A Reflective Spectroscopy Proof-of-Concept Study of Urea for Supporting Investigations of Human Waste in Multiple Forensic Contexts
Abstract
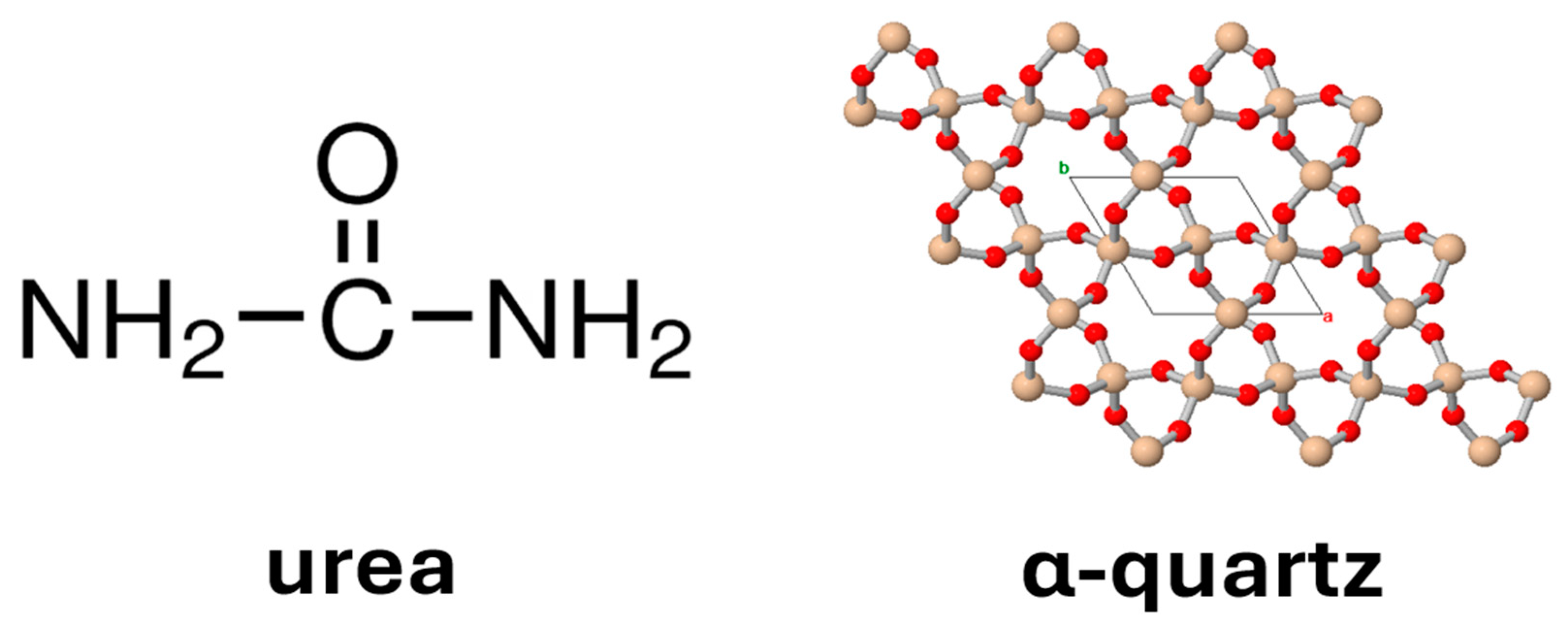
:1. Introduction
2. Materials
3. Methods
3.1. Grain Size Characteristics
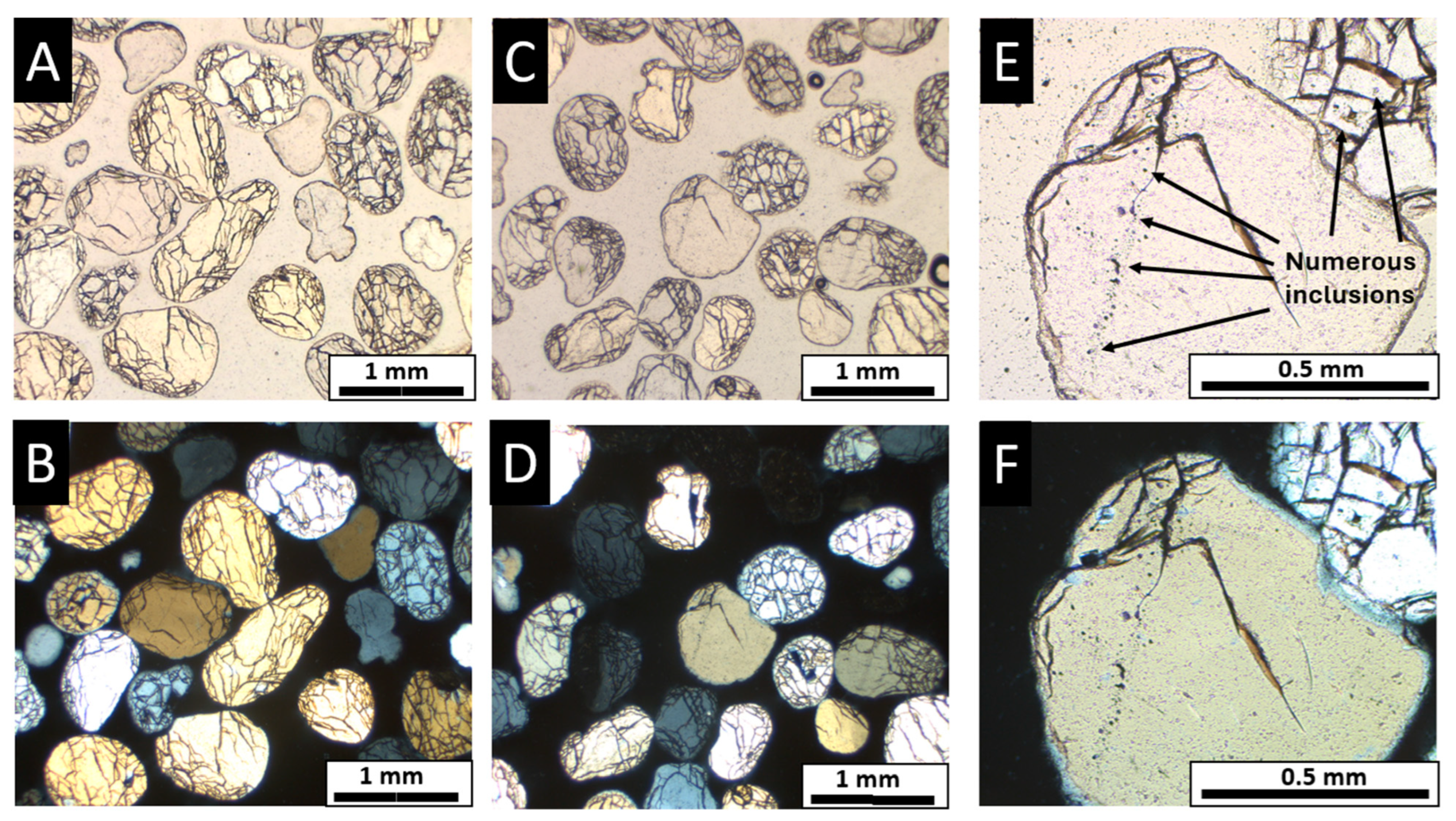
3.2. Polarized Light Microscopy (PLM)
3.3. Scanning Electron Microscopy and Energy Dispersive Spectroscopy
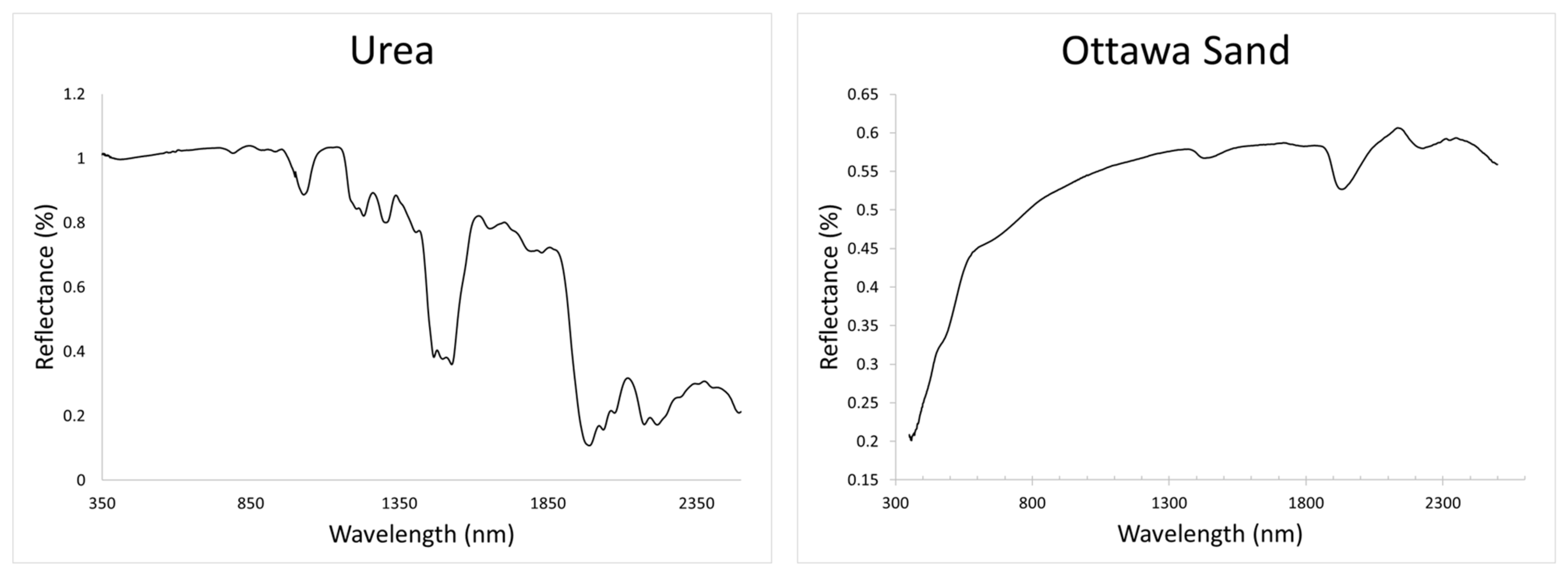
3.4. Reflective Spectroscopy
3.5. Bond Assignments
3.6. Experimental
4. Results
4.1. Ottawa Sand
4.2. Urea Experiments
5. Discussion
5.1. Selection of Materials
5.2. Comments Regarding the Ottawa Sand
5.3. Comments Regarding the Urea Experiments
5.4. Implications for Supporting HRS/IS Approaches for Locating Persons
5.5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.S.; Krekeler, M.P.S. Crude oil, petroleum and water discrimination on terrestrial substrates with airborne imaging spectroscopy. In Active and Passive Signatures, Proceedings of the SPIE Defense, Security, and Sensing, Orlando, FL, USA, 11–29 April 2011; International Society for Optics and Photonics: San Diego, CA, USA, 2011; Volume 8040. [Google Scholar] [CrossRef]
