Abstract
A new species from the genus Cosmolaelaps, with potential to control western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), were collected from litter and remains of plant material on soil of different rose greenhouses in The Netherlands. Collected specimens were used to initiate a laboratory colony. Subsequently, a sample of mites of different developmental stages were analysed morphologically, by means of the measurement of structures and determination of the main morphological characteristics and chaetotaxy of the leg segments. The new species, Cosmolaelaps sabelisi sp. nov., is described and illustrated based on the morphological characters of the adult and immature stages (including the protonymph and deutonymphal stages) and compared with closely related species.
1. Introduction
Cosmolaelaps belongs to the Laelapidae, which is characterized by a high diversity of species that cover a wide range of habitats and have many biotic associations [1,2,3]. This cosmopolitan genus is a member of the subfamily Hypoaspidinae, with 135 valid species [3]. This group has a wide ecological diversity, which includes free-living forms inhabiting soil, litter, moss, humus, and nests of rodents and ants, as well as insect-associated forms, found for cockroaches, beetles, and termites [3,4,5,6,7,8]. Species of this genus have been reported as free-living predators, feeding mainly on nematodes, other soil-dwelling arthropods, and thrips [6,9,10]. Some species are considered to have potential as biological control agents of some agricultural pests [2,10,11].
The purpose of this study is to describe a new species of Cosmolaelaps known to feed on Frankliniella occidentalis (Pergande, 1895) and Carpoglyphus lactis (Linnaeus, 1758) (Muñoz-Cárdenas pers. comm). The discovery of this new species and its potential as a predator of thrips shows that there are several options that can be explored for practical use in agriculture and in the poultry industry (see [6,10,12,13]). Diversity studies and the identification of previously unknown species are the basis for further ecological studies and studies of their potential for biological control. In this paper, we describe and illustrate the adult and immature stages (including protonymph and deutonymph) of the new species Cosmolaelaps sabelisi sp. nov., from rose greenhouses in The Netherlands, which has the potential to control thrips.
2. Materials and Methods
Specimens used in the description of the new species were obtained from a laboratory colony (Laboratory of Entomology and Acarology B4.13 IBED, University of Amsterdam), initiated with specimens collected by K. Muñoz-Cárdenas from old flowers and/or litter from different rose greenhouses in The Netherlands: Litter from Amstelveen (52.314247310461916, 4.870552099634361) (1) October 2011 and (2) December 2013; (3) litter from Stompwijk (52.09622377807346, 4.471125264249524, July 2012; (4) moss from Aalsmeer (52.267116169700024, 4.762337815196021), January 2013; and (5) old flowers from Amstelveen (52.314247310461916, 4.870552099634361), July 2013. Colonies were maintained in plastic containers (125 mL), the bottom of which had a humid layer of plaster of Paris mixed with activated charcoal [13]. Peat mixed with autoclaved rose litter was added on top of the plaster–charcoal layer. We introduced 50 adult females of Cosmolaelaps sp. nov. to each rearing unit. The mites were fed with C. lactis (3 g), which were added with a mixture of bran and fresh yeast. The rearing units were then placed inside a tray with water to increase the humidity, and this tray was covered with another plastic tray to protect it from direct light.
Specimens were mounted in Hoyer’s medium on microscope slides. Illustrations of the taxonomically important structures of the new species collected were made with a camera lucida attached to a phase-contrast microscope and a digital camera connected to a differential interference contrast (DIC) microscope; photos and illustrations were then processed with a digital tablet, using the Adobe Illustrator® program (version 21.0.0, 2017, Adobe, United States). Measurements were taken with a graded ocular. For each structure, measurements are presented in micrometres, with the mean measurement for the specimens examined followed (in parentheses) by the respective ranges, to indicate the variation in the structures.
The length of the shields indicates the maximum distance between the anterior and posterior margins, while the width of the shields indicates the maximum width, except for the sternal shield, whose width indicates the width at the level of coxae II. The length of the epigynal shield includes the anterior hyaline region. Leg lengths indicate the distance between the base of the coxa to the tip of the tarsus (not including the pretarsus). Nomenclature of the idiosomal setae is based on Lindquist and Evans [14]; leg chaetotaxy is based on Evans [15,16] and pore-like structures on Athias-Henriot [17]. The number of teeth in each cheliceral digit does not include the apical ‘hook’.
Voucher specimens of the described species were deposited in the Museum für Naturkunde, Berlin, Germany (one holotype female, <ZMB-Arach 55000>; three paratype females, <ZMB-Arach 55001–55003>; four paratype males, <ZMB-Arach 55004–55007>; one paratype deutonymph, <ZMB-Arach 55008>; and one paratype protonymph, <ZMB-Arach 55008>); the Naturalis Biodiversity Center, Leiden, The Netherlands (two paratype females, <collection numbers RMNH.ACA.P.67608 and RMNH.ACA.P.67609>); and the Acarological Collection of the Tyumen State University Museum of Zoology, Tyumen, Russia (TUMZ) (four paratype females, <TSUMZ.ACA.0001, TSUMZ.ACA.0002, TSUMZ.ACA.0003, TSUMZ.ACA.0004>; two paratype males, <TSUMZ.ACA.0005, TSUMZ.ACA.0006>; two paratype deutonymphs, < TSUMZ.ACA.0007, TSUMZ.ACA.0008>; and two paratypes protonymphs, <TSUMZ.ACA.0009, TSUMZ.ACA.0010>). Type depositories of the new species are also indicated in the “Material examined” section.
3. Results
3.1. Genus Diagnosis
The genus diagnosis of Moreira et al. [18], as modified by Gwiazdowicz et al. [1] and Moraes et al. [3], was followed.
3.2. New Species Description
Cosmolaelaps sabelisi Sierra-Monroy & Rueda-Ramírez sp. nov.
Material examined: one holotype female, six paratype females, six paratype males, three paratypes deutonymph, and three paratype protonymph.
Diagnosis: female with anterior region of epistome acuminated triangular, denticulate; fixed cheliceral digit with 7–9 teeth; deutosternal groove with seven transverse lines, of which the most distal is smooth and smaller and the others with 22–10 denticles, delimited by subparallel lateral lines; dorsal shield almost completely covering the idiosoma, brownish, reticulate, with posterior end rounded, with 39 pairs of setae and three unpaired setae, all scimitar-shaped, except j1 and z1, setiform; two anterior lobes of the sternal shield uniformly as sclerotized as the remainder of the shield; opisthogaster with ten pairs of setiform setae (Jv1–Jv5; Zv1–Zv5) on an unsclerotized cuticle; two pairs of metapodal plates; a pair of rod-shaped platelets next to the edge of the epigynal shield; epigynal shield bearing only st5. Male spermadactyl curved upward and extending slightly beyond the tip of the movable digit, progressively tapering in diameter to a blunt tip; holoventral shield reticulate, anterolateral corners extending between coxae I and II.
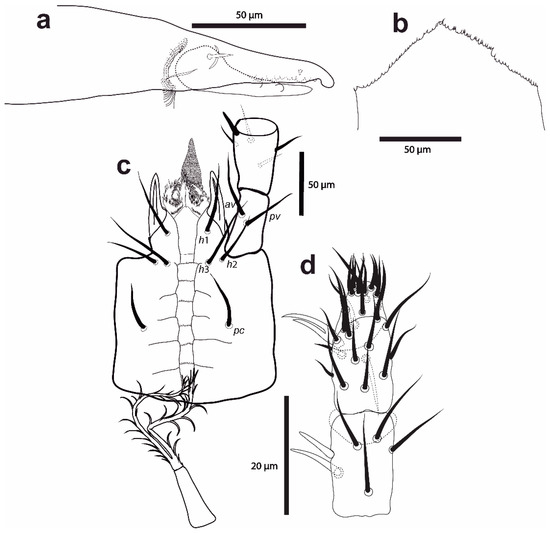
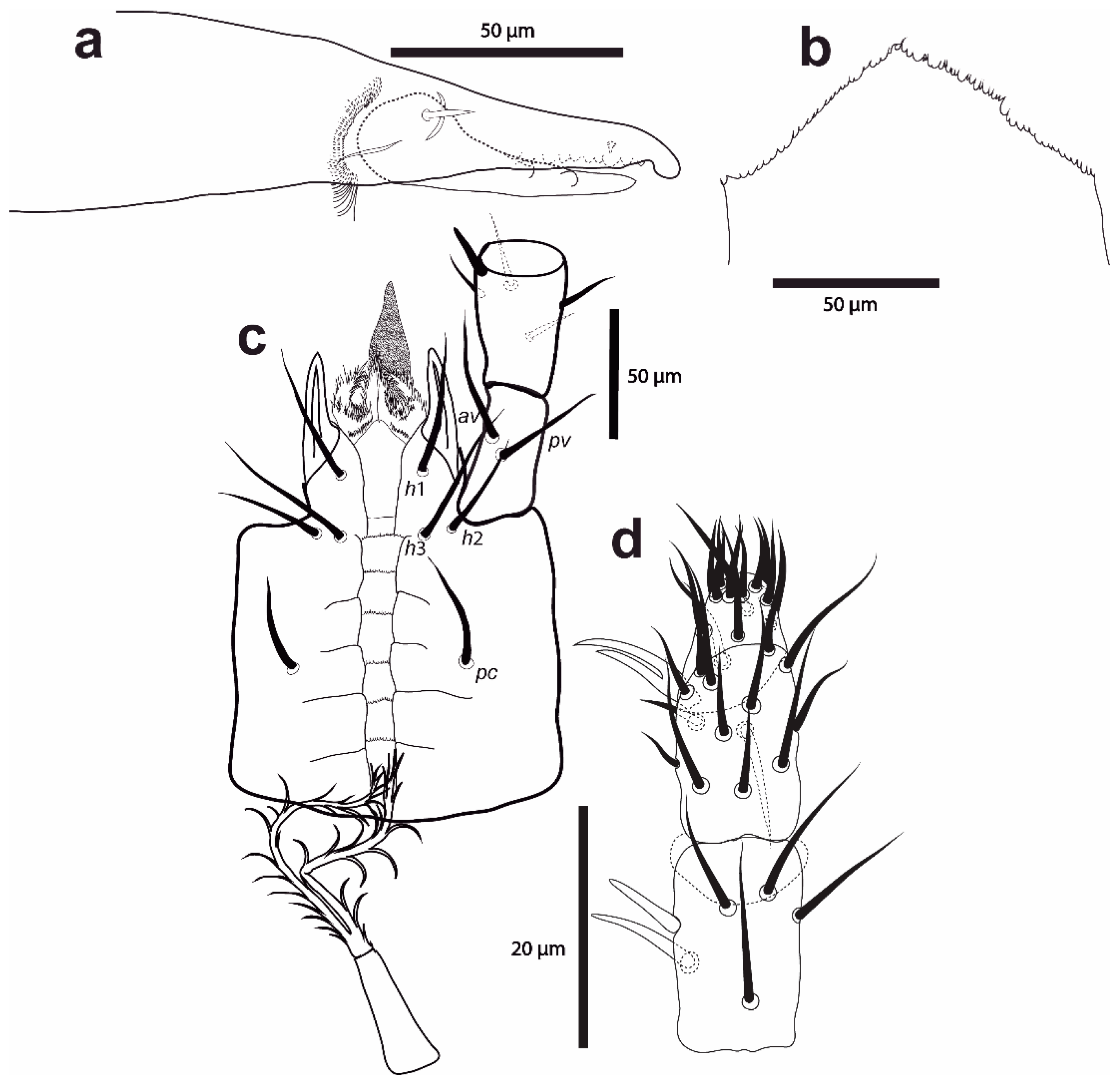
Figure 1.
