Advances in Oral Solid Drug Delivery Systems: Quality by Design Approach in Development of Controlled Release Tablets
Abstract
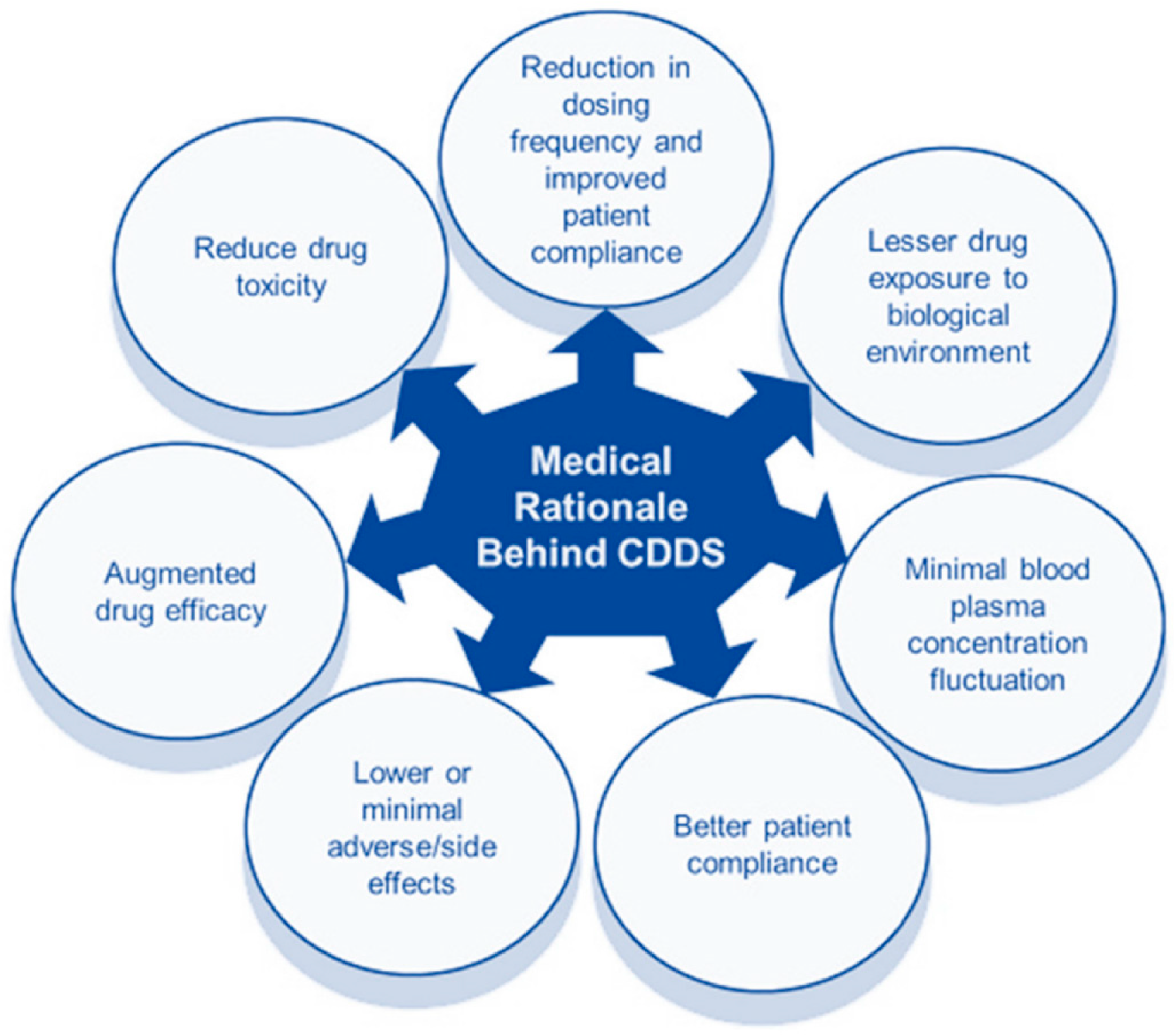
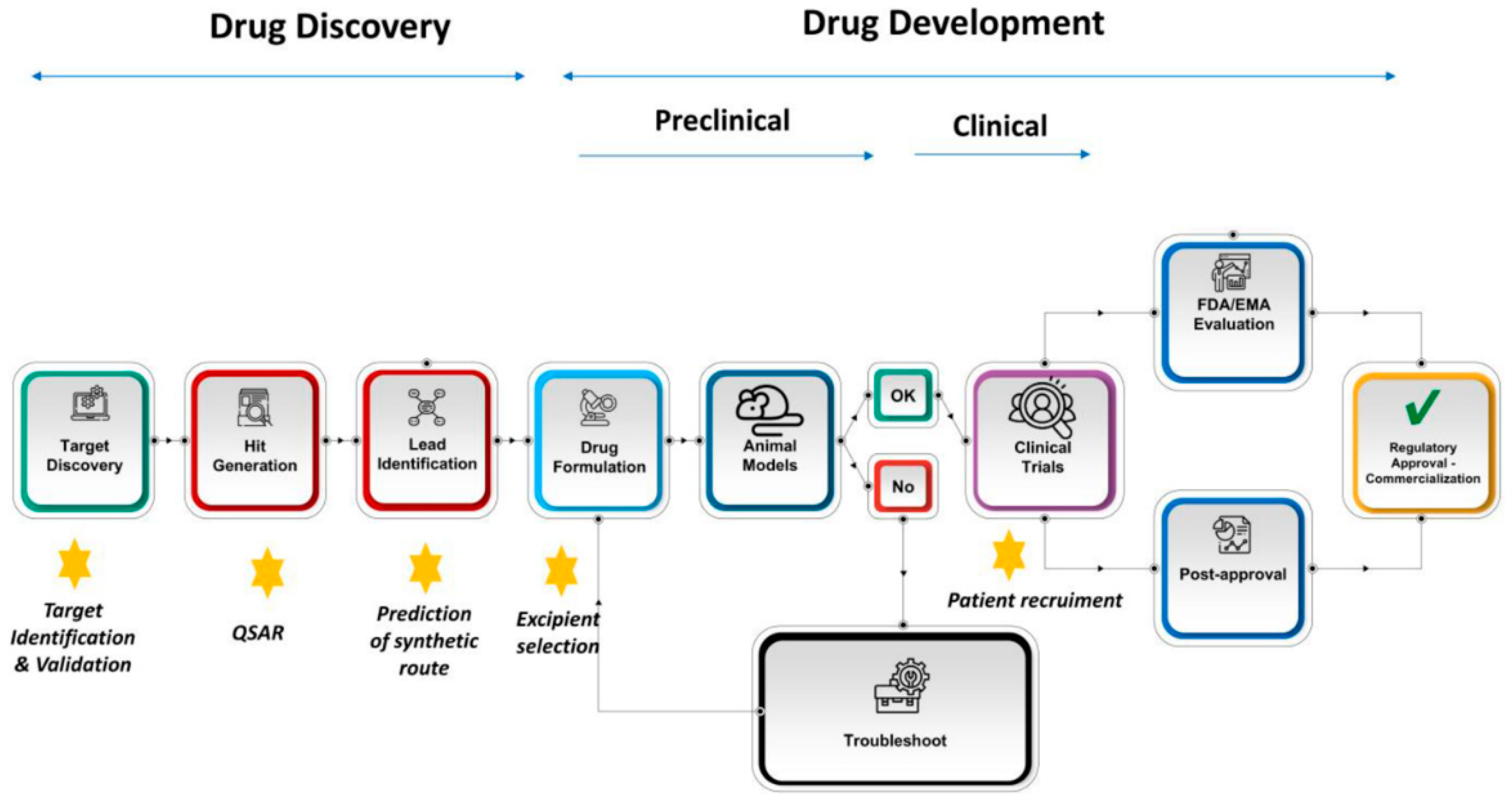
:1. Introduction
2. QbD Guided Development and Product Performance of Controlled Release Matrix Tablets
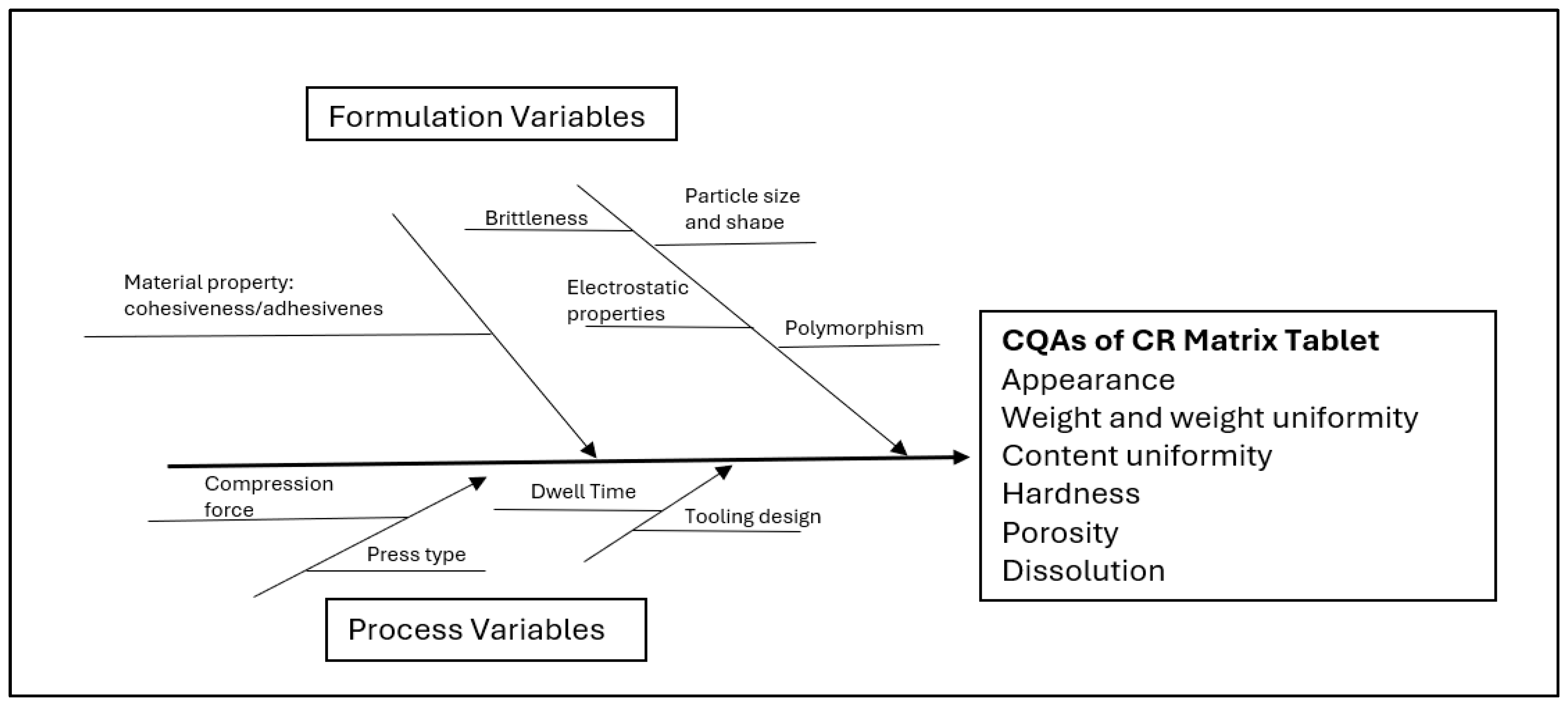
2.1. Identification of QTPP and Risk Assessment Analysis
2.2. Tableting: A Key Process Parameter
2.3. Controlled Release Matrix Tablets
2.3.1. Mechanisms of Controlled Release
2.3.2. Directly Compressed Tablets Formulated with Hydrophilic Matrix
2.3.3. Critical Factors Affecting Drug Release
Polymer Characteristics
Drug Attributes
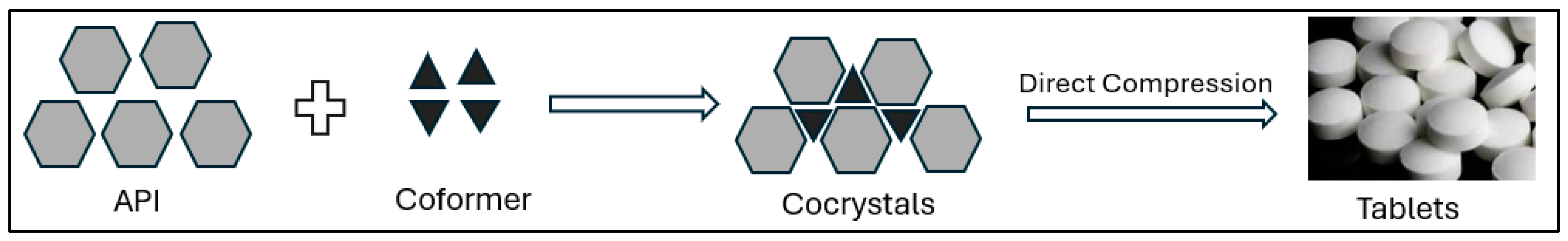
2.4. Alteration of Drug Properties and Drug Release Modulation via Crystal Engineering
- Solvent Evaporation and Recrystallization: These traditional methods involve dissolving an API in a suitable solvent and then carefully evaporating the solvent to form a specific crystal structure. By controlling factors like temperature, solvent choice, and concentration, scientists can influence the resulting crystal form [67].
- Milling and Mechanical Activation: High-energy milling can induce polymorphic transformations or the formation of amorphous materials, which often have higher solubility but may require stabilization. Mechanical activation is used to improve the compressibility and flow, which are essential properties for tablet manufacturing [68].
- Spray Drying: This technique allows for the rapid evaporation of a solvent, leading to the formation of microcrystalline or amorphous forms. Spray drying is particularly effective for creating stable dispersions of APIs with improved dissolution rates [69].
- Hot-Melt Extrusion: In this method, an API and excipients are heated and extruded to form a solid dispersion, often in an amorphous state. This technique is suitable for drugs with poor solubility and can be used to improve the tablet performance [70].
