A Cautionary Note on Linear Measurements and Their Ratios in Taxonomy
Abstract
:1. Introduction
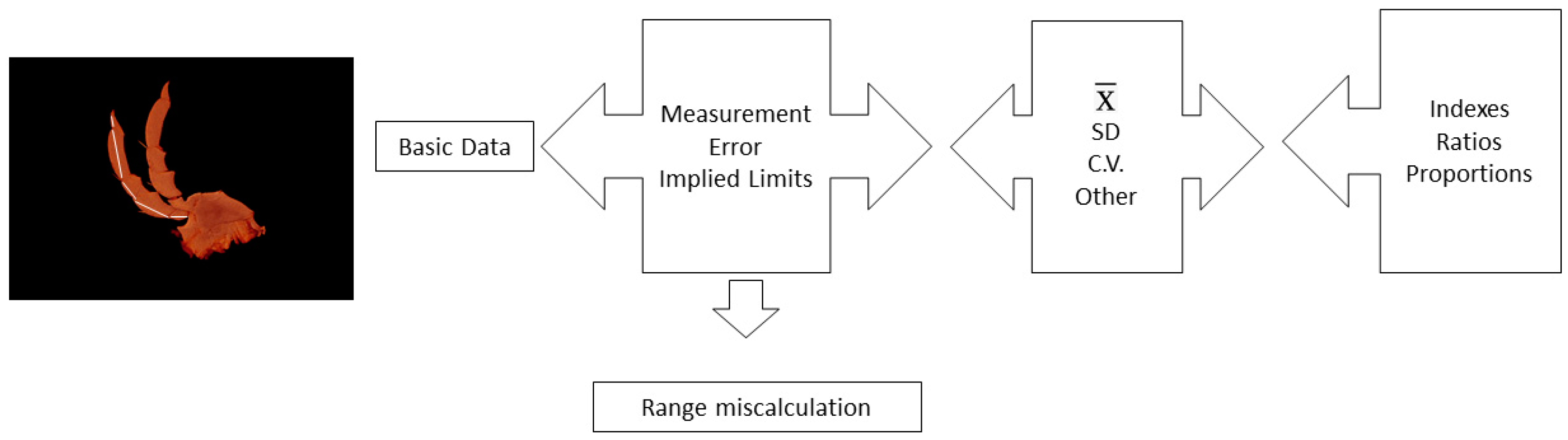
2. On the Origin of Data: Uncertainty and Implied Range
2.1. Measurement of Variability in Species
2.2. Range Calculation
2.3. Mean and Standard Deviation
2.4. Coefficient of Variation
2.5. Ratios and Indexes
- Single figures, before being used in a ratio, should be converted to their implied range.
- The lower limit of the first measurement should be divided by the higher limit of the second measurement.
- The higher limit of the first measurement should be divided by the lower limit of the second measurement.
- Although the ratio cannot have more significant figures than the measurement with the fewest significant figures, it may be acceptable to leave them.
3. Conclusions
- (1)
- Primary data should always be made accessible either in the main publication or as supplementary material or, as is becoming more commonplace, in a research data repository. This point is very important: in order to (re)examine the accuracy of any statistical quantity, the data distribution must be known.
- (2)
- Any direct measurement has implicit uncertainty limits. In the case of microscopic animals, uncertainty derives from the primary error that occurs when calibrating the ocular micrometer with a stage micrometer, followed by secondary errors that can occur during the act of measuring itself.
- (3)
- Along with the traditional measures of variability (i.e., and s), cv may be a useful tool for identifying characters with low variability that could potentially function as diagnostic characters.
