Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System
Abstract
:1. Introduction
2. Materials and Methods
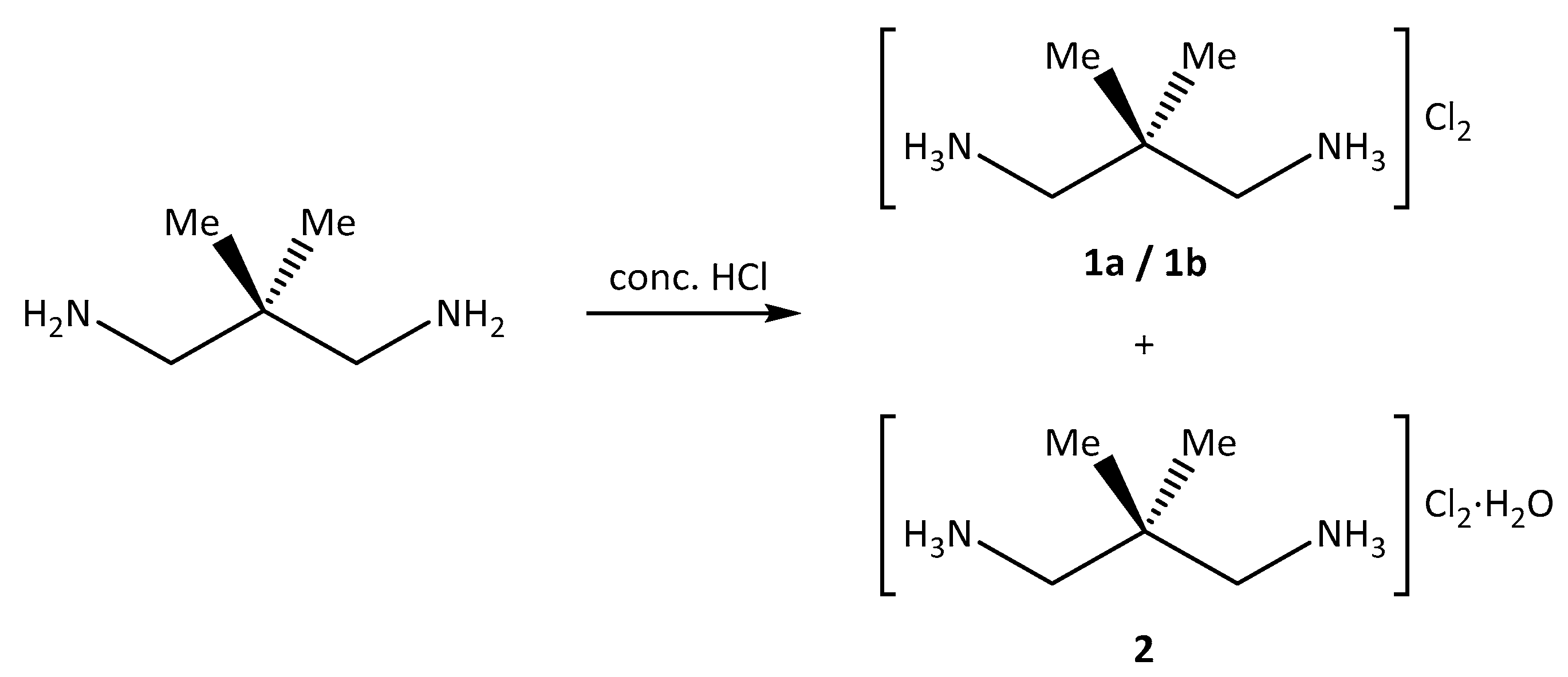
2.1. Synthesis
2.2. IR-and Raman Spectroscopy
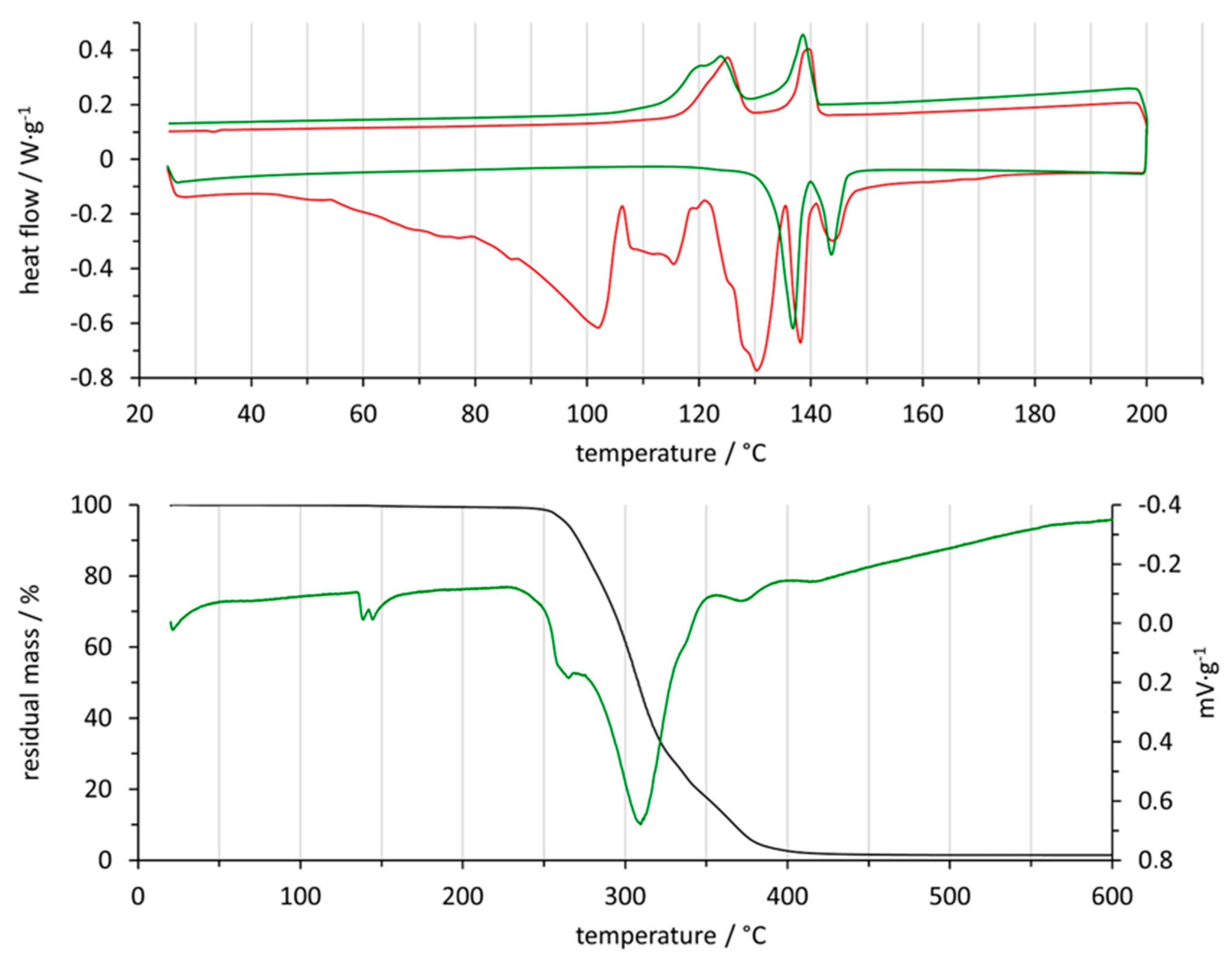
2.3. Thermal Analysis
2.4. Crystal Structure Analysis
3. Results
3.1. Structural Commentary
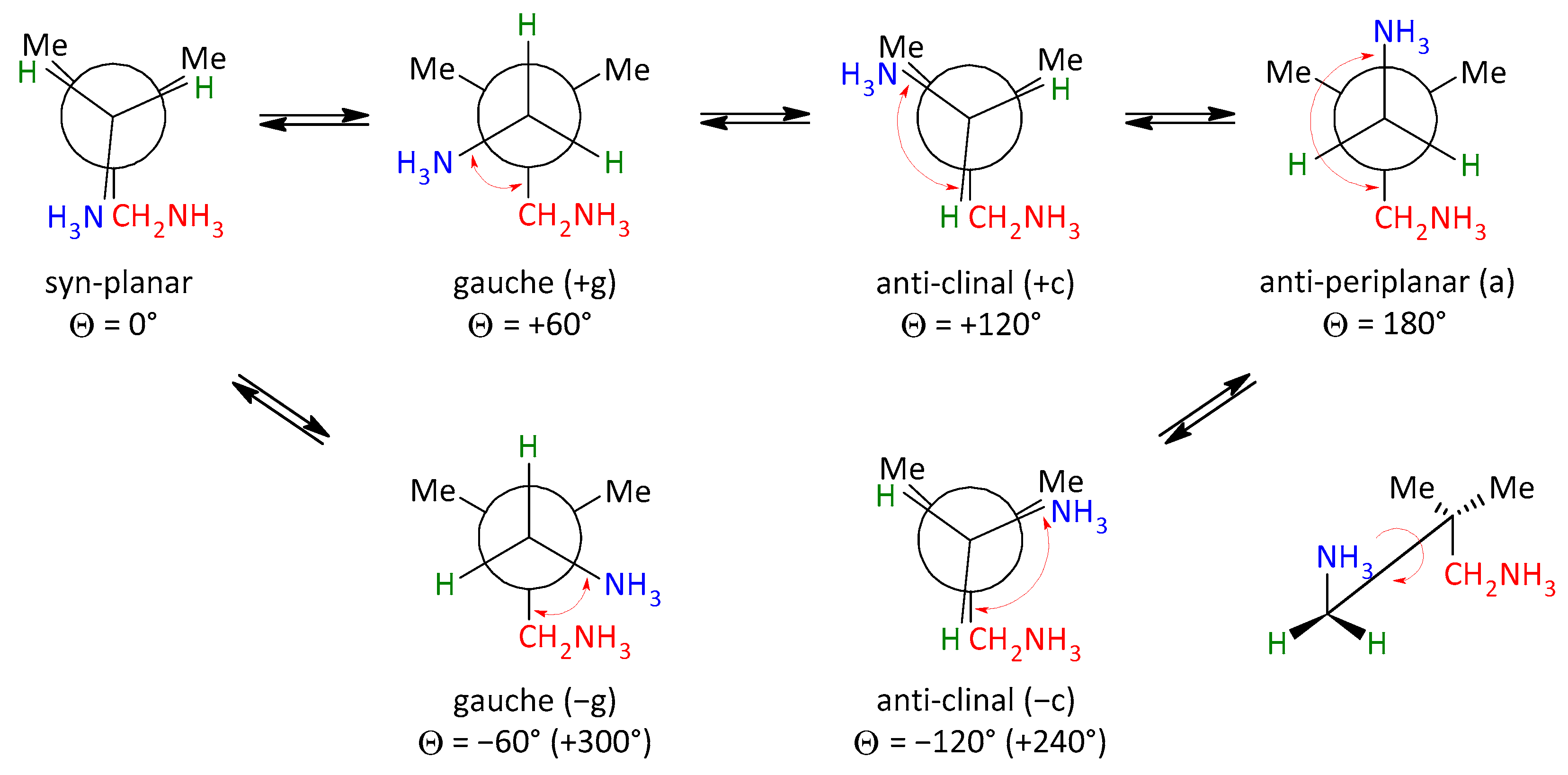
3.2. Conformational Analysis
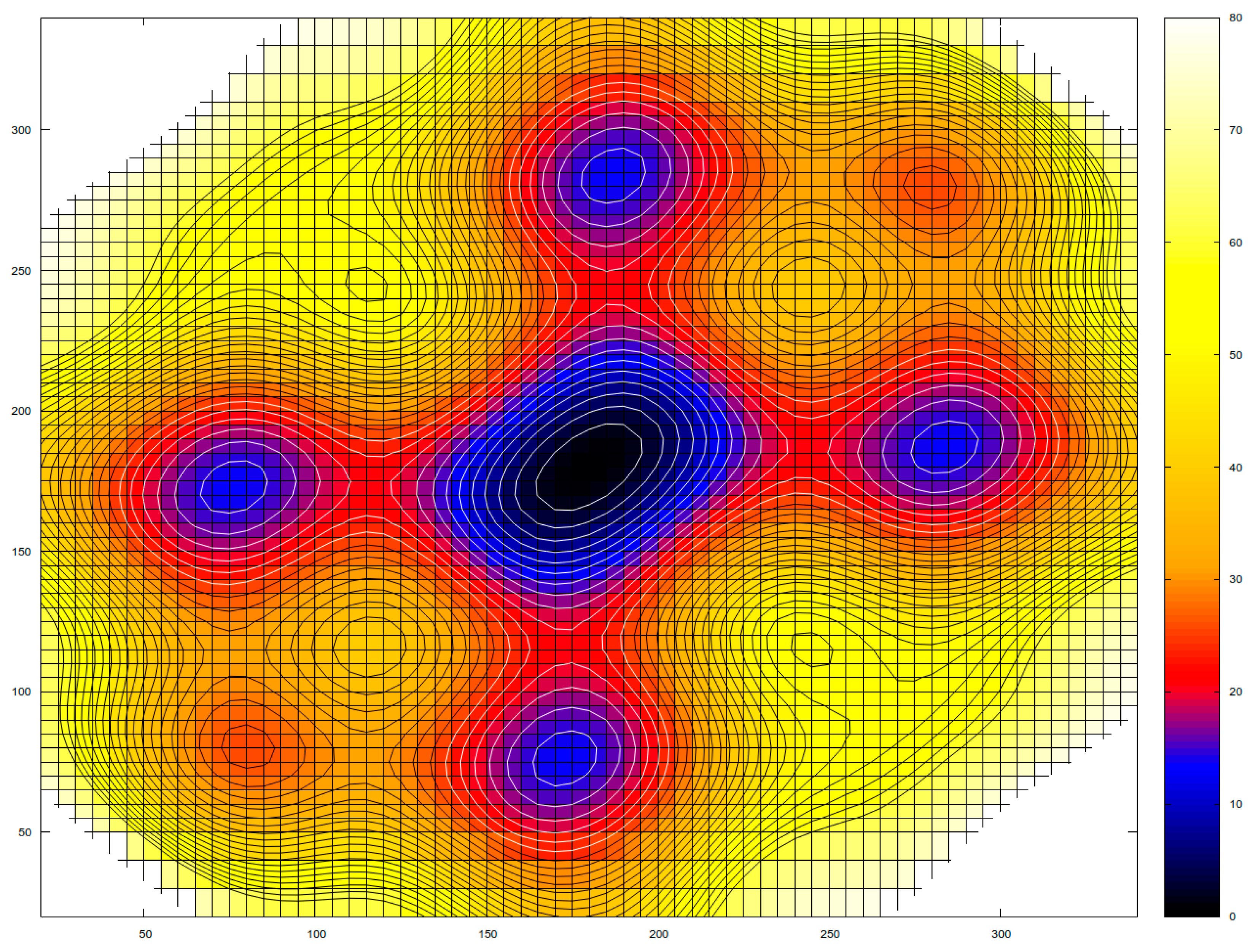
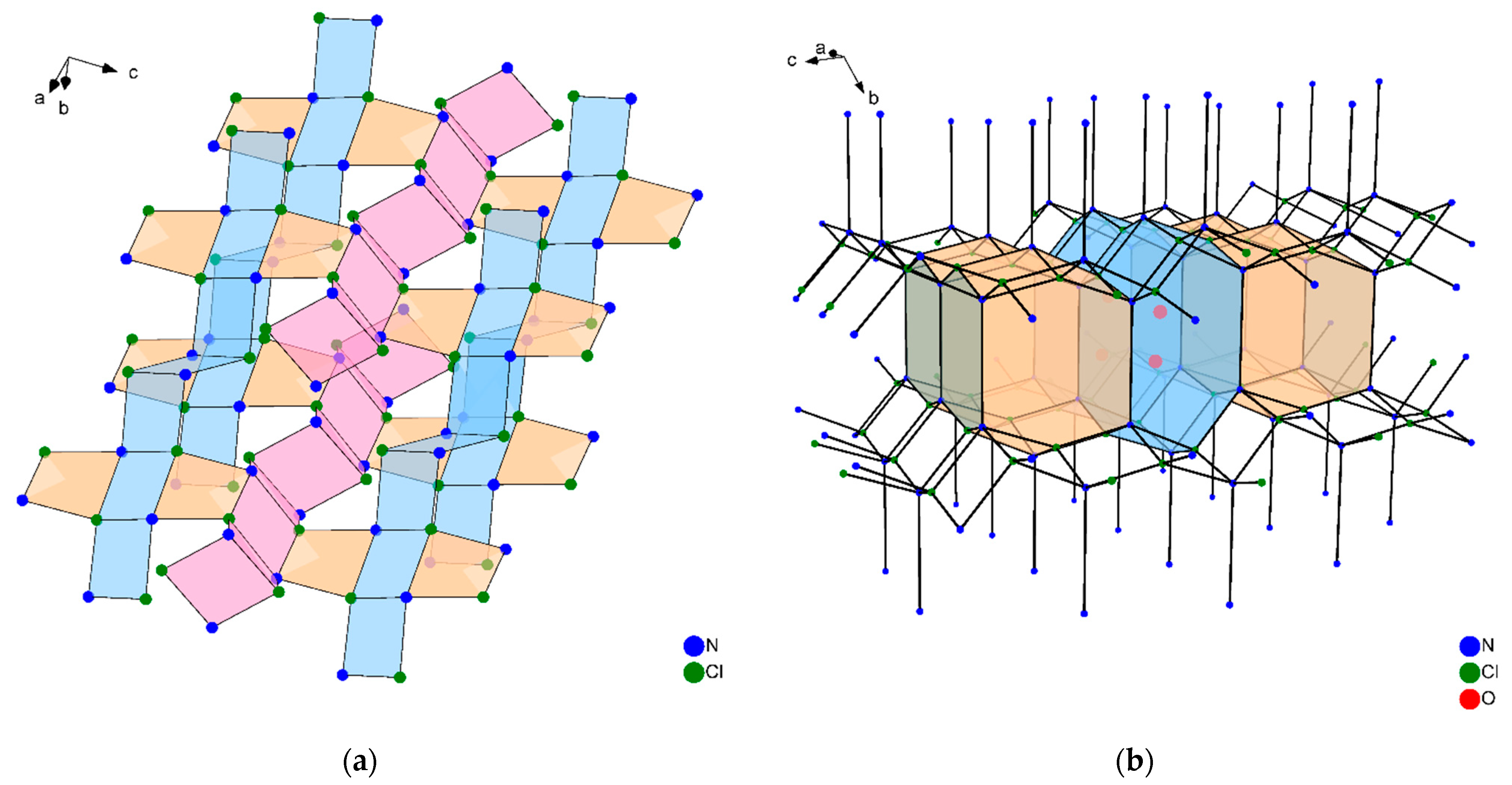
3.3. Topological Classification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senkus, M. Iron reduction of some aliphatic nitro compounds. J. Ind. Eng. Chem. 1948, 40, 506–508. [Google Scholar] [CrossRef]
- Feichtinger, H.; Aschmann, H.; Birnkraut, H.-W.; Brinkmann, L.; Pluta, W. Method for the Preparation of 1,3-Diamino-2,2-Dimethyl Propane. U.S. Patent US4078003A, 1973. [Google Scholar]