- Supercritical Fluid Techniques: Using supercritical CO2 as a solvent, this technique enables precise control over crystallization conditions, allowing for the formation of unique crystal forms. Supercritical fluid technology is particularly useful for producing small, uniform particles with enhanced dissolution rates [71].
Applications of Crystal Engineering in Matrix Tablet Formulation
- Solubility Enhancement: Many new APIs suffer from poor water solubility, limiting their bioavailability and therapeutic effectiveness. Crystal engineering provides several strategies, such as polymorph selection, co-crystallization, and amorphization, to increase the solubility and dissolution rates [72]. For example, by selecting a more soluble polymorph or forming a co-crystal with a water-soluble coformer, the drug’s dissolution rate can be significantly improved, leading to better absorption in the gastrointestinal tract [73].
- Stability Improvement: Chemical and physical stability are crucial for maintaining the efficacy of a drug during its shelf life. Certain polymorphs may be more susceptible to degradation, while others are more stable [74]. Crystal engineering can help identify and select the most stable form of an API, reducing the risk of polymorphic transformations. Co-crystals and salts can also offer enhanced stability under different environmental conditions, protecting the API from degradation due to moisture, temperature, or light [75].
- Optimization of Mechanical Properties: Tablet formulation requires APIs with specific mechanical properties, such as compressibility and hardness. Some crystal forms are more brittle or difficult to compress, posing challenges in the tableting process. By designing crystals with improved mechanical properties, crystal engineering can enhance tablet manufacturability, reducing the likelihood of capping, lamination, or other tableting defects [76].
