Classifying Cockroaches According to Forewings: Pitfalls and Implications for Fossil Systematics
Abstract
1. Introduction
2. Materials and Methods
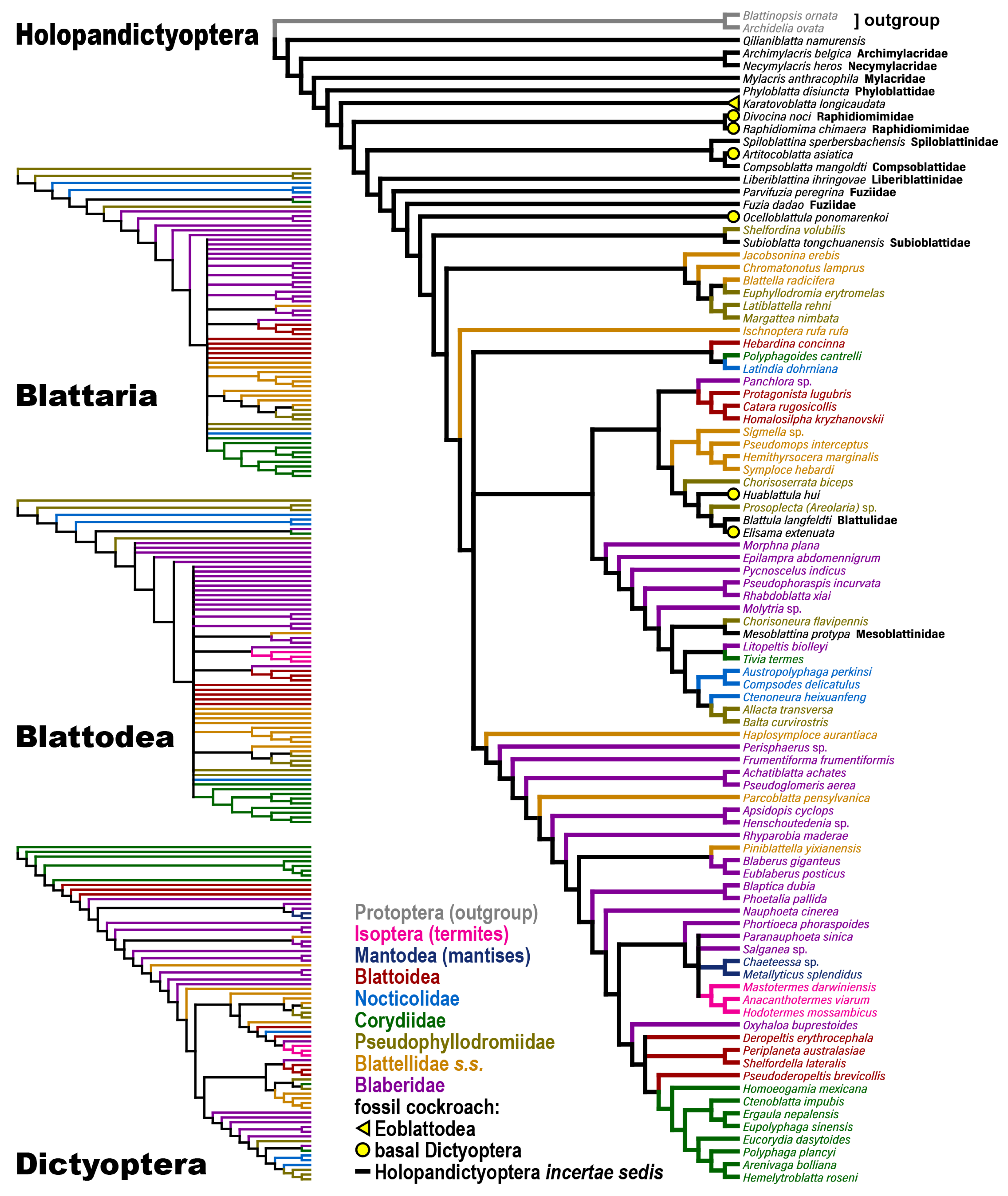
2.1. Taxonomy and Sampling
2.2. Phylogenetic Reconstruction
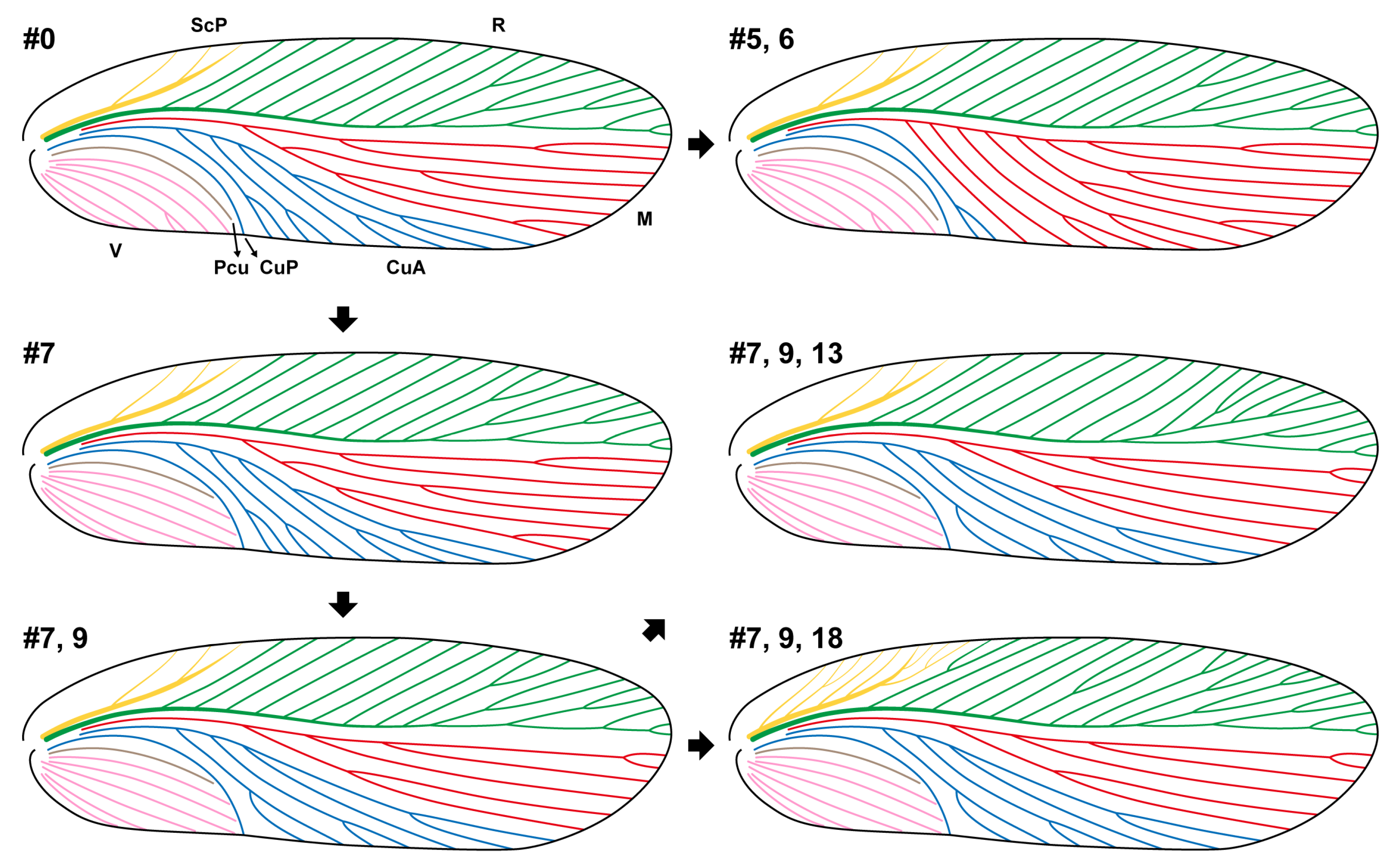
3. Results
4. Discussion
4.1. How Much Can We Learn from Forewings?
4.2. Why Is Forewing Not a Good Character System?
4.3. Implications for Paleontological Studies
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
- Narrowing of R area
- Cross-veins well developed
- Diagonal channel
- Fused stem of M+CuA
- M more developed than CuA
- M becomes pectinate
- Claval veins become oblique or diagonal
- CuA absent
- Mediocubital veins become parallel
- Mediocubital veins essentially longitudinal
- Anterior proximal branches of M reduced, leaving some incomplete branches and sometimes a fusiform space between R and M
- Dichotomy more developed
- Apical branches of R divide into two parts, between which is an obvious long area
- Short characteristic branch of R
- Claval veins become longitudinal
- Characteristic branch of R elongates
- Apical branches of R become pectinate
- ScP and R more developed and somewhat irregular
- Pectinate CuA
- Multi-branched ScP
- Distal end of R pseudostem toward costal margin of the wing
- Straight R pseudostem ends at costal margin, with some posterior branches
- Clavus elongates
- ScP and R even more developed and more irregular
- CuA more developed than M
- R even more irregular; proximal pectinate branches become branched and even branch again
- Clavus shortens
- Preclavus expands backwards
- Apical branches of R curve backwards
- Wing apex with backward branches that comprise nearly pectinate posterior branches of R and suddenly bent anterior branches of M
References
- McKittrick, F.A. Evolutionary studies of cockroaches. Cornell Univ. Agric. Exp. Stn. Mem. 1964, 389, 1–197. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014. [Google Scholar]
- Klass, K.-D.; Meier, R. A phylogenetic analysis of Dictyoptera (Insecta) based on morphological characters. Entomol. Abh. 2006, 63, 3–50. [Google Scholar]
- Anisyutkin, L.N. A review of the genus Euphyllodromia Shelford, 1908 (Dictyoptera: Ectobiidae), with description of three new species. Proc. Zool. Inst. RAS 2011, 315, 369–398. [Google Scholar] [CrossRef]
- Bohn, H.; Beccaloni, G.; Dorow, W.H.O.; Pfeifer, M.A. Another species of European Ectobiinae travelling north – the new genus Planuncus and its relatives (Insecta: Blattodea: Ectobiinae). Arthropod Syst. Phylogeny 2013, 71, 139–168. [Google Scholar] [CrossRef]