- Aumann, H.H.; Gregorich, D.; Gaiser, S. AIRS hyper-spectral measurements for climate research: Carbon dioxide and nitrous oxide effects. Geophys. Res. 2011, 32, L05806. [Google Scholar] [CrossRef]
- Cracknell, M.J.; Reading, A.M. Geological mapping using remote sensing data: A comparison of five machine learning algorithms, their response to variations in the spatial distribution of training data and the use of explicit spatial information. Comput. Geosci. 2014, 63, 22–33. [Google Scholar] [CrossRef]
- Crouvi, O.; Ben-Dor, E.; Beyth, M.; Avigad, D.; Amit, R. Quantitative mapping of arid alluvial fan surfaces using field spectrometer and hyperspectral remote sensing. Remote Sens. Environ. 2016, 104, 103–117. [Google Scholar] [CrossRef]
- Hörig, B.; Kühn, F.; Oschütz, F.; Lehmann, F. HyMap hyperspectral remote sensing to detect hydrocarbons. Int. J. Remote Sens. 2001, 22, 1413–1422. [Google Scholar] [CrossRef]
- Krekeler, M.P.S.; Allen, C.S. Remote sensing spectra of cesium chloride provide a potential emergency management tool for response to a radiological dispersal device detonation. J. Emerg. Manag. 2008, 6, 60–64. [Google Scholar] [CrossRef]
