A Scintillating One-Dimensional Coordination Polymer Based on Cadmium(II), N,N′-(1,4-Phenylenedicarbonyl)diglycinate, and 2,2′-Bipyridine: Crystal Structure, Hirshfeld Surface Analysis, and Luminescence Lifetime Properties †
Abstract
:1. Introduction
2. Results
2.1. Description of the Crystal Structure
2.2. Hirshfeld Surface Analysis
2.3. X-ray Excited Optical Luminescence (XEOL) and Fluorescence Properties
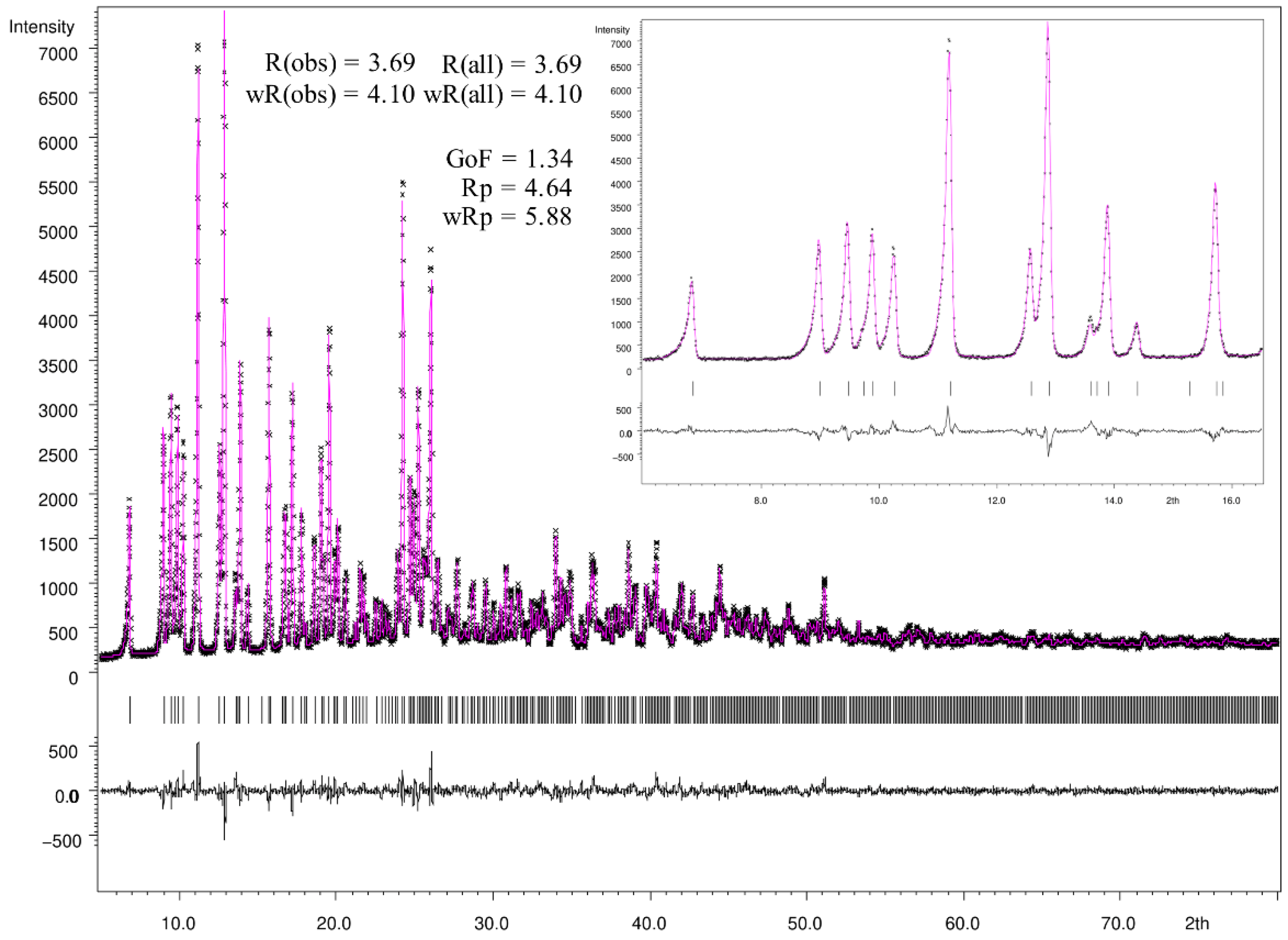
2.4. Rietveld Refinement
3. Materials and Methods
3.1. Syntheses of the Compound
3.2. Single-Crystal X-ray Crystallography
3.3. Powder X-ray Diffraction (PXRD)
3.4. X-ray Excited Optical Luminescence (XEOL) and Time-Resolved Spectroscopy
3.4.1. Sample Preparation for Photoluminescence and Scintillation Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Röntgen, W.C. On a new kind of rays. Science 1896, 3, 227–231. [Google Scholar] [CrossRef]
- Edison, T. Communication to Lord Kelvin. Nature 1896, 53, 470–474. [Google Scholar] [CrossRef]
- Boyes, W. Instrumentation Reference Book, 4th ed.; Butterworth-Heinemann/Elsevier: Amsterdam, Boston, 2010; ISBN 978-0-7506-8308-1. [Google Scholar]
- Fabjan, C.W.; Schopper, H. Particle Physics Reference Library: Volume 2: Detectors for Particles and Radiation, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-35318-6. [Google Scholar]
- Lecoq, P. Development of new scintillators for medical applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Pook, N.-P.; Fruhner, C.-J.; Franzl, T.; Denzer, U.; Adam, A. Instrumentation for X-ray excited and laser induced fluorescence lifetime spectroscopy using two-dimensional photon counting. IEEE Trans. Nucl. Sci. 2012, 59, 2319–2323. [Google Scholar] [CrossRef]
- Pook, N.-P.; Fruhner, C.-J.; Franzl, T.; Denzer, U.; Adam, A. Further performance tests of a picosecond X-ray and laser induced streak camera system with fast scintillation materials. Radiat. Meas. 2013, 56, 281–284. [Google Scholar] [CrossRef]
- Nikl, M.; Yoshikawa, A. Recent R&D trends in inorganic single-crystal scintillator materials for radiation detection. Adv. Opt. Mater. 2015, 3, 463–481. [Google Scholar] [CrossRef]
- Yamada, T.; Otsubo, K.; Makiura, R.; Kitagawa, H. Designer coordination polymers: Dimensional crossover architectures and proton conduction. Chem. Soc. Rev. 2013, 42, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. Engl. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Adarsh, N.N. Porous coordination polymers. In Functional Polymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 181–223. ISBN 978-3-319-95986-3. [Google Scholar]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal-organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Foster, M.E.; Léonard, F.; Stavila, V.; Feng, P.L.; Doty, F.P.; Leong, K.; Ma, E.Y.; Johnston, S.R.; Talin, A.A. Guest-induced emergent properties in metal–organic frameworks. J. Phys. Chem. Lett. 2015, 6, 1182–1195. [Google Scholar] [CrossRef]