- (4)
- Finally, the ratios of characters should be properly calculated by taking into consideration the degree of uncertainty, particularly if they are to be used in species diagnoses.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houle, D.; Pélabon, C.; Wagner, G.P.; Hansen, T.F. Measurement and meaning in biology. Q. Rev. Biol. 2011, 86, 3–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdecasas, A.G.; Pelaéz, M.L.; Wheeler, Q.D.; de Carvalho, M.R. Evidence-Based Taxonomy: Labels as Illocutionary Acts. Taxonomy 2022, 2, 339–346. [Google Scholar] [CrossRef]
- Brower, A.V.Z. A slippery reality: The epistemological shifting sands of tokogeny, phylogeny, lineages and species delimitation. Syst. Biodivers. 2021, 19, 782–796. [Google Scholar] [CrossRef]
- Simpson, G.G.; Roe, A.; Lewontin, R.C. Quantitative Zoology; Harcourt, Brace & World, Inc.: New York, NY, USA, 1960. [Google Scholar]
- Sokal, R.R.; Rohlf, J. Biometry: Principles and Practice of Statistics in Biological Research; W.H. Freeman and Company: San Francisco, CA, USA, 2011. [Google Scholar]
- Jasieński, M.; Bazzaz, F.A. The fallacy of ratios and the testability of models in biology. Oikos 1999, 1, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Davids, K.; Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. On the taxonomy of water mites (Acari: Hydrachnidia) described from the Palaearctic, part 1: Hydrachnidae, Limnocharidae and Eylaidae. Zootaxa 2005, 1061, 35–64. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. On the taxonomy of water mites (Acari: Hydrachnidia) described from the Palaearctic, part 2: Hydryphantoidea and Lebertioidea. Zootaxa 2009, 2266, 1–34. [Google Scholar] [CrossRef]
- Smit, H.; Gerecke, R.; Pešić, V.; Gledhill, T. On the taxonomic state of water mite taxa (Acari: Hydrachnidia) described from the Palaearctic, part 3, Hygrobatoidea and Arrenuroidea with new faunistic data. Zootaxa 2015, 3981, 542–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davids, C.; Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H.; Van der Hammen, H. Acari: Hydrachnidia. In Süßwasserfauna Mitteleuropas; 7/2-1, Chelicerata, Acari I: 241-376; Gerecke, R., Ed.; Spektrum Elsevier: München, Germany, 2007. [Google Scholar]
- Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. Chelicerata: Acari II. In Süßwasserfauna von Mitteleuropa 7/2-2: I-IX, 1-134; Spektrum Akademischer Verlag: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gerecke, R.; Gledhill, T.; Pesic, V.; Smit, H. Chelicerata: Acari III. In Süßwasserfauna von Mitteleuropa 7/2-3; Springer Specktrum: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pešić, V.; Smit, H. A second Palaearctic species of the genus Wettina Piersig, 1892 based on morphological and molecular data (Acari, Hydrachnidia: Wettinidae). Syst. Appl. Acarol. 2018, 23, 724–732. [Google Scholar] [CrossRef]
