Synthesis and Characterisation of Metal Oxide Nanostructures Using Choline/Linear Alkyl Carboxylate Deep Eutectic Solvents
Abstract
1. Introduction
2. Results
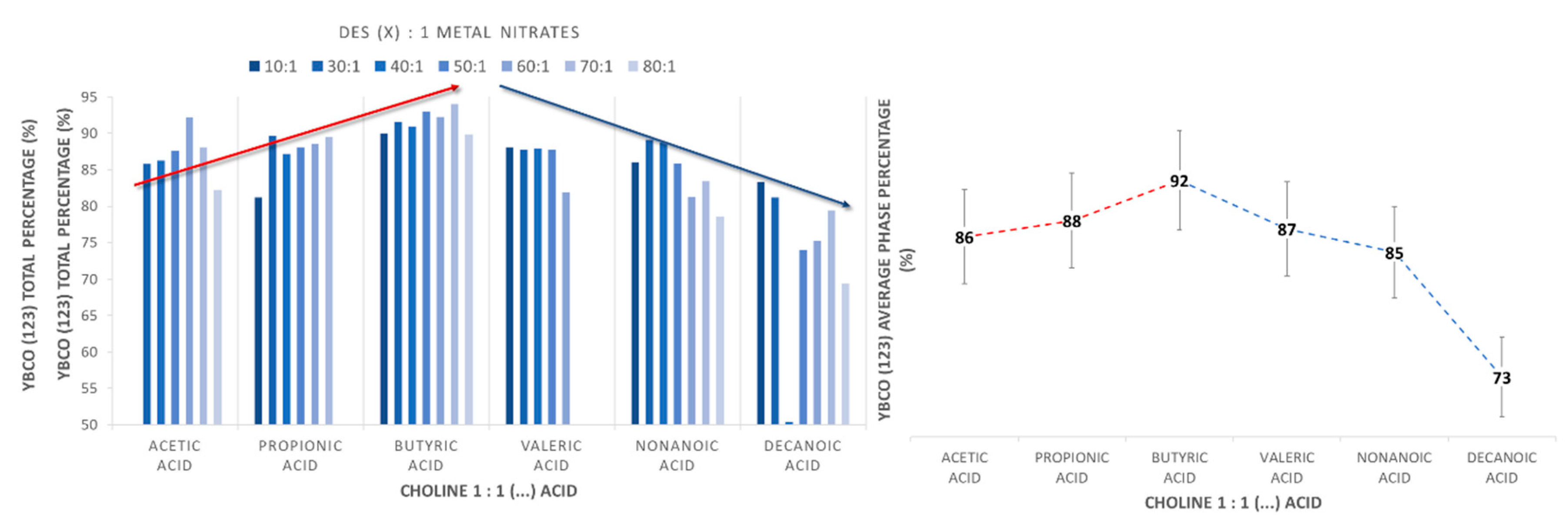
2.1. Synthetic Performance of DES (X):1 YBaCu Metal Nitrates Mixes, through Variation of the Carboxylic Acid Alkyl Chain Lengths
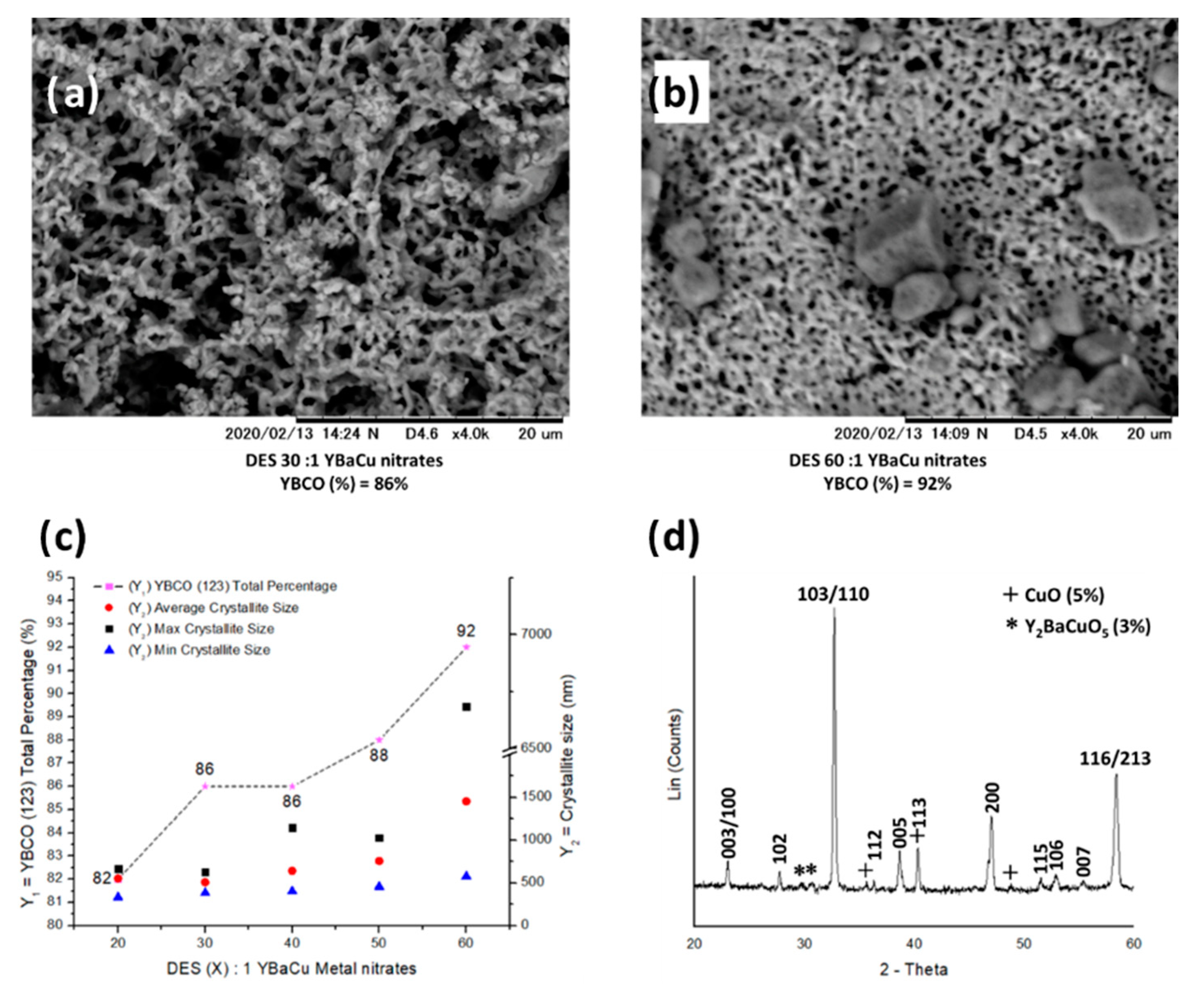
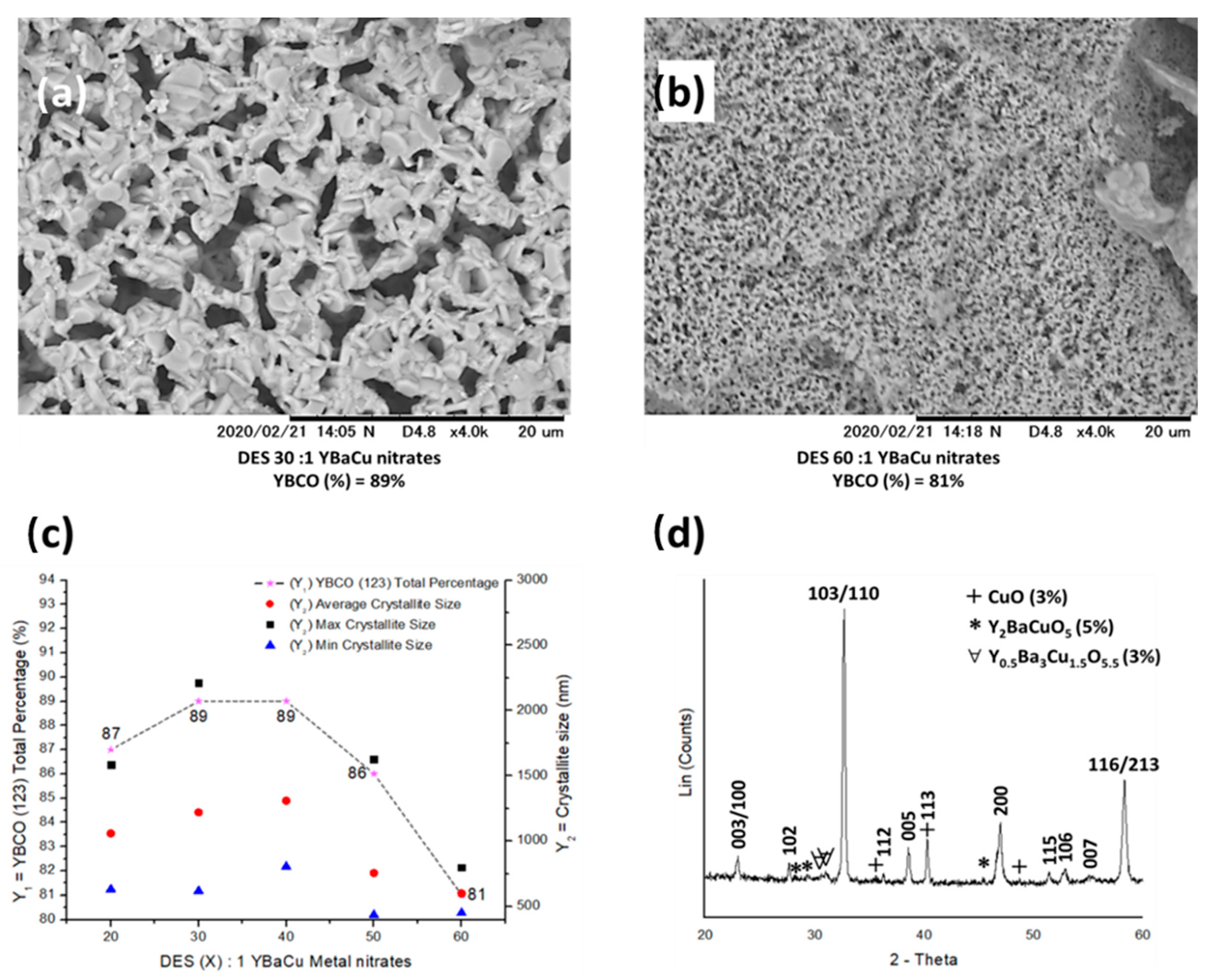
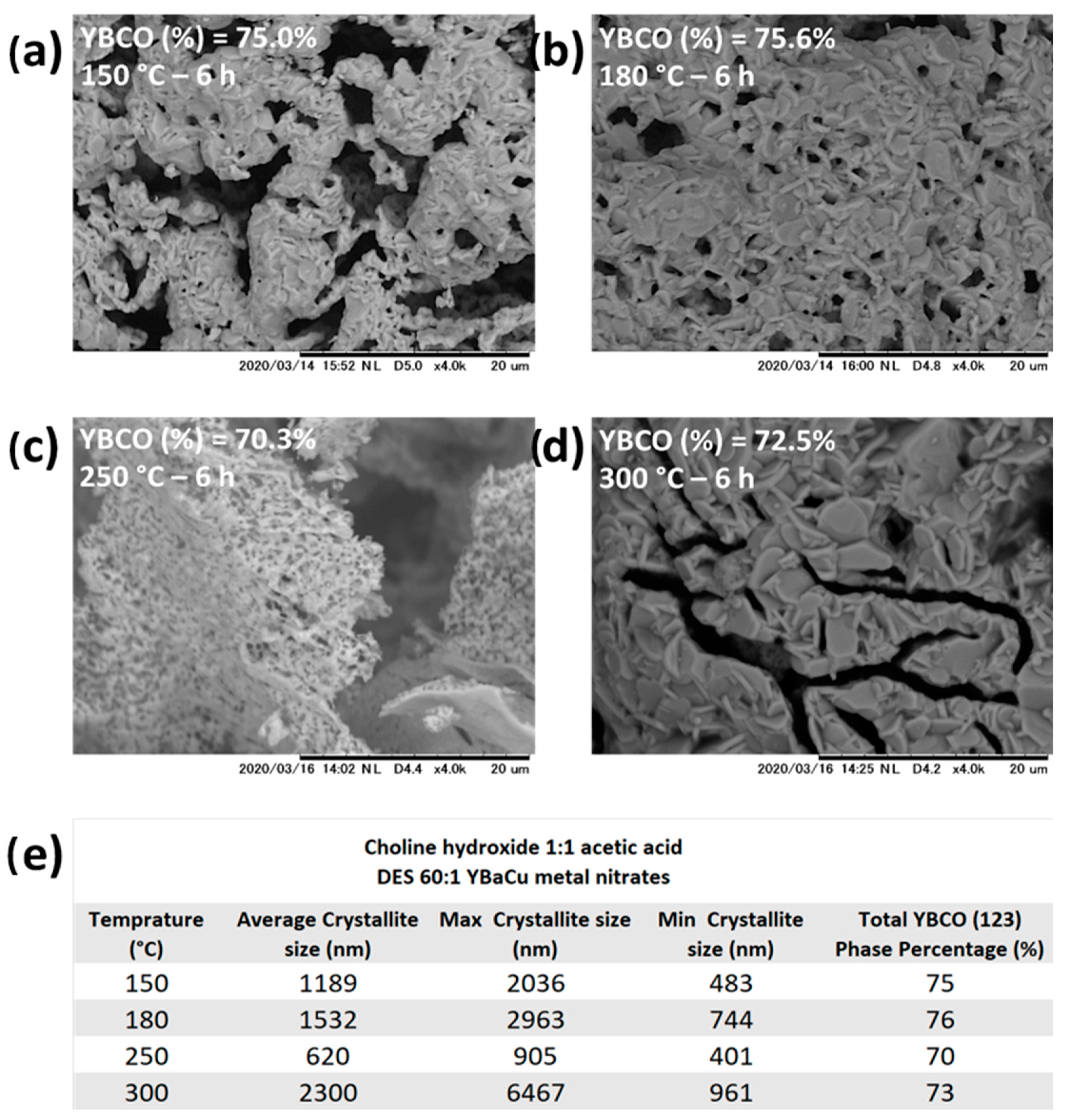
2.2. Synthetic Performance of Choline Hydroxide 1:1 Acetic Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60
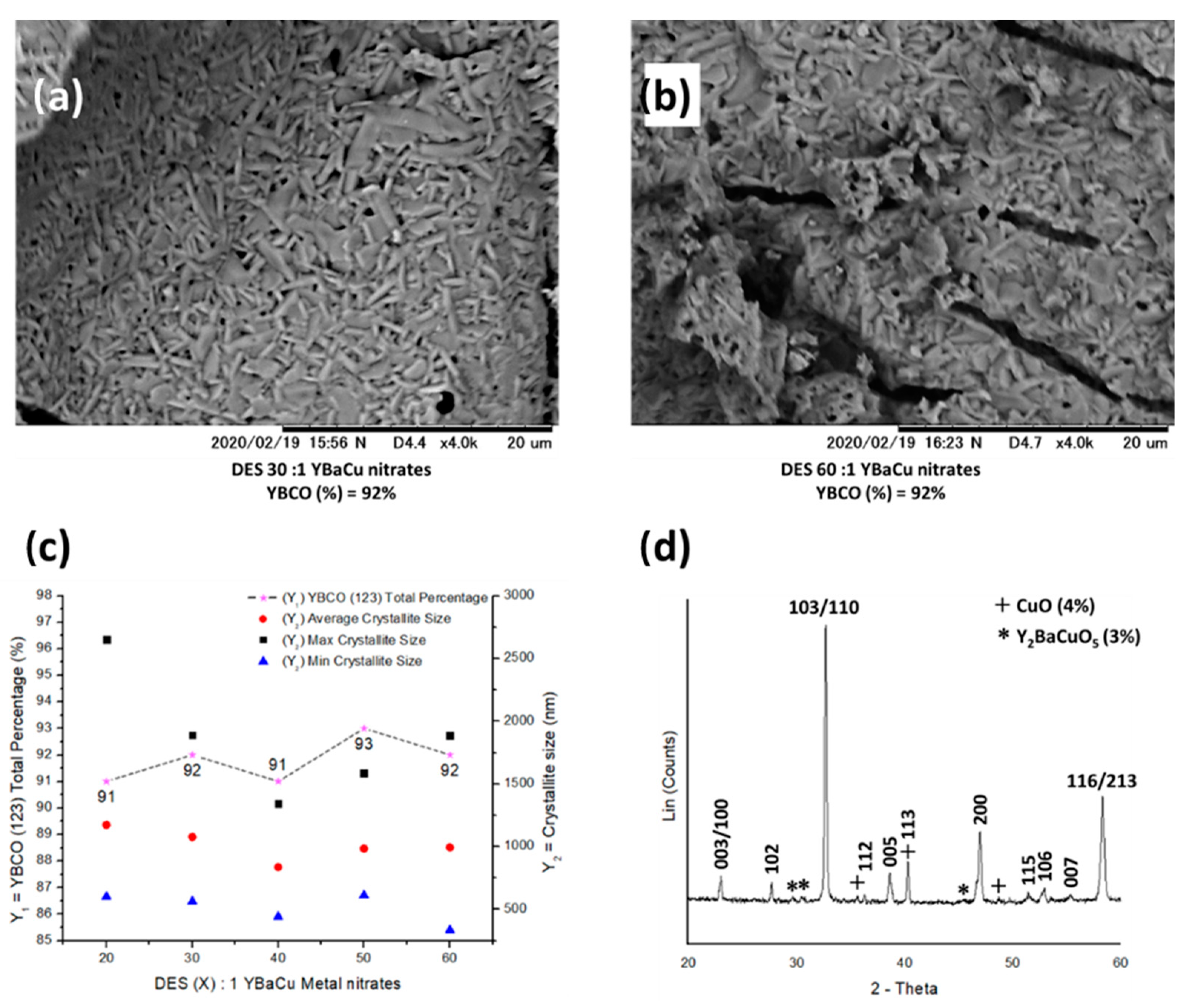
2.3. Synthetic Performance of Choline Hydroxide 1:1 Propionic Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60
2.4. Synthetic Performance of Choline Hydroxide 1:1 Butyric Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60
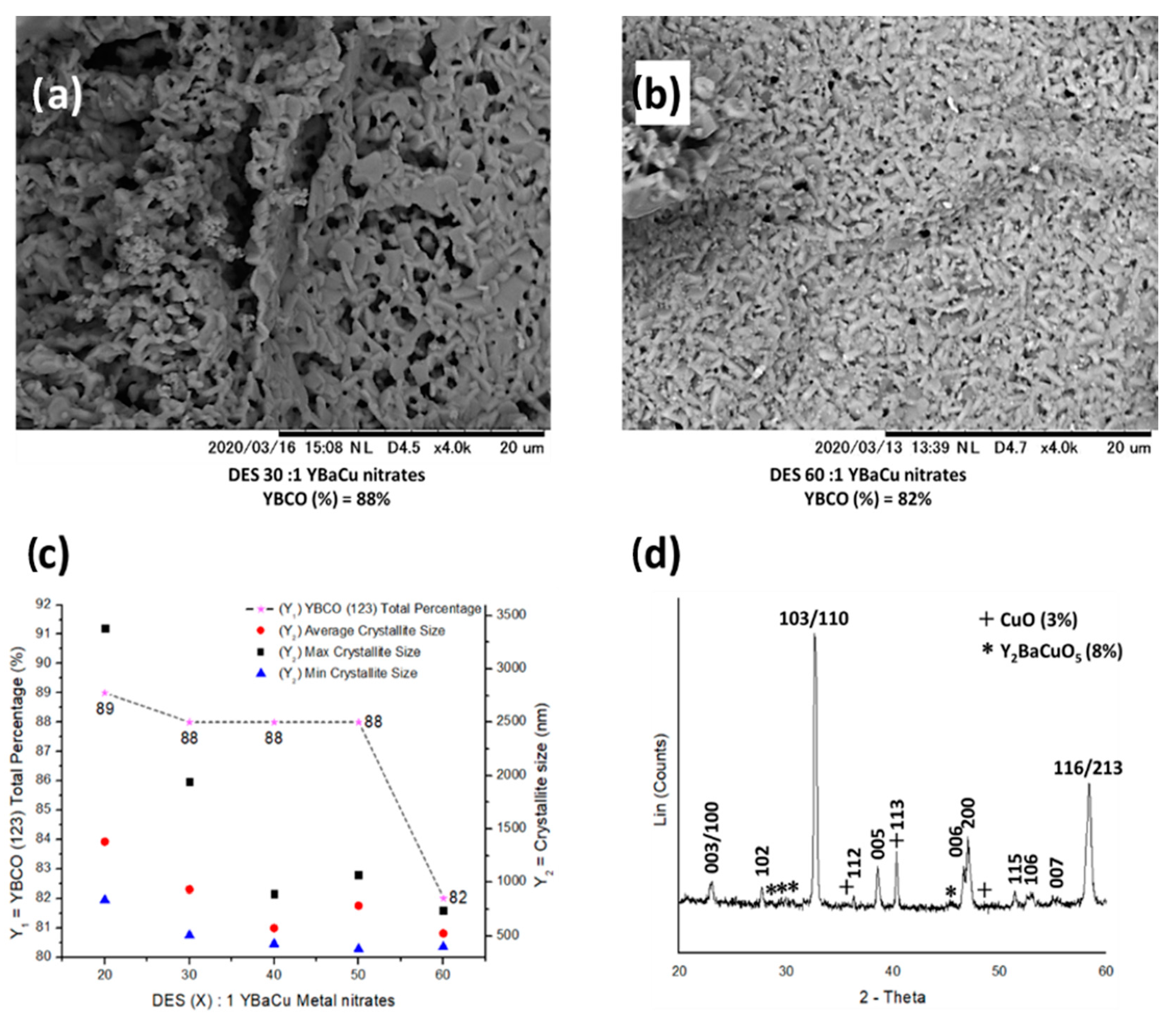
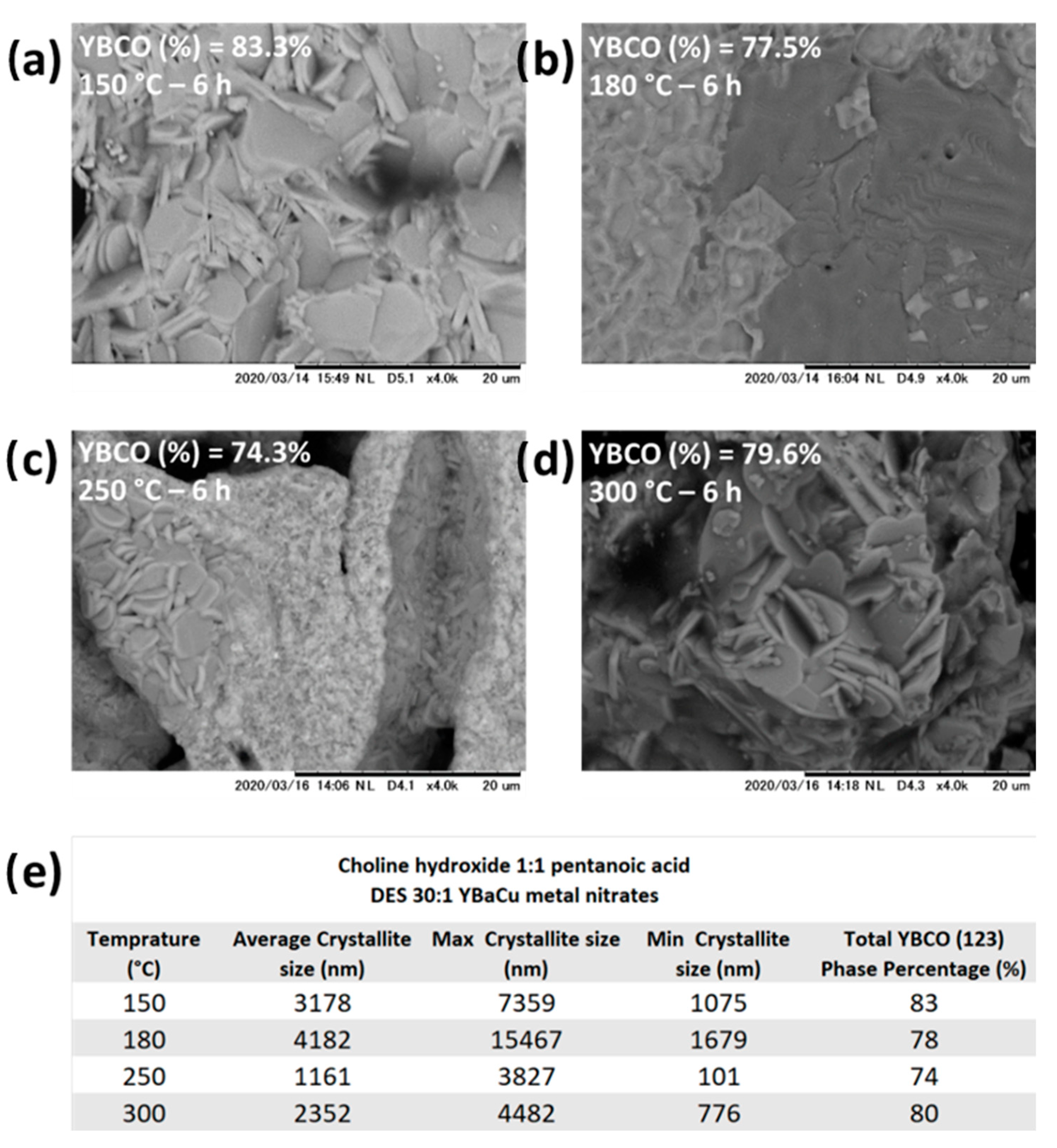