- Doty, F.P.; Bauer, C.A.; Skulan, A.J.; Grant, P.G.; Allendorf, M.D. Scintillating metal-organic frameworks: A new class of radiation detection materials. Adv. Mater. 2009, 21, 95–101. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.-H.; Li, Y.; Wang, W.-F.; Sun, C.; Li, P.-X.; Zheng, F.-K.; Guo, G.-C. Heat-resistant Pb(ii)-based X-ray scintillating metal-organic frameworks for sensitive dosage detection via an aggregation-induced luminescent chromophore. Dalton Trans. 2020, 49, 7309–7314. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, Y.; Liu, H.; Zhang, Y.; Liu, W.; Wang, X.; Wang, S. Color-tunable X-ray scintillation based on a series of isotypic lanthanide-organic frameworks. Chem. Commun. 2019, 56, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xin, X.-H.; Lin, Y.-J.; Wang, S.-H.; Xu, J.-G.; Zheng, F.-K.; Guo, G.-C. Efficient X-ray scintillating lead(ii)-based MOFs derived from rigid luminescent naphthalene motifs. Dalton Trans. 2019, 48, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Volotskova, O.; Lu, K.; Ahmad, M.; Sun, C.; Xing, L.; Lin, W. Synergistic assembly of heavy metal clusters and luminescent organic bridging ligands in metal-organic frameworks for highly efficient X-ray scintillation. J. Am. Chem. Soc. 2014, 136, 6171–6174. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The Early years of 2,2′-Bipyridine-A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [Green Version]
- Pook, N.-P. Supramolecular architecture in a Ni(II) complex with a weakly bonded N,N′-(1,4-phenylenedi- carbonyl)diglycinate counter-anion: Crystal structure investigation and hirshfeld surface analysis. Crystals 2019, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Pook, N.-P.; Adam, A.; Gjikaj, M. Crystal structure and Hirshfeld surface analysis of (μ-2-{4-(carboxyl-atometh-yl)carbamo-ylbenz-amido}-acetato-κ2O:O′)bis-bis-(1,10-phenanthroline-κ2N,N′)copper(II) dinitrate N,N′-(1,4-phenyl-enedicarbon-yl)diglycine monosolvate octa-hydrate. Acta Crystallogr. E Crystallogr. Commun. 2019, 75, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pook, N.-P.; Gjikaj, M.; Adam, A. Bisbis-(2,2′-bi-pyridine-κ(2) N,N′)(carbon-ato-κ(2) O,O′)cobalt(III) 2-{4-(carboxyl-atometh-yl)carbamo-ylbenz-amido}-acetate hexa-hydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m160–m161. [Google Scholar] [CrossRef] [Green Version]