- Hopkins, H. A revision of the genus Arenivaga (Rehn) (Blattodea, Corydiidae), with descriptions of new species and key to the males of the genus. ZooKeys 2014, 384, 1–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, Y.; Wang, C.; Che, Y.; Wang, Z. The species of Symplocodes Hebard (Blattodea: Ectobiidae: Blattellinae) with description of a new species from China. J. Nat. Hist. 2015, 50, 339–361. [Google Scholar] [CrossRef]
- Lucañas, C.C.; Lit, I.L., Jr. Cockroaches (Insecta, Blattodea) from caves of Polillo Island (Philippines), with description of a new species. Subterr. Biol. 2016, 19, 51–64. [Google Scholar] [CrossRef]
- Li, X.-R.; Wang, Z.-Q. Updating the knowledge of assassin bug cockroaches (Blattodea: Blaberidae: Paranauphoeta Brunner von Wattenwyl): Species from China and taxonomic changes. Entomol. Sci. 2017, 20, 302–317. [Google Scholar] [CrossRef]
- Qiu, L.; Che, Y.-L.; Wang, Z.-Q. A taxonomic study of Eupolyphaga Chopard, 1929 (Blattodea: Corydiidae: Corydiinae). Zootaxa 2018, 4506, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, D.A.; Kotyková Varadínová, Z.; Jůna, F.; Grandcolas, P.; Legendre, F. New cockroaches (Dictyoptera: Blattodea) from French Guiana and a revised checklist for the region. Neotrop. Entomol. 2019, 48, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Álvarez, J.C.; Sormani, C.G.; Cano, E.B. Contribution to the knowledge of the Neotropical cockroaches of the family Ectobiidae Brunner von Wattenwyl, 1865 (Blattodea: Ectobiidae). Bol. Soc. Entomol. Aragonesa 2020, 66, 129–143. [Google Scholar]
- Luo, X.-X.; Li, Q.-Q.; Zamani, A.; Che, Y.-L.; Wang, Z.-Q. Redescription of Periplaneta arabica (Bey-Bienko, 1938) (Blattodea, Blattidae), with a comparative analysis of three species of Periplaneta Burmeister, 1838 (sensu stricto). ZooKeys 2023, 1146, 165–183. [Google Scholar] [CrossRef]
- Polizeli, L.; Pinto, Â.P. A taxonomic revision of the south American trilobite cockroaches of Parahormetica Brunner von Wattenwyl 1865 (Blattodea: Blaberidae), with description of Parahormetica museunacional sp. nov. from the atlantic forest. Neotrop. Entomol. 2024, 53, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neto, R.G.; Mancuso, A.; Gallego, O.F. The Triassic insect fauna from Argentina. Blattoptera from the Los Rastros Formation (Bermejo Basin), La Rioja Province. Ameghiniana 2005, 42, 705–723. [Google Scholar]
- Martin, S.K. Early Jurassic cockroaches (Blattodea) from the Mintaja insect locality, Western Australia. Alavesia 2010, 3, 55–72. [Google Scholar]
- Barna, P. Low diversity cockroach assemblage from Chernovskie Kopi in Russia confirms wing deformities in insects at the Jurassic/Cretaceous boundary. Biologia 2014, 69, 651–675. [Google Scholar] [CrossRef]
