Abstract
Forensically important blow flies, Diptera: Calliphoridae, are among the first organisms to colonize carrion. After eggs hatch, the larvae of most blow fly species feed in an aggregation or “mass”. While in this mass larvae may experience periods of hypoxia/anoxia, but the tolerance of blow fly larvae to anoxic conditions is poorly studied. We tested the anoxia tolerance of four species of calliphorids (Calliphora vicina, Cochliomyia macellaria, Lucilia sericata, and Phormia regina), by examining actively feeding third-stage larvae across five temperatures. Experiments were conducted by exposing larvae to pure nitrogen environments and determining mortality at set time intervals. All species show significant linear relationships between survival time and temperature under anoxic conditions. Of species tested, C. macellaria had the greatest tolerance to anoxia (LT50 of 9 h at 20 °C). In contrast, C. vicina was the least tolerant (LT50 of 2.2 h at 40 °C). With all species, survivorship decreased with increasing temperature. Unlike many other insects tested in severe hypoxia, the larvae of the calliphorids tested, which included members of three subfamilies, were not tolerant of anoxic conditions. From these findings, it seems likely that hypoxia is a significant limitation for maggots in a maggot mass, particularly when the mass temperature is high (>40 °C). Forensically, these data provide a limit on potential maggot survival on bodies that have been submerged or otherwise experience severe hypoxia before discovery.
1. Introduction
When an animal dies, an intense competition for the nutrients contained in its body commences. Within minutes of death, internal bacteria proliferate creating volatiles that are attractive to necrophores including vertebrate scavengers, and a predictable succession of insects [,]. As the animal decomposes, it creates a nutrient island that provides ecological niches for the organisms and microbes that utilize its resources. Beyond these important ecological roles, understanding the ecology of human decomposition has proven essential as a technique for estimating time since death in some criminal investigations [,].
In most instances, excluding winter and periods of cold temperatures, blow flies, Diptera: Calliphoridae, are the first insects to arrive at carrion []. Typically, female calliphorids lay eggs on carrion within hours after death. These eggs hatch into maggots, which rapidly develop through two stages, and molt into a third stage where most feeding occurs. On larger carcasses, blow fly larvae form an aggregation, or “mass”, in which they feed. By the third stage this maggot mass can be substantially warmer than ambient temperatures (e.g., 10 °C or more) and the maggots continue to feed until most of the soft tissue has been consumed [,].
Because blow fly larvae have mouthparts consisting only of small hooks, they secrete extra-oral enzymes to help soften and digest the carrion. These oral secretions also may have antibiotic properties, suggesting that maggots compete with bacteria for access to carrion []. These aspects of blow fly larval life history are important when considering why maggots form masses. One hypothesis is that the mass improves feeding efficiency, because the accumulated action of oral secretions works synergistically in breaking down tissue []. Another, non-exclusive, hypothesis is that maggot masses increase maggot development rates by increasing temperature []. Faster development may be beneficial in giving larvae a competitive advantage in using an ephemeral resource []. However, conditions in a mass also appear to require individuals to move despite the presumption that maggot movement would be minimal, because it delays feeding and has an energetic cost [].
Several potential disadvantages are likely associated with aggregation. Temperatures in the center of a mass can reach or exceed lethal limits of a species [,]. In addition, the feeding area under the mass usually becomes so liquefied that feeding larvae can be completely submerged, creating potential drowning. Finally, the high rates of insect and bacterial metabolism also generate high concentrations of carbon dioxide (hypercapnia) and limited oxygen availability (hypoxia) or even anoxia [].
Individual maggots within a mass rotate frequently [], which is a key indication that one or more limiting factors occur within maggot masses. In addition to avoiding lethal temperatures, movement could be associated with the need to obtain oxygen and/or avoid high concentrations of carbon dioxide which can cause death [,].
Hoback and Stanley [] reviewed anoxia tolerance among insects and showed that a number of terrestrial insects are adapted to survive hypoxic conditions. Some larval flies, including Chironomidae and Gastrophillidae, show considerable adaptation to low oxygen; however, relatively little is known about response of Calliphoridae larvae to severe hypoxia or anoxia. Keister and Buck [] performed some of the earliest experiments on Phormia regina life stages and found metabolism to be oxygen dependent at oxygen concentrations between 0–21%. Metabolically active stages had lower tolerance to hypoxic conditions and third stage larvae were most sensitive []; however, survival times were not reported.