- Kruse, F.A.; Boardman, J.A.; Huntington, J.F. Comparison of airborne hyper- spectral data and EO-1 hyperion for mineral mapping. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1388–1400. [Google Scholar] [CrossRef]
- Mars, J.C.; Crowley, J.K. Mapping mine wastes and analyzing areas affected by selenium-rich water runoff in southeast Idaho using AVIRIS imagery and digital elevation data. Remote Sens. Environ. 2003, 84, 422–436. [Google Scholar] [CrossRef]
- Swayze, G.A.; Smith, K.S.; Clark, R.N.; Sutley, S.J.; Pearson, R.M.; Vance, J.S.; Hageman, P.L.; Briggs, P.H.; Meier, A.L.; Singleton, M.J.; et al. Using imaging spectroscopy to map acidic mine waste. Environ. Sci. Technol. 2000, 34, 47–54. [Google Scholar] [CrossRef]
- Ustin, S.L.; Valko, P.G.; Kefauver, S.C.; Santos, M.J.; Zimpfer, J.F.; Smith, S.D. Remote sensing of biological soil crust under simulated climate change manipulations in the Mojave Desert. Remote Sens. Environ. 2009, 113, 317–328. [Google Scholar] [CrossRef]
- Valdovinos, M.; Specht, J.; Zeunik, J. Community Policing & Unmanned Aircraft Systems (UAS) Guidelines to Enhance Community Trust. 2016. Available online: https://portal.cops.usdoj.gov/resourcecenter/RIC/Publications/cops-w0822-pub.pdf (accessed on 1 July 2024).
