Abstract
Virtual autopsies (VAs) are non-invasive, bypassing many of the challenges posed by traditional autopsies (TAs). This is a literature review about the sensitivity of the main VA techniques: post mortem (PM) computed tomography (PMCT) and PM magnetic resonance (PMMR). This could help to identify the most appropriate uses for VA, and where future research should focus. A review was performed, searching for literature from the last 10 years regarding how sensitive VA is at detecting common lesions that could cause or contribute to death. 33 studies were included. There was strong agreement that PMCT had strengths in detecting: free gas; fractures; large fluid accumulations; and calcifications. PMCT’s weaknesses included missing: pulmonary emboli; myocardial infarctions; and visceral/soft tissue lesions. The strengths of PMMR were less widely agreed, but included detecting: large fluid collections; myocardial infarctions; and visceral/soft tissue lesions. There were no wide agreements on PMMR’s weaknesses due to a lack of literature. Therefore, VA is a useful adjunct to TA; however, its drawbacks in reliably detecting common causes of death restrict its ability to fully replace TA. Novel imaging techniques are being developed in order to bridge the current gaps of VA, and make autopsies even less invasive.
1. Introduction
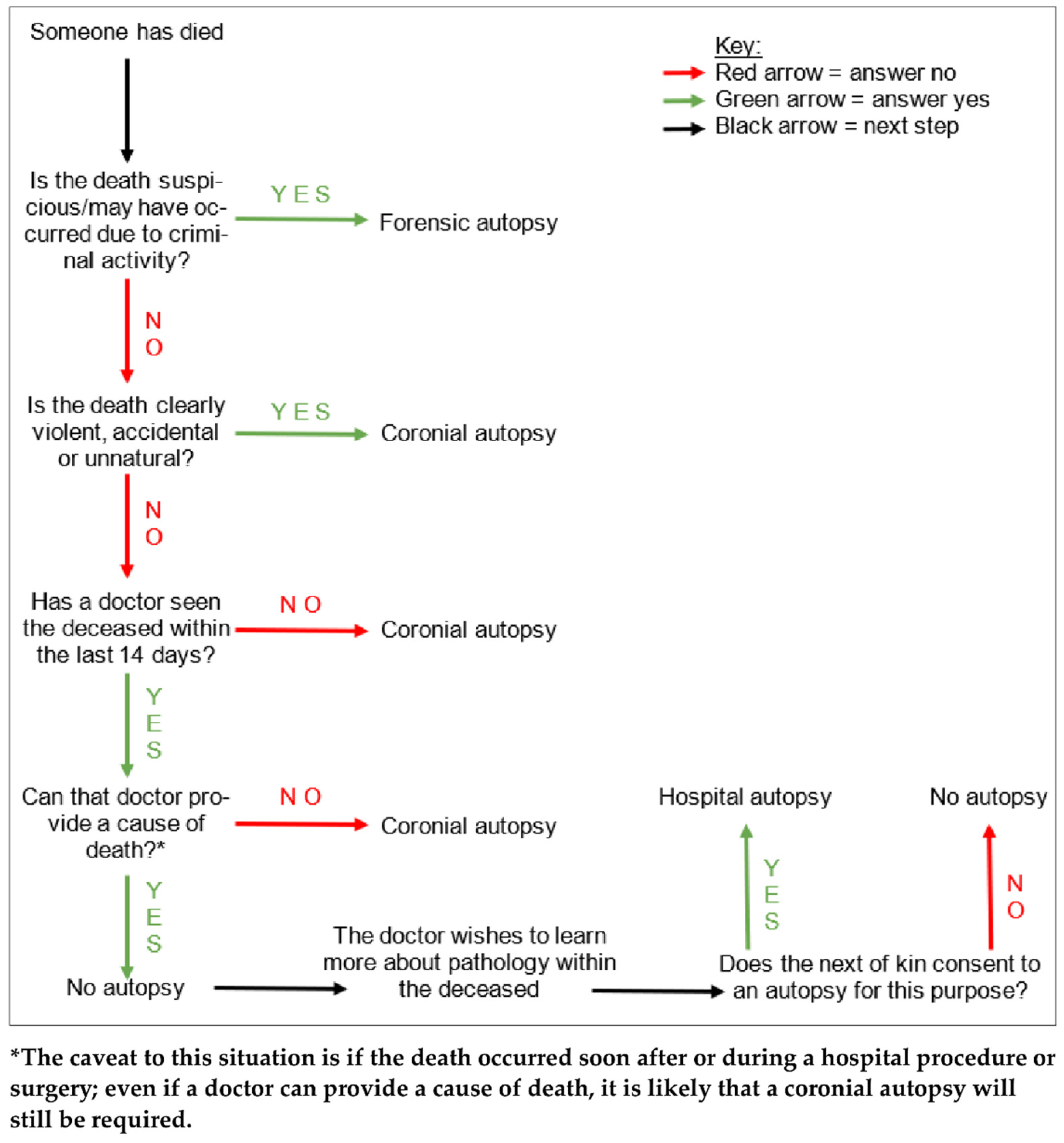
Autopsies are the examinations of deceased patients which are performed in order to determine the cause of death. As outlined in Figure 1, autopsies in England, Wales, and Northern Ireland are carried out for three main reasons. Firstly, if the cause of death has been clinically certified, but the attending doctor to the deceased has obtained consent from the next of kin, an autopsy may be performed in order to learn more about the pathology (hospital autopsy). Secondly, if there is no clinician involved in the care of the deceased who can certify the cause of death, but the death is not suspicious, the Coroner may order an examination (coronial autopsy). Thirdly, if the death was suspicious or potentially due to criminal activity, it may involve both a police investigation as well as coronial interest, and a more thorough autopsy would be performed in order to find the cause of death, and to gain evidence to present in a court of law (forensic autopsy) [1].
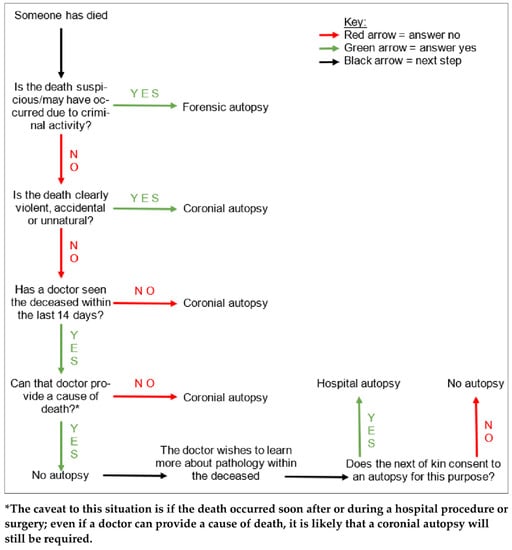
Figure 1.
A tree diagram demonstrating the simplified general decision pathway undertaken when ascertaining whether an autopsy is required, and if so, what type. There are various caveats to these conditions but this diagram provides a general overview.
Traditional autopsies (TAs) are invasive procedures, typically involving an external examination of the body, and an examination of the internal organs which are obtained by evisceration of the thorax, abdomen, and skull. This is coupled with a report surrounding the circumstances of the death, provided by the Coroner’s office, as well as the medical history of the deceased. If the cause of death cannot be found through macroscopic examination of the viscera, samples may have to be retained for further histological, toxicological, biochemical, and/or microbiological analysis.
It is worth noting that coronial autopsies do not require informed consent from the deceased’s next of kin in order to take place. However, the family and friends of the deceased may be able to negotiate a limited TA on the basis of certain lesions within the body. For example, if a clear cause of death has already been found upon thoracoabdominal examination, the head and skull may not need to be opened in order to examine the brain. In general though, if the next of kin object to an autopsy taking place on whatever grounds, this will not affect the Coroner’s decision for one to be performed.
Due to a variety of reasons surrounding the challenges and disadvantages of TAs, the use of radiological imaging for non-invasive examinations, or virtual autopsies (VAs), is gaining traction. Most commonly, post mortem (PM) computed tomography (PMCT) and PM magnetic resonance (PMMR) are being used in various applications to garner more information surrounding the cause of death. VAs also increase the use of non-invasive and minimally invasive examination techniques available to pathologists, thereby reducing the necessity to fully open the body in order to perform an autopsy [2]. As radiological technology advances, the idea of using VA techniques as a substitute, rather than just an adjunct, for TA is gaining popularity.
This paper aims to review the literature and ascertain the current position of how PM imaging is being used in the context of coronial autopsies, and where both the limitations and potential advances to these novel examination practices may lie at this time. This paper will not be addressing the use of PM imaging in specialist paediatric or forensic autopsies.
The Challenges of Traditional Autopsies
There are a variety of different reasons behind the drive to find less invasive autopsy examination techniques. Historically, an important rationale for this has been the reception of TA by the public. In particular, among the Jewish and Muslim communities, the disfigurement and dissection of the deceased is seen as desecration, and there is a requirement to bury the body as soon as possible without any unnecessary delay [2,3]. That said, studies have shown that the wider general public also prefers the concept of non-invasive VA in comparison to invasive TA [4,5]. There may still be lingering public objection to TAs following the Alder Hey and Bristol organ scandals in the 1990s, where inappropriate and unconsented tissue retention took place. There is also evidence that the public may have cosmetic concerns regarding the large incisions made during TA [6]. Finally, it seems that VA images are perceived to be more acceptable than photographs taken at TA by non-medicolegal professionals in the Coroner’s court and the autopsy report, if images are to be included [7,8,9,10].