More-recently, Singh and Bala [] examined survival of calliphorid maggots submerged in water. Two species in the Genus Chrysomya died within 5 h with older larvae surviving slightly longer than earlier stages at 26 °C. With some terrestrial insect species, dissolved oxygen increases survival when submerged, while in others, there is no effect []. Because the liquid at a carcass is likely to contain no oxygen, it is important to characterize blowfly tolerance to severe hypoxia and test responses across other forensically important species at different temperatures. We tested the hypothesis that survival times in anoxic conditions is inversely related to temperature for four North American calliphorid species.
2. Materials and Methods
2.1. Flies and Rearing Conditions
Four species of calliphorids were used in experiments: Calliphora vicina, Cochliomyia macellaria, Lucilia sericata, and Phormia regina. These species are among the most common and forensically important blow flies in North America [], and all four species routinely produce maggot masses on carrion [].
All flies used in the experiments were from colonies maintained in the laboratory. These colonies were established and have been maintained to minimize genetic variation within the colony. Our purpose in this effort is to obtain genetic homogeneity among test subjects, so we can get an indication of physiological variation in response without confounding from population variation. Thus, results here are intended as a baseline against which potential variation among populations can be tested. The chief danger in using such inbred lines experimentally is the potential for inadvertent selection. With insects, inadvertent selection in colonies most frequently occurs in oviposition behavior and in reduced fecundity, however, no indications of change in either of these factors were observed in any of our colonies over many generations.
The C. vicina colony was established in October 2012 from a single field-collected female from Lincoln, NE. At the time of these experiments the colony had been maintained through a minimum of 20 generations. The C. macelleria colony was established in August 2011 from a single female, collected from the field in Lincoln, NE. At the time of experiments the colony had been maintained through a minimum of 75 generations. The L. sericata colony was established from insects provided by Dr. Jeff Wells (at West Virginia University) in Oct. 2010, and this colony was established with field-collected insects from near Morgantown, West Virginia. At the time of experiments our colony had been maintained through a minimum of 100 generations. The P. regina colony was established in Aug. 2011 from a single female field-collected from Lincoln, NE. At the time of experiments the colony had been maintained through a minimum of 75 generations.
Adult flies were maintained in cages in a rearing room with temperature maintained at 27.5 °C (±3 °C), with a 16:8 light:dark cycle. Multiple generations were maintained in a single cage, and ca. 1000 adult flies were introduced every 1–2 wk (adult lifespan of the flies in colony is approximately one month). Adults were provided sugar water as a carbohydrate source, and raw beef liver for protein and as an ovipositional substrate. After egg-laying, eggs and liver were maintained in 1.7 L plastic boxes in I30-BLL Percival biological incubators (Percival Scientific, Inc., Perry, IA, USA) set at 26 °C (which was ± 1.5 °C of this set temperature based on internal temperature measurements). Within the plastic box, liver and feeding maggots were placed in a smaller 0.8 L plastic cup, which rested on pine shavings. The pine shavings provided an area for larval migration at the end of the third larval stage and as a substrate for pupation (larvae bury themselves within the pine shavings after migratory movement).
2.2. Experimental Design and Conditions
A series of preliminary experiments were conducted to determine the potential range of survival times, best method for producing anoxic conditions, and methods to minimize mortality in controls. From these trials, final protocols were established.
All experiments were conducted with feeding, third-stage larvae (typically collected from colonies 3–5 d after molting). The experimental unit was a vial with one third-stage larva. The experimental design was a factorial arrangement of oxygen environment x temperature, with five replications. Treatments were with oxygen (normoxic, the controls) and without oxygen (anoxic, nitrogen gas only). Temperature treatments were 20, 25, 30, 35, and 40 °C. Treatments were evaluated by sampling at set (1 h) intervals, so a complete set of experimental units (2 treatments × 5 replications) were used for each sampling period. In principle, a total of 10 sampling periods were anticipated, requiring 50 experimental units per species-temperature combination. In practice, 100% mortality usually occurred well before 10 h, and experiments were terminated when 100% mortality in the anoxia treatments occurred.