Cosmolaelaps sabelisi sp. nov. adult female: (a) chelicera (antiaxial view); (b) epistome; (c) hypostome and proximal palp segments; (d) dorsal view of the distal palp segments and apotele.
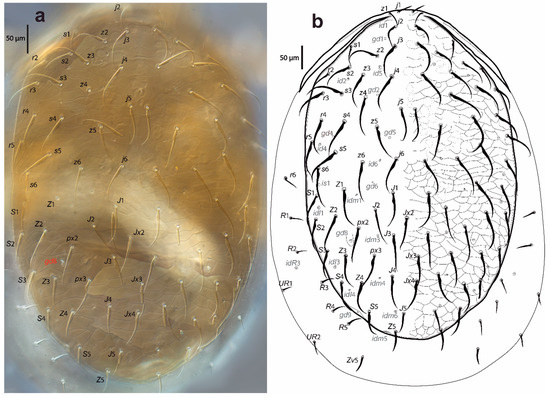
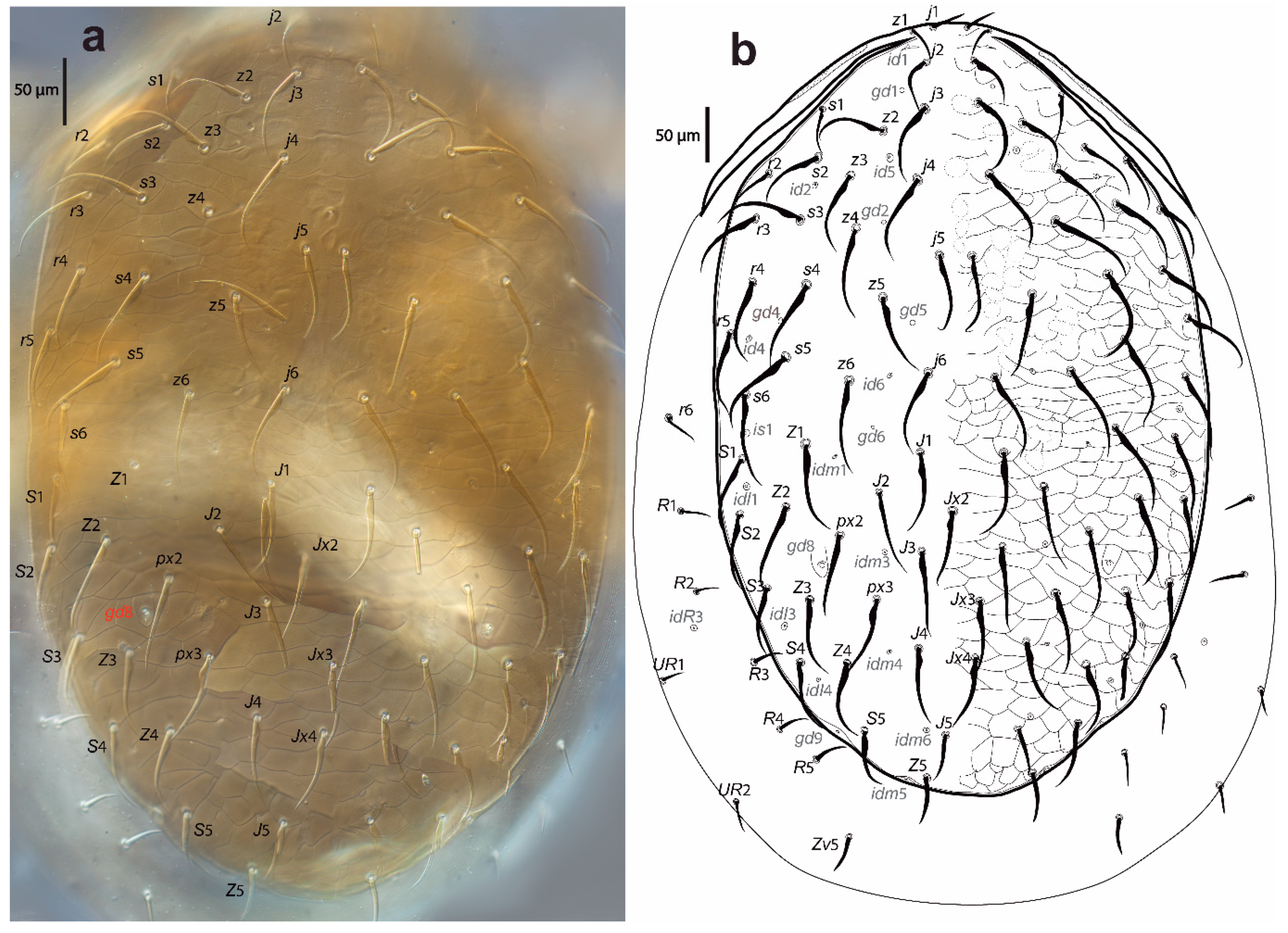
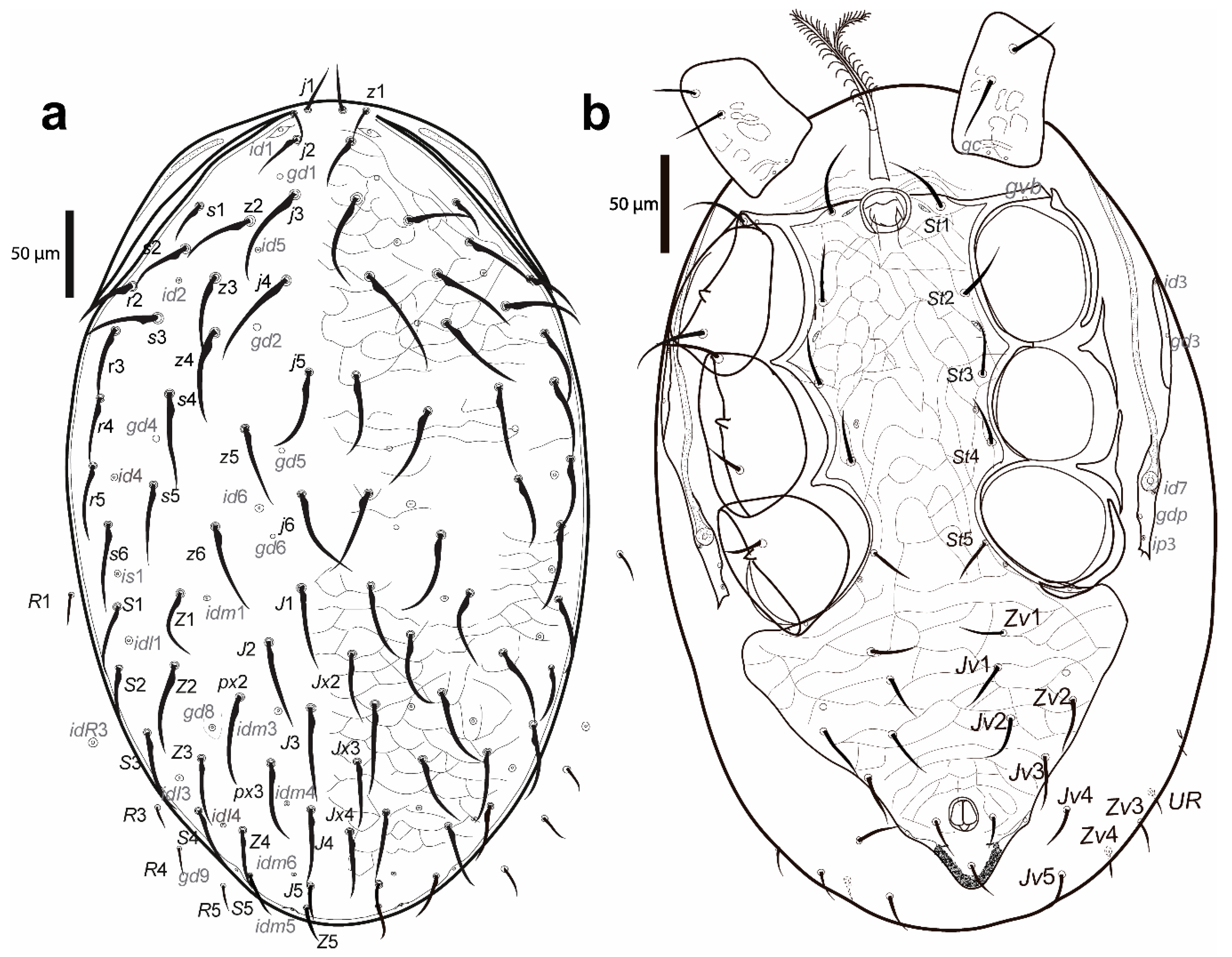
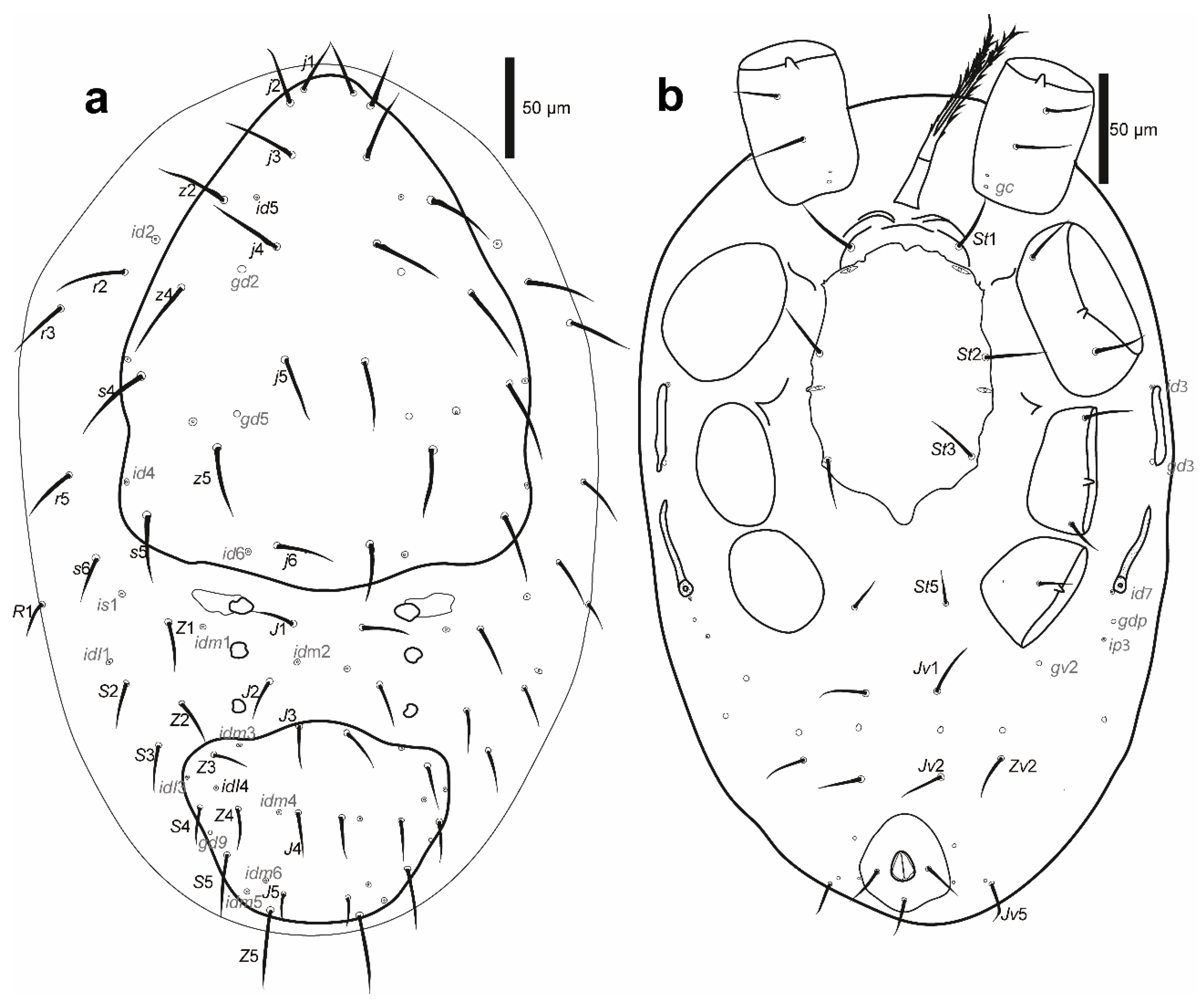
Figure 2.