- Challenges and Limitations: While crystal engineering offers substantial benefits, it also faces challenges. Developing new crystal forms requires extensive screening and characterization, which can be time-consuming and costly [77]. Additionally, the formation of novel polymorphs or co-crystals may raise intellectual property concerns, as existing patents may restrict the use of certain crystal forms [78]. Regulatory challenges are also a significant concern. Regulatory agencies, such as the FDA and EMA, require thorough documentation of the crystal form used in a drug product, and any changes to this form may necessitate re-evaluation. This requirement can make it difficult to implement crystal engineering strategies after a drug has already reached the market [79].
2.5. Compression and Porosity
2.6. Kinetic Modeling of Oral Drug Release
- Zero-Order Model: Drug dissolution from pharmaceutical dosage systems from which the drug is released slowly can be represented by the following equation:where ft represents the fraction of drug dissolved in time t, and K0 is a zero-order release constant or a dissolution rate constant [21,85]. This relationship can be used to explain drug dissolution from transdermal systems, matrix tablets, coated forms, osmotic systems, etc. The dosage forms that follow the zero-order model release the same amount of drug per unit time.
- First-Order Model: The application of the first-order model to drug dissolution studies was first proposed by Gibaldi and Feldman [87] and later by Wagner [88]. Absorption and elimination phases have been described by this model. Pharmaceutical dosage forms containing water-soluble drugs in porous matrices follow first-order release kinetics [89]. The drug release is proportional to the amount of drug remaining in the interior of the porous matrix. The first-order model can be described by the following equation:where Qt is the amount of drug released in time t, Q0 is the initial amount of drug in the solution, and K1 is the first-order release constant.