2.5. Synthetic Performance of Choline Hydroxide 1:1 Pentanoic Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60
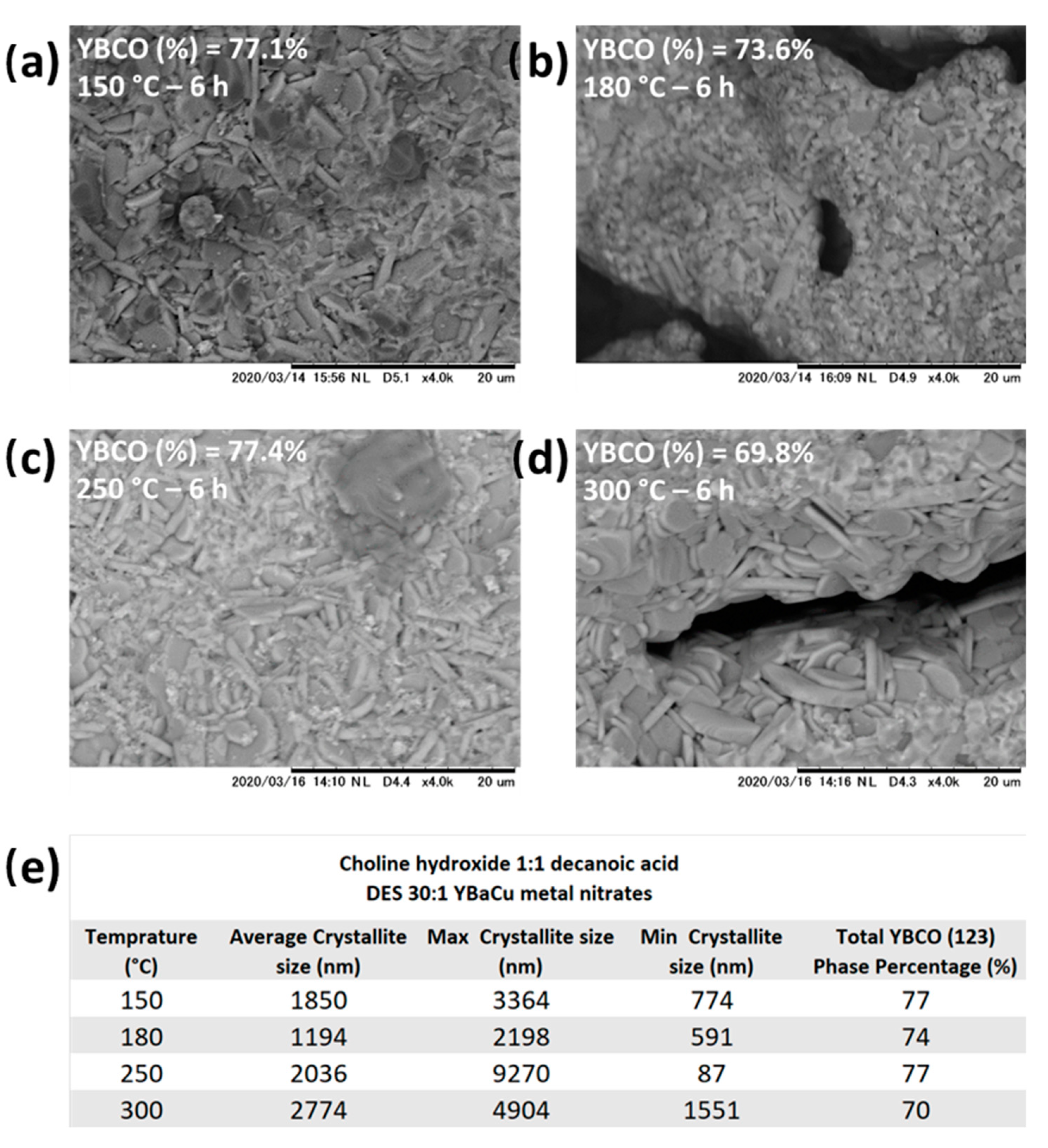
2.6. Synthetic Performance of Choline Hydroxide 1:1 Nonanoic Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60, and Choline Hydroxide 1:1 Decanoic Acid, DES (X):1 YBaCu Metal Nitrates Mixes, with x Values of 20 ≤ x ≤ 60.
2.7. Synthetic Performance of Choline Hydroxide 1:1 Acetic Acid, DES 60:1 YBaCu Metal Nitrates, Choline Hydroxide 1:1 Pentanoic Acid, DES 30:1 YBaCu Metal Nitrates, and Choline Hydroxide 1:1 Nonanoic Acid, DES 20:1 YBaCu Metal Nitrates Mixes, in Extraordinary Initial Conditions
2.7.1. Synthetic Performance of Choline Hydroxide 1:1 Acetic Acid, DES 60:1 YBaCu Metal Nitrates
2.7.2. Synthetic Performance of Choline Hydroxide 1:1 Pentanoic Acid, DES 30:1 YBaCu Metal Nitrates
2.7.3. Synthetic Performance of Choline Hydroxide 1:1 Nonanoic Acid, DES 20:1 YBaCu Metal Nitrates