- Hares, G.B.; Fernelius, W.C.; Douglas, B.E. Equilibrium constants for the formation of complexes between metal ions and polyamines. J. Am. Chem. Soc. 1956, 78, 1816–1818. [Google Scholar] [CrossRef]
- Skinner, G.S.; Wunz, P.R. 2,5,5-Trialkyl-l,4,5,6-tetrahydropyrimidines. J. Am. Chem. Soc. 1951, 73, 3814–3815. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Gebala, A.E.; Levey, A.; Tasker, P.A. Conversion of aliphatic and alicyclic polyalcohols to the corresponding primary polyamines. J. Org. Chem. 1971, 36, 3042–3044. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.K.; Shim, J.H.; Kim, M.; Han, M.; Livinghouse, T.; Lee, P.H. Internal alkene hydroaminations catalyzed by Zirconium(IV) complexes and asymmetric alkene hydroaminations catalyzed by Yttrium(III) complexes. Adv. Synth. Catal. 2006, 348, 2609–2618. [Google Scholar] [CrossRef]
- Biswas, A.; Jana, A.; Sarkar, S.; Sparkes, H.A.; Howard, J.A.K.; Aliaga-Alcalde, N.; Mohanta, S. Discrete systems and two-dimensional coordination polymers containing potentially multidentate and bridging inorganic anions: Observation of a new type of two-dimensional topology. Polyhedron 2014, 74, 57–66. [Google Scholar] [CrossRef]