- Vršanský, P. Cockroaches from Jurassic Sediments of the Bakhar Formation in Mongolia; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Li, X.-R.; Zheng, Y.-H.; Wang, C.-C.; Wang, Z.-Q. Old method not old-fashioned: Parallelism between wing venation and wing-pad tracheation of cockroaches and a revision of terminology. Zoomorphology 2018, 137, 519–533. [Google Scholar] [CrossRef]
- Schneider, J. Zur variabilität der flügel paläozoischer Blattodea (Insecta), teil II. Freiberg. Forschungsh. 1978, 334, 21–39. [Google Scholar]
- Ross, A.J. Testing decreasing variabililty of cockroach forewings through time using four Recent species: Blattella germanica, Polyphaga aegyptiaca, Shelfordella lateralis and Blaberus craniifer, with implications for the study of fossil cockroach forewings. Insect Sci. 2012, 19, 129–142. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Wang, Z.; Che, Y. Revision of the genus Salganea Stål (Blattodea, Blaberidae, Panesthiinae) from China, with descriptions of three new species. ZooKeys 2014, 412, 59–87. [Google Scholar]
- Rehn, J.W.H. Classification of the Blattaria as indicated by their wings (Orthoptera). Mem. Am. Entomol. Soc. 1951, 14, 1–134. [Google Scholar]
- Klass, K.-D. The external male genitalia and the phylogeny of Blattaria and Mantodea. Bonn. Zool. Monogr. 1997, 42, 1–341. [Google Scholar]
- Bourguignon, T.; Tang, Q.; Ho, S.Y.W.; Juna, F.; Wang, Z.; Arab, D.A.; Cameron, S.L.; Walker, J.; Rentz, D.; Evans, T.A.; et al. Transoceanic dispersal and plate tectonics shaped global cockroach distributions: Evidence from mitochondrial phylogenomics. Mol. Biol. Evol. 2018, 35, 970–983. [Google Scholar] [PubMed]
- Evangelista, D.A.; Wipfler, B.; Béthoux, O.; Donath, A.; Fujita, M. An integrative phylogenomic approach illuminates the evolutionary history of cockroaches and termites (Blattodea). Proc. R. Soc. B Biol. Sci. 2019, 286, 20182076. [Google Scholar] [CrossRef]
- Durden, C.J. Pennsylvanian correlation using blattoid insects. Can. J. Earth Sci. 1969, 6, 1159–1177. [Google Scholar] [CrossRef]
- Schneider, J.W.; Lucas, S.G.; Barrick, J.E. The Early Permian age of the Dunkard Group, Appalachian basin, U.S.A., based on spiloblattinid insect biostratigraphy. Int. J. Coal Geol. 2013, 119, 88–92. [Google Scholar] [CrossRef]
- Schneider, J.W.; Lucas, S.G.; Scholze, F.; Voigt, S.; Marchetti, L.; Klein, H.; Opluštil, S.; Werneburg, R.; Golubev, V.K.; Barrick, J.E.; et al. Late Paleozoic–early Mesozoic continental biostratigraphy—Links to the standard global chronostratigraphic scale. Palaeoworld 2020, 29, 186–238. [Google Scholar]
- Schneider, J.W.; Scholze, F.; Ross, A.J.; Blake, B.M.; Lucas, S.G. Improved blattoid insect and conchostracan zonation for the Late Carboniferous, Pennsylvanian, of Euramerica. Geol. Soc. Spec. Publ. 2021, 512, 865–891. [Google Scholar]