- Higuchi Model: Higuchi [90,91] developed theoretical models to study the release of poorly water-soluble and water-soluble drugs from semi-solid or solid matrices. Higuchi describes drug release as a diffusion process based on Fick’s law, square-root time dependent. The Higuchi model can be expressed as follows:where ft is the fraction of dissolved drug in time t, and KH is the Higuchi dissolution rate constant.
- Korsemeyer–Peppas Model: Korsemeyer et al. [92] developed a simple, semi-empirical model, exponentially relating the drug release to the elapsed time (t):where Mt/M∞ is the fraction of drug released at time t, k is the release rate constant, and n is the release exponent. The value of the release exponent n characterizes the drug release mechanism. A value of n = 0.45, 0.45 < n < 0.89, and 0.89 < n < 1 indicates Fickian (Case I), non-Fickian (anomalous), and zero-order (Case II) transport, respectively [52,93].
3. Emerging Approaches for the Customization of Drug Release
3.1. Three-Dimensional (3D) Printing Technology
3.1.1. Layer-by-Layer Printing
3.1.2. Multi-Drug Delivery
3.1.3. Types of 3D Printing Techniques Used in Pharmaceutical Applications
3.2. Use of Artificial Intelligence in Controlled Release Formulation Development
- Predicting Drug Release Profiles: AI algorithms, particularly machine learning (ML) and deep learning, can be trained to predict the release profiles of drugs based on a set of input variables, such as the physicochemical properties of the drug, excipients, and formulation methods. These models can optimize the release rates and duration, ensuring that the formulation meets the therapeutic goals [107].
- Data-Driven Formulation Strategies: AI can analyze large datasets from various stages of formulation development, including experimental results, clinical trials, and real-time manufacturing data. By recognizing patterns in these data, AI can provide insights into the most effective formulation strategies, including optimal excipients for controlled release systems based on their properties, such as solubility, permeability, and biodegradability. AI models can also optimize the concentration and combinations of excipients to control drug release over time [108].
- Process Optimization: Machine learning models can be used to optimize the manufacturing processes, including hot-melt extrusion, granulation, and spray drying, which are commonly used in controlled release formulations. AI can identify the optimal parameters (e.g., temperature, pressure, and speed) to improve the efficiency, reduce batch variability, and ensure consistent drug release [109].
- Simulation of Release Mechanisms: AI-powered in silico models simulate the drug release process within different environments, such as varying pH levels in the gastrointestinal tract. This can help predict how the drug will behave in vivo, allowing for a better formulation design without the need for extensive in vivo testing [106].
- Stability Studies: AI can assist in predicting the stability of controlled release formulations over time under various storage conditions (e.g., temperature, humidity). This helps to design more stable formulations, ensuring the drug’s efficacy is maintained throughout its shelf life [110].
- Improving Bioavailability: AI models can optimize controlled release formulations to improve the bioavailability of poorly soluble drugs. By predicting the optimal particle size, excipient composition, and release kinetics, AI can enhance drug absorption and therapeutic outcomes [111].
- Virtual Screening and Testing: Before physical testing of the formulations, AI can be used to perform virtual screening of the excipients, drug compounds, and formulation strategies. This reduces the time and cost of physical experimentation and helps identify the most promising candidates for further development [111,112].
- Animal Testing Alternatives: AI models can simulate the pharmacokinetics (PK) and pharmacodynamics (PD) of controlled release formulations, reducing the need for extensive animal testing. This aligns with the growing trend toward reducing animal use in research and development [113].
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, J.; Yuen, K.H.; Ng, B.H.; Wong, J.W.; Al-Dhalli, S.; Elhassan, G.O.; Chitneni, M.; Kaleemullah, M.; Hami, J.A.; Yusuf, E.; et al. Bioequivalence evaluation of two different controlled release matrix formulations of ketoprofen tablets in healthy malaysian volunteers. Lat. Am. J. Pharm. 2011, 30, 1991–1998. [Google Scholar]
- Ummadi, S.; Shravani, B.; Rao, N.G.R.; Reddy, M.S.; Nayak, B.S. Overview on Controlled Release Dosage Form. Int. J. Pharma. Sci. 2013, 3, 258–269. [Google Scholar]
- Tiwari, S.B.; Rajabi-Siahboomi, A.R. Extended-release oral drug delivery technologies: Monolithic matrix systems. Methods Mol. Biol. 2008, 437, 217–243. [Google Scholar]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Patil, S.A.; Aminabhavi, T.M. Evaluation of acrylamide-grafted-xanthan gum copolymer matrix tablets for oral controlled delivery of antihypertensive drugs. Carbohydr. Polym. 2007, 69, 130–141. [Google Scholar] [CrossRef]
- Hoffman, A. Pharmacodynamic aspects of sustained release preparations. Adv. Drug Deliv. Rev. 1998, 33, 185–199. [Google Scholar] [CrossRef]
- Martin, C.; De Baerdemaeker, A.; Poelaert, J.; Madder, A.; Hoogenboom, R.; Ballet, S. Controlled-release of opioids for improved pain management. Mater. Today 2016, 19, 491–502. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Schneider, L.S.; Dunner, D.L.; Davies, J.T.; Pitts, C.D. Efficacy of controlled-release paroxetine in the treatment of late-life depression. J. Clin. Psychiatry 2003, 64, 1065–1074. [Google Scholar] [CrossRef]