Cosmolaelaps sabelisi sp. nov., adult female: (a) DIC image of the dorsal shield; (b) drawing of the dorsal idiosoma.
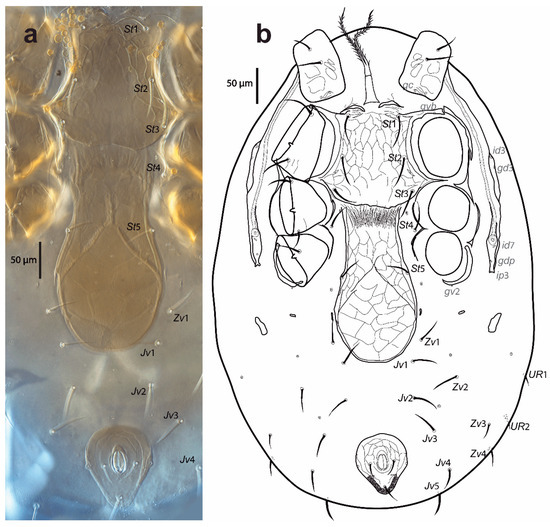
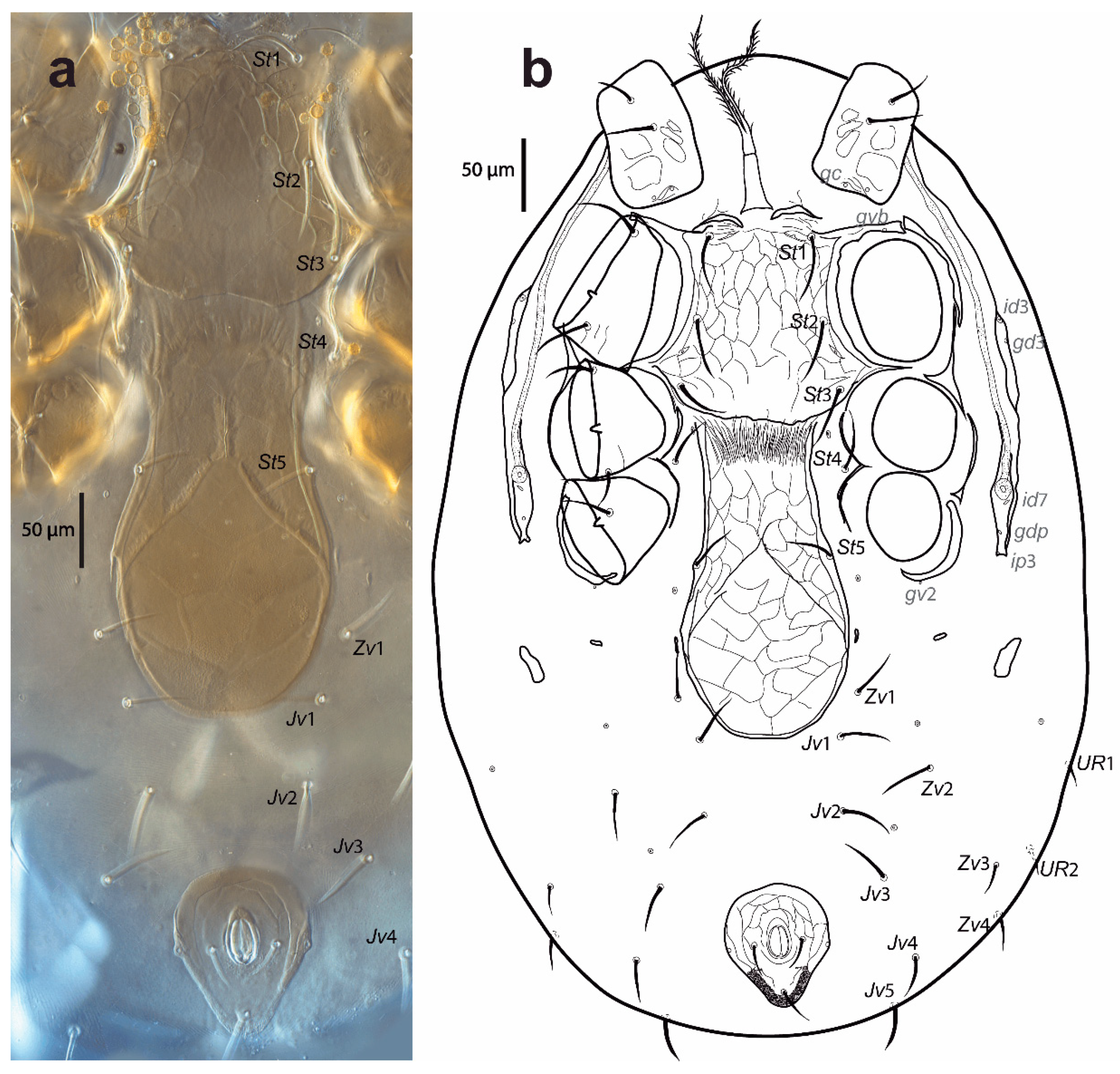
Figure 3.
Cosmolaelaps sabelisi sp. nov., adult female: (a) DIC image of ventral idiosoma; (b) drawing of the ventral idiosoma.
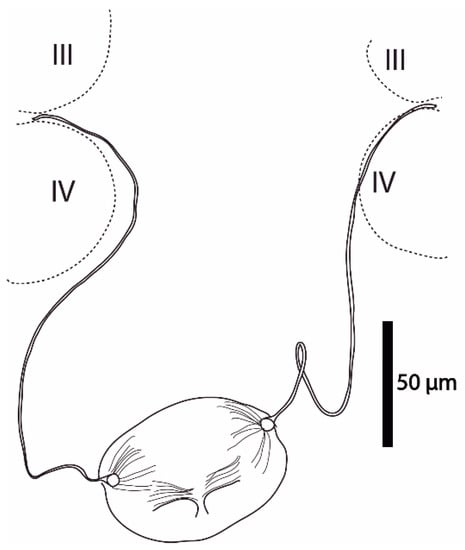
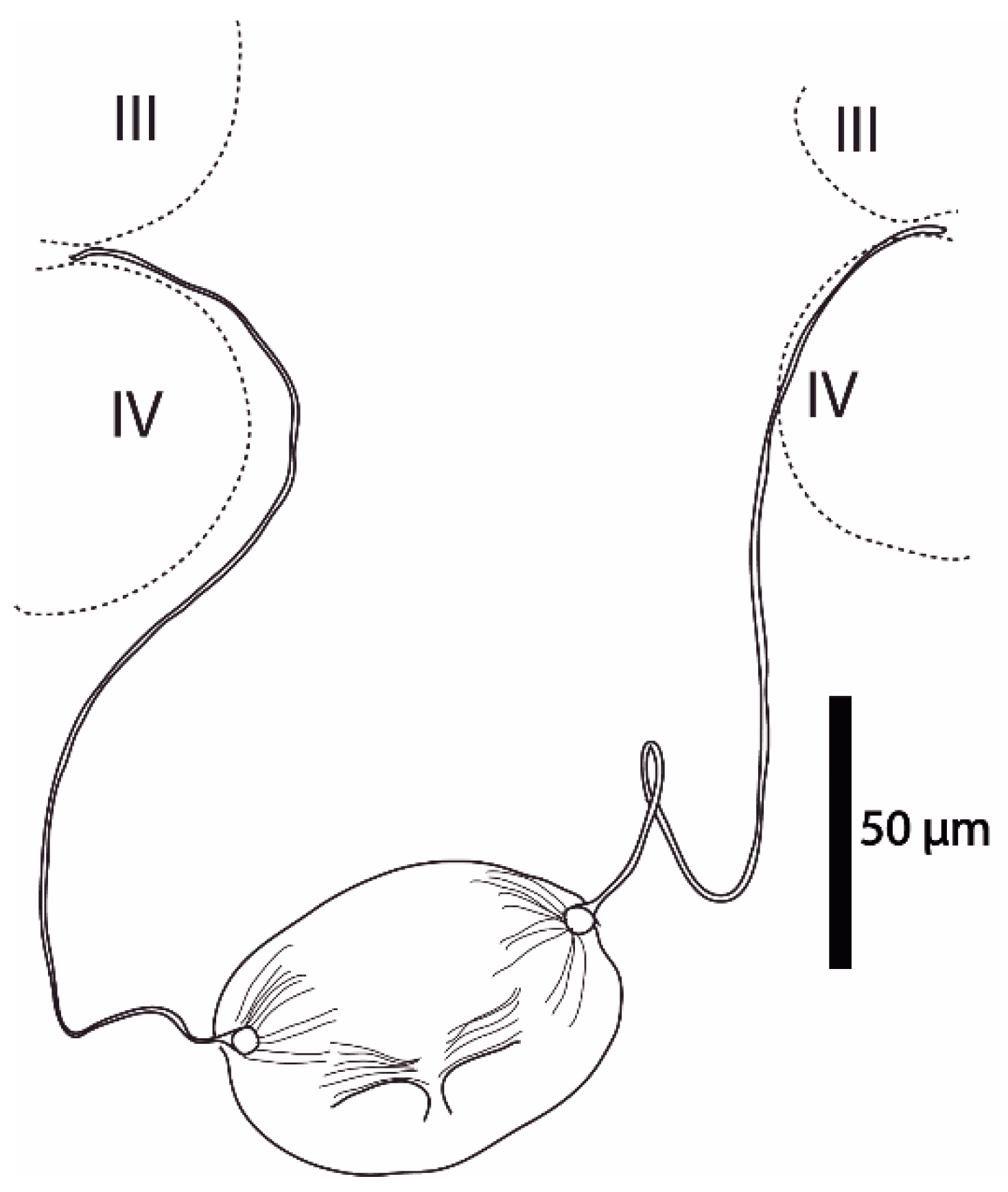
Figure 4.