Further, the outbreak of the ongoing COVID-19 pandemic in early 2020 demonstrated a need for safer autopsy alternatives to TA, where VA often provided efficient and dignified examinations for potentially infected cases. This achieved faster turnaround times for the release of the deceased into the care of the nominated undertaker, and also minimised the contact between mortuary staff and the deceased; these issues were of grave importance at a time when both the pathogenicity and transmissibility of the SARS-CoV-2 virus were poorly understood. Scanning the deceased for VA may also have helped save precious time and resources as health services were pushed to their capacity and staffing limits during the pandemic [11].
2. General Overview of Imaging in Autopsy
One of the main methods for PM imaging is the use of X-ray images, a technique that has been used for a relatively long time now [10]. PM X-ray images allow clear visualisation of fractures and radio-opaque foreign bodies within the deceased, and in this way are useful in guiding TAs in certain circumstances, for example in traumatic deaths. This imaging can also limit the TA, perhaps by providing information that would be difficult to access during the invasive examination, such as fractures in areas like the base of the skull which would require extensive, time-consuming, and delicate dissection. Another benefit for taking X-ray images is the portability and speed. However, a pitfall for this imaging method is its two-dimensional quality; to gain any appreciation of structures in a three-dimensional state, multiple X-ray images must be obtained, often through laborious manoeuvring and balancing of the body into many different positions for each image [10]. This is a tedious process and in terms of coronial investigations, its usage is limited to specific circumstances and deaths.
2.1. PMCT
As such, the evolution of more modern, three-dimensional clinical imaging techniques has taken place, and these imaging methods have emerged into PM investigations, instead of being used only with living patients. The current availability of imaging methods for VA are largely PMCT techniques. Full body PMCT scanning is growing in usage for coronial autopsies, for example where there are large Muslim or Jewish communities [12]. PMCT is also available privately in Wales and the South of England for families who wish to avoid a TA for their loved ones [12]. The cost of a private PMCT for the next of kin in these areas is quoted as £500–£1500 [13,14,15].
2.2. PMMR
Whilst PMCT techniques are currently at the forefront of VA in England and Wales, PMMR methods are also available. The difference in the use of current VA methods may explain the disparity in the amount of studies reported on PMCT and PMMR; namely, there is a lot more literature on the former than the latter. Practically, there are many areas in which PMCT is superior to PMMR. PMCT is both cheaper and faster to perform than PMMR, with a PMCT taking around five to ten minutes to perform in comparison to the approximate hour taken for PMMR [4,16,17]. PMCT is also more widely available across England and Wales; together, these may be the main reasons for the currently more frequent use of PMCT over PMMR [12,16,18,19]. However, one advantage of PMMR over PMCT is that MRI scanning does not use radiation in order to acquire images [20], rendering it a safer VA technique for mortuary technicians, who tend to be the team members who manoeuvre the bodies in and out of the scanners. On the other hand, one issue posed by the use of PMMR is metal artefacts within the body; the presence of metal can not only distort the images on the scan, but may even disrupt the body if ripped out by the strong magnetic fields used to image [21].
2.3. Overview of the VA Procedure
Typically, the current method for VA is to perform a full-body scan; this would be undertaken alongside an external examination of the body by a pathologist. As with TA, this is in conjunction with scrutiny of the Coroner’s office report detailing the circumstances surrounding the death, as well as the medical records of the deceased [22]. The imaging would then be interpreted by a radiologist, who then offers either a cause of death to the pathologist, or a recommendation for TA; a TA would be recommended in the instance that the scan is inconclusive regarding cause of death, or when there is a degree of uncertainty regarding any significant lesions on VA. The radiologist’s report will be considered by the pathologist, who will either proceed with a TA anyway, or complete the cause of death form on the basis of the lesions seen at VA and the opinion of the radiologist.
2.4. VA vs. TA
There are many advantages of VA over TA. The images obtained through VA serve as a permanent record of the autopsy, unlike during TA, where the body goes on to be destroyed by cremation or decomposition in burial without any evidence of the lesions except in the pathologist’s report. Not only would the records of VA enable pathologists to more readily seek second opinions, but they would also allow more scrutiny of autopsies, which could help to improve the quality of autopsy reports being produced by pathologists [23,24,25].
Further, nearly 40% of all deaths being reported to the Coroner in England and Wales require an autopsy [26]; the increased speed of an accurate VA in comparison to TA has the potential to help with the management of this huge workload for the mortuary and pathologist. This could also improve turnaround times to release the body, a positive for the loved ones of the deceased, but also regarding mortuary storage capacity [8]. This time-saving and service expansion is also of importance considering the current shortage of pathologists across England and Wales who are willing to perform TAs [27]. Indeed, perhaps the emergence of non-invasive investigations would encourage more pathologists to continue performing PM investigations instead of choosing to opt out.
Lastly, another advantage of VA over TA is the lack of physical contact involved with the body, since the scans are performed with the deceased remaining zipped in a body-bag. As aforementioned, this proved to be essential during the COVID-19 pandemic, in order to reduce the risk of the SARS-CoV-2 virus infecting mortuary staff and pathologists; the transmission of bloodborne and airborne diseases would be reduced by a lower number of TAs. Further, this practice contains any entomological infestations of a decomposing corpse within the body-bag, reducing the risk of infestation spreading to other bodies as well as within the mortuary itself, particularly during the hot summer months. A non-invasive autopsy also means that fewer sharp instruments are used, reducing the risk of needlestick injuries for mortuary staff and pathologists, as well as further transmission of bloodborne diseases [28].
However, there is a caveat: these advantages of VA can only be achieved through accurate diagnoses. If a VA is performed and no cause of death can be found, the TA may have to be performed anyway. This combination of TA following VA would result in an invasive examination regardless, as well as prolonged turnaround times in comparison to TA alone, not to mention the cost of both autopsies instead of one. As such, the accuracy and effectiveness of VA techniques must be optimised in order to justify using VA as a first-line investigation into the cause of death, as well as the financial and spatial investments in setting up such services.
Finally, it is an unfortunate fact that not every mortuary, particularly public mortuaries which are not part of a hospital, has the space or funding to realistically consider on-site installation of VA scanning facilities. This would mean transferring bodies to other facilities in order to perform scanning, which is timely and costly. If mortuaries do have scanners on site, they may have to share the usage with scans for living patients; this juggling could mean longer waiting times for autopsies to take place, depending on how busy the hospital is.
3. Materials and Methods
A lot of the literature surrounding VA provides opinions on the strengths and weaknesses of both PMCT and PMMR, particularly surrounding PMCT owing to its relative ubiquity and lower costs. The results of the studies are rarely consistent; some provide statistics, whereas others provide less specific results, and more of a general overview. Table 1 demonstrates trends of opinions on the individual sensitivity of both PMCT and PMMR to detect specific lesions found commonly at coronial autopsy.

Table 1.
A broad summary of conclusions and findings from studies regarding the strengths and weaknesses of both PMCT and PMMR in their sensitivity in detecting various clinical and pathological PM findings that may contribute to finding the cause of death. This is not in comparison to autopsy, but rather as a general overview of whether each imaging method is able to detect certain pathologies when performing VA. This table also clearly demonstrates an unfavourable imbalance in the amount of literature examining PMMR in comparison to PMCT, which may reflect fewer PMMR facilities available for VA use.
For the contents of Table 1, the University of Manchester online library (https://www.library.manchester.ac.uk/) (accessed from 19 April 2022 to 10 June 2022) was used to find studies which researched the strengths and weaknesses of each VA method in detecting various lesions which could contribute to ascertaining the cause of death in the deceased. Papers were searched between 2012 and present, with search terms including: “autopsy”; “post mortem”; “imaging”; “CT”; and “MR”. Any literature reviews found were also used as a source for references, but conclusions from reviews were not directly used as a data source. Earlier studies published before 2012 with large sample sizes were used as a source for literature through their citation lists of later studies which had referenced them. Only studies published in English were used.
Omitted literature included: studies that included only paediatric or foetal autopsies; studies including under 20 cases per mode of imaging; and studies which only performed and analysed combined PMCT/PMMR VA instead of comparing both imaging methods individually.
Some of the studies did include a small number of bodies belonging to children above the age of one; however, since these studies were primarily adults, they were included. Additionally, although this paper does focus on coronial autopsies only, studies that researched forensic autopsies were also read, before being cherry-picked if any of their results concerned natural or non-suspicious findings that could apply to coronial autopsies too.
Further, many studies focussed on detecting lesions within specific areas of the body, instead of performing a general VA. Some of these more targeted studies were included if their focus was appropriate for application in a general VA. For example, a study researching PM neuroimmunology would have been deemed too specialised for day-to-day VA application in coronial autopsies.
Some studies included a sufficient sample size, but the numbers of individual pathologies found within the bodies were small; any conclusions made by the studies in relation to these sparse lesions were not included in these data. For example, if a study reported a 100% detection rate for aortic dissection by PMMR, but only two of the bodies from their sample had this lesion, this rate of detection was deemed insufficient to include in the results since it reflected the 100% detection of only two present lesions. Further, if a study demonstrated or claimed no clear strength or weakness for a VA method, these results were not included in the table either. For example, if only 50% of myocardial infarctions found at TA were detected by PMMR, this was deemed to be neither a demonstrable strength nor weakness of the imaging technique.