3. Scientific Discussion and Conclusions
4. Experimental Section
4.1. Materials
4.2. Generation of Aqueous Precursors
4.3. Generation of Metal-Containing Deep Eutectic Solvents (DESs)
4.4. Dehydration of DES X:1 YBaCu Metal Nitrates Mix
4.5. Heating Protocols
4.6. Characteriszation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogale, S.B.; Venkatesan, T.V.; Blamire, M.G. Functional Metal Oxides: New Science and Novel Applications; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33179-6. [Google Scholar]
- Xiang, X.-D.; Takeuchi, I. Combinatorial Materials Synthesis; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Jorgensen, J.D.; Veal, B.W.; Paulikas, A.P.; Nowicki, L.J.; Crabtree, G.W.; Claus, H.; Kwok, W.K. Structural properties of oxygen-deficient YBa2Cu3O7−δ. Phys. Rev. B 1990, 41, 1863–1877. [Google Scholar] [CrossRef]
- Longo, E.; la Porta, F.D.A. Recent Advances in Complex Functional Materials: From Design to Application; Springer: New York, NY, USA, 2017; ISBN 3319538985. [Google Scholar]
- Lin, Y.H.; Nelson, J.; Goldman, A.M. Superconductivity of very thin films: The superconductor-insulator transition. Phys. C Supercond. Appl. 2015, 514, 130–141. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Lai, Y.; Chi, L. Advanced colloidal lithography: From patterning to applications. Nano Today 2018, 22, 36–61. [Google Scholar] [CrossRef]
- Fecht, H.J.; Hellstern, E.; Fu, Z.; Johnson, W.L. Nanocrystalline metals prepared by high-energy ball milling. Metall. Trans. A 1990, 21, 2333–2337. [Google Scholar] [CrossRef]
- Desbiolles, B.X.E.; Bertsch, A.; Renaud, P. Ion beam etching redeposition for 3D multimaterial nanostructure manufacturing. Microsyst. Nanoeng. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Khan, S.A.; Shah, J.; Kotnala, R.K.; Mohapatra, S. Nanostructured TiO2 thin films prepared by RF magnetron sputtering for photocatalytic applications. Appl. Surf. Sci. 2017, 422, 953–961. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Qu, S.; Yu, Y.; Lin, K.; Liu, P.; Zheng, C.; Wang, L.; Xu, T.; Wang, Z.; Wu, H. Easy hydrothermal synthesis of multi-shelled La2O3 hollow spheres for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 1232–1237. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated: Via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.G.; Sudoh, I.; Nakayama, T.; Hall, S.R. The role of ionic liquids in the synthesis of the high-temperature superconductor YBa2Cu3O7−δ. CrystEngComm 2018. [Google Scholar] [CrossRef]
- Celorrio, V.; Calvillo, L.; van den Bosch, C.A.M.; Granozzi, G.; Aguadero, A.; Russell, A.E.; Fermín, D.J. Mean Intrinsic Activity of Single Mn Sites at LaMnO3 Nanoparticles Towards the Oxygen Reduction Reaction. ChemElectroChem 2018. [Google Scholar] [CrossRef]
- Rojas, O.G.; Nakayama, T.; Hall, S.R. Green and cost-effective synthesis of the superconductor BSCCO (Bi-2212), using a natural deep eutectic solvent. Ceram. Int. 2019. [Google Scholar] [CrossRef]
- Rojas, O.G.; Song, G.; Hall, S.R. Fast and scalable synthesis of strontium niobates with controlled stoichiometry. CrystEngComm 2017, 19, 5351–5355. [Google Scholar] [CrossRef]
- Maugeri, Z.; de María, P.D. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Rojas, O.G.; Hall, S.R.; Nakayama, T. Synthesis of a Metal Oxide by Forming Solvate Eutectic Mixtures and Study of Their Synthetic Performance under Hyper- and Hypo-Eutectic Conditions. Crystals 2020, 10, 414. [Google Scholar] [CrossRef]
- Green, D.C.; Glatzel, S.; Collins, A.M.; Patil, A.J.; Hall, S.R. A New General Synthetic Strategy for Phase-Pure Complex Functional Materials. Adv. Mater. 