- Kar, R.K.; Mohapatra, S.; Barik, B.B. Design and characterization of controlled release matrix tablets of zidovudine. Asian J. Pharm. Clin. Res. 2009, 2, 54–61. [Google Scholar]
- Barzeh, H.; Sogali, B.S.; Shadvar, S.A. Review on Extended Release Matrix Tablet. J. Pharm. Res. 2016, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, N.G.; Richard, K.; Raj, P.; Sanjeev Nayak, B. Review on Matrix Tablet as Sustained Release. Int. J. Pharm. Res. Allied Sci. 2013, 2, 1–17. [Google Scholar]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Bretti, G.; McGinty, S.; Pontrelli, G. Modelling smart drug release with functionally graded materials. Comput. Biol. Med. 2023, 164, 107294. [Google Scholar] [CrossRef]
- Mohaniya, P.; Modi, D.; Pawar, R. A review on sustained release matrix tablet. Int. J. Pharm. Sci. Med. 2024, 9, 17–29. [Google Scholar] [CrossRef]
- Bisht, T.; Rishiwer, P.; Kumar, P. Review on matrix tablet. Indo Glob. J. Pharm. Sci. 2016, 6, 38–42. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Raja, S.; Patel, P.; Asare-Addo, K. The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts 2012, 2, 175–187. [Google Scholar]
- Colombo, P. Swelling-controlled release in hydrogel matrices for oral route. Adv. Drug Deliv. Rev. 1993, 11, 37–57. [Google Scholar] [CrossRef]
- Shahzad, Y.; Ibrar, N.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Rizvi, S.A.A. Relevancy of Nizatidine’s Release from Floating Tablets with Viscosity of Various Cellulose Ethers. Sci 2021, 3, 22. [Google Scholar] [CrossRef]
- Kim, H.; Fassihi, R. A new ternary polymeric matrix system for controlled drug delivery of highly soluble drugs: I. Diltiazem hydrochloride. Pharm. Res. 1997, 14, 1415–1421. [Google Scholar] [CrossRef]
- Zahirul, M.; Khan, I. Dissolution testing for sustained or controlled release oral dosage forms and correlation with in vivo data: Challenges and opportunities. Int. J. Pharm. 1996, 140, 131–143. [Google Scholar] [CrossRef]
- Kharat, A.R. Mathematical Models of Drug Dissolution: A Review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar]
- Atre, P.; Rizvi, S.A.A. A brief overview of quality by design approach for developing pharmaceutical liposomes as nano-sized parenteral drug delivery systems. RSC Pharm. 2024, 1, 675–688. [Google Scholar] [CrossRef]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef]
- Hart, M.L. Brief Overview of Various Approaches to Enhance Drug Solubility. J. Dev. Drugs 2013, 2, 1000115. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S.L.; Rizvi, S.A.A.; Velaga, S.P. Pharmaceutical cocrystals of nitrofurantoin: Screening, characterization and crystal structure analysis. CrystEngComm 2012, 14, 5078–5088. [Google Scholar] [CrossRef]
- Nijhawan, M.; Dhyagala, S.; Sailaja, G.; Aleti, R.; Saxena, T. Dipyridamole Cocrystal Tablets with Enhanced Solubility and Dissolution at Intestinal pH. Fabad J. Pharm. Sci. 2024, 49, 37–50. [Google Scholar] [CrossRef]
- Friić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Vinod, K.P.; Anshu, S. Pharmaceutical Co-Crystal Tablet Formulation: An Overview. J. Gujarat Res. Soc. 2019, 21, 2703–2717. [Google Scholar]
- Zhou, S.; Yang, T.; Qian, C.; Wu, F.; Hong, Y.; Lin, X. An update on solid-state form, crystal modification and transition issues related to tablet manufacturing. J. Drug Deliv. Sci. Technol. 2024, 100, 106135. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; de Oliveira da Rocha, A.B.; Gonçalves Cardoso, S.; Rodríguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef]
- Gan, Y.; Baak, J.P.A.; Chen, T.; Ye, H.; Liao, W.; Lv, H.; Wen, C.; Zheng, S. Supersaturation and Precipitation Applicated in Drug Delivery Systems: Development Strategies and Evaluation Approaches. Molecules 2023, 28, 2212. [Google Scholar] [CrossRef]
- Childs, S.L.; Kandi, P.; Lingireddy, S.R. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Mol. Pharm. 2013, 10, 3112–3127. [Google Scholar] [CrossRef]
- Ansari, M.T.; Alahmed, T.A.A.; Sami, F. Quality by Design (QbD) Concept for Formulation of Oral Formulations for Tablets. In Introduction to Quality by Design (QbD) from Theory to Practice; Springer: Singapore, 2024; pp. 161–184. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int. J. Pharm. 2011, 419, 52–59. [Google Scholar] [CrossRef]
- Joshi, D.; Choudhary, N.K. Enhancing Sublingual Tablet-Quality Through Quality-by-Design Principles: Current Trends and Insights. Precis. Nanomed. 2023, 6, 1099–1108. [Google Scholar] [CrossRef]
- Jagan, B.G.V.S.; Narasimha Murthy, P.; Mahapatra, A.K.; Patra, R.K. Quality by design (QBD): Principles, underlying concepts, and regulatory prospects. Thai J. Pharm. Sci. 2021, 45, 54–69. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Singh, P.K.; Shuaib Mohd Iqubal, A.; Singh, M. Recent Advances in Direct Compression Technique for Pharmaceutical Tablet Formulation. Int. J. Pharm. Res. Dev. (IJPRD) 2014, 6, 49–57. [Google Scholar]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar]
- Hodsdon, A.C.; Mitchell, J.R.; Davies, M.C.; Melia, C.D. Structure and behaviour in hydrophilic matrix sustained release dosage forms: 3. The influence of pH on the sustained-release performance and internal gel structure of sodium alginate matrices. J. Controll. Release 1995, 33, 143–152. [Google Scholar] [CrossRef]