- Karaca, Y.; Cicek, M.; Tatli, O.; Sahin, A.; Pasli, S.; Beser, M.F.; Turedi, S. The potential use of unmanned aircraft systems (drones) in mountain search and rescue operations. Am. J. Emerg. Med. 2018, 36, 583–588. [Google Scholar] [CrossRef]
- Eyerman, J.; Crispino, G.; Zamarro, A.; Durscher, R. Drone Efficacy Study (DES): Evaluating the Impact of Drones for Locating Lost Persons in Search and Rescue Events; DJI and European Emergency Number Association: Brussels, Belgium, 2018; Available online: https://unode1.s3.amazonaws.com/assets/6946/VA4uymUuQYaFmDMt9jLA_Durscher_Romeo_08222019_v1Handout_5.pdf (accessed on 1 July 2024).
- Goda, N.; Soules, J. Testing AI-Enabled Drones for Search and Rescue. 2024. Available online: https://www.colorado.edu/today/2024/06/14/testing-ai-enabled-drones-search-and-rescue (accessed on 1 July 2024).
- O’Donnell, J. AI-Directed Drones Could Help Find Lost Hikers Faster. 2024. Available online: https://www.technologyreview.com/2024/05/30/1092988/ai-directed-drones-could-help-find-lost-hikers-faster/ (accessed on 1 July 2024).