2012, 24, 5767–5772. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of Inorganic Materials Using Ionic Liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Perry, D.L. Handbook of Inorganic Compounds; CRC Press: London, UK, 1995; ISBN 9781439814611. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]

| Average Crystallite Size (nm) | ||||||
|---|---|---|---|---|---|---|
| DES (X):1 YBaCu Metal Nitrates | Choline Hydroxide 1:1 Acetic Acid | Choline Hydroxide 1:1 Propionic Acid | Choline Hydroxide 1:1 Butyric Acid | Choline Hydroxide 1:1 Pentanoic Acid | Choline Hydroxide 1:1 Nonanoic Acid | Choline Hydroxide 1:1 Decanoic Acid |
| 20 | 553 | 650 | 1173 | 1380 | 1059 | 1059 |
| 30 | 507 | 1151 | 1077 | 933 | 1220 | 1218 |
| 40 | 640 | 1009 | 837 | 572 | 1310 | 1310 |
| 50 | 758 | 623 | 985 | 783 | 754 | 493 |
| 60 | 1454 | 465 | 995 | 523 | 600 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Rojas, O.; Nakayama, T. Synthesis and Characterisation of Metal Oxide Nanostructures Using Choline/Linear Alkyl Carboxylate Deep Eutectic Solvents. Solids 2020, 1, 31-46. https://doi.org/10.3390/solids1010004
Gómez Rojas O, Nakayama T. Synthesis and Characterisation of Metal Oxide Nanostructures Using Choline/Linear Alkyl Carboxylate Deep Eutectic Solvents. Solids. 2020; 1(1):31-46. https://doi.org/10.3390/solids1010004
Chicago/Turabian StyleGómez Rojas, Omar, and Tadachika Nakayama. 2020. "Synthesis and Characterisation of Metal Oxide Nanostructures Using Choline/Linear Alkyl Carboxylate Deep Eutectic Solvents" Solids 1, no. 1: 31-46. https://doi.org/10.3390/solids1010004
APA StyleGómez Rojas, O., & Nakayama, T. (2020). Synthesis and Characterisation of Metal Oxide Nanostructures Using Choline/Linear Alkyl Carboxylate Deep Eutectic Solvents. Solids, 1(1), 31-46. https://doi.org/10.3390/solids1010004