The treatment (anoxic) vials contained N2(g). The N2(g) was introduced into XX ml glass screwcap vials by first submerging the vial completely in water and then streaming the N2(g) into the vial while it was upside down until all the air was displaced. Then, the maggot was placed in the vial, while it was still upside down, under the water, and the vial was capped. The control (normoxic) vials were submerged under water as well, lifted out to remove the water, the maggot was placed into the vial, and then the vial was capped. Small volumes of water were left in both the control and treated vials (<2 mL) which helped minimize any desiccation of the maggots.
Temperature treatments were established in incubators. Our incubators were customized model SMY04-1 DigiTherm® CirKinetics Incubators (TriTech Research, Inc., Los Angeles, CA, USA). The DigiTherm® CirKinetics Incubator has microprocessor-controlled temperature regulation, internal lighting, recirculating air system (to help maintain humidity), and use a thermoelectric heat pump (rather than coolant and condenser as is typical with larger incubators and growth chambers). Our customizations included addition of a data port, vertical lighting (so all shelves were illuminated), and an additional internal fan. The manufacturer’s specifications indicate an operational range of 10–60 °C ± 0.1 °C.
Often anoxia measurements are conducted in water baths to ensure constant temperature, and in our initial trials we compared water bath treatments to our incubators. The great advantage with incubators was that we could examine all treatments for a given species-temperature combination simultaneously. Although growth chambers have been shown to display substantial differences between programmed temperatures and actual internal temperatures [], A. Fujikawa [] tested the incubators with internal thermocouples in a replicated study and determined that internal temperatures on all shelves within incubators never varied more than 0.1 °C from the programmed temperature, in agreement with the manufacturer’s specifications [].
Each vial contained one larva so that individual variation in response could be measured. Treatments were placed in incubators in a completely randomized order. Each hour a subset of five larvae were removed. Each maggot was removed from the vial and checked for movement. Because other insects that appear lifeless can recover from anoxia, the maggot was placed back into the vial, which was left open to the air, capped to maintain humidity, and rechecked 24 h later []. Scores of no movement for the initial removal and 24 h post-removal resulted in a response of dead, while maggots that had either initial movement or movement at the 24 h check, were recorded as alive. Sampling continued until all maggots were recorded as no movement in the initial test. Throughout our experiments, no maggots recorded as dead at the first check recovered after 24 h.
2.3. Analysis
Although our experimental design follows a factorial treatment arrangement (oxygenation × temperature) which might imply use of analysis of variance, the idea that anoxia response differs with temperature was not central to the experiments. Instead, the key questions were how survivorship differed at different temperatures, and what was the mathematical relationship between anoxia survivorship and temperature.
To address the question of survivorship, we used Kaplan–Meier survivorship analysis through the Life module of the XLSTAT (Addinsoft, Inc., Paris, France) plugin to Microsoft Excel 2010 (Microsoft, Inc., Redmond, WA, USA). As part of the Kaplan–Meier analysis XLSTAT provides comparisons of survivorship distribution functions through three different tests: Log-rank, Wilcoxon, and Tarone-Ware tests. The appropriateness of these comparisons depends upon when most mortality occurs, and as we had no prior indication of the nature of our survivorship curves, we decided to use all three tests in evaluating differences in distribution functions. Because the control would necessarily be different from treatments (i.e., the control survivorship “curve” should be a flat line at 1.0), control data were excluded from comparisons of survivorship distribution functions.
For regression analyses, we chose the LT25, LT50, and LT75 values (the lethal temperatures at 25%, 50%, and 75% of the population) as the most appropriate response variables (in the literature the LT50 is most commonly used), and we conducted regression analyses with Prism 6.0 software (Graphpad Software, San Diego, CA, USA). We chose these values to provide an indication of potential population variability in response. In these analyses we used ‘runs testing’ to identify potential departures from linearity.
3. Results
Proportional survival in anoxia for larvae of all four calliphorid species were determined at five temperatures, 20–40 °C (Figure 1 and Figure 2). Control treatments showed essentially no mortality over the period of the experiments; consequently, no corrections were made in analyzing survival in anoxia.
