liang, H.K.; Huang, C.M.; Wang, T.Y.; Yang, Y.H.; Chu, C.H.; Huang, H.D.; Ko, M.T.; Hwang, J.K. KinasePhos 2.0: A web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 2007, 35, W588–W594. [Google Scholar] [CrossRef]
- Fukasawa, T.; Enomoto, A.; Miyagawa, K. Serine–Threonine Kinase 38 regulates CDC25A stability and the DNA damage-induced G2/M checkpoint. Cell. Signal. 2015, 27, 1569–1575. [Google Scholar] [CrossRef]
- Lehtinen, M.K.; Yuan, Z.; Boag, P.R.; Yang, Y.; Villén, J.; Becker, E.B.; DiBacco, S.; de la Iglesia, N.; Gygi, S.; Blackwell, T.K.; et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 2006, 125, 987–1001. [Google Scholar] [CrossRef]
- Sanphui, P.; Biswas, S.C. FoxO3a is activated and executes neuron death via Bim in response to β-amyloid. Cell Death Dis. 2013, 4, e625. [Google Scholar] [CrossRef] [PubMed]
- Valis, K.; Prochazka, L.; Boura, E.; Chladova, J.; Obsil, T.; Rohlena, J.; Truksa, J.; Dong, L.F.; Ralph, S.J.; Neuzil, J. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res 2011, 71, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Nagai, M.; Miyazaki, K.; Kurata, T.; Takehisa, Y.; Ikeda, Y.; Kamiya, T.; Okazawa, H.; Abe, K. Progressive decrease in the level of YAPdeltaCs, prosurvival isoforms of YAP, in the spinal cord of transgenic mouse carrying a mutant SOD1 gene. J. Neurosci. Res. 2009, 87, 928–936. [Google Scholar] [CrossRef]
- He, Y.; Emoto, K.; Fang, X.; Ren, N.; Tian, X.; Jan, Y.N.; Adler, P.N.; Kurischko, C.; Kuravi, V.K.; Wannissorn, N.; et al. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol. Biol. Cell 2005, 16, 4139–4152. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K.; He, Y.; Ye, B.; Grueber, W.B.; Adler, P.N.; Jan, L.Y.; Jan, Y.N. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 2004, 119, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Norkett, R.; del Castillo, U.; Lu, W.; Gelfand, V.I. Ser/Thr kinase Trc controls neurite outgrowth in Drosophila by modulating microtubule-microtubule sliding. eLife 2020, 9, e52009. [Google Scholar] [CrossRef]
- Wang, L.H.; Baker, N.E. Salvador-Warts-Hippo pathway regulates sensory organ development via caspase-dependent nonapoptotic signaling. Cell Death Dis. 2019, 10, 669. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsieh, M.; Fan, S.S. The promotion of neurite formation in Neuro2A cells by mouse Mob2 protein. FEBS Lett. 2011, 585, 523–530. [Google Scholar] [CrossRef]
- Campbell, M.; Ganetzky, B. Identification of Mob2, a novel regulator of larval neuromuscular junction morphology, in natural populations of Drosophila melanogaster. Genetics 2013, 195, 915–926. [Google Scholar] [CrossRef]
- O’neill, A.C.; Kyrousi, C.; Einsiedler, M.; Burtscher, I.; Drukker, M.; Markie, D.M.; Kirk, E.P.; Götz, M.; Robertson, S.P.; Cappello, S. Mob2 Insufficiency Disrupts Neuronal Migration in the Developing Cortex. Front. Cell. Neurosci. 2018, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Hondius, D.C.; Eigenhuis, K.N.; Morrema, T.H.J.; van der Schors, R.C.; van Nierop, P.; Bugiani, M.; Li, K.W.; Hoozemans, J.J.M.; Smit, A.B.; Rozemuller, A.J.M. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.I.; Kawana, N.; Yamamoto, Y. Pathway Analysis of ChIP-Seq-Based NRF1 Target Genes Suggests a Logical Hypothesis of their Involvement in the Pathogenesis of Neurodegenerative Diseases. Gene Regul. Syst. Biol. 2013, 7, 139–152. [Google Scholar] [CrossRef] [PubMed]
- McInnes, J. Insights on altered mitochondrial function and dynamics in the pathogenesis of neurodegeneration. Transl. Neurodegener. 2013, 2, 12. [Google Scholar] [CrossRef]
- Oláh, J.; Vincze, O.; Virók, D.; Simon, D.; Bozsó, Z.; Tőkési, N.; Horváth, I.; Hlavanda, E.; Kovács, J.; Magyar, A.; et al. Interactions of pathological hallmark proteins: Tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein. J. Biol. Chem. 2011, 286, 34088–34100. [Google Scholar] [CrossRef]
- Hidalgo, A.; Ffrench-Constant, C. The control of cell number during central nervous system development in flies and mice. Mech. Dev. 2003, 120, 1311–1325. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Q.; Brooks, D.; Geisbrecht, E.R. A conserved STRIPAK complex is required for autophagy in muscle tissue. Mol. Biol. Cell 2023, 34, ar91. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.B.; Garrido-Maraver, J.; Gonçalves, C.; Oliveira, B.I.; Tavares, Á.A. Role of MOB4 in Cell Proliferation and Neurogenesis. BioChem 2023, 3, 182-196. https://doi.org/10.3390/biochem3040013
Santos IB, Garrido-Maraver J, Gonçalves C, Oliveira BI, Tavares ÁA. Role of MOB4 in Cell Proliferation and Neurogenesis. BioChem. 2023; 3(4):182-196. https://doi.org/10.3390/biochem3040013
Chicago/Turabian StyleSantos, Inês B., Juan Garrido-Maraver, Carolina Gonçalves, Bruna I. Oliveira, and Álvaro A. Tavares. 2023. "Role of MOB4 in Cell Proliferation and Neurogenesis" BioChem 3, no. 4: 182-196. https://doi.org/10.3390/biochem3040013