- Krekeler, M.P.S.; Burke, M.; Allen, S.; Sather, B.; Chappell, C.; McLeod, C.L.; Loertscher, C.; Loertscher, S.; Dawson, C.; Brum, J.; et al. A novel hyperspectral remote sensing tool for detecting and analyzing human materials in the environment: A geoenvironmental approach to aid in emergency response. Environ. Earth Sci. 2023, 82, 109. [Google Scholar] [CrossRef]
- Lim, H.T.; Murukeshan, V.M. Hyperspectral imaging of polymer banknotes for building and analysis of spectral libraries. Opt. Las. Eng. 2017, 98, 168–175. [Google Scholar] [CrossRef]
- Silva, C.S.; Pimentel, M.F.; Amigo, J.M.; Honorato, R.S.; Pasquini, C. Detecting semen stains on fabrics using near infrared hyperspectral images and multivariate models. TRAC-Trend. Anal. Chem. 2017, 95, 23–35. [Google Scholar] [CrossRef]
- Cadd, S.; Li, B.; Beveridge, P.; O’Hare, W.T.; Islam, M. Age determination of blood stained fingerprints using visible wavelength reflectance hyperspectral imaging. J. Imaging 2018, 4, 141. [Google Scholar] [CrossRef]
- De Carvalho, M.A.; Talhavini, M.; Pimentel, M.F.; Amigo, J.M.; Pasquini, C.; Alves, S.; Weber, I.T. NIR hyperspectral images for identification of gunshot residue from tagged ammunition. Anal. Methods UK 2018, 10, 4711–4717. [Google Scholar] [CrossRef]
- Edelman, G.J.; Aalders, M.C. Photogrammetry using visible, infrared, hyper- spectral and thermal imaging of crime scenes. Forensic Sci. Int. 2019, 292, 181–189. [Google Scholar] [CrossRef]
- Glomb, P.; Romaszewski, M.; Cholewa, M.; Domino, K. Application of hyper- spectral imaging and machine learning methods for the detection of gunshot residue patterns. Forensic Sci. Int. 2018, 290, 227–237. [Google Scholar] [CrossRef]
- Khan, M.J.; Khan, H.S.; Yousaf, A.; Khurshid, K.; Abbas, A. Modern trends in hyperspectral image analysis: A review. IEEE Access 2018, 6, 14118–14129. [Google Scholar] [CrossRef]
- Murray, B.; Anderson, D.T.; Wescott, D.J.; Moorhead, R.; Anderson, M.F. Survey and Insights into unmanned aerial-vehicle-based detection and documentation of clandestine graves and human remains. Hum. Biol. 2018, 90, 45–61. [Google Scholar] [CrossRef]
- Brito, L.R.E.; Braz, A.; Honorato, R.S.; Pimentel, M.F.; Pasquini, C. Evaluating the potential of near infrared hyperspectral imaging associated with multivariate data analysis for examining crossing ink lines. Forensic Sci. Int. 2019, 298, 169–176. [Google Scholar] [CrossRef]
- Xu, J.Y.; Fang, S.B.; Zhou, J. Application of hyperspectral imaging and mass spectrometry imaging technique to fingerprinting visualization and trace analysis. Acta Phys. Sin. 2019, 68, 068701. [Google Scholar] [CrossRef]
- Qureshi, R.; Uzair, M.; Khurdid, K.; Yan, H. Hyperspectral document image processing: Applications, challenges and future prospects. Pattern Recogn. 2019, 90, 12–22. [Google Scholar] [CrossRef]
- Książek, K.; Romaszewski, M.; Głomb, P.; Grabowski, B.; Cholewa, M. Blood stain classification with hyperspectral imaging and deep neural networks. Sensors 2020, 20, 6666. [Google Scholar] [CrossRef]
- Devassy, B.M.; George, S. Dimensionality reduction and visualization of hyperspectral ink data using t-SNE. Forensic Sci. Int. 2020, 311, 110194. [Google Scholar] [CrossRef]
- Devassy, B.M.; George, S.; Nussbaum, P. Unsupervised Clustering of Hyperspectral Paper Data Using t-SNE. J. Imaging 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Devassy, B.M.; George, S. Forensic analysis of beverage stains using hyperspectral imaging. Sci Rep. 2021, 11, 6512. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2021, 26, 1237–1248. [Google Scholar] [CrossRef]