Cosmolaelaps sabelisi sp. nov., adult female. Spermathecal apparatus.
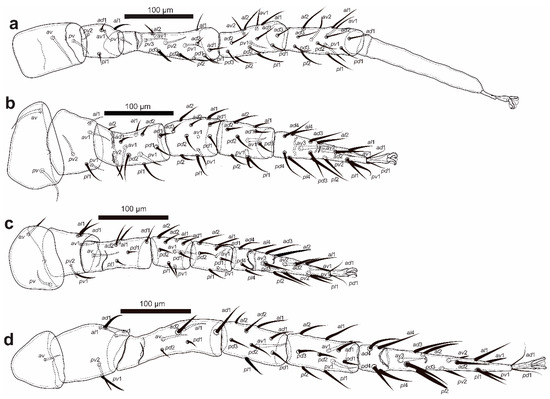
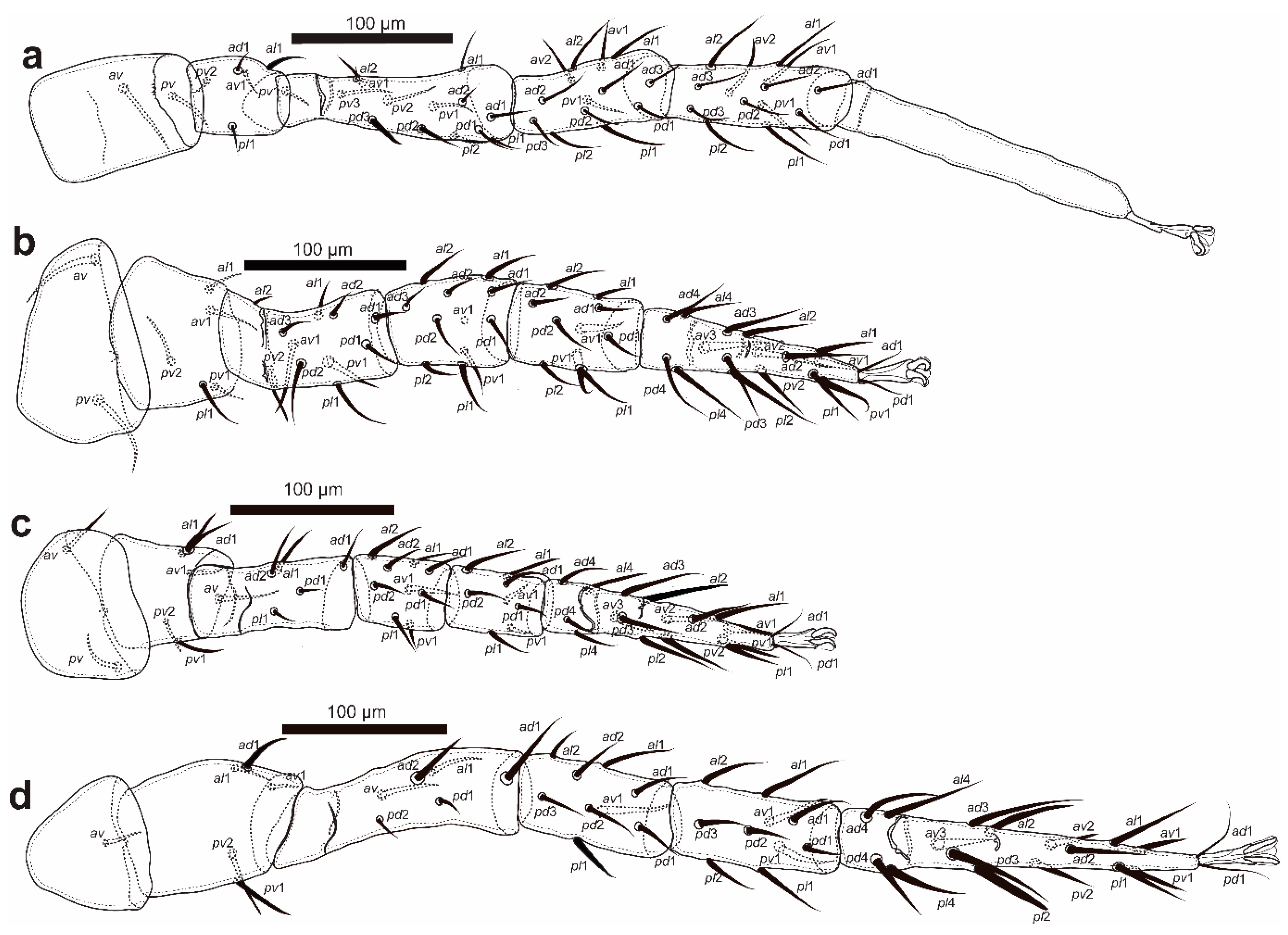
Figure 5.
Cosmolaelaps sabelisi sp. nov., adult female: (a) leg I; (b) leg II; (c) leg III; (d) leg IV.
Gnathosoma. Chelicera with a fringed coronet-like arthrodial process. Fixed cheliceral digit 72 (70–75) long, with two subapical teeth, followed by a large (lobe-like) tooth at the level of the spine-shaped pilus dentilis, and a row of 7–9 irregular teeth; movable digit 68 (66–70) long, with two teeth; dorsal seta thick and setiform, dorsal and antiaxial lyrifissures distinct (Figure 1a). Anterior region of epistome triangular, denticulate (Figure 1b).
The deutosternal groove (Figure 1c) has seven transverse lines, of which the most distal one is smooth and smaller, and the others with 22–10 denticles; it is delimited by subparallel lateral lines, which are constricted and separated along the most distal row; it has four pairs of rows of laterad of lateral line (between lines 3 and 4, between lines 4 and 5, between lines 5 and 6, and posteriorad of line 7). Internal malae are broad, distinctly separated, adjacent to each other, and ventrally fimbriate, laterad by a pair of curved structures with an internal surface and distal end coarsely fimbriate. Corniculi are normal and horn-shaped, about twice as long as their basal width, well separated from each other, and subparallel. Hypostomal seta h3 about in longitudinal line with h1 and laterad and slightly posteriad of h2. Numbers of setae on the palp trochanter–tarsus: 2, 5, 6, 14, 15; with acicular setae, except al on femur and al1 and al2 on genu thickened and spatulated; palp tarsal apotele bifurcate (Figure 1d). Measurements of setae: h1 44 (38–53), h2 29 (26–31), h3 56 (51–65), pc 34 (32–36), palp trochanter av 48 (48–50), and palp trochanter pv 37 (38–43); setae aciculate and smooth.
Dorsal idiosoma (Figure 2). Idiosoma 623 (590–650) long and 428 (380–485) wide at the widest level. Holodorsal shield, 598 (560–630) long and 377 (360–390) wide at the widest level, almost completely covering the dorsal surface of the idiosoma, brownish, reticulate, with the posterior end rounded. The podonotal region of the shield has 22 pairs of setae (including j1–6, z1–6, s1–6 r2–5), six pairs of lyrifissures (id1, id2, id4–id6 and is1), and five pairs of pores (gd1, gd2, gd4–6).
The opisthonotal region of the shield has 17 pairs of setae (including J1–5, Z1–5, S1–5, px2 and px3) and three unpaired Jx (between J2 and J3, between J3 and J4, and in transverse line with J4), eight pairs of lyrifissures (idl1, idl3–4, idm1, idm3–6), and two pairs of pores (gd8 and gd9). Setae r6, R1–5, UR1, and UR2 and lyrifissure idR3 are on the unsclerotized lateral cuticle. All setae are scimitar-shaped, except j1, z1, and Z5, which are setiform; seta J5 is almost imperceptible scimitar-shaped. Setal measurements are shown in Table 1.

Table 1.
Length of the dorsal setae of Cosmolaelaps sabelisi sp. nov.; mean (minimum–maximum). – = seta absent.
Ventral idiosoma (Figure 3). Base of tritosternum 40 (35–43) long and 18 (13–23) wide proximally; laciniae 111 (98–141), separated for about 80% of their total length, pilose. The pre-sternal area as two anterior lobes of the sternal shield, slightly sclerotized, and adjacent to the anterior margin of the sternal shield, with two well-sclerotized curved strips as the remainder of the shield. The sternal shield is mostly reticulate, lateral, and the anterolateral margins have a line of smooth thickening; the posterior margin is slightly convex, with anterolateral corners extending between coxae I and II, distally abutting to the anterior exopodal and bearing pores gvb; 156 (140–163) long and 159 (154–164) wide at level of coxae II, with three pairs of setae (st1–st3) and two pairs of lyrifissures (iv1, sligthly posterior and mesad to st1, and iv2, between st2 and st3); distances st1–st3 119 (115–121), st2–st2 97 (93–100). The fourth pair of sternal setae (st4) and the third pair of lyrifissure (iv3) are on the unsclerotized cuticle.
The epigynal shield is not fused with the anal shield, and is a bottle-shaped, reticulate surface with an inverted v-shaped pattern line at the central portion, which encloses 18–22 cells, and lateral margins with a line of smooth thickening; 278 (260–285) long and 130 (123–135) wide at the widest level; distance st5–st5 101 (96–105); seta st5 inserted on the margin of the shield and lyrifissure iv5 on the unsclerotised cuticle, posterolaterad of st5. A pair of rod-shaped platelets next to the edge of the epigynal shield. With two pairs of metapodal plates, the anterior is elongate and small whereas the posterior irregular and bigger. The opisthogaster has ten pairs of setae on an unsclerotised cuticle (Jv1–Jv5, Zv1–Zv5; Zv5 sometimes is dorsally displaced), three lyrifissures, and a pore (gv2). The anterior section of the endopodal shield is fused with the sternal shield; the section behind the sternal shield is fragmented into a boomerang-shaped section between coxae III and IV. The exopodal shield is represented by strips between coxae I and II and coxae III and IV, a triradiate fragment between coxae II and III, and an arched fragment partially surrounding the external margin of coxa IV. The anal shield is subtriangular (wider section anterior), reticulate; 90 (85–93) long and 76 (73–80) wide, with a pair of marginal pores about in transverse line with the para-anal setae, the latter slightly shorter than the post-anal seta and inserted on the mid-length of the anal opening; anal opening almost 1/3 as long as the shield, located at the centre of the shield; 23 (21–25) long and 17 (13–19) wide. Cribrum well-developed, extending to level off and anterolaterad to post-anal seta and extending along the margin, and anteriorly to the mid-length between the posterior margin of the anal shield and the insertion of para-anal setae. All ventral setae are setiform. Setal measurements shown in Table 2.