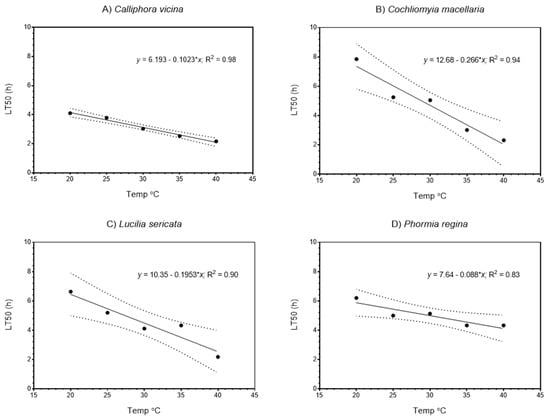
Figure 1.
Relationships between LT50 (lethal time to 50% survivorship) and temperature (°C) for four species of blow fly: (A) Calliphora vicina, (B) Cochliomyia macelleria, (C) Lucilia sericata, and (D) Phormia regina. Dotted lines indicate 95% confidence limits.
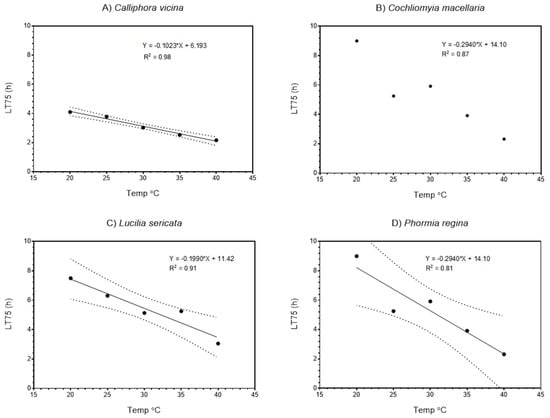
Figure 2.
Relationships between LT75 (lethal time to 75% survivorship) and temperature (°C) for 3rd-stage larvae of four species of blow fly: (A) Calliphora vicina, (B) Cochliomyia macelleria, (C) Lucilia sericata, and (D) Phormia regina. Dotted lines indicate 95% confidence limits.
As expected, survival was substantially greater at lower temperatures than higher temperatures. The range of survival times across species and temperature were a minimum of 2.2 h at 40 °C for C. vicina (Figure 1a) and a maximum of 9.0 h at 20 °C for C. macellaria (Figure 1a). Most survivorship distributions differed with temperature (Figure 1 and Figure 2), however, with P. regina survivorship distributions were more similar than those with other species, and the end point (0% survivorship) occurred at ca. 6 h, irrespective of temperature (Figure 1b).
Tests of differences among survival distributions within species are shown in Table 1 and Table 2. Because identifying differences among survival distributions depends on assumptions regarding when deaths occur, different hypothesis testing procedures are used for different assumptions. To avoid potential bias, we used log-rank, Wilcoxon, and Tarone-Ware non-parametric tests to examine potential differences, specifically. As Table 1 indicates, differences in survival distributions (excluding the control) were observed with all three tests for C. vicina, C. macellaria, and L. sericata (p < 0.0001). In contrast, P. regina showed no differences in survival distribution functions, indicating that the pattern of survival was similar across tested temperatures.

Table 1.
Tests of equality of survival distribution functions by Log-rank, Wilcoxon, and Tarone-Ware tests. Survival distribution functions from Kaplan–Meier survivorship analysis of anoxia survivorship by time at different temperatures, for 3rd-stage larvae of each of four species: Calliphora vicina, Cochliomyia macelleria, Lucilia sericata, and Phormia regina.

Table 2.
Estimates of LT50 and LT75 (lethal time to 50% or 75% survivorship) and 95% confidence limits, at 5 temperatures for 3rd-stage larvae under anoxia for each of 4 species: Calliphora vicina, Cochliomyia macelleria, Lucilia sericata, and Phormia regina.