Various steps were taken in order to minimise the effect of bias. Confirmation bias was minimised by not collating all data into a table from the beginning, but instead drawing conclusions of strengths and weaknesses from individual studies before adding all the data together at the end. This was to avoid spotting certain trends within the data and thereby seeking results from the literature to agree with these emerging patterns. Selection bias was minimised by reading the abstracts of every study in both the citation lists of earlier literature, as well as all of the studies which were presented by the online library when these specific search terms were inputted; the abstracts of studies with titles that were clearly not relevant for the purposes of this paper were not read, for example those mentioning foetal autopsies.
4. Results
Table 1 provides an outline of how widely-acknowledged the levels of sensitivity for different lesions for both PMCT and PMMR VA methods are. This table is also a clear demonstration of the imbalance between the number of studies reporting on PMCT and PMMR.
4.1. VA: The Strengths
As outlined in Table 1, PMCT and PMMR both have different strengths regarding their sensitivity in detecting different pathologies. It is widely agreed that PMCT is particularly sensitive when detecting: fractures; large fluid collections including blood and effusions; ectopic air; calcifications; and intracranial findings. Most of these specific strengths render PMCT a particularly useful tool for investigating traumatic deaths. Indeed, PMCT has been found to actually be more sensitive than TA in the detection of bony injuries [56], excluding rib fractures. However, it is worth mentioning that this is often due to the location of the fractures, many of which may not be found in routine dissections, such as in the limbs, face, and neck [8,38,57]. However, because PMCT allows the documentation of bony injuries located in these hard-to-reach areas, in this way, a more thorough examination of fractures in traumatic deaths can be performed without performing a more invasive dissection. This also provides a huge benefit for the next of kin of the deceased who may wish to view the body following autopsy; dissection of the face is often a disfiguring process [10]. If these procedures can be avoided, it can only be a positive outcome for the bereaved.
Further, through its ability to differentiate between densities, PMCT allows the differentiation of bone, fat, water, and gases [17]. In particular, the ability of PMCT to image free gas accumulations renders it an extremely useful tool in the diagnosis of deaths involving ectopic air which would be immediately lost upon evisceration, demonstrating a strong advantage of PMCT over TA [23,54,58,59]. Namely, the pathologist must have a high degree of suspicion for a pneumothorax, pneumoperitoneum, or gas embolism in order to test for these findings prior to commencing full evisceration of the body; the use of PMCT would detect the gas accumulations within the body in these cases, and therefore could act as a screening tool for such causes of death which may be easily missed at TA. Even if tests for these accumulations of free gases were performed prior to full evisceration at TA, the PMCT would still be useful in detecting smaller collections of gases.
Conversely, PMMR complements PMCT in its sensitivity of contrasting pathologies. PMMR has been shown to have use in the detection of soft tissue abnormalities and lesions, as well as intracranial findings, although these conclusions are far less widely agreed than the strengths of PMCT due to there being fewer studies reported. Nonetheless, this strength in imaging pathological soft tissues renders PMMR a valuable tool in the non-invasive examination of intracranial and cardiac deaths [16,60]. For example, PMMR could allow the examination of the most superior region of the spinal cord, which is often severed and damaged at TA during removal of the brain [10]. This is particularly helpful given the generally poor performance of PMCT to detect soft tissue lesions and cardiac pathologies, with the exception of arterial calcifications.
Further, intramuscular haemorrhages and lymph node swellings have been found to be detectable by PMMR, demonstrating its potential use in investigating deaths by hanging or applied pressure to the neck [41,61]. However, it is worth mentioning that PMCT would likely be necessitated in conjunction with PMMR in such deaths, since bony and cartilaginous fractures, for which PMMR has a lower sensitivity, are valuable diagnoses when considering death due to neck injury [41,61]. That said, one retrospective study did find that PMCT often missed fractures of the hyoid bone and laryngeal cartilages; whether this was down to inexperience of the PM radiographic interpretation, at least in part, was unclear, although in most cases the lesions were still not detected even during revision of the imaging [23].
4.2. VA: The Weaknesses
As summarised in Table 1, the glaring Achilles’ heels of PMCT seem to be the detection of: pulmonary emboli; visceral and soft tissue lesions; and cardiac pathologies (excepting vascular calcifications). Unfortunately, together, these are common causes of death encountered by the autopsy pathologist. This means that in many cases, PMCT will be unhelpful in detecting any lesions at all relating to the cause of death, so either a PMMR or TA would need to take place afterwards anyway.
As outlined above, PMMR is widely acknowledged to be superior to PMCT when it comes to detecting pathology within soft tissues and organs. This suggests that together, PMCT and PMMR could cover a lot of pathological ground when performing a VA. However, since there is a lot less literature on PMMR than on PMCT, these conclusions are not as widely acknowledged. In fact, there was such a sparsity of literature surrounding the weaknesses of PMMR that no wide agreements or clear conclusions could confidently be drawn from the data. For example, one study comparing both PMCT and PMMR diagnoses with TA results found that PMCT was unable to detect a single cause of death due to myocardial infarction or pulmonary embolism out of a possible 27 cases [16]. Within the same study, PMMR only detected half of the deaths due to myocardial infarction. Another study found that PMMR had strengths in identifying a cardiac cause of death, but that detection of an acute myocardial infarction over other cardiac causes of death was less sensitive [55]. As such, it seems that more research may need to be performed in order to set a standard against which lesions by PMMR could be confidently determined.
Unfortunately, there are many common pathologies that have been noted by multiple studies to commonly missed upon both PMCT and PMMR, including: pulmonary emboli; intestinal infarction; sepsis; metabolic conditions; intoxication; meningitis; pancreatitis; perforated peptic ulcers; and coronary heart disease [21,24,27,29]. Again, since these are not especially rare PM findings, the role for either VA method, or even using both together, to perform an entire examination does seem limited.
5. Discussion
5.1. Sensitivity vs. Specificity
Whilst PMCT and PMMR may be sensitive in detecting certain findings, many are not specific and do require further dissection at TA in order to ascertain the cause of death and avoid misdiagnosis. For example, PMCT may detect a haemopericardium, but it is not able to reliably indicate whether this was caused by a vascular rupture or a ruptured transmural myocardial infarction [1,24,62]. Again, PMCT can detect a pneumoperitoneum, but will often not be able to locate the perforation [1].
Another common issue was the ability of VA to differentiate between causes of high pulmonary density, although there is seemingly no consensus on this matter within the literature. Some studies claim that whilst PMCT can detect an increased attenuation in pulmonary parenchyma, its ability to differentiate between pulmonary oedema, perhaps due to heart failure, pneumonic consolidation, and even hypostasis, is poor [1,24,34,50,63]. One of these studies mentioned that although they found that lobar pneumonia could often be detected by PMCT due to its localisation, there was a risk of mistaking other pulmonary pathologies for infection [1]. Other studies reported that PMCT was able to accurately differentiate between pneumonia and pulmonary oedema [30,46,52].
5.2. The Current Potential Uses for VA
Whilst a high sensitivity with low specificity may mean that VA cannot replace TA in many cases, it can certainly serve a purpose to guide the TA, informing the pathologist where lesions may lie. For example, even if PMCT cannot provide the aetiology of increased pulmonary density, its ability to detect this finding could guide the pathologist to perform a more thorough examination of the lungs. Similarly, VA findings may be able to indicate the presence of certain pathologies, even if those pathologies themselves cannot be detected. For example, although PMCT cannot reliably detect visceral lesions themselves, its ability to locate blood collections may help to suggest that organ damage may be present nearby, since haemorrhage often accompanies many significant visceral injuries [64].
On the other hand, using similar methods, the VA could also potentially rule out other areas of the body where no obvious findings were detected; for example, PMMR could play a role in ruling out pathological intracranial findings, meaning that an invasive examination into the skull would not be necessary. Therefore, the VA could limit the TA to one anatomical region: a more acceptable autopsy for the bereaved, but also a method for streamlining the pathologist’s dissection and examination.
There are also clear strengths for VA, promoting its use as an adjunct to TA in particular circumstances of death. For example, the great ability of PMCT to detect fractures would enable the pathologist to provide a far more thorough report regarding injuries to the deceased in traumatic deaths, without having to spend time dissecting hard-to-reach areas. This would save time and also render the TA less invasive.
VA could also be a useful screening tool to test for causes of death which are commonly missed at TA. For example, should the case history suggest that a pneumothorax may be the cause of death, a test may be performed at TA before full evisceration is completed and the ectopic air is lost. However, PMCT could screen for pneumothoraces in cases where there may be a scanty case history, or where there is no high suspicion of pneumothorax. Given its high sensitivity for free gas accumulations within the body, a PMCT could therefore either flag up a pneumothorax for the pathologist to confirm at TA, or if the scan is conclusive enough to prove a deadly pneumothorax, avoid TA altogether.
VA could also have use as a triage tool; this may have been useful during the COVID-19 pandemic, especially at the start when the dangers of infection from an infected body posed to pathologists and mortuary staff were largely unknown. For example, an option could be to scan all bodies arriving at the mortuary in order to check for common pulmonary changes found in individuals infected with the virus. Namely, these are most commonly bilateral ground glass opacities, often with a ‘crazy-paving’ appearance, and consolidation [65,66,67,68]. This could have helped to triage cases with high suspicion of SARS-CoV-2 infection, prompting a nasopharyngeal swab of the deceased for virological PCR testing to confirm the pulmonary cause of death, thereby avoiding a full TA. Unfortunately, this would not have been possible at first, since it would have taken time to identify what specific pathological changes should be expected in the bodies of those infected with the SARS-CoV-2 virus, through performing TAs on deceased, infected patients, as well as through ante mortem (AM) scans obtained from living, infected patients. Further, we must acknowledge that, particularly in the cases of new and emerging diseases, TA will always serve to support scientific research and help us to understand new illnesses through histological examination; a mortuary service performing VAs alone would not have provided information surrounding the histological changes seen in deceased patients infected with the SARS-CoV-2 virus.