- Shamjuddin, A.; Sinka, C. Modelling of Coupled Diffusion-Deformation for Swelling-Assisted Drug Delivery Mechanism in Hydroxypropyl Methylcellulose-Controlled Release Tablet. Chem. Eng. Trans. 2023, 106, 1063–1068. [Google Scholar]
- Khan, K.A.; Khan, G.M.; Muzammal, M.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Ahmad, A.; Niazi, Z.R.; Shah, K.U.; Farid, A. Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents. Molecules 2022, 27, 864. [Google Scholar] [CrossRef]
- Vyshnavi, A.; Anjaneyulu, V.; Kumar, G.V. Formulation and evaluation of osmotic tablets of Ranolazine. Int. J. Pharm. Res. Technol. (IJPRT) 2023, 13, 1–6. [Google Scholar]
- Sriamornsak, P.; Thirawong, N.; Korkerd, K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur. J. Pharm. Biopharm. 2007, 66, 435–450. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Peppas, N.A. (Ed.) Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Almazrou, S.; Alqarni, A.; Alghamdi, A.; Alzahrani, M.; Alghamdi, S. Drug loading methods and kinetic release models using mesoporous silica nanoparticles as a drug delivery system: A review. S. Afr. J. Chem. Eng. 2024, 50, 261–280. [Google Scholar]
- Vergnaud, J.M. Liquid transport controlled release processes in polymeric materials: Applications to oral dosage forms. Int. J. Pharm. 1993, 90, 89–94. [Google Scholar] [CrossRef]
- Ebube, N.K.; Hikal, A.H.; Wyandt, C.M.; Beer, D.C.; Miller, L.G.; Jones, A.B. Sustained release of acetaminophen from heterogeneous matrix tablets: Influence of polymer ratio, polymer loading, and co-active on drug release. Pharm. Dev. Technol. 1997, 2, 161–170. [Google Scholar] [CrossRef]
- Sinha Roy, D.; Rohera, B.D. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur. J. Pharm. Sci. 2002, 16, 193–199. [Google Scholar] [CrossRef]
- Lee, P.I.; Peppas, N.A. Prediction of polymer dissolution in swellable controlled-release systems. J. Control. Release 1987, 6, 207–215. [Google Scholar] [CrossRef]
- Harland, R.S.; Gazzaniga, A.; Sangalli, M.E.; Colombo, P.; Peppas, N.A. Drug/Polymer Matrix Swelling and Dissolution. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1988, 5, 488–494. [Google Scholar]
- Ghori, M.U.; Conway, B.R. Hydrophilic Matrices for Oral Control Drug Delivery. Am. J. Pharmacol. Sci. 2015, 3, 103–109. [Google Scholar]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Maggi, L.; Machiste, E.O.; Torre, M.L.; Conte, U. Formulation of biphasic release tablets containing slightly soluble drugs. Eur. J. Pharm. Biopharm. 1999, 48, 37–42. [Google Scholar] [CrossRef]
- Wan, L.S.C.; Heng, P.W.S.; Wong, L.F. The effect of hydroxypropylmethylcellulose on water penetration into a matrix system. Int. J. Pharm. 1991, 73, 111–116. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Murthy, T.K.; Pai, M.R.; Mehta, P.R.; Chowdary, P.B. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech 2003, 4, 18–23. [Google Scholar] [CrossRef]
- Samani, S.M.; Montaseri, H.; Kazemi, A. The effect of polymer blends on release profiles of diclofenac sodium from matrices. Eur. J. Pharm. Biopharm. 2003, 55, 351–355. [Google Scholar] [CrossRef]
- Phatak, A.; Joshi, D.; Bhadgale, M.; Chaudhari, P. Development and optimization of naproxen sodium controlled release tablets: Qbd approach. Indian J. Pharm. Educ. Res. 2020, 54, s108–s116. [Google Scholar] [CrossRef]
- Barik, S.; Soni, P.; Kharia, A.A. Controlled Release Formulation of Tofacitinib Citrate Tablets Evaluated Using Quality by Design (QBD) Approach. J. Pharm. Res. Int. 2021, 33, 290–306. [Google Scholar] [CrossRef]
- Talukdar, M.M.; Michoel, A.; Rombaut, P.; Kinget, R. Comparative study on xanthan gum and hydroxypropylmethyl cellulose as matrices for controlled-release drug delivery I. Compaction and in vitro drug release behaviour. Int. J. Pharm. 1996, 129, 233–241. [Google Scholar]
- Ford, J.L.; Rubinstein, M.H.; McCaul, F.; Hogan, J.E.; Edgar, P.J. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Int. J. Pharm. 1987, 40, 223–234. [Google Scholar] [CrossRef]
- Furlanetto, S.; Cirri, M.; Maestrelli, F.; Corti, G.; Mura, P. Study of formulation variables influencing the drug release rate from matrix tablets by experimental design. Eur. J. Pharm. Biopharm. 2006, 62, 77–84. [Google Scholar] [CrossRef]
- Velasco, M.V.; Ford, J.L.; Rowe, P.; Rajabi-Siahboomi, A.R. Influence of drug:hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J. Control. Release 1999, 57, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chewle, S.; Emmerling, F.; Weber, M. Effect of choice of solvent on crystallization pathway of paracetamol: An experimental and theoretical case study. Crystals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Martínez, L.M.; Cruz-Angeles, J.; Vázquez-Dávila, M.; Martínez, E.; Cabada, P.; Navarrete-Bernal, C.; Cortez, F. Mechanical Activation by Ball Milling as a Strategy to Prepare Highly Soluble Pharmaceutical Formulations in the Form of Co-Amorphous, Co-Crystals, or Polymorphs. Pharmaceutics 2022, 14, 2003. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Patil, H.; Vemula, S.K.; Narala, S.; Lakkala, P.; Munnangi, S.R.; Narala, N.; Jara, M.O.; Williams, R.O.; Terefe, H.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation—Where Are We Now? AAPS PharmSciTech 2024, 25, 1–25. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E.; Ahmadipour, S. Crystal Engineering for Enhanced Solubility and Bioavailability of Poorly Soluble Drugs. Curr. Pharm. Des. 2018, 24, 2473–2496. [Google Scholar] [CrossRef]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical co-crystals: A green way to enhance drug stability and solubility for improved therapeutic efficacy. J. Pharm. Pharmacol. 2024, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Di Martino, P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Yousef, M.A.E.; Vangala, V.R. Pharmaceutical cocrystals: Molecules, crystals, formulations, medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Wang, Z.; Solomos, M.; Axnanda, S.; Chen, C.; Figus, M.; Schenck, L.; Sun, C.C. Varied Bulk Powder Properties of Micro-Sized API within Size Specifications as a Result of Particle Engineering Methods. Pharmaceutics 2022, 14, 1901. [Google Scholar] [CrossRef]