- Milner, P.J.; Siegelman, R.L.; Forse, A.C.; Gonzalez, M.I.; Runcevski, T.; Martell, J.D.; Reimer, J.A.; Long, J.R. A diaminopropane-appended metal−organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc. 2017, 139, 13541–13553. [Google Scholar] [CrossRef] [Green Version]
- Heimgert, J.; Neumann, D.; Reiss, G.J. (3-Ammonio-2,2-dimethyl-propyl)carbamate dihydrate. Molbank 2018, 2018, M1015. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Kim, Y.; Park, S. Quantum chemical calculations, spectroscopic studies and biological activity of organic–inorganic hybrid compound (2,2-dimethylpropane-1,3-diammonium) tetrachlorozincate(II). Arab. J. Sci. Eng. 2019, 44, 631–645. [Google Scholar] [CrossRef]
- Dou, S.; Fuess, H.; Paulus, H.; Weiss, A. Halogen NQR and crystal structure of ammonium halides (R-NH3)+X− and (X−)(+H3NR’NH3+)(X−). R=(HOCH2)3C, R’=CH2C(CH3)2CH2; X=I, Br *. Z. Naturforsch. 1994, 49, 174–184. [Google Scholar] [CrossRef]
- Megen, M.v.; Jablonka, A.; Reiss, G.J. Synthesis, structure and thermal decomposition of a new iodine inclusion compound in the 2,2-dimethylpropane-1,3-diamine/HI/I2 System. Z. Naturforsch. 2014, 69, 753–760. [Google Scholar] [CrossRef]
- Reiss, G.J.; Heimgert, J.; Morsbach, F. CCDC 1901965: Experimental Crystal Structure Determination. 2019. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc21nrfx&sid=DataCite (accessed on 1 April 2022).
- Chebbi, H.; Hajem, A.A.; Driss, A. Chromate de 2,2-diméthylpropyléne-diammonium. Acta Cryst. C 2000, 56, e333–e334. [Google Scholar] [CrossRef]
- Al-Resayes, S.I.; Azam, M.; Alam, M.; Kumar, R.S.; Adil, S.F. Synthesis, crystal structure and Hirschfeld surface analyses of an alkyl amine based salt, [C5H16N2][ZnCl4] and its enzyme inhibition activity. J. Saudi Chem. Soc. 2017, 21, 481–486. [Google Scholar] [CrossRef]
- Ishihara, H.; Dou, S.; Horiuchi, K.; Krishnan, V.G.; Paulus, H.; Fuess, H.; Weiss, A. Isolated versus condensed anion structure: The influence of the cation size and hydrogen bond on structure and phase transition in MX42− complex salts. 2,2-dimethyl-1,3-propanediammonium tetrabromocadmate(II) monohydrate, dimethylammonium tetrabromozincate(II), and dimethylammonium tetrabromocadmate(II). Z. Naturforsch. 1996, 51, 1027–1036. [Google Scholar]
- Iwakiri, T.; Terao, H.; Lork, E.; Gesing, T.M.; Ishihara, H. X-ray and NQR studies of bromoindate(III) complexes: [C2H5NH3]4InBr7, [C(NH2)3]3InBr6, and [H3NCH2C(CH3)2CH2NH3]InBr5. Z. Naturforsch. 2017, 72, 141–151. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Cryst. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Reiss, G.J.; Heimgert, J.; Morsbach, F. CCDC 1896779: Experimental Crystal Structure Determination. 2019. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc21nrfx&sid=DataCite (accessed on 1 April 2022).