- Tuzovskij, P.V. Description of a new water mite species of the genus Kongsbergia Thor, 1899 (Acari, Hydrachnidia: Aturidae) from Kazakhstan. Zootaxa 2021, 4999, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, R. Further studies on hydryphantoid water mites in the W Palaearctic region (Acari, Actinedida). Arch. Hydrobiologie. Supplementband. Monogr. Beiträge 1999, 121, 119–158. [Google Scholar]
- Galton, F.R. Memories of My Life; Methuen: London, UK, 1908. [Google Scholar]
- Quesnel, L.B. Chapter I Microscopy and Micrometry. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1971; Volume 5, Part A, pp. 1–103. [Google Scholar]
- Goodwin, P.C. A primer on the fundamental principles of light microscopy: Optimizing magnification, resolution, and contrast. Mol. Reprod. Dev. 2015, 82, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Van Valen, L. The statistics of variation. In Variation; Hallgrímsson, B., Hall, B.K., Eds.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Holmes, S.; Huber, W. Modern Statistics for Modern Biology; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Lewontin, R.C. On the measurement of relative variability. Syst. Zool. 1966, 15, 141–142. [Google Scholar] [CrossRef]
- Minelli, A. Species diversity vs. morphological disparity in the light of evolutionary developmental biology. Ann. Bot. 2016, 117, 781–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lande, R. On comparing coefficients of variation. Syst. Zool. 1977, 26, 214–217. [Google Scholar] [CrossRef]
- Goldschmidt, T. Studies on Latin American water mites of the genus Torrenticola Piersig, 1896 (Torrenticolidae, Hydrachnidia, Acari). Zool. J. Linn. Soc. 2007, 150, 443–678. [Google Scholar] [CrossRef]
- Gerecke, R. Water mites of the genus Atractides Koch, 1837 (Acari: Parasitengona: Hygrobatidae) in the western Palaearctic region: A revision. Zool. J. Linn. Soc. 2003, 138, 141–378. [Google Scholar] [CrossRef]
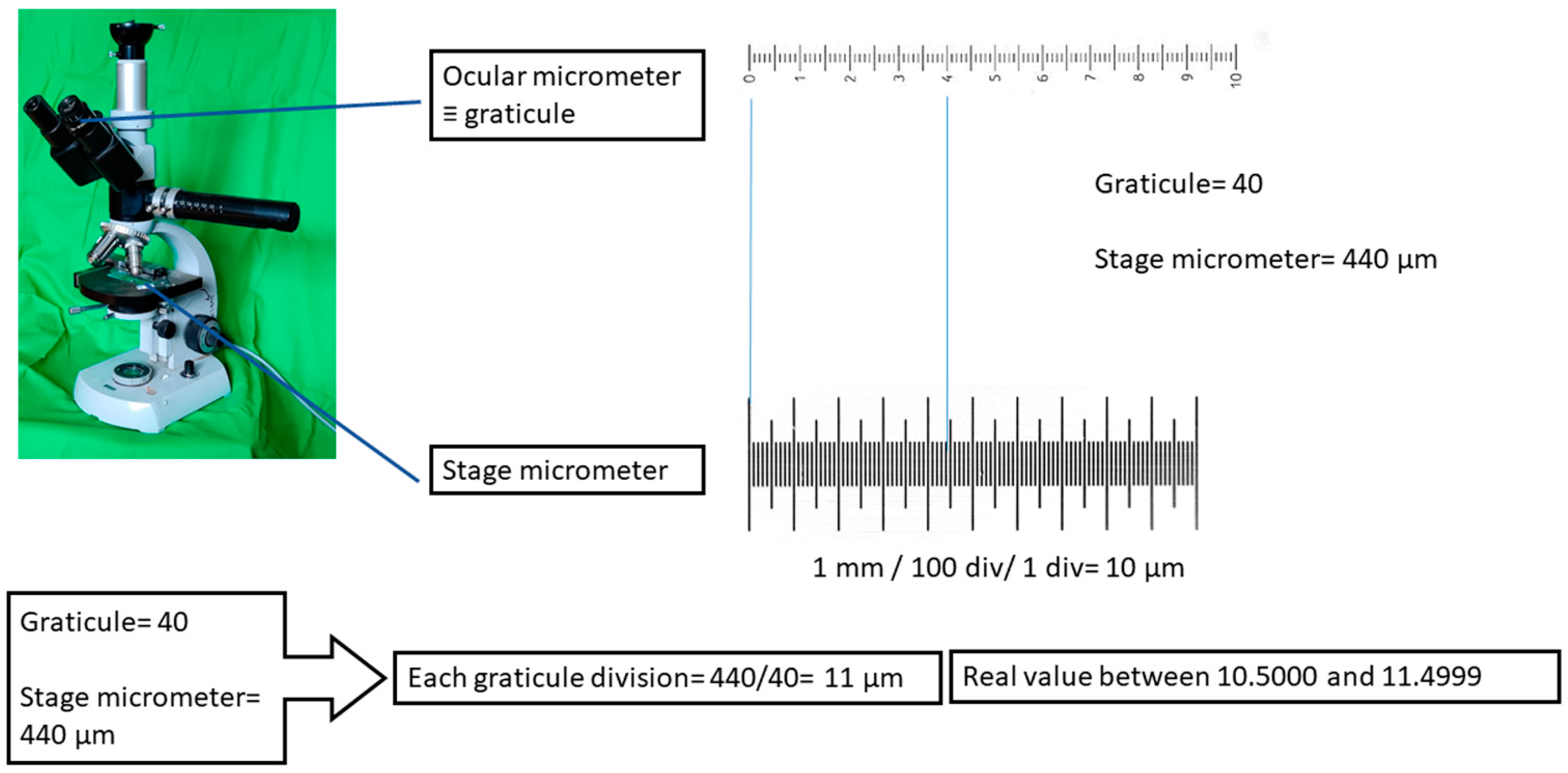
| Stage Micrometer | Ocular Micrometer | Ocular 1 div | Significant Numbers |
|---|---|---|---|
| 100 µm | 13 div | 7.69 µm | 7.7 µm |
| 150 µm | 19 div | 7.89 µm | 7.9 µm |
| 250 µm | 32 div | 7.81 µm | 7.8 µm |
| 350 µm | 45 div | 7.77 µm | 7.8 µm |
| 490 µm | 63 div | 7.77 µm | 7.8 µm |
| Torrenticola | elliptica | 100 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 92.35 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 92.35 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 92.33 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 92.11 | Torrenticola | meridionalis |
| Torrenticola | elliptica | 91.9 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.9 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.9 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.74 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.74 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.71 | Torrenticola | elliptica |
| Torrenticola | meridionalis | 91.67 | Torrenticola | elliptica |
| Torrenticola | elliptica | 91.59 | Torrenticola | meridionalis |