In conclusion, despite the pitfalls of PMCT and PMMR, VA can serve as a useful adjunct to TA in certain cases, providing extra information or guiding the TA. VA may be able to limit, or even replace a full TA, in cases with specific causes of death where VA is found to be highly sensitive and specific for certain lesions. Perhaps in the interest of saving time and money, cases could be cherry-picked through careful scrutiny by the pathologist of both the medical history of the deceased and the report of circumstances surrounding the death. This triaging system could allow an informed decision as to whether PMCT or PMMR would be most appropriate for each specific VA, in order to reduce the necessity for subsequent VA or TA in order to find the cause of death. Further, VA could also be used as an adjunct to screen for deaths and lesions that may be commonly missed at TA, providing more accurate causes of death in such cases, but also aiding the pathologist in performing a more complete and thorough autopsy.
5.3. The Challenges of VA
Despite these potential uses for VA as an adjunct to TA, an issue which must be considered should be the role of interpreting VA images. Due to a number of PM changes that occur in the human body, the interpretation of both PMCT and PMMR imaging is not identical to that of AM scans. These significant PM changes detected by VA include phenomena such as: increased pulmonary parenchymal density due to hypostasis [63]; increased levels of intrarectal and intrahepatic gas [69]; shrinking of the adrenal glands, kidneys, and spleens [70]; presentation of bilateral opacities and bronchovascular bundle thickening in lungs without pulmonary pathologies [71]; differences in the presence of intravascular air, intestinal distension, and pleural effusions between patients who received CPR and those who did not [44,60]; the false-positive appearance of a subarachnoid haemorrhage upon imaging [1,72]; loss of grey-white matter differentiation within the brain [44]; and the production of intraosseous, intracranial, and intraperitoneal gas by decomposition [24,73]. Indeed, one study found that 9.7% of all clinically relevant PMCT findings were due to PM changes in the body [30]. These changes, by their nature of occurring after death, are not encountered routinely by clinical radiologists; as such, their detection could mimic or mask other pathologies, and therefore mislead VA interpretation when utilised by a radiologist unfamiliar with or untrained in PM imaging [23,45,60,74]. As such, the high incidence of such PM changes as well as their potential implications regarding misdiagnosis may suggest that further training into PM radiology is required for routine VAs to take place [30].
Therefore, the challenge remains with both pathologists having little radiological training, and radiologists having little training in the interpretation of PM images [7]. Even the clinical approach of the radiologist has been noted to differ between AM and PM cases; the focus of AM scans is to interpret and guide treatment for the future, whereas the interpretation of PM scans centres on looking backwards as to how the pathological findings came to arise [74]. Currently, multi-disciplinary collaboration and good co-operation between pathologists and radiologists is recommended for the interpretation and application of VA [7,27]. It is also imperative that the reporting radiologist should have adequate experience in the interpretation of PM scans as well as knowledge of PM changes that occur within the body. However, whether this would mean more radiological training for pathologists, more PM training for radiologists, or even the union of both specialities and conception of a new role, such as a “necroradiologist”, remains to be answered [19,74,75,76,77].
That said, further research into novel techniques used to combat PM changes in the deceased may help to negate the need for more radiological specialisation. For example, experimental use of PMCT coupled with continuous positive airway pressure ventilation has been trialled in order to combat the collapse of pulmonary parenchyma in order to better differentiate between pathology and PM changes [7]. Artificially filling the lungs with air seems to improve the detection of smaller lesions and pathologies by reducing the impact of hypostatic changes on the images of pulmonary parenchyma [78].
A further challenge for VA is financial cost, which is always an important consideration for any service funded by the public authority. One study investigating the feasibility of performing VA in daily practice observed that the financial hospital cost of a single PMCT scan was the same as the clinical AM CT scanning of four body regions [79]. This was the same cost as performing minimally invasive CT-guided needle biopsies, regardless of how many samples were taken. The specific figures for the aforementioned procedures were not published within the paper; nonetheless, it raises the issue of whether VA replacing TA is financially viable for many institutions.
Similarly, another issue lies in the fact that even though many lesions can be detected by one method of VA or another, this would require two scans: a PMCT and a PMMR. This would be a huge expense, more time-consuming, and also is unrealistic in many instances; there is already a shortage of either scanner for mortuaries to access, let alone two types of scanner per VA. Even if both a PMCT and a PMMR scan could be performed, there are still a number of common pathologies that are commonly missed by both VA methods. In these cases, three examinations would need to be carried out: one PMCT scan; one PMMR scan; and finally one TA. When a single TA would be sufficient to determine cause of death, more work must be done to ensure the reliable diagnosis of these common causes of deaths by VA before it can be considered a replacement for TA.
Lastly, one further weakness of VA is simply the lack of colour in the images. Colour, and indeed smell, can both be valuable tools for the pathologist when determining the cause of death [10]. For example: the faint smell of alcohol often present when the deceased was intoxicated at the time of death; the classic ‘blue bag’ appearance of a haemopericardium upon removal of the sternum; or the dark discolouration of an ischaemic bowel.
5.4. Limitations of This Review
One issue concerning the data collection for this review was the variety throughout the content of the literature. The studies varied hugely between one another in many areas: whether they were testing sensitivity or specificity; whether or not the studies were blinded; whether the studies were looking for causes of death only or recording all lesions; how many radiologists and pathologists were working on the autopsies; the experience that those particular staff members had in the interpretation of VA; the experience that those same staff members had regarding PM changes within bodies; the extent and variety of PM changes present within the bodies; and whether the various findings were reported as data or as general conclusions. To add to this variety, the sheer number of different but specific lesions made the data difficult to group and present in a way that was not just a single claim from a single study; hence Table 1 demonstrates a broader overview of grouped pathologies. The disparities in the different focuses and methods of the literature made more specific conclusions difficult.
A further limitation to this review was the inability to assess the overall accuracy of VA to find the correct cause of death. There were so many different standards against which research groups worked and based their conclusions upon: some studies reported varying degrees of certainty and possibility decided by the radiologists, whereas others used more of a binary decision as to whether VA had found the cause of death. Further, a small number of studies, in the event of their VA finding a certain cause of death according to their methods, would then not perform a TA. Whilst we do not question the expertise of the radiologists or pathologists regarding these decisions, it does raise the issue of no control autopsy being available against which to compare the VA findings and their specificity.
Similarly, in the specific cases of ectopic air, there remains the issue of the blind studies. The detection of gas accumulations was the second most widely agreed strength of PMCT within the literature, following fractures. However, in the blind studies, where TA was performed by pathologists who had not had access to the PMCT results, these gas accumulations would likely have often been missed. This is due to the testing of ectopic air not being a particularly routine procedure performed in every single TA. As such, the detection of any gas accumulations at VA may not have frequently had a control, since it would have been immediately lost upon evisceration if not specifically tested for beforehand.
6. Emerging Techniques for VA
Despite the array of challenges surrounding VA, there are undoubtedly strengths for both PMCT and PMMR, and their various advantages over TA are driving the development of new techniques. These novel methods may help to tackle the weaknesses of both VA methods, as well as help them to provide a more reliable and thorough examination of the deceased.
6.1. PM Angiography
Cardiovascular diseases are very common causes of sudden death in adults living in developed countries [9,29,80]. This fact, along with the pitfalls of both PMCT and in many cases PMMR to identify coronary vascular pathology, has helped to drive the evolution of VA into angiographic use. There has been great development in the practice of PM angiography (PMA), whereby PMCT coronary angiography (PMCTA) and PMMR coronary angiography (PMMRA) can be performed by combining PM scanning with the injection of intravascular contrast agents. These methods help to bypass the limitations of PMCT and PMMR by providing detailed coronary imaging in the cases of cardiac-related deaths [7,81]. PMCTA and PMMRA allow minimally invasive opacification imaging of the cardiac vasculature, through intravascular infusion of contrast agent into the coronary arteries via a small incision into the left common carotid artery and subsequent catheterisation. The main benefits of PMA include the same general advantages that VA has over TA, and also the ability to detail vascular occlusions and stenoses without the destruction of the cardiac tissues from dissection, as is the case during TA [81].
Further, PMA allows the visualisation of hard-to-reach areas along with those which may prove difficult to dissect due to post-surgical scarring and anastomoses [81]. PMA can also be used to visualise vasculature of other regions, including the abdomen, head, and extremities, potentially in the investigations of haemorrhagic or ischaemic pathologies [11,81]. Some VAs even include whole-body PMA to visualise the vasculature of the entire body [81].
Some studies have found that PMMR has strengths lying in identifying areas of myocardial ischaemia, with evidence that PMMR can detect even hyperacute myocardial damage which may not be visible at both TA and histological examination at such early stages [60,81,82]. It is worth noting that the pathology seen in PMMRA would also be found using PMCTA, and so in the interest of finances and scanner availability, PMCTA would seem to be the preferable choice for PMA [81]; however, the complementary strengths of PMMR and PMA make them useful techniques for thorough cardiac examination during VA. Notably, the strength of PMCT in locating arterial calcifications may be used to associate heavy calcifications with an increased probability of significant vascular occlusion; however, this method cannot accurately determine the degree of occlusion and therefore may warrant further research [11]. PMCT may sometimes be able to detect ischaemic myocardial thickening found in cases of chronic infarction, but it is not a sensitive method for investigating deaths caused by myocardial infarction [81].