- Nesse, W. Introduction to Optical Mineralogy; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Allen, A.; McLeod, C.L.; Velázquez Santana, L.; Zimmerer, M.; Lytle, M.L.; Krekeler, E.; Amick, W.; Tegge, J.; Ventura-Valentín, W.; Vest, J.; et al. Mineralogy and Geochemistry of Sands from Playa las Golondrinas, Puerto Rico: Establishing a Regional Geogenic Background. Environ. Ear. Sci. 2024; minor revisions submitted. [Google Scholar]
- Allen, A.; Dietrich, M.; McLeod, C.L.; Gillis, M.; Gokey, K.; Fouh, M.; Krekeler, M.P.S. Investigating mercury in road sediment in Michigan City, Indiana: A new type of environmental pollution record. Environ. Adv. 2024, 15, 100483. [Google Scholar] [CrossRef]
- Wudke, H.; Brown, K.; Murchland, M.; Gillis, M.; Gokey, K.; Bank, J.; Lytle, M.; McLeod, C.L.; Krekeler, M.P.S. Mineralogical and geochemical characterization of Johnson’s baby powder from 1985: Evidence of contamination. Appl. Clay Sci. 2024, 250, 107252. [Google Scholar] [CrossRef]
- Flett, L.; McLeod, C.L.; McCarty, J.L.; Shaulis, B.J.; Fain, J.J.; Krekeler, M.P.S. Monitoring uranium mine pollution on Native American lands: Insights from tree bark particulate matter on the Spokane Reservation, Washington, USA. Environ. Res. 2021, 194, 110619. [Google Scholar] [CrossRef]
- O’Shea, M.J.; Krekeler, M.P.; Vann, D.R.; Gieré, R. Investigation of Pb-contaminated soil and road dust in a polluted area of Philadelphia. Environ. Monit. Assess. 2021, 193, 440. [Google Scholar] [CrossRef] [PubMed]
- Cymes, B.A.; Almquist, C.B.; Krekeler, M.P.S. Europium-doped cryptomelane: Multi-pathway synthesis, characterization, and evaluation for the gas phase catalytic oxidation of ethanol. Appl. Catal. A Gen. 2020, 589, 117310. [Google Scholar] [CrossRef]
- Klein, E.; Krekeler, M.P.S. The occurrence of Hg, Se, S, Ni, Cr, and Th in Talc Ore: A scanning electron microscopy (SEM) study of historical samples from the Willow Creek Mine, Montana. Results Geochem. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Oglesbee, T.; McLeod, C.; Chappell, C.; Vest, J.; Sturmer, D.; Krekeler, M.P.S. A Mineralogical and Geochemical Investigation of Modern Aeolian Sands near Tonopah, Nevada: Sources and Environmental Implications. Catena 2020, 194, 104640. [Google Scholar] [CrossRef]
- Velázquez Santana, L.V.; McLeod, C.L.; Blakemore, D.; Shaulis, B.; Hill, T. Bolivian hornblendite cumulates: Insights into the depths of Central Andean arc magmatic systems. Lithos 2020, 370, 105618. [Google Scholar] [CrossRef]
- Dietrich, M.; Wolfe, A.; Burke, M.; Krekeler, M.P.S. The first pollution investigation of road sediment in Gary, Indiana: Anthropogenic metals and possible health implications for a socioeconomically disadvantaged area. Environ. Intern. 2019, 128, 175–192. [Google Scholar] [CrossRef]
- Dietrich, M.; Huling, J.; Krekeler, M.P.S. Metal pollution investigation of Goldman Park, Middletown Ohio: Evidence for steel and coal pollution in a high child use setting. Sci. Tot. Environ. 2018, 618, 1350–1362. [Google Scholar] [CrossRef]
- Burke, M.; Rakovan, J.; Krekeler, M.P.S. A study by electron microscopy of gold and associated minerals from Round Mountain, Nevada. Ore Geol. Rev. 2017, 91, 708–717. [Google Scholar] [CrossRef]
- Paul, K.C.; Silverstein, J.; Krekeler, M.P.S. New insights into rare earth element (REE) particulate generated by cigarette lighters: An electron microscopy and materials science investigation of a poorly understood indoor air pollutant and constraints for urban geochemistry. Environ. Earth Sci. 2017, 76, 369. [Google Scholar] [CrossRef]
- Curtis, J.; Stitle, L.; Certain, J.; Murchland, M.; Piszel, C.; Vest, J.; McLeod, C.L.; Krekeler, M.P.S. A reflective spectroscopy and mineralogical investigation of cosmetic blush (Wet’N’Wild) potentially for forensic investigations related to interpersonal violence—An experimental feasibility study. Forensic Sci. 2023, 3, 544–559. [Google Scholar] [CrossRef]