- Pook, N.-P.; Hentrich, P.; Gjikaj, M. Crystal structure of bis-tris-(1,10-phenanthroline-κ(2) N,N′)cobalt(II) tetra-nitrate N,N′-(1,4-phenyl-enedicarbon-yl)diglycine solvate octa-hydrate. Acta Crystallogr. E Crystallogr. Commun. 2015, 71, 910–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherni, S.; Abdessatar, C.; Driss, A. Crystal structure of bicapped trigonal-antiprismatic coordinated Cd(II) complex [Cd(C10H8N2)(NO3)2(H2O)]. X-Ray Struct. Anal. Online 2012, 28, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Huang, X.; Li, J. Assembly of new coordination frameworks in a pH-controlled medium: Syntheses, structures, and properties of 3∞[Cd(Hpdc)(H2O)] and 3∞[Cd3(pdc)2(H2O)2]. J. Solid State Chem. 2000, 152, 236–246. [Google Scholar] [CrossRef]
- He, Y.C.; Xiao, L.Y.; Yuan, Z.H.; Zhang, J.; Wang, Y.; Xu, N. Two coordination polymers constructed by 5-(4-carboxyphenoxy)methylbenzene-1,3-dicarboxylic acid and 2,2′-bipyridine: Syntheses, structures and luminescence properties. Acta Crystallogr. C Struct. Chem. 2019, 75, 1562–1568. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Zhao, X.-L.; Liu, F.-Y.; Kou, J.-F. Synthesis and crystal structures of two coordination polymers and a binuclear cadmium(II) complex containing 3- and 4-aminobenzoate ligands. Acta Crystallogr. C Struct. Chem. 2015, 71, 673–678. [Google Scholar] [CrossRef]
- Granifo, J.; Suarez, S.; Baggio, R. Structure of a dinuclear cadmium complex with 2,2′-bi-pyridine, monodentate nitrate and 3-carb-oxy-6-methyl-pyridine-2-carboxyl-ate ligands: Intra-molecular carbon-yl(lone pair)⋯π(ring) and nitrate(π)⋯π(ring) inter-actions. Acta Crystallogr. E Crystallogr. Commun. 2015, 71, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. Engl. 2003, 42, 1210–1250. [Google Scholar] [CrossRef]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Amide-pi interactions between formamide and benzene. J. Comput. Chem. 2009, 30, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ramanathan, V.; Sankararamakrishnan, R. Lone pair … pi interactions between water oxygens and aromatic residues: Quantum chemical studies based on high-resolution protein structures and model compounds. Protein Sci. 2009, 18, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Miao, H.; Hay, B.P. Crystal structure evidence for the directionality of lone pair−π interactions: Fact or fiction? Cryst. Growth Des. 2019, 19, 6806–6821. [Google Scholar] [CrossRef] [Green Version]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Rezende, M.V.D.S.; Montes, P.J.R.; Andrade, A.B.; Macedo, Z.S.; Valerio, M.E.G. Mechanism of X-ray excited optical luminescence (XEOL) in europium doped BaAl2O4 phosphor. Phys. Chem. Chem. Phys. 2016, 18, 17646–17654. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Impact, C. Diamond—Crystal and Molecular Structure Visualization. Crystal Impact-Dr. H. Putz and Dr. K. Brandenburg GbR, Bonn, Germany. Available online: http://www.crystalimpact.com/diamond (accessed on 15 September 2021).
- Persistence of Vision Raytracer (Version 3.6) [Computer Software]. Persistence of Vision Pty. Ltd. 2004. Available online: http://www.povray.org/download/ (accessed on 15 September 2021).