| Torrenticola | elliptica | 91.59 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.59 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.55 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.51 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.51 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.47 | Torrenticola | meridionalis |
| Torrenticola | meridionalis | 91.46 | Torrenticola | meridionalis |
| Holotype | Mean | Min | Max | sd | cv | |
|---|---|---|---|---|---|---|
| IdiosomaL | 701 | 753 | 667 | 849 | 44.3 | 5.88 |
| Idiosoma W | 594 | 618 | 544 | 755 | 52.6 | 8.51 |
| Idiosoma L/W | 1.18 | 1.2 | 1.11 | 1.3 | 0.05 | 4.17 |
| Cx-It L | 314 | 314 | 284 | 358 | 14.5 | 4.62 |
| Cx-III W | 412 | 412 | 368 | 500 | 28.9 | 7.01 |
| Cx-ItL/Cx-III W | 0.76 | 0.75 | 0.67 | 0.82 | 0.04 | 5.33 |
| Ds L | 608 | 652 | 559 | 760 | 46.6 | 7.15 |
| Dp L | 574 | 618 | 525 | 706 | 44.8 | 7.25 |
| Ds W | 481 | 527 | 461 | 628 | 38.8 | 7.36 |
| Ds L/W | 1.27 | 1.24 | 1.16 | 1.29 | 0.03 | 2.42 |
| Dp L/W | 1.19 | 1.17 | 1.08 | 1.23 | 0.03 | 2.56 |
| A-mplatelet L | 184 | 189 | 159 | 218 | 13.1 | 6.93 |
| A-mplatelet W | 61 | 69 | 58 | 83 | 5.9 | 8.55 |
| A-mplatelet L/W | 3 | 2.69 | 2.44 | 3 | 0.1 | 3.72 |
| Capitularbay L | 164 | 164 | 135 | 181 | 10.2 | 6.22 |
| Capitularbay W | 69 | 76 | 64 | 102 | 8.1 | 10.66 |
| Cb L/W | 2.39 | 2.13 | 1.51 | 2.44 | 0.2 | 9.39 |
| Distcb–gf | 216 | 224 | 203 | 279 | 15.7 | 7.01 |
| Cx-Im L | 152 | 157 | 135 | 194 | 10.6 | 6.75 |
| Cx-II + IIIm L | 54 | 59 | 39 | 78 | 8 | 13.56 |
| Cx-ItL/Cx-II/IIIm L | 5.82 | 5.35 | 4 | 8.63 | 0.9 | 16.82 |
| Cx-I/Cx-II + IIIm L | 2.82 | 2.67 | 1.87 | 4.25 | 0.4 | 14.98 |
| Palp Segment | Published Measurements of L/H (in μm) | Published Ratio Range | Corrected Ratio Range | Calculation Correcting for Uncertainty |
|---|---|---|---|---|
| P-1 | 400–420/590–640 | 0.66–0.68 | 0.62–0.71 | 400/640 = 0.62 420/590 = 0.71 |
| P-2 | 670–710/370–380 | 1.81–1.87 | 1.76–1.92 | 670/380 = 1.76 710/370 = 1.92 |
| P-3 | 850–930/190–195 | 4.47–4.77 | 4.35–4.89 | 850/195 = 4.35 930/190 = 4.89 |
| P-4 | 250/110 | 2.27 | 2.27 | 250/110 |
| P-5 | 100–110/50–60 | 1.67–2.20 | 1.64–2.23 | 100/60 = 1.67 110/50 = 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdecasas, A.G. A Cautionary Note on Linear Measurements and Their Ratios in Taxonomy. Taxonomy 2023, 3, 1-9. https://doi.org/10.3390/taxonomy3010001
Valdecasas AG. A Cautionary Note on Linear Measurements and Their Ratios in Taxonomy. Taxonomy. 2023; 3(1):1-9. https://doi.org/10.3390/taxonomy3010001
Chicago/Turabian StyleValdecasas, Antonio G. 2023. "A Cautionary Note on Linear Measurements and Their Ratios in Taxonomy" Taxonomy 3, no. 1: 1-9. https://doi.org/10.3390/taxonomy3010001
APA StyleValdecasas, A. G. (2023). A Cautionary Note on Linear Measurements and Their Ratios in Taxonomy. Taxonomy, 3(1), 1-9. https://doi.org/10.3390/taxonomy3010001