- Li, X.R. Disambiguating the scientific names of cockroaches. Palaeoentomology 2019, 2, 390–402. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhang, J.-W.; Han, W.; Wang, Y.-S.; He, S.-L.; Wang, Z.-Q. Advances in the understanding of Blattodea evolution: Insights from phylotranscriptomics and spermathecae. Mol. Phylogenet. Evol. 2023, 182, 107753. [Google Scholar] [CrossRef]
- Deng, W.; Luo, X.; Ho, S.Y.W.; Liao, S.; Wang, Z.; Che, Y. Inclusion of rare taxa from Blattidae and Anaplectidae improves phylogenetic resolution in the cockroach superfamily Blattoidea. Syst. Entomol. 2023, 48, 23–39. [Google Scholar] [CrossRef]
- Han, W.; Qiu, L.; Zhang, J.; Wang, Z.; Che, Y. Phylogenetic reconstruction of Corydioidea (Dictyoptera: Blattodea) provides new insights on the placement of Latindiinae and supports the proposal of the new subfamily Ctenoneurinae. Syst. Entomol. 2023, 49, 156–172. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Zhang, J.-W.; Lo, N.; Bourguignon, T.; Guo, L.; Li, B.-L.; Che, Y.-L.; Wang, Z.-Q. Phylogenetic analysis of Blaberoidea reveals non-monophyly of taxa and supports the creation of multiple new subfamilies. Cladistics 2023, 39, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Krell, F.-T.; Cranston, P.S. Which side of the tree is more basal? Syst. Entomol. 2004, 29, 279–281. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, US, 2005. [Google Scholar]
- Prokop, J.; Krzeminski, W.; Krzeminska, E.; Hörnschemeyer, T.; Ilger, J.-M.; Brauckmann, C.; Grandcolas, P.; Nel, A. Late Palaeozoic Paoliida is the sister group of Dictyoptera (Insecta: Neoptera). J. Syst. Palaeontol. 2013, 12, 601–622. [Google Scholar] [CrossRef]
- Liang, J.-H.; Vršanský, P.; Ren, D. Variability and symmetry of a Jurassic nocturnal predatory cockroach (Blattida: Raphidiomimidae). Rev. Mex. Cienc. Geol. 2012, 29, 411–421. [Google Scholar]
- Wei, D.D.; Liang, J.H.; Ren, D. A new species of Fuziidae (Insecta, Blattida) from the Inner Mongolia, China. ZooKeys 2012, 217, 53–61. [Google Scholar]
- Vishnyakova, V.N. Мезoзoйские тараканы с наружным яйцекладoм и oсoбеннoсти их размнoжения (Blattodea) [= Mezozoyskiye tarakany s naruzhnym yaytsekladom i osobennosti ikh razmnozheniya (Blattodea)]. In Юрские Насекoмые Каратау [= Yurskiye Nasekomyye Karatau]; Rohdendorf, B.B., Ed.; Издательствo Наука [= Izdatel’stvo Nauka]: Moscow, USSR, 1968; pp. 55–86. [Google Scholar]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System For Evolutionary Analysis. Version 3.61. 2019. Available online: http://www.mesquiteproject.org (accessed on 13 June 2020).