Table 2.
Length of the hyposthomal and ventral setae of Cosmolaelaps sabelisi sp. nov.; mean (minimum–maximum). – = seta absent.
Peritreme and peritrematic plate (Figure 3b). Peritremes extending anteriorly at the level of id1. Peritrematic plate only fused with the dorsal shield near z1, bearing a pore (gdp) and two lyrifissures (id7 and ip3) behind each stigma, with a lyrifissure (id3) and pore (gd3) dorsad of the peritreme and between coxae II and III.
Spermathecal apparatus (Figure 4). Laelapid-type. Insemination pore apparently located at the anterior margin at the base of coxa IV; infundibulum indistinct; tubulus elongated, whose ends are differentiated in a sclerotized ramus, attached to a globular structure (sacculus) with a sperm duct.
Legs (Figure 5). Tarsi I–IV with claws and ambulacra. Lengths: I: 665 (620–697); II: 488 (460–520); III: 473 (430–500); IV: 698 (650–730).
Setation (legs I–IV): coxae: 0-0/1 0/1-0, 0-0/1 0/1-0, 0-0/1 0/1-0, 0-0/1 0/0-0; trochanters: 1-1/1 0/2-1 (al1 thicker and longer than the others), 1-0/1 0/2-1 (av1, pv2 and pl1 slightly thicker and longer than the others), 1-1/1 0/2-0 (al1 and ad1 slightly thicker and longer than the others), 1-1/1 0/2-0 (ad1, pv1 and pv2 thicker and longer than the others); femora: 2-2/1 3/3-2 (pd3 thickened), 2-3/1 2/2-1 (av1, pd2, pl1, pv1 and pv2 slightly thickened), 1-2/1 1/0-1 (ad2, al1 and av1 slightly thickened and longer than the others), 1-2/1 2/0-0 (ad1, ad2, al1 and av slightly thicker and longer than the others); genua: 2-3/2 3/1-2, 2-3/1 2/1-2, 2-2/1 2/1-1, 2-2/1 3/0-1 (pl1 thickened); tibiae: 2-3/2 3/1-2, 2-2/1 2/1-2, 2-1/1 2/1-1, 2-1/1 3/1-2; tarsi: I not counted, 18 (av3, av1 and pv1 thickened), 18 (av1 and pv1 thickened), 18 (av3 and pl2 thickened).
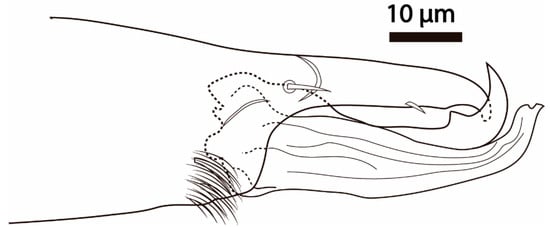
Figure 6.
Cosmolaelaps sabelisi sp. nov., adult male. Chelicera (antiaxial view).
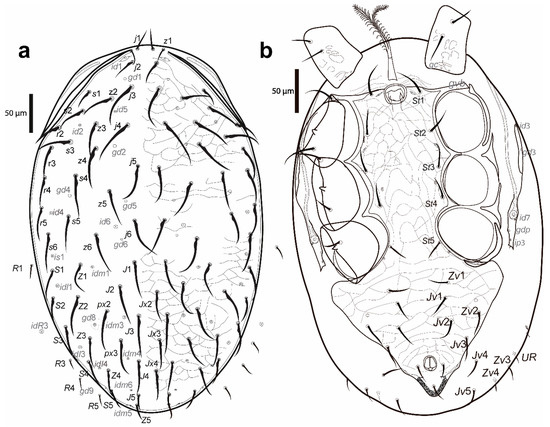
Figure 7.
Cosmolaelaps sabelisi sp. nov., adult male: (a) dorsal idiosoma; (b) ventral idiosoma.
Gnathosoma. Fixed cheliceral digit 43 (42–44) long, with a small tooth, anterior to a small setiform pilus dentilis; movable digit 39 (37–40) long, with one large tooth; spermadactyl 48 (47–49) long, curved upward, extending slightly beyond the tip of the movable digit, and progressively tapering in diameter to a blunt tip; dorsal seta thick and setiform; dorsal and antiaxial lyrifissures distinct (Figure 6). Internal malae distinctly separated, adjacent to each other, triangular, and with a serrate margin. Arthrodial process of chelicera, palp chaetotaxy, apotele, epistome, deutosternum, internal malae, corniculus, and position of hypostomal setae as in adult female. Measurements of setae: h1 34 (30–40), h2 23 (22–25), h3 42 (40–45), pc 22 (20–23); setae aciculate and smooth, except al on the femur and al1 and al2 on the genu, which are thickened and spatulated.
Dorsal idiosoma (Figure 7a). Holodorsal shield, 455 (442–483) long and 298 (281–318) wide, completely covering the dorsal surface of the idiosoma, brownish, reticulate, with posterior end rounded. Podonotal and opisthonotal region with the same setae, lyrifissures and pores as adult female. Setae r6 and R1, R3–R5 (R2 and UR2 absent) on the unsclerotized lateral cuticle, not visible dorsally in most of the specimens. Other features similar to the adult female. Setal measurements shown in Table 1.
Ventral idiosoma (Figure 7b). Base of the tritosternum 28 (25–30) long and 13 (12–15) wide proximally; laciniae 76 (74–79) long, 80% separated and pilose, same as the adult female. Pre-sternal area as two anterior lobes of the sternal shield slightly sclerotized adjacent to the anterior margin of the sternal shield. Sternogenital and ventrianal shields fused in a holoventral shield, reticulate, with lateral and anterolateral margins with a line of smooth thickening, anterolateral corners extending between coxae I and II, distally bearing pores gvb; 379 (373–388) long and 209 (198–223) wide at the level of coxae IV; with ten pairs of setae (st1–5, Jv1–3, Zv1 and Zv2) in addition to circumanal setae, four pairs of distinguishable lyrifissures, and three pairs of pores (gv2 behind coxa IV, a pore anteriorad to Zv2 and a marginal pore about in transverse line with or slightly posteriad of para-anal setae). Cribrum as adult female. Unsclerotised cuticle posterolaterad of the ventrianal region with four pairs of setae (Jv4–5 and Zv3–4). Shape of the ventral idiosomal setae, same as the adult female. Setal measurements are shown in Table 2.
Peritreme and peritrematic plate. As in adult female.
Legs. Lengths: I: 518 (503–527); II: 375 (360–385); III: 368 (350–405); IV: 517 (495–570). Shape of the setae as with the adult female.
Deutonymph (Figure 8, three specimens measured).
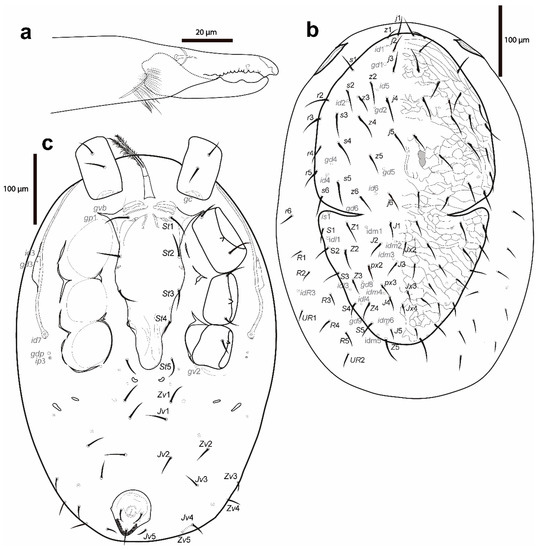
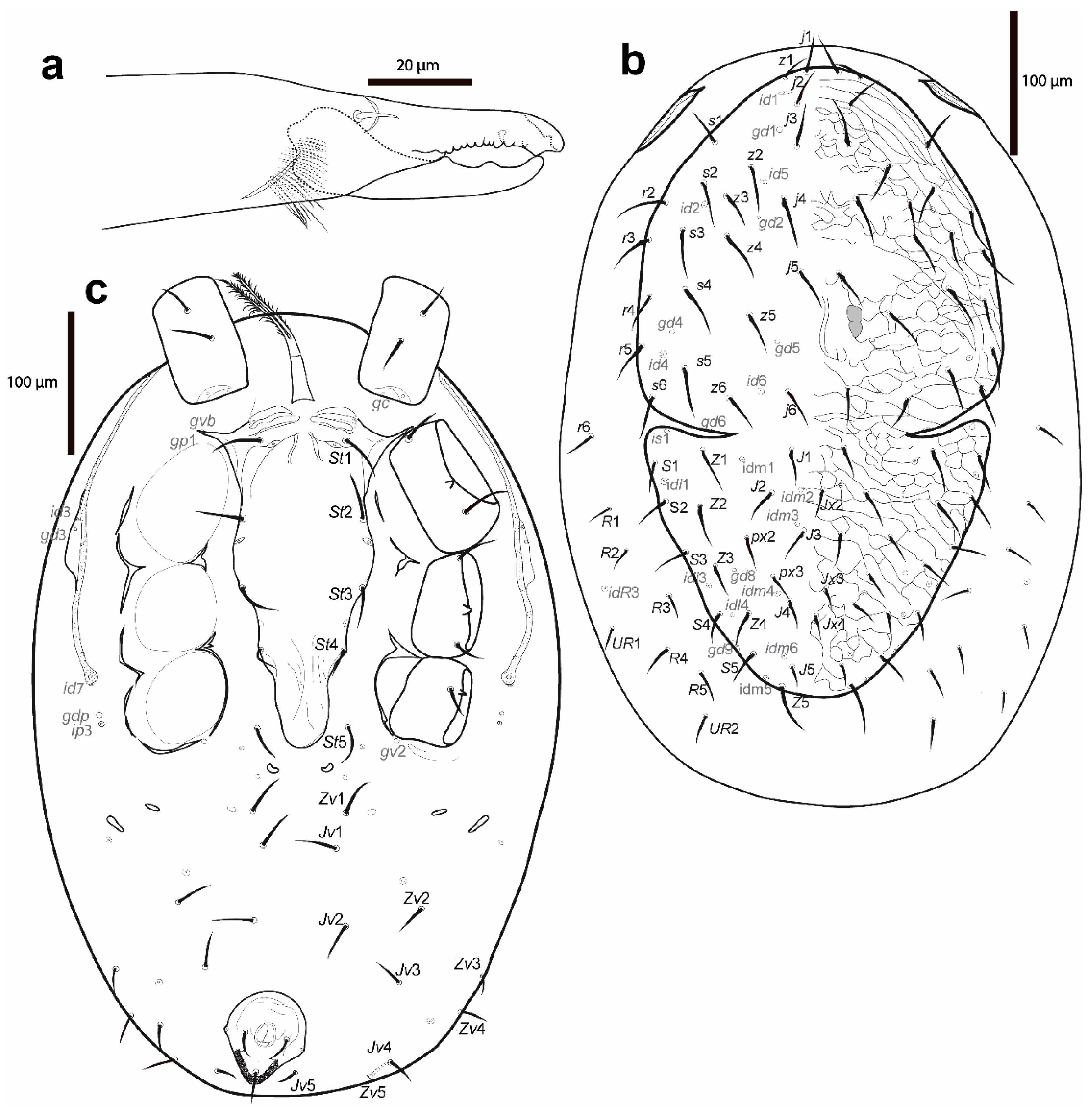
Figure 8.