Table 2 shows LT25, LT50, and LT75 values for each species, where LT refers to the lethal time to a given % mortality. A significant linear relationship between LT50 and temperature was observed in all four species (Figure 1), with R2 varying between 0.98 and 0.83 among species. Very similar linear relationships were observed for LT75 (Figure 2). For both LT50 and LT75 runs testing was used to determine if significant non-linearity occurred; these tests were not significant for any species.
Table 3 reports mean survival times, however, given the substantial variation in these times across temperatures, the means are much less informative than LT50 and LT75 data.

Table 3.
Estimates of mean survival times, standard deviation, and 95% confidence limits, at 5 temperatures for 3rd-stage larvae under anoxia for each of 4 species: Calliphora vicina, Cochliomyia macelleria, Lucilia sericata, and Phormia regina.
4. Discussion
Third stage maggots of the four calliphorid species tested showed relatively limited abilities to tolerate anoxia. At temperatures associated with maggot masses (typically in excess of 30 °C), none of the species survived anoxia longer than 6.5 h. Moreover, at higher temperatures, survival times were much more limited (ca. 2–3 h).
Although some differences may exist in anoxia tolerance among species (e.g., Figure 1 and Figure 2, slopes of LT50 and LT75 versus temperature of C. macellaria compared with slopes for other species), species generally had similar relationships. Species differences in anoxia tolerance are likely related to differences in metabolic rates (i.e., metabolic demand for oxygen) and their potential for anaerobic respiration. The species used represent various subfamilies of the Calliphoridae; specifically, Calliphorinae (C. vicina), Chrysomyinae (C. macellaria and P. regina), and Luciliinae (L. sericata). Because the variation within the Chrysomyinae (slopes of C. macellaria versus P. regina) is greater than that observed among subfamilies, it seems likely that the responses observed here may be broadly characteristic of the Calliphoridae. Indeed, our results are like those obtained from Chrysomya megacephala and Chrysomya rufifacies in water at 26 °C [] suggesting that submersion and atmospheric anoxia quickly result in mortality for metabolically active larvae.
Certainly, the relatively low tolerance to anoxia seen here could contribute to the need for larval movement in maggot masses. Tiger beetle (Coleoptera: Carabidae) have been model organisms for examining the effects of flood-induced hypoxia on terrestrial insects. Larvae of many species face occasional prolonged hypoxia during habitat flooding. In response to anoxia, larvae exhibit metabolic depression of more than 98% and surviving larvae appear dead and require many hours to resume normal activity []. Population differences have been observed between species that are flooded daily by ocean tides and those flooded occasionally by river flooding with the larvae flooded daily surviving shorter periods and recovering more quickly from exposure to anoxia [].
Within a mass, larvae face potentially lethal temperatures [], submersion in severely hypoxic liquid, and atmospheres with high levels of CO2. The very short survival times of maggots tested here appears to be explained by the need for third stage maggots to maintain active metabolism and be able to escape lethal conditions.
The anoxia tolerances observed here set one limit to the ability of larvae to remain while in a mass. Additionally, maggot masses can reach temperatures exceeding 45 °C, and at these temperatures maggots clearly have limited ability to withstand prolonged anoxia. Maggot mass temperatures at or above 45 °C are near lethal limits for many species, however, it is noteworthy that in our experiments, we saw virtually no mortality in control treatments even at temperatures of 40 °C. Like anoxia tolerance, temperature tolerance is a function of time of exposure, and the environmental cues for maggot movement could represent a combination of hypoxia and temperature and more research, including determination of larval cooling through evaporation in a mass is needed [].
Figure 1 and Figure 2 show strong linear relationships between survival times and temperature. These data also illustrate variation in responses. Because we deliberately tested maggots from flies with presumably uniform genetic backgrounds, the variation we observed is not attributable to underlying genetic differences. Instead, this variation represents the intrinsic physiological variation associated with anoxia tolerance. Naturally, we would expect greater variation to be observed were we to conduct the same tests with wild flies (i.e., flies with greater genetic variability). However, we would not expect the underlying linear relationship to be appreciably different (given that linear relationships were observed in all species, across subfamilies of Calliphoridae).