- Roth, L.M. Systematics and phylogeny of cockroaches (Dictyoptera: Blattaria). Orient. Insects 2003, 37, 1–186. [Google Scholar]
- Li, X.-R.; Huang, D. A new mid-Cretaceous cockroach of stem Nocticolidae and reestimating the age of Corydioidea (Dictyoptera: Blattodea). Cretaceous Res. 2019, 106, 104202. [Google Scholar]
- Roth, L.M. The cockroach genera Sundablatta Hebard, Pseudophyllodromia Brunner, and Allacta Saussure & Zehntner (Blattaria: Blattellidae, Pseudophyllodromiinae). Tijdschr. Entomol. 1996, 139, 215–242. [Google Scholar]
- Vidlička, Ľ. New cockroach species of the genus Panchlora Burmeister (Blaberidae, Panchlorinae) from Ecuador. Zootaxa 2016, 4121, 181–186. [Google Scholar] [CrossRef]
- Senraj, M.; Packiam, S.M.; Prabakaran, S.; Lucanas, C.C.; Jaiswal, D. Review of Indian Allacta Saussure & Zehntner, 1895 (Blattodea: Ectobiidae: Pseudophyllodromiinae), with description of three new species. Zootaxa 2021, 4920, 254–266. [Google Scholar]
- Evangelista, D.A.; Djernæs, M.; Kohli, M.K. Fossil calibrations for the cockroach phylogeny (Insecta, Dictyoptera, Blattodea), comments on the use of wings for their identification, and a redescription of the oldest Blaberidae. Palaeontol. Electron. 2017, 20, 1FC. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Che, Y.-L.; Wang, Z.-Q. Contribution to the cockroach genus Ctenoneura Hanitsch, 1925 (Blattodea: Corydioidea: Corydiidae) with descriptions of seven new species from China. Zootaxa 2017, 4237, 265–299. [Google Scholar] [CrossRef] [PubMed]
- Anisyutkin, L.N. Notes on the genus Ctenoneura Hanitsch, 1925 with description of six new species (Dictyoptera: Corydiidae). Rev. Suisse Zool. 2021, 128, 455–468. [Google Scholar] [CrossRef]
- Li, X.R. Phylogeny and age of cockroaches: A reanalysis of mitogenomes with selective fossil calibrations. Dtsch. Entomol. Z. 2022, 69, 1–18. [Google Scholar] [CrossRef]
- Anisyutkin, L.N.; Gorochov, A.V. A new genus and species of the cockroach family Blattulidae from Lebanese amber (Dictyoptera, Blattina). Paleontol. J. 2008, 42, 43–46. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Z.-Q.; Che, Y.-L. First record of Blattulidae from mid-Cretaceous Burmese amber (Insecta: Dictyoptera). Cretaceous Res. 2019, 99, 281–290. [Google Scholar] [CrossRef]
- Vršanský, P. Cretaceous Gondwanian cockroaches (Insecta: Blattaria). Entomol. Probl. 2004, 34, 49–54. [Google Scholar]
- Vršanský, P. Late Jurassic cockroaches (Insecta, Blattaria) from the Houtiyn-Hotgor locality in Mongolia. Paleontol. J. 2008, 42, 36–42. [Google Scholar] [CrossRef]
- Vršanský, P. Transitional Jurassic/Cretaceous cockroach assemblage (Insecta, Blattaria) from the Shar-Teg in Mongolia. Geol. Carpath. 2004, 55, 457–468. [Google Scholar]
- Vršanský, P. Lower Cretaceous cockroaches and mantids (Insecta: Blattaria, Mantodea) from the Sharin-Gol in Mongolia. Entomol. Probl. 2005, 35, 163–167. [Google Scholar]
- Vršanský, P. New blattarians and a review of dictyopteran assemblages from the Lower Cretaceous of Mongolia. Acta Palaeontol. Pol. 2008, 53, 129–136. [Google Scholar] [CrossRef]
- Roth, L.M. Revision of the cockroach genus Homopteroidea Shelford (Blattaria, Polyphagidae). Tijdschr. Entomol. 1995, 138, 103–116. [Google Scholar]
- Wang, X.; Wang, Z.; Che, Y. A taxonomic study of the genus Panesthia (Blattodea, Blaberidae, Panesthiinae) from China with descriptions of one new species, one new subspecies and the male of Panesthia antennata. ZooKeys 2014, 466, 53–75. [Google Scholar]
- Schneider, J. Zur Taxonomie und Biostratigraphie der Blattodea (lnsecta) des Karbon und Perm der DDR. Freiberg. Forschungsh. 1978, 340, 7–152. [Google Scholar]