Cosmolaelaps sabelisi sp. nov., deutonymph: (a) chelicera (antiaxial view); (b) dorsal idiosoma; (c) ventral idiosoma.
Gnathosoma. Fixed cheliceral digit 59 (58–60) long, with two subapical teeth, followed by a large (lobe-like) tooth at the level of the spine-shaped pilus dentilis, and a row of 7–8 irregular teeth; movable digit 56 (55–58) long, with two large teeth; dorsal seta thick and setiform; dorsal and antiaxial lyrifissures distinct (Figure 8a). Internal malae distinctly separated, adjacent to each other, triangular, and with serrate margin. Arthrodial process of the chelicera, palp chaetotaxy, apotele, epistome, deutosternum, internal malae, corniculus, and position of hypostomal setae same as the adult female. Measurements of setae: h1 38 (38–40), h2 28 (28–28), h3 53 (53–55), pc 29 (28–30); setae aciculate and smooth, except al on femur and al1 and al2 on the genu, which are thickened and spatulated.
Idiosoma (Figure 8b,c). Oval, whitish, 592 (558–638) long and 402 (388–428) wide.
Dorsal idiosoma (Figure 8b). Dorsal shield with deep, curved lateral incisions almost reaching the level between z6 and idm1 (schizodorsal shield), 486 (480–495) long and 294 (280–320) wide, covering only part of the dorsal surface of the idiosoma, tapered posteriad of r3–4, whitish, and reticulate. Podonotal region with 22 pairs of setae (including j1–6, z1–6, s1–6 and r2–5), six pairs of lyrifissures (id1, id2, id4–id6 and is1; is1 posteriad of lateral incision), and five pairs of pores (gd1, gd2, gd4–6; gd6 in margin of the lateral incision). Opisthonotal region with 17 pairs of setae (including J1–5, Z1–5, S1–5, px2 and px3) and three unpaired Jx (in transverse line with J2, between px3 and J4 and slightly posterior J4), with nine pairs of lyrifissures (idl1, idl3–4, idm1–6) and two pairs of pores (gd8 and gd9). Setae r6 and R1–5 and UR1–UR2 on an unsclerotized lateral cuticle. Other features similar to the adult female. Setal measurements are shown in Table 1.
Ventral idiosoma (Figure 8c). Base of tritosternum 34 (33–35) long and 15 (15–15) wide proximally; laciniae 93 (93–95), 80% separated and pilose, same as the adult female. Pre-sternal area similar to the adult female, but slightly less sclerotized. Sternal shield lightly sclerotized, smooth, except for a slight reticulation laterad of st1 and on the epigynial area, tapering posteriad of st3, anterolateral corners extending between coxae I and II, distally and marginally bearing pores gvb and gp1, 264 (260–273) long and 108 (105–110) wide, with four pairs of setae (st1–st4) and three pairs of lyrifissures (iv1–iv3); distances st1–3 117 (115–118), st2–st2 98 (93–100). Seta st5 and lyrifissure iv5 on an unsclerotized cuticle, the latter posterolaterad of st5. Two pairs of bacillate metapodal platelets. Opisthogaster with ten pairs of setae on an unsclerotised cuticle (Jv1–5, Zv1–5), and three pairs of lyrifissures. Exopodal plate reduced to some strip fragments next to the coxae and behind coxa IV, and triradiate fragments between coxae II and III and III and IV; gv2 on an unsclerotized cuticle behind coxa IV. Anal shield small, inversely pear-shaped, slightly reticulate, 19 (18–20) long and 13 (13–15) wide, with a pair of marginal pores about in transverse line with para-anal setae. Cribrum as adult female. Shape of the ventral idiosomal setae same as the adult female. Setal measurements shown in Table 2.
Peritreme and peritrematic plate. Peritreme same as the adult female. Peritrematic plate lightly sclerotized, reduced to a small distal section between j2 and r2, not fused with the dorsal shield, and with a platelet between coxae II and III, bearing a lyrifissure (id3) and a pore (gd3). Post-stigmatic peritrematic poroid (gdp) and lyrifissures (ip7 and ip3) on an unsclerotized cuticle; id7 abutting the peritreme.
Legs. Lengths: I: 580 (575–588); II: 428 (420–443); III: 394 (393–398); IV: 588 (580–605). Shape of setae same as the adult female.
Protonymph (Figure 9, three specimens measured).
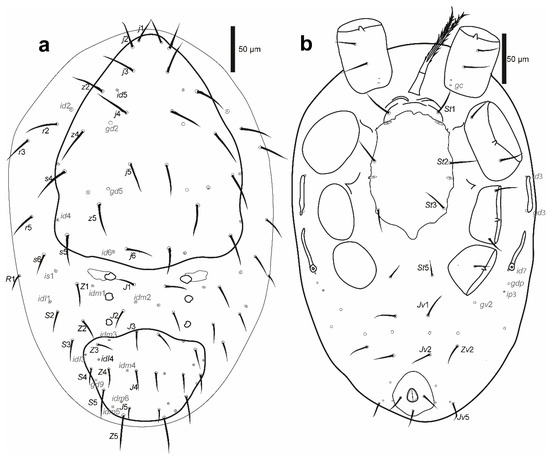
Figure 9.
Cosmolaelaps sabelisi sp. nov., protonymph: (a) dorsal idiosoma; (b) ventral idiosoma.
Gnathosoma. Fixed cheliceral digit 44 (40–48) long, with two subapical teeth, followed by a large (lobe-like) tooth at the level of the spine-shaped pilus dentilis, and a row of 7–8 irregular teeth; movable digit 41 (38–43) long, with two large teeth. Numbers of setae on palp trochanter–tarsus: 1, 4, 5, 12, 15; arthrodial process of chelicera, apotele, epistome, deutosternum, internal malae, corniculus and position of the hypostomal setae same as the adult female. Measurements of the setae: h1 29 (28–30), h2 21 (20–23), h3 36 (33–40), pc 25 (23–28); setae aciculate and smooth, except al on the femur and genu, which are thickened and spatulated.
Idiosoma (Figure 9). Oval, whitish, 378 (315–423) long and 254 (210–295) wide.
Dorsal idiosoma (Figure 9a). Podonotal and opisthonotal shields separate. Podonotal shield 234 (203–253) long and 203 (195–215) wide, whitish, lightly reticulate, with 11 pairs of setae (j1–6, z2, z4, z5, s4, s5), three pairs of lyrifissures (id4–6), and two pairs of pores (gd2 and gd5); unsclerotized cuticule laterad of podonotal shield with four pairs of setae (s6, r2, r3 and r5) and a pair of lyrifissures (id2). Opisthonotal shield 93 (85–98) long and 121 (113–125) wide, lightly reticulate, with eight pairs of setae (J3–5, Z3–5, S4 and S5), six pairs of lyrifissures (idm3–6 and idl3–4), and a pair of pores (gd9). Unsclerotized cuticle between the podonotal and opisthonotal shields with three pairs of lightly sclerotized and rounded sigillar platelets, seven pairs of setae (J1–2, Z1–2, S2–3 and R1), and four pairs of lyrifissures (is1, il1 and idm1–2). Dorsal setae j2–4 and z2 slightly perceptible as scimitar-shaped, with the other setae setiform. Setal measurements are shown in Table 1.
Ventral idiosoma (Figure 9b). Base of tritosternum 24 (20–28) long and 11 (8–13) wide proximally; laciniae 68 (65–75), 80% separated and pilose, same as the adult female. Pre-sternal area similar to the deutonymph. Sternal shield lightly sclerotized, smooth; 133 (130–135) long and 97 (85–105), with three pairs of setae (st1–3) and two pairs of lyrifissures (iv1–iv2); distances st1–3 110 (100–118), st2–st2 88 (80–93); setae st4 absent. Seta st5 on an unsclerotized cuticle. Unsclerotized opisthogastric cuticle with four pairs of setae on an unsclerotised cuticle (Jv1, Jv2, Jv5, Zv2), six pairs of lyrifissures, and two pairs of pores (a pair of pores about in transverse line with the para-anal setae, near the margin of the anal shield and gv2 behind coxa IV). Endopodal and exopodal plates indistinguishable; on an unsclerotized cuticle. Anal shield small, semi-ovoid, 13 (13–15) long and 49 (48–50) wide. Shape of the ventral idiosomal setae same as the adult female. Setal measurements are shown in Table 2.
Peritreme and peritrematic plate. Peritreme short, reaching the area next to the middle of coxa III. The peritrematic shield lightly sclerotized, reduced to a small fragment between coxae II and III; lyrifissure (ip2) and a pore (gp2) in the exterior margin of the plate. Post-stigmatic peritrematic poroid (gp3) and lyrifissures (ips and ip3) on a soft cuticle.
Legs. Lengths: I: 410 (373–448); II: 286 (263–310); III: 256 (253–260); IV: 351 (338–365). Chaetotaxy (legs I–IV): coxae: 0-0/1 0/1-0, 0-0/1 0/1-0, 0-0/1 0/1-0, 0-0/1 0/0-0; trochanters: 1-0/0 0/2-1, 1-0/0 0/2-1, 1-1/0 0/2-0, 1-1/1 0/1-0; femora: 2-3/1 1/1-2, 1-2/1 2/1-1,1-2/1 1/0-0, 1-2/0 1/0-0; genua: 1-2/1 2/1-1,1-2/0 2/0-1, 1-2/0 2/0-1, 1-2/0 2/0-0; tibiae: 1-2/1 2/1-1, 1-1/1 2/1-1, 1-1/1 2/1-1, 1-1/1 2/1-1; tarsal setation: I not counted, 17, 17, 17. Shape of setae as in adult female.
3.3. Etymology
The specific name “sabelisi” is a noun in apposition and is named after and in memoriam of Maurice Sabelis, eminent acarologist, and excellent person.