One possible forensic application of these findings pertains to bodies with maggots found in conditions where anoxia or hypoxia is expected. For example, if a submerged body is found with live maggots, based on survival time-temperature relationships determined here, we could calculate a limit on the time of submergence (given the temperature of the water in which the body was found). As a rule of thumb, we would not expect to find live maggots on bodies that had been submerged longer than 10 h after colonization, even at temperatures below 20 °C (based on extrapolations of the linear models in Figure 1 and Figure 2). Indeed,
Regarding the central question of the role of anoxia tolerance in behavior of third stage larvae in maggot masses, various conclusions emerge from this study. First, anoxia tolerance among the blow fly species tested shows that a maggot could not remain in anoxic or hypoxic conditions for extended periods (more than a few hours). Therefore, access to oxygen is necessary while feeding, and some movement by maggots in a mass is likely associated with oxygen access (assuming hypoxic/hypercapnic conditions exist in maggot masses, which needs to be further established).
Second, in comparing the relative important and potential interaction of oxygen access and thermoregulation as factors in maggot behavior while in a maggot mass, these results show that the influence of oxygen deficiency occurs over a time frame of only a few hours. If thermoregulatory responses occur at a similar time frame, then it seems likely these factors interact to influence larval movement. In contrast, if thermoregulatory responses occur at a shorter time frame (minutes rather than hours), then temperature would be the key influence on larval movement in a maggot mass [].
Third, the variation in survival times we observed within species and experiments was unexpected given the lack of genetic variation in our experimental fly populations. Because oxygen use is directly tied to metabolism in Phormia [], the simplest explanation for our observations is that there are metabolic differences among individuals of the same species and with similar genetics. Presumably these differences arise from individual differences in feeding (specifically, variation in how much food has been consumed). Although blow flies are among the first insects to arrive at carrion and seem optimized for rapid development (because they are feeding on what is a transient resource), our results suggest feeding behavior is not optimized—all maggots may not feed as fast as they could. If this conclusion regarding variability in feeding rates proves accurate, then interesting possibilities emerge: is variation in larval feeding rates a reflection of intraspecific competition, is variation a consequence of differences in thermogulatory movement (perhaps maggots close to the center of a mass feed less and move more because they experience higher temperatures), or is variation in feeding somehow evolutionarily advantageous, perhaps as a form of spreading the risk to avoid developmental synchrony that might benefit parasites or predators [].
Additional research should also examine survival times of other stages of forensically important species. After feeding, maggots enter a wandering stage prior to pupation, and it is possible that reduced metabolism for this non-feeding stage would increase survival times. Indeed, Cavallero and Hoback [] found differences in survival between aquatic and terrestrial stages of a caddisfly. Interestingly, they also found that the pupal stage was most sensitive to hypoxia. Among forensically important fly species, survival times of immersion for pupae of Lucilia sericata and Calliphora vomitoria were tested by Magni et al. []. The authors tested survival in both fresh and salt water and found survival of some individuals of more than 120 h with LC50 times of around 8 to 9 h. In a forensic context, storage of larvae or pupae in a hermetic container may not result in immediate death [] and additional research should examine whether fly stages survive for some period in preservative which could influence PMI estimates.