Perhaps surprisingly, it has been found that PMCTA is still possible even in heavily decomposing bodies and those infested with maggots, although the diagnostic value does decrease; this is due to the destruction of tissues due to autolysis and putrefaction [73,83]. Whilst there is not much literature in this area, the findings may provide an avenue for future studies.
In terms of where we may see PMA explore in the future, the use of lipophilic contrast agents has been found to enhance infarcted myocardium [82,84]; this could be extremely useful in obtaining a more comprehensive understanding of the cardiac pathology through PMA techniques alone, without having to perform further scans or even a TA as well. These specialist methods for non-invasive cardiac PM examination are great steps towards bridging the gap of soft tissue imaging in VA.
6.2. Pulmonary Emboli—A Major Pitfall of VA
Pulmonary emboli are a relatively common cause of death encountered by the coronial autopsy pathologist [1,85,86]. Unfortunately, this means that the ability of VA to detect clots within the pulmonary vasculature remains a huge challenge.
One recent study has trialled the use of PMCTA in order to better visualise the pulmonary arteries, and thereby attempt to tackle the weakness of VA in its ability to detect pulmonary emboli [87]. In this study, using CT-guidance to inject contrast medium into the right ventricle allowed the visualisation of the pulmonary arteries and therefore highlighted any filling defects. This is similar to an earlier study where again, PMCTA was used to identify large clots within the pulmonary arteries [86]. Another singular case study showed that performing chest compressions on the deceased shortly after death (within an hour or so) could restore blood flow to the pulmonary arteries and therefore distribute peripherally injected contrast medium to the area for PMCTA [88]. However, this method is not practicable for application on bodies requiring a coronial autopsy, since these cases need time-consuming administration before an autopsy can be performed. This is also alongside the fact that very few people die, are found, and then are brought to the public mortuary within such a short space of time. Overall, whilst these studies were successful in detecting any large arterial blockages, there was still the issue of differentiating between clots formed during either AM or PM coagulation; this would often result in a TA being performed anyway in order to examine the embolus. However, the presence and visualisation of filling defects was a useful tool in flagging up cases with suspicions of a pulmonary embolism being the cause of death.
Further studies have researched PMMR and its use in cases of fatal pulmonary embolism. One small study used PMMR to detect pulmonary emboli in eight cases [89]; whilst they found that the VA was useful for detecting filling defects within the pulmonary arteries, again, the challenge to differentiate between AM and PM clots persisted. Another case study has demonstrated a potential use of proton density PMMR images to differentiate between AM and PM clots in cases of potential pulmonary embolism [85]; this was achieved by providing more detailed soft tissue differentiation, and thereby helped to differentiate more uniform pulmonary emboli from more disorganised PM clots. It is encouraging to see work tackling the diagnosis of the fatal pulmonary embolism by VA, but there is still a long way to go before this gap is bridged.
6.3. VA-Guided Biopsies
An issue remains that even if VA can identify regions of pathology within a body, tissue samples may still be required for full histological examination by the pathologist. As such, even if the VA has successfully identified lesions, an invasive examination of the body may still be required in order to retrieve visceral samples. However, new methods are being developed in order to sample tissues from inside the body, without fully opening the body up. One strategy to make autopsies as minimally invasive as possible has been the use of CT-guided needle biopsies. This method relies on performing a VA and using the pathological findings on the scan to indicate where histological samples should be removed, before taking small pieces of tissue via needle biopsies from the viscera without fully opening the body.
One recent study compared the quality of histological samples taken at TA with pulmonary and hepatic tissue samples from the same bodies taken via CT-guided needle biopsies [90]. It found that the lung biopsies missed the right diagnosis in over one third of cases in which there was pulmonary pathology to find; however, for the hepatic biopsies, over 90% of the samples showed findings that were consistent with the pathology found in the samples taken at TA. Another similar study compared needle biopsy tissue samples with tissue samples taken at TA, but from more organs: cardiac, pulmonary, hepatic, renal, and splenic as standard, plus other tissues if lesions were also suspected elsewhere within the body [91]. Here, it was found that the results of the minimally invasive autopsy agreed with the cause of death found during TA in 92% of cases. However, this latter study used both PMCT and PMMR for the VA and imaging-guided biopsies, which may explain in part the higher yield of diagnostic histological samples. The results of both studies show promise for VA-guided needle biopsies, and perhaps suggest that with further VA training, wider access to both CT and MRI facilities, and improved sampling techniques, the sensitivity and specificity of CT-guided needle biopsies may increase the use and availability of minimally invasive autopsies.
6.4. PM Micro-CT
PM micro-CT (PMmCT) is the use of CT at a resolution high enough to image micrometres [81]; the limited resolution of hospital CT scanners means that micro-CT is performed on specialised machines, although their availability is currently limited [92]. The current PM use of micro-CT lies mainly in foetal autopsies, as well as providing detailed analysis of subtle cartilaginous and bony fractures in forensic investigations [92,93]. However, PMmCT has been found to provide cardiac detail to nearly the same level of histological examination of tissue samples [81]. Since micro-CT can allow such high resolution imaging of internal structures without any invasion or dissection of the specimen [94], perhaps future advances in PMmCT and specialist training could one day lead to a completely non-invasive autopsy without compromise of the detail and quality of the pathologist’s investigation.
6.5. PM Ultrasonography
In want of a less invasive, fast autopsy technique due to the COVID-19 pandemic, a recent study has examined the use of PM ultrasonography (PMUS) as a non-invasive autopsy examination method [95]. A much older study demonstrated that certain lesions can be detected by PMUS, including: cardiac hypertrophy; fatty changes to the liver; and ascites or intra-abdominal bleeding [96]. Whilst it is acknowledged that PMUS cannot provide anywhere near the detail of PMCT and PMMR, perhaps it has use as a back-up for non-invasive examinations in specific circumstances, given its dynamic nature and contemporaneous imaging. It could also help to guide the TA in certain situations, for example by locating haemorrhage in traumatic deaths and thereby potentially limiting the extent of the invasive autopsy. However, given that ultrasound waves cannot travel through air, it remains to be ascertained how much the gas production in decomposition would hamper PMUS results [95]. That said, the ability of ultrasonography to detect air through the fact that air very much disrupts its signal could perhaps work as a quick, cheap, and easy screen for pneumothoraces in bodies before commencing TA. However, given the prevalence, speed, and low cost of ultrasonography, the fact that there is very little literature on its use in autopsies may betray an extremely limited capacity for PMUS.
6.6. Toxicological Deaths
Another current limitation to VA has been deaths involving drug toxicity, where blood samples are required for further specialist toxicological analysis and often, little can be seen upon imaging [12]. Whilst these toxicology samples can be taken as a minimally invasive procedure through using a syringe to remove blood, typically from the femoral vein, the general consensus is that VA has limited use regarding the examination of these toxicological deaths [34,64]. However, there is potentially an opening for the use of PMCT in deaths by oral drug overdose; one study showed that in a cohort of 23 deaths by known oral drug overdose, 87% of the PMCT scans performed showed high gastric radiodensity [97]. In a different study, another case was found to have high density gastric contents visible on PMCT which was later correlated with ingestion of a large volume of organic solvent [31]. These findings of high gastric X-ray absorption could merit further research into the use of PMCT for cases of death by suspected oral drug ingestion.
Another case study demonstrated the appearance of a large number of pills (which turned out to be Bupropion) within the duodenum of a body, detected by PMCT [98]. Although the cause of death turned out to be intoxication by other substances, the presence of so many tablets within the deceased on the scan was a clear indication of suicidal intent, and could have been able to guide the autopsy in regard to sampling blood for toxicological analysis through minimally invasive means. Again, whilst PMCT may not be able to ascertain a cause of death in cases of lethal intoxication, there is a potential for its use as an adjunct to evolve.
7. Conclusions
PMCT and PMMR each have individual strengths and weaknesses, in practical, financial, and imaging terms. VA can provide detailed, non-invasive information about the whole body, although for truly thorough imaging, at present, one method would not be sufficient to adequately analyse all areas. There are still flaws in the ability of VA to detect very common causes of death when used in general coronial investigations. As such, VA is currently a useful adjunct or preliminary investigation method to TA, but not a replacement just yet; the usefulness of VA seems to depend on the circumstances and pathologies surrounding the death. However, together, VA and TA may be able to achieve a more complete and thorough autopsy than either method alone. That said, since the role of the coronial autopsy is to simply determine the cause of death, incidental lesions, no matter how detailed, which do not contribute to the overall cause of death may often be of little relevance to the pathologist and therefore the Coroner.
That said, VA is a useful tool in its unique advantages over autopsy in detecting specific lesions common in certain circumstances of death, and so may have more useful applications when cherry-picked for use in specific situations, such as traumatic deaths. VA could also be used to focus a limited TA, or to enable a minimally invasive autopsy through VA-guided biopsies; these uses acknowledge the ability of VA to detect lesions, as well as its lower reliability in ascertaining their aetiology or specific nature.