3.4. Remarks
Cosmolaelaps is a genus with 135 described species [3], which has been extensively studied in Europe. The conclusion that this was a new species was made after an extensive review of the descriptions of the species described until now, especially in Europe, and of material of Cosmolaelaps weeversi (Oudemans, 1926) [19] deposited in the Naturalis Biodiversity Center, Leiden, The Netherlands. We consider the species described here to be similar to Cosmolaelaps hortensis (Ishikawa, 1986) [20], Cosmolaelaps lutegiensis (Shcherbak, 1971) [21], Cosmolaelaps longus (Hafez, Elbadry & Nasr, 1982) [22], Cosmolaelaps transvaalensis Ryke, 1963 [23], and C. weeversi.
Cosmolaelaps hortensis, described from specimens collected on millipedes in Japan, differs by having a shorter dorsal shield, although with very high variation (female: 490–605 long and 332–385 wide; male: 355–398 long and 232–260 wide); some setae are shorter (j3 (58.5), j4 (67.5), j5 (60.8), j6 (61.5), J1 (63), and J5 (32.5)), Z5 is weakly pilose with a convex epistome margin, the epigynal shield extends posteriorly (distance between the epigynal shield and anal shield shorter than the length of the anal shield), and it has a wide spermatodactyl (according to the illustration). According to the illustration of the original description, C. hortensis has two unpaired setae; however, according to our observations and those of other authors [2,4,12,24], this characteristic may vary and has no taxonomic value.
Cosmolaelaps lutegiensis, described from specimens collected in litter in Ukraine, differs by having smaller idiosoma (female: 530–580 long and 300–390 wide; male: 420 long and 200 wide), a fixed cheliceral digit with three teeth, an epigynal shield extending close to anal shield, the epigynal shield broadened posteriorly past the level of setae st5 (Jv1 and Zv1–2 lateral to epigynal shield), and with apparently shorter and delicate setae (as observed in the illustration of the original description, the setae do not reach the base of the next setae, which occurs in the new species). A deeply incised tectum is described as a diagnostic feature of C. lutegiensis; however, we believe that the author observed the ornamentation of this structure rather than the anterior margin of the epistome, as observed by Joharchi et al. [25]. The illustration from the original description also shows 13 pairs of setae in the opisthogastric region. We believe that these correspond to 10 pairs of ventral setae (Jv1–5) and three pairs of dorsal lateral setae (R or UR). In the examined specimens and others observed from the colony maintained in the laboratory of the species described here was observed the presence of three unpaired setae among the J series. According to the original description of C. lutegiensis, this species can have 2–3 unpaired setae.
Cosmolaelaps longus described from specimens collected in litter under Lantana camara (Plantae: Lamiales: Verbenaceae) in Egypt differs by having a dorsal shield narrowing from the level of setae r3, progressively tapering until being rounded posteriorly, with a sternal shield smooth medially (or faintly reticulated) and reticulated laterally, and femur I with ad1, pd1–3 thickened and inserted on small tubercles [22,26].
Cosmolaelaps transvaalensis described from specimens collected in the litter of a blue-gum (Eucalyptus sp.) plantation in South Africa differs by having smaller idiosoma (female: 400–420 long and 235–250 wide), eight pairs of setiform setae (we consider that they correspond to Jv1–Jv5; Zv1–Zv3, according to the illustration) on an unsclerotized cuticle, a deutosternal groove with five rows of denticles, a fixed cheliceral digit with five subequal teeth, and the pre-sternal area anterior to st1 is sclerotized. According Illustration 20 of the original description of C. transvaalensis [23], the sternal and epigynial shields are punctate. No ornamentation was described or illustrated on the dorsal shield of this species.
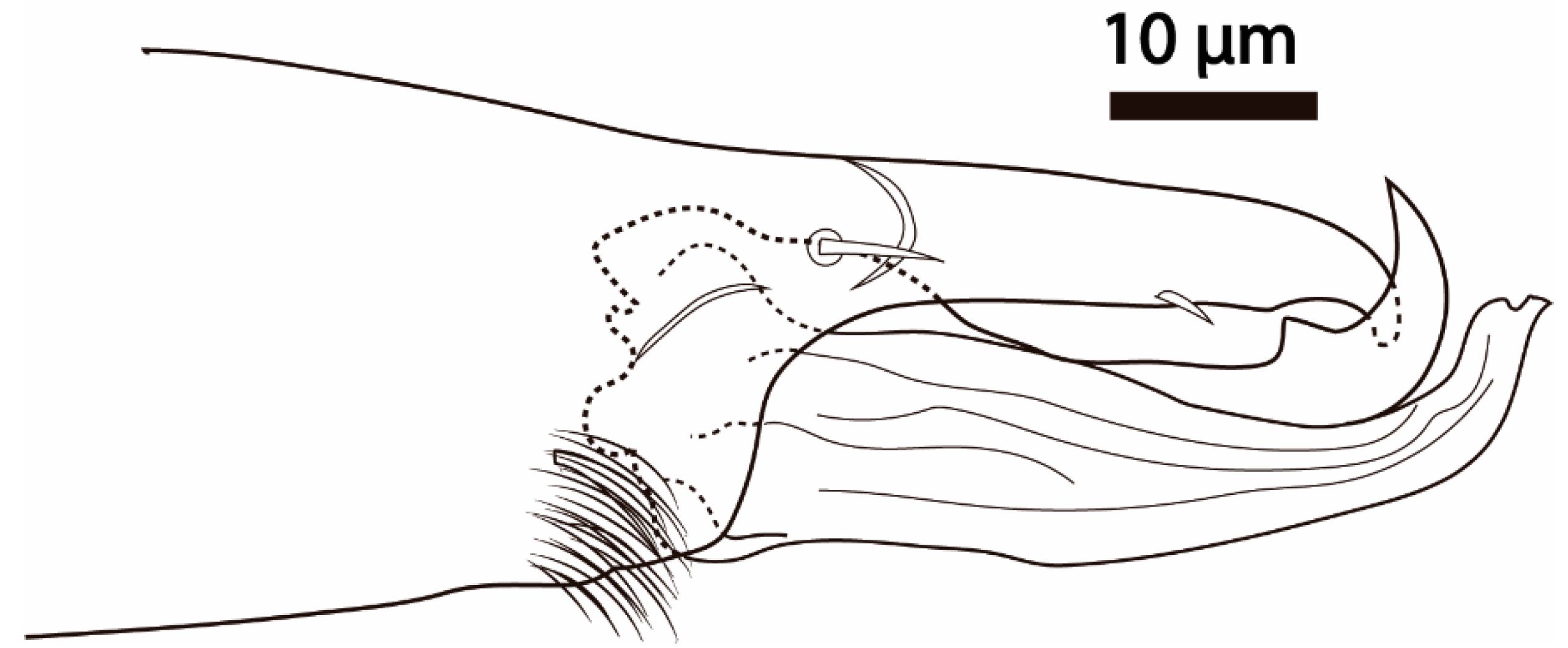
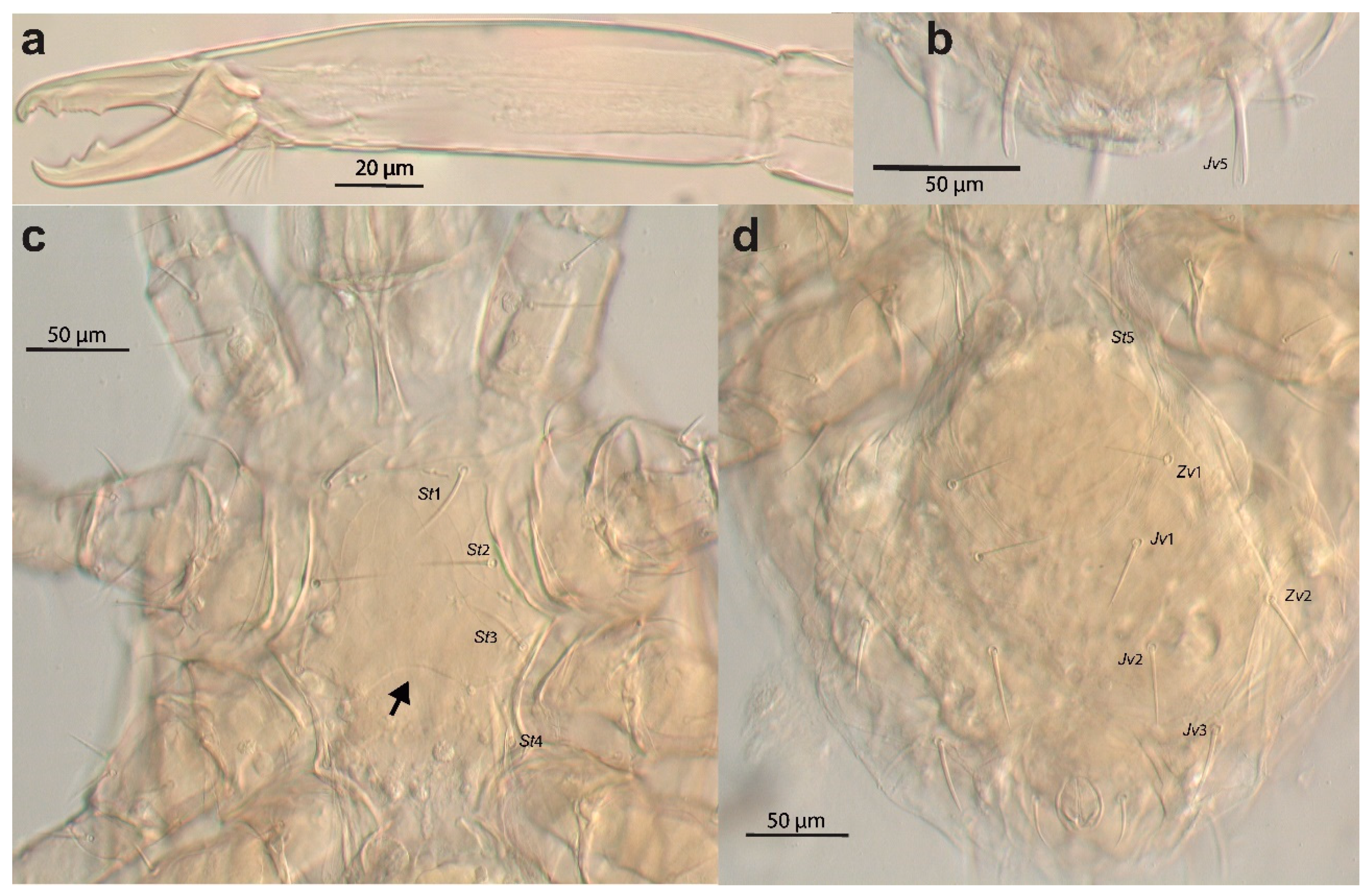
Cosmolaelaps weeversi was described from specimens collected on rotten fruit of Phytelephas sp. (Plantae: Arecales: Arecaceae) in Brazil. Although the original description is not very detailed [19], Oudemans’ illustration published by Stranstmann [27] showed the fixed cheliceral digit had two large teeth and a row of small teeth between them (Figure 10a), the presence of only 37 pairs of setae on the dorsal shield (r5 and px2 missing), the presence of some posterior spatulated setae (apparently some R, UR, Jv5, and Zv5) (Figure 10b), the posterior margin of the sternal shield being distinctly concave (arrow, Figure 10c), and no metapodal plates (Figure 10d), which differs from the species described here. We have examined the holotype of this species (deposited in the Naturalis Biodiversity Center under the code RMNH.ACA.P 3477 and 3476, corresponding to the complete mite and to a separate chelicera of the specimen, respectively, and whose slides label bears the same site and date of collection as the holotype, the sole specimen cited in the original description). Based on examination of the type specimen, we confirm the characteristics observed in the illustration of Oudemans published by Stranstmann [27] (Figure 10).