- Schneider, J. Die Blattodea (Insecta) des Paläozoikums. Teil 1: Systematik, Ökologie und Biostratigraphie. Freiberg. Forschungsh. 1983, 382, 106–145. [Google Scholar]
- Handlirsch, A. Die Fossilen Insekten und die Phylogenie der Rezenten Formen; Wilhelm Engelmann: Leipzig, Germany, 1906. [Google Scholar]
- Vršanský, P. Decreasing variability—from the Carboniferous to the present! (Validated on independent lineages of Blattaria). Paleontol. J. 2000, 34 (Suppl. S3), S374–S379. [Google Scholar]
- Vršanský, P.; Ansorge, J. Lower Jurassic cockroaches (Insecta: Blattaria) from Germany and England. Afr. Invertebr. 2007, 48, 103–126. [Google Scholar]
- Roth, L.M. The cockroach genera Anaplecta, Anaplectella, Anaplectoidea, and Malaccina Blattaria, Blattellidae: Anaplectinae and Blattellinae). Orient. Insects 1996, 30, 301–372. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Gui, S.-H.; Che, Y.-L.; Wang, J.-J. The species of Allacta (Blattodea: Ectobiidae: Pseudophyllodromiinae) occurring in China, with a description of a new species. Fla. Entomol. 2014, 97, 439–453. [Google Scholar] [CrossRef]
- Qiu, Z.-W.; Che, Y.-L.; Zheng, Y.-H.; Wang, Z.-Q. The cockroaches of Balta Tepper from China, with the description of four new species (Blattodea, Ectobiidae, Pseudophyllodromiinae). ZooKeys 2017, 714, 13–32. [Google Scholar] [CrossRef]
- Laurentiaux, D. Le problème des blattes paléozoïques a ovipositeur externe. Ann. Paléontol. 1951, 37, 3–12. [Google Scholar]
- Laurentiaux, D. La reproduction chez les Insectes blattaires du Carbonifère: Facteurs du panchronisme et classification naturelle de l’ordre. Bull. Soc. Geol. Fr. 1959, S7-I, 759–766. [Google Scholar] [CrossRef]
- Vishnyakova, V.N. Стрoение придаткoв брюшка Мезoзoйских тараканoв (Insecta: Blattodea) [= Stroyeniye pridatkov bryushka Mezozoyskikh tarakanov (Insecta: Blattodea)]. Curr. Probl. Palaeontol. 1971, 130, 174–186. [Google Scholar]
- Vishnyakova, V.N. Нoвые тараканы (Insecta: Blattodea) из верхнеюрских oтлoжений хребта Каратау [= Novyye tarakany (Insecta: Blattodea) iz verkhneyurskikh otlozheniy khrebta Karatau]. Чтения Памяти Н. А. Хoлoдкoвскoгo [= Chteniya Pamyati N. A. Kholodkovskogo] 1973, 1971, 64–77. [Google Scholar]
- Vršanský, P.; Liang, J.-H.; Ren, D. Advanced morphology and behaviour of extinct earwig-like cockroaches (Blattida: Fuziidae fam. nov.). Geol. Carpath. 2009, 60, 449–462. [Google Scholar] [CrossRef]
- Shelford, R. On a collection of Blattidae preserved in amber, from Prussia. J. Linn. Soc., Zool. 1910, 30, 336–355. [Google Scholar] [CrossRef]
- Gorokhov, A.V. New and little known orthopteroid insects (Polyneoptera) from fossil resins: Communication 2. Paleontol. J. 2007, 41, 156–166. [Google Scholar] [CrossRef]
- Anisyutkin, L.N. Paraeuthyrrhapha groehni gen. et sp. nov., a new genus of the family Polyphagidae (Dictyoptera) from Baltic amber and its phylogenetical position. Alavesia 2008, 2, 77–85. [Google Scholar]
- Anisyutkin, L.N.; Gröhn, C. New cockroaches (Dictyoptera: Blattina) from Baltic amber, with the description of a new genus and species: Stegoblatta irmgardgroehni. Proc. Zool. Inst. RAS 2012, 316, 193–202. [Google Scholar] [CrossRef]
- Gao, T.; Shih, C.; Labandeira, C.C.; Liu, X.; Wang, Z.; Che, Y.; Yin, X.; Ren, D. Maternal care by Early Cretaceous cockroaches. J. Syst. Palaeontol. 2018, 17, 379–391. [Google Scholar] [CrossRef]
- Li, X.-R.; Huang, D. A new Cretaceous cockroach with heterogeneous tarsi preserved in Burmese amber (Dictyoptera, Blattodea, Corydiidae). Cretaceous Res. 2018, 92, 12–17. [Google Scholar] [CrossRef]