As the availability of VA facilities, particularly PMMR, increases, it may be useful to produce similar studies comparing VA using PMMR with TA, in order to balance the literature, which currently heavily favours PMCT; this could give a wider insight into the applications of VA and when they may best be used to complement TA. As technology advances and imaging techniques continue to emerge and improve, VA may grow in its use as an adjunct to TA, and autopsies may continue to become less and less invasive.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burton, J.L.; Kitsanta, P. Daily application of post-mortem computed tomography digital autopsy in a public mortuary. Diagn. Histopathol. 2020, 26, 358–367. [Google Scholar] [CrossRef]
- Herath, J.C.; Herath, S.O. Is it time for targeted and minimally invasive post-mortem examination using total body computed tomography in a medicolegal autopsy? Forensic Sci. Med. Pathol. 2021, 17, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Latif, Z.; Hill, M.; Riddington, M.; Lakhanpaul, M.; Arthurs, O.J.; Hutchinson, J.C.; Chitty, L.S.; Sebire, N.J. “We might get a lot more families who will agree”: Muslim and Jewish perspectives on less invasive perinatal and paediatric autopsy. PLoS ONE 2018, 13, e0202023. [Google Scholar] [CrossRef] [PubMed]
- Wagensveld, I.M.; Weustink, A.C.; Kors, J.A.; Blokker, B.M.; Hunink, M.G.M.; Oosterhuis, J.W. Effect of minimally invasive autopsy and ethnic background on acceptance of clinical postmortem investigation in adults. PLoS ONE 2020, 15, e0232944. [Google Scholar] [CrossRef]
- Rutty, G.N.; Rutty, J.E. Perceptions of near virtual autopsies. J. Forensic Leg. Med. 2011, 18, 306–309. [Google Scholar] [CrossRef]
- Patowary, A. The fourth incision: A cosmetic autopsy incision technique. Am. J. Forensic Med. Pathol. 2010, 31, 37–41. [Google Scholar] [CrossRef]
- Norberti, N.; Tonelli, P.; Giaconi, C.; Nardi, C.; Focardi, M.; Nesi, G.; Miele, V.; Colagrande, S. State of the art in post-mortem computed tomography: A review of current literature. Virchows Arch. 2019, 475, 139–150. [Google Scholar] [CrossRef]
- Ukpo, O.; Boger, D. Chapter 4—The role of virtual autopsy and use of a CT scanner in medico-legal death investigations. In Multidisciplinary Medico-Legal Death Investigation: Role of Consultants; Sathyavagiswaran, L., Rogers, C.B., Eds.; Academic Press: London, UK, 2018; pp. 69–73. [Google Scholar]
- Moskała, A.; Woźniak, K.; Kluza, P.; Romaszko, K.; Lopatin, O. The importance of post-mortem computed tomography (PMCT) in confrontation with conventional forensic autopsy of victims of motorcycle accidents. Leg. Med. 2016, 18, 25–30. [Google Scholar] [CrossRef]
- Bolliger, S.A.; Thali, M.J. Imaging and virtual autopsy: Looking back and forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140253. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Kitsanta, P.; Burton, J.L. The effects of postmortem CT scanning all cases entering a UK public mortuary: A 3-month pilot. J. Clin. Pathol. 2017, 70, 903–905. [Google Scholar] [CrossRef]
- Smith, A.P.T.; Traill, Z.C.; Roberts, I.S.D. Post-mortem imaging in adults. Diagn. Histopathol. 2018, 24, 365–371. [Google Scholar] [CrossRef]
- Hendon Mosque & Islamic Centre. CT Scan. Available online: https://hendonmosque.co.uk/funeral-services/ct-scan/ (accessed on 1 June 2022).
- Haringey Council. Oxford Minimally Invasive Autopsy Service. Available online: https://www.haringey.gov.uk/sites/haringeygovuk/files/oxford_mia_service.pdf (accessed on 1 June 2022).
- Watts, G. Imaging the dead. Br. Med. J. 2010, 341, 1130–1131. [Google Scholar] [CrossRef] [PubMed]
- Femia, G.; Langlois, N.; Raleigh, J.; Gray, B.; Othman, F.; Perumal, S.R.; Semsarian, C.; Puranik, R. Comparison of conventional autopsy with post-mortem magnetic resonance, computed tomography in determining the cause of unexplained death. Forensic Sci. Med. Pathol. 2021, 17, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dedouit, F.; Campana, L.; Uldin, T.; Grabherr, S. Post-mortem forensic imaging. In P5 Medicine and Justice: Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 544–559. [Google Scholar]
- Ross, S.; Ebner, L.; Flach, P.; Brodhage, R.; Bolliger, S.A.; Christe, A.; Thali, M.J. Postmortem whole-body MRI in traumatic causes of death. Am. J. Roentgenol. 2012, 199, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Genet, P.; Sabatasso, S.; Grabherr, S. Postmortem imaging as a complementary tool for the investigation of cardiac death. Forensic Sci. Res. 2019, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Woodford, N. Post-mortem radiology—A new sub-speciality? Clin. Radiol. 2008, 63, 1189–1194. [Google Scholar] [CrossRef]
- Suvarna, S.K. Teaching and examining for post-mortem CT-scanned autopsies. Diagn. Histopathol. 2020, 26, 343–349. [Google Scholar] [CrossRef]
- Lancashire County Council. Non-Invasive Post-Mortem Examination. Available online: https://www.lancashire.gov.uk/births-marriages-and-deaths/deaths/coroners/non-invasive-post-mortem-examination/ (accessed on 12 September 2022).
- Graziani, G.; Tal, S.; Adelman, A.; Kugel, C.; Bdolah-Abram, T.; Krispin, A. Usefulness of unenhanced post mortem computed tomography—Findings in postmortem non-contrast computed tomography of the head, neck and spine compared to traditional medicolegal autopsy. J. Forensic Leg. Med. 2018, 55, 105–111. [Google Scholar] [CrossRef]
- Roberts, I.S.D.; Benamore, R.E.; Benbow, E.W.; Lee, S.H.; Harris, J.N.; Jackson, A.; Mallett, S.; Patankar, T.; Peebles, C.; Roobottom, C.; et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: A validation study. Lancet 2012, 379, 136–142. [Google Scholar] [CrossRef]
- National Confidential Enquiry into Patient Outcome and Death. The Coroner’s Autopsy: Do We Deserve Better? A Report of the National Confidential Enquiry into Patient Outcome and Death. Available online: http://www.ncepod.org.uk/2006Report/Downloads/Coronial Autopsy Report 2006.pdf (accessed on 1 June 2022).
- Gov.uk. National Statistics: Coroners Statistics 2020: England and Wales. Available online: https://www.gov.uk/government/statistics/coroners-statistics-2020/coroners-statistics-2020-england-andwales#:~:text=There%20were%2079%2C357%20post%2Dmortem,2%2C715%20(3%25)%20from%202019 (accessed on 1 June 2022).
- The Royal College of Pathologists. Guidelines for Post-Mortem Cross-Sectional Imaging in Adults for Non-Forensic Deaths July 2021. Available online: https://www.rcpath.org/uploads/assets/666dbf95-de06-44ad-89c3b4e5f1ceab79/G182-Guidelines-for-post-mortem-cross-sectional-imagingFor-Publication.pdf (accessed on 1 June 2022).