Author Contributions
Conceptualization, M.L.A. and L.G.H.; methodology, L.G.H. and W.W.H.; formal analysis, M.L.A. and L.G.H.; investigation, M.L.A.; resources, L.G.H.; data curation, L.G.H.; writing—original draft preparation, M.L.A.; writing—review and editing, L.G.H. and W.W.H.; visualization, M.L.A. and L.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institute of Justice, Office of Justice Programs, U.S. Department of Justice grant number 2010-DN-BX-K231.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data reported are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iancu, L.; Dean, D.E.; Purcarea, C. Temperature Influence on Prevailing Necrophagous Diptera and Bacterial Taxa with Forensic Implications for Postmortem Interval Estimation: A Review. J. Med. Entomol. 2018, 55, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Factors That Influence Insect Succession on Carrion. In Forensic Entomology; CRC Press: Boca Raton, FL, USA, 2019; pp. 103–139. [Google Scholar] [CrossRef]
- Tibbett, M.; Carter, D.O. Soil Analysis in Forensic Taphonomy: Chemical and Biological Effects of Buried Human Remains; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gennard, D.E. Forensic Entomology: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Higley, L.G.; Haskell, N.H. Insect development and forensic entomology. In Forensic Entomology: The Utility of Arthro-Pods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 389–405. [Google Scholar]
- Heaton, V.; Moffatt, C.; Simmons, T. The movement of fly (Diptera) larvae within a feeding aggregation. Can. Èntomol. 2018, 150, 326–333. [Google Scholar] [CrossRef]
- Greenberg, B. Flies as Forensic Indicators. J. Med. Èntomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Green, P.W.C.; Simmonds, M.S.J.; Blaney, W.M. Diet nutriment and rearing density affect the growth of black blowfly larvae, Phormia regina (Diptera: Calliphoridae). Eur. J. Èntomol. 2003, 100, 39–42. [Google Scholar] [CrossRef]
- Johnson, A.P.; Wallman, J.F. Effect of massing on larval growth rate. Forensic Sci. Int. 2014, 241, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Èntomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Van Der Linde, T.C.; Anderson, G.S. The Influence of Clothing and Wrapping on Carcass Decomposition and Arthropod Succession During the Warmer Seasons in Central South Africa. J. Forensic Sci. 2009, 54, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Frazier, M.R.; Henry, J.R.; Kaiser, A.; Klok, C.; Rascón, B. Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 2006, 154, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.C.; Barnhart, M.C.; Hoback, W.W. Causes of Rapid Carrion Beetle (Coleoptera: Silphidae) Death in Flooded Pitfall Traps, Response to Soil Flooding, Immersion Tolerance, and Swimming Behavior. Environ. Èntomol. 2017, 46, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Hoback, W.; Stanley, D. Insects in hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Keister, M.; Buck, J. Respiration of Phormia regina in relation to temperature and oxygen. J. Insect Physiol. 1961, 7, 51–72. [Google Scholar] [CrossRef]
- Singh, D.; Bala, M. Larval survival of two species of forensically important blowflies (Diptera: Calliphoridae) after submergence in water. Èntomol. Res. 2011, 41, 39–45. [Google Scholar] [CrossRef]
- Haskell, N.A.; Williams, R.E. Entomology and Death: A Procedural Guide, 2nd ed.; East Park Printing: Clemson, SC, USA, 2008. [Google Scholar]
- Nabity, P.D.; Higley, L.G.; Heng-Moss, T.M. Light-induced variability in the development of the forensically important blow fly, Phormia regina (Diptera: Calliphoridae). J. Med. Entomol. 2007, 44, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Roe, A. (College of Saint Mary, Omaha, NE, USA). Personal Communication, 2020.
- Brust, M.L.; Hoback, W.W. Hypoxia Tolerance in Adult and Larval Cicindela Tiger Beetles Varies by Life History but Not Habitat Association. Ann. Èntomol. Soc. Am. 2009, 102, 462–466. [Google Scholar] [CrossRef]
- Hoback, W.W.; Podrabsky, J.E.; Higley, L.G.; Stanley, D.W.; Hand, S.C. Anoxia tolerance of con-famial tiger beetle larvae is associated with differences in energy flow and anaerobiosis. J. Comp. Physiol. B 2000, 170, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.L.; Hoback, W.W.; Skinner, K.M.; Knisley, C.B. Differential immersion survival by populations of Cicindela hirticollis Say (Coleoptera: Cicindelidae). Ann. Entomol. Soc. Am. 2005, 98, 973–979. [Google Scholar] [CrossRef]
- Cavallaro, M.C.; Hoback, W.W. Hypoxia Tolerance of Larvae and Pupae of the Semi-Terrestrial Caddisfly (Trichoptera: Limnephilidae). Ann. Èntomol. Soc. Am. 2014, 107, 1081–1085. [Google Scholar] [CrossRef]
- Mądra-Bielewicz, A.; Frątczak-Łagiewska, K.; Matuszewski, S. Blow fly puparia in a hermetic container: Survival under decreasing oxygen conditions. Forensic. Sci. Med. Pathol. 2017, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Reigada, C.; Gião, J.Z.; Galindo, L.A.; Godoy, W.A.C. Survival of submerged blowfly species and their parasitoids: Implications for postmortem submersion interval. Forensic. Sci. Intl. 2011, 212, 126–129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).