| Compound | {[Cd(C12H10N2O6)(C12H8N2)(H2O)]·5H2O}n |
|---|---|
| Empirical formula | C22H30CdN4O12 |
| Formula weight | 654.90 |
| Temperature (K) | 223 |
| Diffractometer | Stoe IPDS II |
| Wavelength (Å) | 0.71073 |
| Crystal system | triclinic |
| Color | colorless |
| Space group | Pī (No. 2) |
| a, b, c (Å) | 10.329(3), 11.424(2), 14.067(3) |
| α, β, γ (°) | 98.266(17), 106.557(19), 115.422(18) |
| V (Å3) | 1367.5(6) |
| Z | 2 |
| Dcalc (g·cm3) | 1.591 |
| µ (mm−1) | 0.866 |
| F(000) | 668.0 |
| Crystal size (mm) | 0.500, 0.367, 0.150 |
| θ Range (°) | 2.190–26.499 |
| Index ranges | −12 ≤ h ≤ 12 −12 ≤ k ≤ 14 −17 ≤ l ≤ 17 |
| Reflection collected/unique | 18,035/5647 |
| Completeness to θ (%) | 0.998 |
| Absorption correction | Numerical; X-AREA, X-RED (2008) |
| Max. and min. transmission | 0.7177/0.9016 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/parameters/restrains | 5647/394/11 |
| Goodness-of-Fit on F2 | 1.054 |
| R1 [I ≥ 2σ(I)]/R1 (all data) | 0.0358/0.0481 |
| wR2 [I ≥ 2σ(I)]/wR2 (all data) | 0.0766/0.0807 |
| Largest diff. peak and hole (e·Å3) | 0.986/−0.578 |
| Deposition number | 2062505 |
| Bond | Lengths | Bond | Lengths |
| Cd1–O1 | 2.345 (2) | Cd1–O7 | 2.298 (3) |
| Cd1–O2 | 2.542 (2) | Cd1–N1 | 2.344 (3) |
| Cd1–O4 | 2.298 (5) | Cd1–N2 | 2.328 (3) |
| Cd1–O5 | 2.574 (2) | ||
| Bond | Angle | Bond | Angle |
| N1–Cd1–N2 | 70.31 (10) | O1–Cd1–O2 | 53.43 (7) |
| N1–Cd1–O1 | 86.10 (9) | O1–Cd1–O4 | 78.04 (8) |
| N1–Cd1–O2 | 92.55 (9) | O1–Cd1–O5 | 130.21 (8) |
| N1–Cd1–O4 | 108.19 (14) | O1–Cd1–O7 | 104.59 (10) |
| N1–Cd1–O5 | 102.02 (9) | O2–Cd1–O4 | 125.80 (9) |
| N1–Cd1–O7 | 158.77 (10) | O2–Cd1–O5 | 165.04 (8) |
| N2–Cd1–O1 | 140.11 (8) | O2–Cd1–O7 | 79.93 (9) |
| N2–Cd1–O2 | 94.81 (8) | O4–Cd1–O5 | 52.57 (9) |
| N2–Cd1–O4 | 139.11 (9) | O4–Cd1–O7 | 92.13 (14) |
| N2–Cd1–O5 | 87.06 (8) | O5–Cd1–O7 | 85.22 (9) |
| N2–Cd1–O7 | 90.41 (11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pook, N.-P.
A Scintillating One-Dimensional Coordination Polymer Based on Cadmium(II), N,N′-(1,4-Phenylenedicarbonyl)diglycinate, and 2,2′-Bipyridine: Crystal Structure, Hirshfeld Surface Analysis, and Luminescence Lifetime Properties
Pook N-P.
A Scintillating One-Dimensional Coordination Polymer Based on Cadmium(II), N,N′-(1,4-Phenylenedicarbonyl)diglycinate, and 2,2′-Bipyridine: Crystal Structure, Hirshfeld Surface Analysis, and Luminescence Lifetime Properties
Pook, Niels-Patrick.
2021. "A Scintillating One-Dimensional Coordination Polymer Based on Cadmium(II), N,N′-(1,4-Phenylenedicarbonyl)diglycinate, and 2,2′-Bipyridine: Crystal Structure, Hirshfeld Surface Analysis, and Luminescence Lifetime Properties
Pook, N.-P.
(2021). A Scintillating One-Dimensional Coordination Polymer Based on Cadmium(II), N,N′-(1,4-Phenylenedicarbonyl)diglycinate, and 2,2′-Bipyridine: Crystal Structure, Hirshfeld Surface Analysis, and Luminescence Lifetime Properties