- Li, X.-R.; Huang, D. Predators or herbivores: Cockroaches of Manipulatoridae revisited with a new genus from Cretaceous Myanmar amber (Dictyoptera: Blattaria: Corydioidea). Insects 2022, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Huang, D. Atypical ‘long-tailed’ cockroaches arose during Cretaceous in response to angiosperm terrestrial revolution. PeerJ 2023, 11, e15067. [Google Scholar] [CrossRef] [PubMed]
- Geinitz, F.E. Ueber die fauna des Dobbertiner Lias. Z. Dtsch. Geol. Ges. 1884, 36, 566–583. [Google Scholar]
- Vishniakova, V.N. Jurassic cockroaches of the new family Blattulidae from Siberia. Paleontol. J. 1982, 1982, 67–77, [Translation from Russian original, pp. 69–79]. [Google Scholar]
- Geinitz, F.E. Die Flözformationen Mecklenburgs. Arch. Ver. Freunde Naturg. Mecklenb. 1883, 37, 6–151. [Google Scholar]
- Andersen, T.; Kjærandsen, J. Three new species of Nocticola Bolívar from Ghana, West Africa (Blattaria: Nocticolidae). J. Afr. Zool. 1995, 109, 377–385. [Google Scholar]
- Anisyutkin, L.N. A description of a new species of the cockroach genus Prosoplecta Saussure, 1864 (Dictyoptera, Ectobiidae) from South Vietnam. Entomol. Obozr. 2012, 91, 742–756. [Google Scholar] [CrossRef]
- Anisyutkin, L.N. New and little known Epilamprinae (Dictyoptera: Blaberidae) from the collections of the Muséum d’histoire naturelle de Genève and the Zoological Institute of Saint Petersburg. Part 1. Rev. Suisse Zool. 2015, 122, 283–296. [Google Scholar] [CrossRef]
- Brannoch, S.K.; Wieland, F.; Rivera, J.; Klass, K.-D.; Béthoux, O.; Svenson, G.J. Manual of praying mantis morphology, nomenclature, and practices (Insecta, Mantodea). ZooKeys 2017, 696, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Chopard, L. Un cas de microphtalmie liée à l’atrophie des ailes chez une blatte cavernicole. In Livre du Centenaire; Société Entomologique de France, Ed.; Société Entomologique de France: Paris, France, 1932; pp. 485–496. [Google Scholar]
- Cui, Y.; Evangelista, D.A.; Béthoux, O. Prayers for fossil mantis unfulfilled: Prochaeradodis enigmaticus Piton, 1940 is a cockroach (Blattodea). Geodiversitas 2018, 40, 355–362. [Google Scholar] [CrossRef]
- Gravely, F.H. Alluaudella himalayensis, a new species of degenerate (♂) cockroach. With an account of the venation found in the genera Cardax and Alluaudella. Rec. Indian Mus. 1910, 5, 307–311. [Google Scholar] [CrossRef]
- He, J.J.; Zheng, Y.H.; Qiu, L.; Che, Y.L.; Wang, Z.Q. Two new species and a new combination of Allacta (Blattodea, Ectobiidae, Pseudophyllodromiinae) from China, with notes on their behavior in nature. ZooKeys 2019, 836, 1–14. [Google Scholar] [CrossRef]
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the world. Volume 1. Introduction. Bull. Am. Mus. Nat. Hist. 2013, 377, 1–200. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Wang, Z.Q. A taxonomic study of the beetle cockroaches (Diploptera Saussure) from China, with notes on the genus and species worldwide (Blattodea: Blaberidae: Diplopterinae). Zootaxa 2015, 4018, 35–56. [Google Scholar] [CrossRef]
- Li, X.R.; Wang, L.L.; Wang, Z.Q. Rediscovered and new perisphaerine cockroaches from SW China with a review of subfamilial diagnosis (Blattodea: Blaberidae). Zootaxa 2018, 4410, 251–290. [Google Scholar] [CrossRef]
- Mackerras, M.J. Polyphagidae (Blattodea) from eastern Australia. Aust. J. Entomol. 1968, 7, 147–154. [Google Scholar] [CrossRef]
- Qiu, L.; Che, Y.L.; Wang, Z.Q. Revision of Eucorydia Hebard, 1929 from China, with notes on the genus and species worldwide (Blattodea, Corydioidea, Corydiidae). ZooKeys 2017, 709, 17–56. [Google Scholar] [CrossRef]