- Lemmerer, A.; Fernandes, M.A. Adventures in co-crystal land: High Z′, stoichiometric variations, polymorphism and phase transitions in the co-crystals of four liquid and solid cyclic carboxylic acids with the supramolecular reagent isonicotinamide. New J. Chem. 2012, 36, 2242–2252. [Google Scholar] [CrossRef]
- Lemmerer, A.; Bernstein, J.; Spackman, M.A. Supramolecular polymorphism of the 1:1 molecular salt (adamantane-1-carboxylate-3,5,7-tricarboxylic acid)·(hexamethylenetetraminium). A “failed” crystal engineering attempt. Chem. Commun. 2012, 48, 1883–1885. [Google Scholar] [CrossRef]
- vario MICRO 3.1.12, Elementar Analysesysteme GmbH: Langenselbold, Germany, 2015.
- MestReNova 14.2.0, Mestrelab Research S.L.: Santiago de Compostela, Spain, 2015.
- Spectrum 10™, PerkinElmer Inc.: Waltham, MA, USA, 2008.
- Feustel, M. Grundlagen der ATR-Technik; Resultec Analytical Equipment: Illerkirchberg, Germany, 1999. [Google Scholar]
- OPUS 6.5, Bruker Corp.: Billerica, MA, USA, 2009.
- Bienz, S.; Bigler, L.; Fox, T.; Meier, H. Spektroskopische Methoden in der Organischen Chemie, 9th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Kowalczyk, I. Synthesis, molecular structure and spectral properties of quaternary ammonium derivatives of 1,1-dimethyl-1,3-propylenediamine. Molecules 2008, 13, 379–390. [Google Scholar] [CrossRef]
- CrysAlisPRO 1.171.34.44, Oxford Diffraction Ltd.: Abingdon, UK, 2011.
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Cryst. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, K. DIAMOND 4.6.4, Crystal Impact GbR: Bonn, Germany, 2018.
- Steiner, T. Hydrogen-bond distances to halide ions in organic and organometallic crystal structures: Up-to-date database study. Acta Cryst. 1998, 54, 456–463. [Google Scholar] [CrossRef]
- TURBOMOLE. Program Package for ab Initio Electronic Structure Calculations. V7.1; 1989–2007, TURBOMOLE GmbH, since 2007; University of Karlsruhe and Forschungszentrum Karlsruhe GmbH: Karlsruhe, Germany, 2016. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryts. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr CompComm Newsl. 2006, 7, 4–38. [Google Scholar]
- Kirillova, M.V.; Santos, C.I.M.; André, V.; Fernandes, T.A.; Dias, S.S.P.; Kirillov, A.M. Self-assembly generation, structural features, and oxidation catalytic properties of new aqua-soluble copper(II)-aminoalcohol derivatives. Inorg. Chem. Front. 2017, 4, 968–977. [Google Scholar] [CrossRef]
- Blatov, V.A.; Proserpio, D.M. Topological relations between three-periodic nets. II. Binodal nets. Acta Cryst. 2009, 65, 202–212. [Google Scholar] [CrossRef]
- Kumar, M.; Qiu, C.-Q.; Zareba, J.K.; Frontera, A.; Jassal, A.K.; Sahoo, S.C.; Liu, S.-J.; Sheikh, H.N. Magnetic, luminescence, topological and theoretical studies of structurally diverse supramolecular lanthanide coordination polymers with flexible glutaric acid as a linker. New J. Chem. 2019, 43, 14546–14564. [Google Scholar] [CrossRef]
- Jansen, C.; Küper, J.; Krebs, B. Na2B2S5 and Li2B2S5: Two novel perthioborates with planar 1,2,4-trithia-3,5-diborolane rings. Z. Anorg. Allg. Chem. 1995, 621, 1322–1329. [Google Scholar] [CrossRef]