- O’sullivan, A.; Spoletti, E.; Ross, S.A.; Lusi, M.; Douroumis, D.; Ryan, K.M.; Padrela, L. Screening, synthesis, and characterization of a more rapidly dissolving celecoxib crystal form. ACS Omega 2024, 9, 29710–29722. [Google Scholar] [CrossRef] [PubMed]
- Sakhiya, D.C.; Borkhataria, C.H. A review on advancement of cocrystallization approach and a brief on screening, formulation and characterization of the same. Heliyon 2024, 10, e29057. [Google Scholar] [CrossRef]
- Samuel Rigilin Kunjal, K.K.; Thayyil, A.R.; Shabaraya, R.F.D.A. Regulatory Implications for Co-crystals and recent Co-crystal Patents. Int. J. Drug Regul. Aff. 2022, 10, 10–18. [Google Scholar] [CrossRef]
- Adeleye, O.A.; Olutayo, A.; Adeleye, A.F. Address for correspondence Funding sources the Creative Commons Attribution 3.0 Unported (CC BY 3.0) Relationship between compression pressure, mechanical strenghth and release properties of tablets. Polym. Med. 2019, 49, 27–33. [Google Scholar] [CrossRef]
- Adeleye, O.A.; Femi-Oyewo, M.N.; Odeniyi, M.A. Effect of compression pressure on mechanical and release properties of tramadol matrix tablets. Curr. Issues Pharm. Med. Sci. 2015, 28, 120–125. [Google Scholar] [CrossRef]
- Markl, D.; Strobel, A.; Schlossnikl, R.; Bøtker, J.; Bawuah, P.; Ridgway, C.; Rantanen, J.; Rades, T.; Gane, P.; Peiponen, K.-E.; et al. Characterisation of pore structures of pharmaceutical tablets: A review. Int. J. Pharm. 2018, 538, 188–214. [Google Scholar] [CrossRef]
- Vanveen, B.; Pajander, J.; Zuurman, K.; Lappalainen, R.; Poso, A.; Frijlink, H.; Ketolainen, J. The effect of powder blend and tablet structure on drug release mechanisms of hydrophobic starch acetate matrix tablets. Eur. J. Pharm. Biopharm. 2005, 61, 149–157. [Google Scholar] [CrossRef]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andaç, Ö. Mathematical modelling of drug release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [PubMed]
- Wagner, J.G. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J. Pharm. Sci. 1969, 58, 1253–1257. [Google Scholar] [CrossRef]
- Mulye, N.V.; Turco, S.J. A simple model based on first order kinetics to explain release of highly water soluble drugs from porous dicalcium phosphate dihydrate matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release 1. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Milliken, R.L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. Application of 3D printing in early phase development of pharmaceutical solid dosage forms. Int. J. Pharm. 2024, 653, 123902. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication 2024, 16, 032001. [Google Scholar] [CrossRef]
- Bácskay, I.; Ujhelyi, Z.; Fehér, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Roche, A.; Sanchez-Ballester, N.M.; Bataille, B.; Delannoy, V.; Soulairol, I. Fused Deposition Modelling 3D printing and solubility improvement of BCS II and IV active ingredients—A narrative review. J. Control. Release 2023, 365, 507–520. [Google Scholar] [CrossRef]
- Deshmane, S.; Kendre, P.; Mahajan, H.; Jain, S. Stereolithography 3D printing technology in pharmaceuticals: A review. Drug Dev. Ind. Pharm. 2021, 47, 1362–1372. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective laser sintering (Sls), a new chapter in the production of solid oral forms (sofs) by 3d printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Carou-Senra, P.; Ong, J.J.; Castro, B.M.; Seoane-Viaño, I.; Rodríguez-Pombo, L.; Cabalar, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Pérez, G.; Goyanes, A. Predicting pharmaceutical inkjet printing outcomes using machine learning. Int. J. Pharm. X 2023, 5, 100181. [Google Scholar] [CrossRef]
- Skalická, B.; Matzick, K.; Komersová, A.; Svoboda, R.; Bartoš, M.; Hromádko, L. 3D-printed coating of extended-release matrix tablets: Effective tool for prevention of alcohol-induced dose dumping effect. Pharmaceutics 2021, 13, 2123. [Google Scholar] [CrossRef]
- Ghanizadeh Tabriz, A.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 120147. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.; Christofer, M.A.F.; Roy, O.L.; Anne, S. 3D printing of pharmaceutical dosage forms: Recent advances and applications. Adv. Drug Deliv. Rev. 2024, 217, 115504. [Google Scholar]
- Huanbutta, K.; Burapapadh, K.; Kraisit, P.; Sriamornsak, P.; Ganokratanaa, T.; Suwanpitak, K.; Sangnim, T. The Artificial Intelligence-Driven Pharmaceutical Industry: A Paradigm Shift in Drug Discovery, Formulation Development, Manufacturing, Quality Control, and Post-Market Surveillance. Eur. J. Pharm. Sci. 2024, 203, 106938. [Google Scholar] [CrossRef]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial intelligence (AI) applications in drug discovery and drug delivery: Revolutionizing personalized medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Vidhya, K.S.; Sultana, A.; Kumar, N.; Rangareddy, H. Artificial Intelligence’s Impact on Drug Discovery and Development From Bench to Bedside. Cureus 2023, 15, e47486. [Google Scholar] [CrossRef]