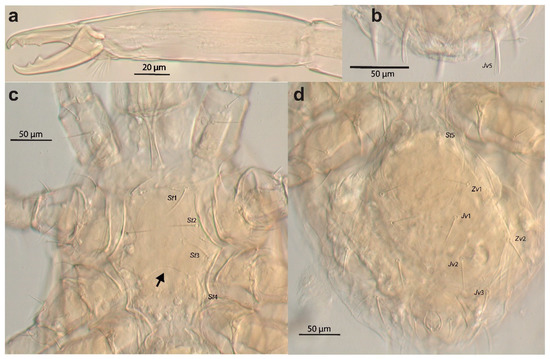
Figure 10.
Cosmolaelaps weeversi (Oudemans, 1926), adult female (holotype?). DIC image of (a) chelicera (antiaxial view); (b) posterior margin of the ventral idiosoma; (c) anterior part of the ventral idiosoma; (d) opistogastric part of the ventral idiosoma.
3.5. Biological Aspects of Cosmolaelaps sabelisi sp. nov.
In laboratory and experimental greenhouse conditions, mites of C. sabelisi sp. nov. were found feeding on eggs, larvae, and adults of C. lactis. In combination with bran and yeast, this prey mite was added to rose plant litter as an alternative food to boost the population of predatory mites (as in Muñoz-Cárdenas et al. [28]). Additionally, they were observed feeding on F. occidentalis larvae and pupae. Cosmolaelaps sabelisi sp. nov was able to develop and reproduce on the two prey species mentioned above. The life cycle of this predatory mite lasted around 9 days from adult to adult in laboratory conditions (T: 20 °C; RH: 70%) (Muñoz-Cárdenas pers. comm). Although the larvae are not described in this work due to the absence of adequate slice mounts, the larvae were observed in the colonies.
Author Contributions
Conceptualization, D.R.-R. and K.M.-C.; writing—original draft preparation, D.R.-R. and J.A.S.-M.; writing—review and editing, D.R.-R., J.A.S.-M., K.M.-C. and O.J.; investigation, D.R.-R. and O.J.; validation, D.R.-R. and O.J.; visualization, D.R.-R. and J.A.S.-M.; supervision, D.R.-R.; project administration, D.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
D.R-R. was supported by the grant RU780/20-1 from the Deutsche Forschungsgemeinschaft (DFG). O.J. was supported by the cooperative agreement No. FEWZ-2021–0004 from the Russian Ministry of Science and Higher Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
To the staff of the Naturalis Biodiversity Center, Leiden, The Netherlands, especially Rob van den Berg (Head Collection Management) and Bram van der Bijl (Department of Invertebrates) for the loan of the slides of Cosmolaelaps weeversi (Oudemans, 1926). To Parm Viktor von Oheimb, Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Berlin, Germany, for assistance in transporting the slides.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gwiazdowicz, D.J.; Nemati, A.; Mohseni, M. A new species of Cosmolaelaps(Acari: Mesostigmata: Laelapidae) from Peru. Int. J. Acarol. 2014, 40, 436–442. [Google Scholar] [CrossRef]
- Nemati, A.; Gwiazdowicz, D.J. Description of a new species of Cosmolaelaps Berlese and the male of C. brevipedestra (Karg) from Iran, with notes on some other species of Cosmolaelaps Berlese (Acari: Laelapidae). Zootaxa 2016, 4066, 535–551. [Google Scholar] [CrossRef]
- de Moraes, G.J.; Moreira, G.F.; Freire, R.A.P.; Beaulieu, F.; Klompen, H.; Halliday, B. Catalogue of the free-living and arthropod-associated Laelapidae Canestrini (Acari: Mesostigmata), with revised generic concepts and a key to genera. Zootaxa 2022, 5184, 1–509. [Google Scholar] [CrossRef]
- Faraji, F.; Halliday, B. Five new species of mites (Acari: Laelapidae) associated with large Australian cockroaches (Blattodea: Blaberidae). Int. J. Acarol. 2009, 35, 245–264. [Google Scholar] [CrossRef]
- Ramroodi, S.; Hajizadeh, J.; Joharchi, O. Two new species of Cosmolaelaps Berlese (Acari: Laelapidae) from Iran. Zootaxa 2014, 3847, 533–544. [Google Scholar] [CrossRef][Green Version]
- Moreira, G.F.; Moraes, G.J. The Potential of Free-Living Laelapid Mites (Mesostigmata: Laelapidae) as Biological Control Agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 77–102. ISBN 978-3-319-15042-0. [Google Scholar]
- Nasr, A.E.K.; Momen, F.M. A new species of the genus Cosmolaelaps Berlese (Acari: Laelapidae) from Egypt. Acarologia 2016, 56, 257–264. [Google Scholar] [CrossRef][Green Version]
- Silva, V.; Moreira, G.; Lopes, J.; Delabie, J.; Oliveira, A.R. A new species of Cosmolaelaps Berlese (Acari: Laelapidae) living in the nest of the ant Neoponera inversa (Smith) (Hymenoptera: Formicidae) in Brazil. Syst. Appl. Acarol. 2018, 23, 13. [Google Scholar] [CrossRef]
- Al Rehiayani, S.M.; Fouly, A.H. Cosmolaelaps simplex (Berlese), a Polyphagous Predatory Mite Feeding on Root-knot Nematode Meloidogyne javanica and Citrus Nematode Tylenchulus semipenetrans. Pak. J. Biol. Sci. 2005, 8, 168–174. [Google Scholar] [CrossRef]
- Moreira, G.F.; de Morais, M.R.; Busoli, A.C.; Moraes, G.J. de Life cycle of Cosmolaelaps jaboticabalensis (Acari: Mesostigmata: Laelapidae) on Frankliniella occidentalis (Thysanoptera: Thripidae) and two factitious food sources. Exp. Appl. Acarol. 2015, 65, 219–226. [Google Scholar] [CrossRef]
- Freire, R.A.P.; Moraes, G.J. de Description of a new species of Cosmolaelaps Berlese (Acari: Laelapidae, Hypoaspidinae) from Brazil and its biological cycle. Int. J. Acarol. 2007, 33, 353–358. [Google Scholar] [CrossRef]
- Beaulieu, F. Review of the mite genus Gaeolaelaps Evans & Till (Acari: Laelapidae), and description of a new species from North America, G. gillespiei n. sp. Zootaxa 2009, 2158, 33–49. [Google Scholar]
- Lesna, I.; Wolfs, P.; Faraji, F.; Roy, L.; Komdeur, J.; Sabelis, M.W. Candidate Predators for Biological Control of the Poultry Red Mite Dermanyssus gallinae. In Control of Poultry Mites (Dermanyssus); Sparagano, O.A.E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 63–80. ISBN 978-90-481-2731-3. [Google Scholar]
- Lindquist, E.E.; Evans, G.O. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can. 1965, 97, 5–66. [Google Scholar] [CrossRef]
- Evans, G.O. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. Nat. Hist. 1963, 10, 275–303. [Google Scholar] [CrossRef]
- Evans, G.O. Observations on the ontogenetic development of the chaetotaxy of the tarsi nomenclature for the chaetotaxy of the tarsi nomenclature for the chaetotaxy of tarsi II-IV of legs II-IV in the Mesostigmata (Acari). In Proceedings of the 2nd International Congress of Acarology, Sutton Bonington, UK, 19–25 July 1967; pp. 195–200. [Google Scholar]
- Athias-Henriot, C. Nouvelles notes sur les Amblyseiini. II. Le releve organotaxique de la face dorsale adulte (Gamasides protoadeniques, Phytoseiidae). Acarologia 1975, 27, 20–29. [Google Scholar]
- Moreira, G.F.G.F.; Klompen, H.; Moraes, G.J. de Redefinition of Cosmolaelaps berlese (acari: Laelapidae) and description of five new species from Brazil. Zootaxa 2014, 3764, 317–346. [Google Scholar] [CrossRef]
- Oudemans, A.C. Acarologische Aanteekeningen, LXXXI. Entomol. Ber. 1926, 149, 97–102. [Google Scholar]
- Ishikawa, K. Gamasid mites (Acarina) associated with Japanese Millipeds. Rep. Res. Matsuyama Shinonome Jr. Coll. 1986, 17, 165–177. [Google Scholar]
- Shcherbak, G.I. New species of Gamasid from the genus Hypoaspis Canestrini (Acarina, Gamasoidea). Vestn. Zool. 1971, 5, 76–79. [Google Scholar]
- Hafez, S.M.; Elbadry, E.A.; Nasr, A.K. Soil mites of the family Laelapidae from Egypt (Acari: Mesostigmata). Ain Shams Res. Bull. 1982, 1759, 1–15. [Google Scholar]
- Ryke, P.A.J. Some free-living Hypoaspidinae (Acari: Mesostigmata) from South Africa. Rev. Biol. 1963, 5, 1–15. [Google Scholar]
- Strong, K.L.; Halliday, R.B. Three new species of Hypoaspis Canestrini (Acarina: Laelapidae) associated with large Australian cockroaches. Aust. J. Entomol. 1994, 33, 87–96. [Google Scholar] [CrossRef]
- Joharchi, O.; Issakova, A.K.; Asyamova, O.S.; Sarcheshmeh, M.A.; Tolstikov, A.V. Some soil-inhabiting mites (Acari: Mesostigmata) from Kazakhstan, with description of a new species of Gaeolaelaps Evans & Till (Acari: Laelapidae). Zootaxa 2020, 4819, 473–498. [Google Scholar] [CrossRef]
- Joharchi, O.; Negm, M.W. Soil-inhabiting mites of the family Laelapidae (Acari: Mesostigmata) from Assiut Governorate, Egypt. Zootaxa 2020, 4759, 488–510. [Google Scholar] [CrossRef] [PubMed]
- Strandtmann, R.W. Some previously unpublished drawings of gamasid mites by the late Dr. A. C. Oudemans. J. Kans. Entomol. Soc. 1963, 36, 2–31. [Google Scholar]
- Muñoz-Cárdenas, K.; Ersin, F.; Pijnakker, J.; van Houten, Y.; Hoogerbrugge, H.; Leman, A.; Pappas, M.L.; Duarte, M.V.A.A.; Messelink, G.J.; Sabelis, M.W.; et al. Supplying high-quality alternative prey in the litter increases control of an above-ground plant pest by a generalist predator. Biol. Control 2017, 105, 19–26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).