- Löhken, A.; Mewis, A. Crystal Structures of Ca4Ir8P7 and Ca4Ir8As7 and the Relationship to the ThCr2Si2 Type Structure. Z. Anorg. Allg. Chem. 2004, 630, 2418–2421. [Google Scholar] [CrossRef]
- Ban, Z.; Sikirica, M. The crystal structure of ternary silicides ThM2Si2 (M=Cr, Mn, Fe, Co, Ni and Cu). Acta Cryst. 1965, 18, 594–599. [Google Scholar] [CrossRef]
- Pfannenschmidt, U.; Rodewald, U.C.; Pöttgen, R. Ce13Ir34.4P24–An intergrowth structure of ThCr2Si2, SrPtSb, and CeMg2Si2 related slabs. Z. Kristallogr. 2011, 226, 229–235. [Google Scholar] [CrossRef]
- Seidel, S.; Hoffmann, R.-D.; Pöttgen, R. SrPdGa3–An orthorhombic superstructure of the ThCr2Si2 type. Z. Kristallogr. 2014, 229, 421–426. [Google Scholar] [CrossRef]
- Hellmann, A.; Löhken, A.; Wurth, A.; Mewis, A. Neue Arsenide mit ThCr2Si2- oder einer damit verwandten Struktur: Die Verbindungen ARh2As2 (A: Eu, Sr, Ba) und BaZn2As2. Z. Naturforsch. 2007, 62, 155–161. [Google Scholar] [CrossRef]
- Van Megen, M.; Reiss, G.J. I62- anion composed of two asymmetric triiodide moieties-A competition between halogen and hydrogen bond. Inorganics 2013, 1, 3–13. [Google Scholar] [CrossRef]


| Parameters | 1a | 1b | 2 |
|---|---|---|---|
| CCDC depository | 1966904 | 1896779 | 1966943 |
| Crystal data | |||
| Color/shape | Colorless/block | Colorless/block | Colorless/block |
| Chemical formula | C5H16N2Cl2 | C5H16N2Cl2 | C5H18N2OCl2 |
| Mr | 175.10 | 175.10 | 193.11 |
| Temperature [K] | 293 (2) | 293 (2) | 293 (2) |
| Wavelength [Å] | Mo Kα 0.71073 | Mo Kα 0.71073 | Mo Kα 0.71073 |
| Crystal system, space group | Monoclinic, C2/c | Monoclinic, Cc | Triclinic, P |
| a, b, c [Å] | 20.7779 (7), 8.2560 (3), 10.9433 (3) | 32.1820 (1), 14.9592 (6), 18.7867 (8) | 6.2170 (3), 8.2491 (9), 11.2617 (8) |
| α, β, γ [°] | 90, 93.800 (3), 90 | 90, 125.532 (7), 90 | 108.876 (8), 91.412 (5), 109.706 (7) |
| Volume [Å3] | 1873.1 (1) | 7360.2 (7) | 508.66 (8) |
| Z | 8 | 32 | 2 |
| ρcalc. [g·cm−3] | 1.24 | 1.26 | 1.26 |
| µ [mm−1] | 0.63 | 0.64 | 0.59 |
| Crystal size (mm3) | 1.09 × 0.76 × 0.53 | 1.09 × 0.80 × 0.46 | 0.69 × 0.55 × 0.39 |
| Data collection | |||
| Diffractometer | Oxford Xcalibur Eos | Oxford Xcalibur Eos | Oxford Xcalibur Eos |
| Absorption correction | Multi-scan (CrysAlisPRO) | Analytical (CrysAlisPRO) | Multi-Scan (CrysAlisPro) |
| Tmin, Tmax | 0.800, 1.000 | 0.596, 0.770 | 0.798, 1.000 |
| F000 | 752 | 3008 | 208 |
| θ range for data collection [°] | 4.53 ≤ θ ≤ 35.11 | 4.12 ≤ θ ≤ 27.50 | 2.79 ≤ θ ≤ 27.50 |
| Completeness [%] | 99.4 | 99.6 | 100.0 |
| No. of collected reflections | 15949 | 57169 | 7957 |
| No. of independent reflections | 4000 | 16816 | 7957 |
| No. of observed reflections | 3248 | 15013 | 6626 |
| Rint | 0.026 | 0.020 | 0.039 |
| Refinement | |||
| R values [F2 > 2σ(F2)] | R1 = 0.032, wR2 = 0.069 | R1 = 0.030, wR2 = 0.065 | R1 = 0.043, wR2 = 0.096 |
| R values (all data) | R1 = 0.044, wR2 = 0.075 | R1 = 0.036, wR2 = 0.069 | R1 = 0.052, wR2 = 0.102 |
| S (Goodness-of-fit) | 1.04 | 1.06 | 1.09 |
| No. of data (m) | 4000 | 16816 | 7957 |