- Burton, J.L. Health and safety at necropsy. J. Clin. Pathol. 2003, 56, 254–260. [Google Scholar] [CrossRef]
- Rutty, G.N.; Morgan, B.; Robinson, C.; Raj, V.; Pakkal, M.; Amoroso, J.; Visser, T.; Saunders, S.; Biggs, M.; Hollingbury, F.; et al. Diagnostic accuracy of post-mortem CT with targeted coronary angiography versus autopsy for coroner-requested post-mortem investigations: A prospective, masked, comparison study. Lancet 2017, 390, 145–154. [Google Scholar] [CrossRef]
- Mentink, M.G.; Latten, B.G.H.; Bakers, F.C.H.; Mihl, C.; Rennenberg, R.J.M.W.; Kubat, B.; Hofman, P.A.M. Clinical relevance of unexpected findings of post-mortem computed tomography in hospitalized patients: An observational study. Int. J. Environ. Res. Public Health 2020, 17, 7572. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Makino, Y.; Hayakawa, M.; Yajima, D.; Ito, H.; Iwase, H. Diagnosable and non-diagnosable causes of death by postmortem computed tomography: A review of 339 forensic cases. Leg. Med. 2012, 14, 239–245. [Google Scholar] [CrossRef]
- Di Paolo, M.; Maiese, A.; dell’Aquila, M.; Filomena, C.; Turco, S.; Giaconi, C.; Turillazzi, E. Role of post mortem CT (PMCT) in high energy traumatic deaths. Clin. Ther. 2020, 171, 490–500. [Google Scholar]
- Legrand, L.; Delabarde, T.; Souillard-Scemama, R.; Sec, I.; Plu, I.; Laborie, J.-M.; Delannoy, Y.; Hamza, L.; Taccoen, M.; de Jong, L.; et al. Comparison between postmortem computed tomography and autopsy in the detection of traumatic head injuries. J. Neuroradiol. 2020, 47, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hueck, U.; Muggenthaler, H.; Hubig, M.; Heinrich, A.; Güttler, F.; Wagner, R.; Mall, G.; Teichgräber, U. Forensic postmortem computed tomography in suspected unnatural adult deaths. Eur. J. Radiol. 2020, 132, 109297. [Google Scholar] [CrossRef]
- Grabherr, S.; Heinemann, A.; Vogel, H.; Rutty, G.; Morgan, B.; Woźniak, K.; Dedouit, F.; Fischer, F.; Lochner, S.; Wittig, H.; et al. Postmortem CT angiography compared with autopsy: A forensic multicenter study. Radiology 2018, 288, 270–276. [Google Scholar] [CrossRef]
- Adelman, A.; Vasserman, M.; Graziani, G.; Kugel, C.; Meir, K.; Bdolah-Abram, T.; Krispin, A. Post-mortem computed tomography compared to medico-legal autopsy—Pathologies in the torso and limbs. J. Forensic Radiol. Imaging 2018, 12, 43–49. [Google Scholar] [CrossRef]
- Makino, Y.; Yokota, H.; Nakatani, E.; Yajima, D.; Inokuchi, G.; Motomura, A.; Chiba, F.; Torimitsu, S.; Uno, T.; Iwase, H. Differences between postmortem CT and autopsy in death investigation of cervical spine injuries. Forensic Sci. Int. 2017, 281, 44–51. [Google Scholar] [CrossRef]
- Leth, P.M.; Struckmann, H.; Lauritsen, J. Interobserver agreement of the injury diagnoses obtained by postmortem computed tomography of traffic fatality victims and a comparison with autopsy results. Forensic Sci. Int. 2013, 225, 15–19. [Google Scholar] [CrossRef]
- Roberts, I.S.D.; Traill, Z.C. Minimally invasive autopsy employing post-mortem CT and targeted coronary angiography: Evaluation of its application to a routine Coronial service. Histopathology 2014, 64, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc-Louvry, I.; Thureau, S.; Duval, C.; Papin-Lefebvre, F.; Thiebot, J.; Dacher, J.N.; Gricourt, C.; Touré, E.; Proust, B. Post-mortem computed tomography compared to forensic autopsy findings: A French experience. Eur. Radiol. 2013, 23, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Deininger-Czermak, E.; Heimer, J.; Tappero, C.; Thali, M.J.; Gascho, D. The added value of postmortem magnetic resonance imaging in cases of hanging compared to postmortem computed tomography and autopsy. Forensic Sci. Med. Pathol. 2020, 16, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Abboud, S.; Ali, Z.; Sliker, C.; Fowler, D. Comparison of whole-body post mortem 3D CT and autopsy evaluation in accidental blunt force traumatic death using the abbreviated injury scale classification. Forensic Sci. Int. 2013, 225, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Hoppe, H.; Schweitzer, W.; Schwendener, N.; Grabherr, S.; Jackowski, C. Rib fractures at postmortem computed tomography (PMCT) validated against the autopsy. Forensic Sci. Int. 2013, 233, 90–98. [Google Scholar] [CrossRef]
- Wagensveld, I.M.; Blokker, B.M.; Wielopolski, P.A.; Renken, N.S.; Krestin, G.P.; Hunink, M.G.; Oosterhuis, J.W.; Weustink, A.C. Total-body CT and MR features of postmortem change in in-hospital deaths. PLoS ONE 2017, 12, e0185115. [Google Scholar] [CrossRef]
- Serinellii, S.; Richardson, T.E.; Destian, S.; Mirchia, K.; Williams, M.; Medina-Perez, M.; Gitto, L. Head and brain postmortem computed tomography—Autopsy correlation in hospital deaths. Am. J. Forensic Med. Pathol. 2020, 41, 163–175. [Google Scholar] [CrossRef]
- Willaume, T.; Farrugia, A.; Keiffer, E.-M.; Charton, J.; Geraut, A.; Berthelon, L.; Bierry, G.; Raul, J.-S. The benefits and pitfalls of post-mortem computed tomography in forensic external examination: A retrospective study of 145 cases. Forensic Sci. Int. 2018, 286, 70–80. [Google Scholar] [CrossRef]
- Álvarez, A.C.; Mancini, J.; Tuchtan-Torrents, L.; Gach, P.; Bartoli, C.; Desfeux, J.; Piercecchi, M.D.; Gorincour, G. Diagnostic value of unenhanced postmortem computed tomography in the detection of traumatic abdominal injuries. Diagn. Interv. Imaging. 2018, 99, 397–402. [Google Scholar] [CrossRef]
- Bedford, P.J. Routine CT scan combined with preliminary examination as a new method in determining the need for autopsy. Forensic Sci. Med. Pathol. 2012, 8, 390–394. [Google Scholar] [CrossRef]
- Femia, G.; Semsarian, C.; Langlois, N.; McGuire, M.; Raleigh, J.; Taylor, A.; Puranik, R. Post-mortem imaging adjudicated sudden death: Causes and controversies. Heart Lung Circ. 2019, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.E.; Aptizsch, J.; Penzkofer, T.; Mahnken, A.H.; Knüche, R. Virtual CT autopsy in clinical pathology: Feasibility in clinical autopsies. Virchows Arch. 2012, 461, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.K.; Lundemose, S.; Banner, J.; Lynnerup, N.; Jacobsen, C. Forensic postmortem computed tomography: Volumetric measurement of the heart and liver. Forensic Sci. Med. Pathol. 2016, 12, 510–516. [Google Scholar] [CrossRef]
- Schwendener, N.; Jackowski, C.; Persson, A.; Warntjes, M.J.; Schuster, F.; Riva, F.; Zech, W.-D. Detection and differentiation of early acute and following age stages of myocardial infarction with quantitative post-mortem cardiac 1.5 T MR. Forensic Sci. Int. 2017, 270, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sonnemans, L.J.; Kubat, B.; Prokop, M.; Klein, W.M. Can virtual autopsy with postmortem CT improve clinical diagnosis of cause of death? A retrospective observational cohort study in a Dutch tertiary referral centre. BMJ Open 2018, 8, e018834. [Google Scholar] [CrossRef]
- Inai, K.; Noriki, S.; Kinoshita, K.; Sakai, T.; Kimura, H.; Nishijima, A.; Iwasaki, H.; Naiki, H. Postmortem CT is more accurate than clinical diagnosis for identifying the immediate cause of death in hospitalized patients: A prospective autopsy-based study. Virchows Arch. 2016, 469, 101–109. [Google Scholar] [CrossRef]
- Lorenzen, J.; Schenzer-Hoffmann, E.; Braun, C.; Lorenzen, M.; Anders, S.; Adam, G.; Püschel, K. Determination of cause of death with blinded coronal whole-body MRI compared to forensic examination. Rechtsmedizin 2019, 29, 287–294. [Google Scholar] [CrossRef]
- Jalalzadeh, H.; Giannakopoulos, G.F.; Berger, F.H.; Fronczek, J.; van de Goot, F.R.W.; Reijnders, U.J.; Zuidema, W.P. Post-mortem imaging compared with autopsy in trauma victims—A systematic review. Forensic Sci. Int. 2015, 257, 29–48. [Google Scholar] [CrossRef]
- Mondello, C.; Baldino, G.; Bottari, A.; Sapienza, D.; Perri, F.; Argo, A.; Asmundo, A.; Spagnolo, E.V. The role of PMCT for the assessment of the cause of death in natural disaster (landslide and flood): A Sicilian experience. Int. J. Leg. Med. 2022, 136, 237–244. [Google Scholar] [CrossRef]
- Lin, M.J.; Barry, N.; Akusoba, I.; Hon, H.H.; Cohen, M.S.; Shukla, P.; Cipolla, J.; Stawicki, S.P.; Hoey, B.A. Traditional autopsy versus computed tomography imaging autopsy in trauma: A case of “synergistic disagreement”. Surgery 2016, 160, 211–219. [Google Scholar] [CrossRef]
- Worasuwannarak, W.; Peonim, V.; Srisont, S.; Udnoon, J.; Chudoung, U.; Kaewlai, R. Comparison of postmortem CT and conventional autopsy in five trauma fatalities. Forensic Imaging 2020, 22, 200389. [Google Scholar] [CrossRef]
- Ruder, T.D.; Thali, M.J.; Hatch, G.M. Essentials of forensic post-mortem MR imaging in adults. Br. J. Radiol. 2014, 87, 20130567. [Google Scholar] [CrossRef] [PubMed]