- Qiu, L.; Che, Y.L.; Wang, Z.Q. Contributions to some Corydiinae genera (Blattodea: Corydioidea: Corydiidae) from China. J. Nat. Hist. 2018, 52, 1433–1461. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Z.Q.; Che, Y.L. New and little known Latindiinae (Blattodea, Corydiidae) from China, with discussion of the Asian genera and species. ZooKeys 2019, 867, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, Z.Q.; Che, Y.L. Minpolyphaga inexpectata, a new genus and species of Polyphagini (Blattodea: Corydiidae: Corydiinae) from southeast China. Acta Entomol. Mus. Natl. Pragae 2019, 59, 513–518. [Google Scholar] [CrossRef]
- Vidlička, Ľ. New genus and species of cockroaches from the tribe Brachycolini (Blattaria: Blaberidae: Blaberinae) and redescription of the Hormetica strumosa. Zootaxa 2019, 4651, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Wu, K.; Qiu, D.; Hu, J.; Liu, D.; Wei, X.; Chen, J.; Cook, C.E. A formal re-description of the cockroach Hebardina concinna anchored on DNA barcodes confirms wing polymorphism and identifies morphological characters for field identification. PLoS ONE 2014, 9, e106789. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Li, X.R.; Wang, Z.Q. A taxonomic report on the cockroach genus Haplosymploce Hanitsch from China including one new species (Blattodea: Ectobiidae: Blattellinae). Zootaxa 2016, 4066, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Handlirsch, A. Les insectes Houillers de la Belgique. Mém. Mus. Roy. Hist. Nat. Belg. 1904, 3, 3–20. [Google Scholar]
- Lin, Q.B. On the fossil Blattoidea of China. Acta Entomol. Sin. 1978, 21, 335–342. [Google Scholar]
- Schlechtendal, D. Untersuchung über die karbonischen Insekten und Spinnen von Wettin unter Berücksichtigung verwandter Faunen. Erster teil: Revision der Originale von Germar, Giebel und Goldenberg. Abh. Kaiserl. Leop.-Carol. Dtsch. Akad. Naturf. 1912, 98, 1–186. [Google Scholar]
- Schneider, J. Zur Entomofauna des Jungpaläozoikums der Boskovicer Furche (ČSSR), Teil II: Phyloblattidae (Insecta, Blattodea). Freiberg. Forschungsh. 1984, 395, 19–37. [Google Scholar]
- Schneider, J.; Werneburg, R. Neue Spiloblattinidae (Insecta, Blattodea)aus dem Oberkarbon und Unterperm von Mitteleuropa sowie die Biostratigraphie des Rotliegend. Veröff. Naturhist. Mus. Schleusingen 1993, 7, 31–52. [Google Scholar]
- Scudder, S.H. Palaeozoic cockroaches: A complete revision of the species of both worlds, with an essay toward their classification. Mem. Boston Soc. Nat. Hist. 1879, 3, 23–134. [Google Scholar]
- Vršanský, P. Origin and the early evolution of mantises. AMBA Proj. 2002, 6, 1–16. [Google Scholar]
- Wang, T.; Ren, D.; Liang, J.H.; Shih, C. New Mesozoic cockroaches (Blattaria: Blattulidae) from Jehol biota of western Liaoning in China. Ann. Zool. 2007, 57, 483–495. [Google Scholar]
- Zhang, Z.; Schneider, J.W.; Hong, Y. The most ancient roach (Blattodea): A new genus and species from the earliest Late Carboniferous (Namurian) of China, with a discussion of the phylomorphogeny of early blattids. J. Syst. Palaeontol. 2012, 11, 27–40. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-R. Classifying Cockroaches According to Forewings: Pitfalls and Implications for Fossil Systematics. Taxonomy 2024, 4, 618-632. https://doi.org/10.3390/taxonomy4030031
Li X-R. Classifying Cockroaches According to Forewings: Pitfalls and Implications for Fossil Systematics. Taxonomy. 2024; 4(3):618-632. https://doi.org/10.3390/taxonomy4030031
Chicago/Turabian StyleLi, Xin-Ran. 2024. "Classifying Cockroaches According to Forewings: Pitfalls and Implications for Fossil Systematics" Taxonomy 4, no. 3: 618-632. https://doi.org/10.3390/taxonomy4030031
APA StyleLi, X.-R. (2024). Classifying Cockroaches According to Forewings: Pitfalls and Implications for Fossil Systematics. Taxonomy, 4(3), 618-632. https://doi.org/10.3390/taxonomy4030031