| No. of parameters (n) | 108 | 703 | 99 |
| No. of restraints | 0 | 2 | 0 |
| Δρmax, Δρmin | 0.44, −0.32 | 0.30, −0.33 | 0.48, −0.50 |
| D-H∙∙∙A | D-H | H∙∙∙A | D∙∙∙A | D-H∙∙∙A |
|---|---|---|---|---|
| Compound 1a | ||||
| N1-H11···Cl1 | 0.87 (2) | 2.30 (2) | 3.1619 (9) | 170 (2) |
| N1-H12···Cl2 | 0.89 (2) | 2.35 (2) | 3.146 (1) | 148 (1) |
| N1-H13···Cl1i | 0.90 (2) | 2.27 (2) | 3.163 (1) | 175 (1) |
| N2-H21···Cl2iv | 0.76 (2) | 2.66 (2) | 3.183 (1) | 128 (2) |
| N2-H22···Cl1ii | 0.88 (2) | 2.49 (2) | 3.272 (1) | 149 (2) |
| N2-H23···Cl2iii | 0.87 (2) | 2.33 (2) | 3.180 (1) | 165 (2) |
| Compound 2 | ||||
| N1-H11···Cl1i | 0.89 | 2.66 | 3.234 (2) | 122.9 |
| N1-H11···Cl2ii | 0.89 | 2.68 | 3.245 (2) | 122.5 |
| N1-H12···Cl1 | 0.89 | 2.31 | 3.195 (2) | 174.3 |
| N1-H13···Cl1iii | 0.89 | 2.35 | 3.173 (2) | 153.7 |
| N2-H21···Cl2iv | 0.89 | 2.33 | 3.206 (3) | 169.2 |
| N2-H22···Cl1v | 0.89 | 2.57 | 3.223 (2) | 131.2 |
| N2-H22···Cl2vi | 0.89 | 2.80 | 3.318 (3) | 118.7 |
| N2-H23···Cl2 | 0.89 | 2.39 | 3.189 (2) | 149.0 |
| O1-H1O···Cl2iv | 1.05 | 2.22 | 3.259 (6) | 175.7 |
| O1-H2O···Cl1vii | 0.91 | 2.22 | 3.136 (7) | 177.6 |
| O2-H3O···O1 | 1.09 | 1.69 | 2.776 (8) | 177.5 |
| Compound | Θ1 | Θ2 | Conformation | Reference |
|---|---|---|---|---|
| C5H16N2Cl2 (1a) | 169.77 (9) | −178.15 (9) | aa | |
| C5H16N2Cl2 (1b) | −174.0 (4) | 76.0 (5) | ag | [19] |
| 54.3 (6) | 174.5 (4) | ga | ||
| 174.1 (4) | 49.7 (6) | ag | ||
| 76.4 (5) | −174.1 (4) | ga | ||
| 54.1 (7) | 172.2 (5) | ga | ||
| 74.9 (5) | −173.4 (4) | ga | ||
| 72.3 (5) | −174.1 (4) | ga | ||
| 52.7 (6) | 175.6 (4) | ga | ||
| C5H16N2Cl2·H2O (2) | 176.8 (2) | −171.2 (2) | aa | |
| C5H16N2Br2 | −171.2 (5) | 177.7 (5) | aa | [11] |
| C5H16N2I2 | −71 (1) | −71 (1) | gg | [11] |
| C5H16N2I2·I2 | 180.0 (3) | 180.0 (3) | aa | [12] |
| C5H16N2 (NO3)2 | −58.8 (2) | −64.0 (2) | gg | [13] |
| C5H16N2 [CrO4] | −178.3 (2) | −169.0 (2) | aa | [14] |
| C5H16N2 [ZnCl4] | 176.38 (8) | 165.16 (8) | aa | [15] |
| C5H16N2 [CdBr4] | 74.6 (8) | −176.2 (8) | ga | [16] |
| (C5H16N2)3 [InBr4] [In2Br11] | −170 (1) | −177 (1) | aa | [17] |
| 178.2 (6) | 74.4 (8) | ag | ||
| 178.4 (6) | 70.3 (8) | ag |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimgert, J.; Morsbach, F.; Kleinschmidt, M.; Reiss, G.J. Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System. Solids 2022, 3, 385-396. https://doi.org/10.3390/solids3030027
Heimgert J, Morsbach F, Kleinschmidt M, Reiss GJ. Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System. Solids. 2022; 3(3):385-396. https://doi.org/10.3390/solids3030027
Chicago/Turabian StyleHeimgert, Jaqueline, Florian Morsbach, Martin Kleinschmidt, and Guido J. Reiss. 2022. "Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System" Solids 3, no. 3: 385-396. https://doi.org/10.3390/solids3030027
APA StyleHeimgert, J., Morsbach, F., Kleinschmidt, M., & Reiss, G. J. (2022). Synthesis, Structural Characterization, Conformational and Topological Classification of Different Salts in the 2,2-Dimethylpropane-1,3-diamine/HCl/H2O-System. Solids, 3(3), 385-396. https://doi.org/10.3390/solids3030027