- Deininger-Czermak, E.; Heimer, J.; Tappero, C.; Thali, M.J.; Gascho, D. Postmortem Magnetic Resonance Imaging and Postmortem Computed Tomography in Ligature and Manual Strangulation. Am. J. Forensic Med. Pathol. 2020, 41, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ampanozi, G.; Flach, P.M.; Ruder, T.D.; Filograna, L.; Schweitzer, W.; Thali, M.J.; Ebert, L.C. Differentiation of hemopericardium due to ruptured myocardial infarction or aortic dissection on unenhanced postmortem computed tomography. Forensic Sci. Med. Pathol. 2017, 13, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Rutty, G.N.; Morgan, B.; Germerott, T.; Thali, M.; Athurs, O. Ventilated post-mortem computed tomography—A historical review. J. Forensic Radiol. Imaging. 2016, 4, 35–42. [Google Scholar] [CrossRef]
- Morgan, B.; Rutty, G.N. How does post-mortem imaging compare to autopsy, is this a relevant question? J. Forensic Radiol. Imaging 2016, 4, 2–6. [Google Scholar] [CrossRef][Green Version]
- Kniep, I.; Heinemann, A.; Edler, C.; Sperhake, J.P.; Püschel, K.; Ondruschka, B.; Schröder, A.S. COVID-19 lungs in post-mortem computed tomography. Rechtsmedizin 2021, 31, 145–147. [Google Scholar] [CrossRef]
- Ducloyer, M.; Gaborit, B.; Toquet, C.; Castain, L.; Bal, A.; Arrigoni, P.P.; Lecomte, R.; Clement, R.; Sagan, C. Complete post-mortem data in a fatal case of COVID-19: Clinical, radiological and pathological correlations. Int. J. Leg. Med. 2020, 134, 2209–2214. [Google Scholar] [CrossRef]
- Helmrich, E.; Decker, L.; Adolphi, N.; Makino, Y. Postmortem CT lung findings in decedents with Covid-19: A review of 14 decedents and potential triage implications. Forensic Imaging 2020, 23, 200419. [Google Scholar] [CrossRef]
- Filograna, L.; Grassi, S.; Manenti, G.; Di Donna, C.; Tatulli, D.; Nardoni, F.; Masini, V.; Ausania, F.; Grassi, V.M.; Floris, R.; et al. Postmortem CT pulmonary findings in SARS-CoV-2-positive cases: Correlation with lung histopathological findings and autopsy results. Int. J. Leg. Med. 2022, 136, 1407–1415. [Google Scholar] [CrossRef]
- Okumura, M.; Usumoto, Y.; Tsuji, A.; Kudo, K.; Ikeda, N. Analysis of postmortem changes in internal organs and gases using computed tomography data. Leg. Med. 2017, 25, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yajima, K.; Otaki, M.; Yoshikawa, Y.; Ishihara, A.; Sato, Y.; Higuchi, T.; Takatsuka, H. Postmortem volume change of the spleen and kidney on early postmortem computed tomography: Comparison with antemortem computed tomography. Jpn. J. Radiol. 2019, 37, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Gonoi, W.; Watanabe, Y.; Shirota, G.; Abe, H.; Okuma, H.; Shintani-Domoto, Y.; Tajima, T.; Fukayama, M.; Abe, O.; Ishida, M. Pulmonary postmortem computed tomography of bacterial pneumonia and pulmonary edema in patients following non-traumatic in-hospital death. Leg. Med. 2020, 45, 101716. [Google Scholar] [CrossRef]
- Shirota, G.; Gonoi, W.; Ikemura, M.; Ishida, M.; Shintani, Y.; Abe, H.; Fukayama, M.; Higashida, T.; Okuma, H.; Abe, O. The pseudo-SAH sign: An imaging pitfall in postmortem computed tomography. Int. J. Leg. Med. 2017, 131, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, D.; Cicero, G.; Asmundo, A.; Mondello, C.; Spagnolo, E.V.; Bottari, A.; Gaeta, M. Intraosseous gas distribution as a marker of postmortem interval. Forensic Imaging 2020, 23, 200414. [Google Scholar] [CrossRef]
- Sutherland, T.; O’Donnell, C. The artefacts of death: CT post-mortem findings. J. Med. Imaging Radiat. Oncol. 2017, 62, 203–210. [Google Scholar] [CrossRef]
- Rutty, G.N.; Morgan, B. Future evidence in forensic imaging. In P5 Medicine and Justice: Innovation, Unitariness and Evidence; Ferrara, S.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 576–585. [Google Scholar]
- Flach, P.M.; Thali, M.J.; Germerott, T. Times have changed! forensic radiology-a new challenge for radiology and forensic pathology. Am. J. Roentgenol. 2014, 202, 323–334. [Google Scholar] [CrossRef]
- Henningsen, M.J.; Harving, M.L.; Jacobsen, C.; Villa, C. Fractures of the neuro-cranium: Sensitivity and specificity of post-mortem computed tomography compared with autopsy. Int. J. Leg. Med. 2022, 136, 1379–1389. [Google Scholar] [CrossRef]
- Germerott, T.; Flach, P.M.; Preiss, U.S.; Ross, S.G.; Thali, M.J. Postmortem ventilation: A new method for improved detection of pulmonary pathologies in forensic imaging. Leg. Med. 2012, 14, 223–228. [Google Scholar] [CrossRef]
- Mentink, M.G.; Bakers, F.C.H.; Mihl, C.; Lahaye, M.J.; Rennenberg, R.J.M.W.; Latten, B.G.H.; Kubat, B.; Hofman, P.A.M. Introduction of postmortem CT increases the postmortem examination rate without negatively impacting the rate of traditional autopsy in daily practice: An implementation study. J. Clin. Pathol. 2021, 74, 177–181. [Google Scholar] [CrossRef]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; Henriques de Gouveia, R.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef] [PubMed]
- De Marco, E.; Vacchiano, G.; Frati, P.; la Russa, R.; Santurro, A.; Scopetti, M.; Guglielmi, G.; Fineschi, V. Evolution of post-mortem coronary imaging: From selective coronary arteriography to post-mortem CT-angiography and beyond. La Radiol. Med. 2018, 123, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Grabherr, S.; Jackowski, C.; Bollmann, M.D.; Doenz, F.; Mangin, P. Postmortem imaging of sudden cardiac death. Int. J. Leg. Med. 2013, 128, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Franckenberg, S.; Flach, P.M.; Gascho, D.; Thali, M.J.; Ross, S.G. Postmortem computed tomography-angiography (PMCTA) in decomposed bodies—A feasibility study. J. Forensic Radiol. Imaging 2015, 3, 226–234. [Google Scholar] [CrossRef]
- Grabherr, S.; Grimm, J.; Dominguez, A.; Vanhaebost, J.; Mangin, P. Advances in post-mortem CT-angiography. Br. J. Radiol. 2014, 87, 20130488. [Google Scholar] [CrossRef]
- Von Both, I.; Bruni, S.G.; Herath, J.C. Differentiation of antemortem pulmonary thromboembolism and postmortem clot with unenhanced MRI: A case report. Forensic Sci. Med. Pathol. 2018, 14, 95–101. [Google Scholar] [CrossRef]
- Burke, M.P.; Bedford, P.; Baber, Y. Can forensic pathologists diagnose pulmonary thromboembolism on postmortem computed tomography pulmonary angiography? Am. J. Forensic Med. Pathol. 2014, 35, 124–131. [Google Scholar] [CrossRef]
- Tian, Z.L.; Wang, Z.-Q.; Liu, N.-G.; Wan, L.; Huang, P.; Li, Z.-D.; Zou, D.-H.; Dong, H.-W.; Zhang, J.; Zhang, J.-H.; et al. Pulmonary PMCT angiography by right ventricle cardiac puncture: A novel, promising approach for investigating pulmonary thromboembolism. Int. J. Leg. Med. 2021, 135, 913–920. [Google Scholar] [CrossRef]
- Alves, M.; Bigé, N.; Maury, E.; Arrivé, L. Pulmonary embolism diagnosed by contrast-enhanced virtopsy. Am. J. Respir. Crit. Care Med. 2014, 189, 358–359. [Google Scholar] [CrossRef]
- Jackowski, C.; Grabherr, S.; Schwendener, N. Pulmonary thrombembolism as cause of death on unenhanced postmortem 3T MRI. Eur. Radiol. 2013, 23, 1266–1270. [Google Scholar] [CrossRef]
- Latten, B.G.H.; Bakers, F.C.H.; Hofman, P.A.M.; Hausen, A.Z.; Kubat, B. The needle in the haystack: Histology of post-mortem computed tomography guided biopsies versus autopsy derived tissue. Forensic Sci. Int. 2019, 302, 109882. [Google Scholar] [CrossRef] [PubMed]
- Blokker, B.M.; Weustink, A.C.; Wagensveld, I.M.; von der Thüsen, J.H.; Pezzato, A.; Dammers, R.; Bakker, J.; Renken, N.S.; den Bakker, M.A.; van Kemenade, F.J.; et al. Conventional autopsy versus minimally invasive autopsy with postmortem MRI, CT, and CT-guided biopsy: Comparison of diagnostic performance. Radiology 2018, 289, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Baier, W.; Mangham, C.; Warnett, J.M.; Payne, M.; Painter, M.; Williams, M.A. Using histology to evaluate micro-CT findings of trauma in three post-mortem samples—First steps towards method validation. Forensic Sci. Int. 2019, 297, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.C.; Shelmerdine, S.C.; Simcock, I.C.; Sebire, N.J.; Arthurs, O.J. Early clinical applications for imaging at microscopic detail: Microfocus computed tomography (micro-CT). Br. J. Radiol. 2017, 90, 20170113. [Google Scholar] [CrossRef]
- Orhan, K. Introduction to micro-CT imaging. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Orhan, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–5. [Google Scholar]
- Kanchan, T.; Shrestha, R.; Krishan, K. Post-mortem ultrasonography: A safer alternative to autopsies in COVID-19 deaths. J. Ultrasound 2021, 24, 577–578. [Google Scholar] [CrossRef]
- Uchigasaki, S. Postmortem ultrasound imaging in forensic pathology. In Forensic Pathology Reviews Volume 4; Tsokos, K., Ed.; Humana: Totowa, NJ, USA, 2006; pp. 405–412. [Google Scholar]
- Usui, A.; Kawasumi, Y.; Usui, K.; Ishizuka, Y.; Takahashi, K.; Funayama, M.; Saito, H. Postmortem computed tomographic analysis of death caused by oral drug intoxication. Tohoku J. Exp. Med. 2017, 242, 183–192. [Google Scholar] [CrossRef]
- Chatzaraki, V.; Heimer, J.; Thali, M.; Dally, A.; Schweitzer, W. Role of PMCT as a triage tool between external inspection and full autopsy—Case series and review. J. Forensic Radiol. Imaging. 2018, 15, 26–38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).