- Munir, N.; Nugent, M.; Whitaker, D.; McAfee, M. Machine learning for process monitoring and control of hot-melt extrusion: Current state of the art and future directions. Pharmaceutics 2021, 13, 1432. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, X.; Ouyang, D.; Williams, R.O. Emerging Artificial Intelligence (AI) Technologies Used in the Development of Solid Dosage Forms. Pharmaceutics 2022, 14, 2257. [Google Scholar] [CrossRef]
- Jena, G.K.; Patra, C.N.; Jammula, S.; Rana, R.; Chand, S. Artificial Intelligence and Machine Learning Implemented Drug Delivery Systems: A Paradigm Shift in the Pharmaceutical Industry. J. BioX Res. 2024, 7, 0016. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.; Singhal, R.; Yadav, J.P. Revolutionizing drug discovery: The impact of artificial intelligence on advancements in pharmacology and the pharmaceutical industry. Intell. Pharm. 2024, 2, 367–380. [Google Scholar] [CrossRef]
- Niazi, S.K. The Coming of Age of AI/ML in Drug Discovery, Development, Clinical Testing, and Manufacturing: The FDA Perspectives. Drug Des. Dev. Ther. 2023, 17, 2691–2725. [Google Scholar] [CrossRef]
- Abbas, M.K.G.; Rassam, A.; Karamshahi, F.; Abunora, R.; Abouseada, M. The Role of AI in Drug Discovery. Chembiochem 2024, 25, e202300816. [Google Scholar] [CrossRef]
- Arora, P.; Behera, M.; Saraf, S.A.; Shukla, R. Leveraging Artificial Intelligence for Synergies in Drug Discovery: From Computers to Clinics. Curr. Pharm. Des. 2024, 30, 2187–2205. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Integrating Artificial Intelligence for Drug Discovery in the Context of Revolutionizing Drug Delivery. Life 2024, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Hornick, T.; Mao, C.; Koynov, A.; Yawman, P.; Thool, P.; Salish, K.; Giles, M.; Nagapudi, K.; Zhang, S. In silico formulation optimization and particle engineering of pharmaceutical products using a generative artificial intelligence structure synthesis method. Nat. Commun. 2024, 15, 9622. [Google Scholar] [CrossRef]
- Saleem, M.T.; Shoaib, M.H.; Yousuf, R.I.; Siddiqui, F. RSM and AI based machine learning for quality by design development of rivaroxaban push-pull osmotic tablets and its PBPK modeling. Sci. Rep. 2025, 15, 7922. [Google Scholar] [CrossRef]


| Type | Objective |
|---|---|
| Conventional Tablets | Immediate drug release |
| Modified Release (extended release, delayed release, and controlled release) Tablets | Controlled drug delivery |
| Orally Disintegrating Tablets (ODTs) | Rapid onset of action |
| Chewable Tablets | Accelerated drug absorption |
| Effervescent Tablets | |
| Sublingual and Buccal Tablets | Direct drug absorption into the blood stream (bypass first-pass metabolism) |
| Coated Tablets | Protection of the active ingredient, taste masking, and controlling the drug release |
| Controlled Release System | Drug Release Mechanism | |
|---|---|---|
| Diffusion | Reservoir | The drug is encapsulated in a core surrounded by a permeable membrane. The drug diffuses through the membrane at a controlled rate. |
| Matrix | The drug is dispersed in a matrix, and release occurs as the drug diffuses out of the matrix material. | |
| Dissolution | The drug or its coating dissolves gradually, releasing the active ingredient at a controlled rate. | |
| Osmotic | Utilizes osmotic pressure to push the drug out through a small orifice in the dosage form. The release rate is independent of external conditions, like pH. | |
| Erosion | The drug is embedded in a matrix, and release occurs as the matrix erodes over time. | |
| Swelling | The dosage form swells in the presence of bodily fluids, creating pathways for the drug to diffuse out gradually. | |
| Stimuli-Responsive | The drug release is triggered by external stimuli, such as pH, temperature, or enzymes, enabling site-specific drug delivery. | |
| 3D Printing Technique | Description |
|---|---|
| Fused Deposition Modeling (FDM) | Heating and extruding material to build the tablet layer by layer. |
| Stereolithography (SLA) | Usage of UV light to cure liquid resin layer by layer to create solid objects. This method allows for the fabrication of highly detailed structures. |
| Selective Laser Sintering (SLS) | Usage of a laser to sinter powdered materials, creating a solid object. |
| Inkjet Printing | Deposition of liquid droplets onto a substrate to build the tablet. It can be used to directly print APIs or excipients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atre, P.; Rizvi, S.A.A. Advances in Oral Solid Drug Delivery Systems: Quality by Design Approach in Development of Controlled Release Tablets. BioChem 2025, 5, 9. https://doi.org/10.3390/biochem5020009
Atre P, Rizvi SAA. Advances in Oral Solid Drug Delivery Systems: Quality by Design Approach in Development of Controlled Release Tablets. BioChem. 2025; 5(2):9. https://doi.org/10.3390/biochem5020009
Chicago/Turabian StyleAtre, Prachi, and Syed A. A. Rizvi. 2025. "Advances in Oral Solid Drug Delivery Systems: Quality by Design Approach in Development of Controlled Release Tablets" BioChem 5, no. 2: 9. https://doi.org/10.3390/biochem5020009
APA StyleAtre, P., & Rizvi, S. A. A. (2025). Advances in Oral Solid Drug Delivery Systems: Quality by Design Approach in Development of Controlled Release Tablets. BioChem, 5(2), 9. https://doi.org/10.3390/biochem5020009