- Barnes, M.; McLeod, C.; Faraci, O.; Chappell, C.; Krekeler, M.P.S. Characterizing the geogenic background of the Midwest: A detailed mineralogical and geochemical investigation of a glacial till in southwestern Ohio. Environ. Earth Sci. 2011, 79, 159. [Google Scholar] [CrossRef]
- Brum, J.; Schlegel, C.; Chappell, C.; Burke, M.; Krekeler, M.P.S. Reflective spectra of gasoline, diesel and jet fuel ion sand substrates under ambient and cold conditions: Implications for detection using hyperspectral remote sensing and development of age estimation models. Environ. Earth Sci. 2020, 79, 463. [Google Scholar] [CrossRef]
- Burke, M.; Dawson, C.; Allen, C.S.; Brum, J.; Roberts, J.; Krekeler, M.P.S. Reflective spectroscopy investigations of clothing items to support law enforcement, search and rescue, and war crime investigations. Forensic Sci. Int. 2019, 304, 109945. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.S.; Krekeler, M.P.S. Reflectance spectra of crude oils and refined petroleum products on a variety of common substrates. In Active and Passive Signatures; Charmaine, G., Chadwick, H., Eds.; SPIE: Bellingham, WA, USA, 2010; Volume 7687, p. 76870L. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Cloutis, E.A. Spectral reflectance properties of hydrocarbons: Remote-sensing implications. Science 1989, 245, 65–168. [Google Scholar] [CrossRef]
- Hunt, G. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks VI: Additional silicates. Mod. Geol. 1973, 4, 85–106. [Google Scholar]
- Hunt, G.R.; Logan, L.M. Variation of Single Particle Mid-Infrared Emission Spectrum with Particle Size. Appl. Opt. 1972, 11, 142–147. Available online: https://opg.optica.org/ao/abstract.cfm?uri=ao-11-1-142 (accessed on 1 July 2024). [CrossRef]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks III: Oxides and hydroxides. Mod. Geol. 1971, 2, 195–205. [Google Scholar]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks IV: Sulphides and sulphates. Mod. Geol. 1971, 3, 1–14. [Google Scholar]
- Ramasahayam, S.; Chowdhury, S.R. Non invasive estimation of blood urea concentration using near infrared spectroscopy. Int. J. Smart Sens. Intell. Syst. 2016, 9, 449–467. [Google Scholar] [CrossRef]
- Piasek, Z.; Urbanski, T. The infra-red absorption spectrum and structure of urea. B Pol. Acad. Sci.-Tech. X 1962, 21, 113–120. [Google Scholar] [CrossRef]
- Fischer, P.H.H.; McDowell, C.A. The infrared absorption spectra of urea–hydrocarbon adducts. Can. J. Chem. 1960, 38, 187–193. [Google Scholar] [CrossRef]
- Johnson, T.J.; Myers, T.L.; Su, Y.-F.; Tonkyn, R.G.; Kelly-Gorham, M.R.K.; Danby, T.O. “IARPA/PNNL Solid Phase IR Spectra” in NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024; Volume 20899. [CrossRef]
- Beales, E. Hyperspectral Analysis of Selected Fabrics Submerged in the Indian Ocean: An Innovative Way to Aid in the Estimation of the Time Human Remains Have Spent in Water. Ph.D. Thesis, Murdoch University, Perth, Australia, 2020. [Google Scholar]
- Olejnik, M.K. Opportunities of adapting spectral imagery in rescue services of the national fire and rescue system. Sci. Rep. Fire Univ. 2023, 87, 325–343. [Google Scholar] [CrossRef]
- Proft, J.; Suarez, J.; Murphy, R. Spectral anomaly detection with machine learning for wilderness search and rescue. In Proceedings of the IEEE MIT Undergraduate Research Technology Conference (URTC), Cambridge, MA, USA, 7–8 November 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Eismann, M.T.; Stocker, A.D.; Nasrabadi, N.M. Automated hyperspectral cueing for civilian search and rescue. Proc. IEEE 2009, 97, 1031–1055. [Google Scholar] [CrossRef]
- Weerakoon, D.; Bansal, B.; Padhye, L.P.; Rachmani, A.; Wright, L.J.; Roberts, G.S.; Baroutian, S. A critical review on current urea removal technologies from water: An approach for pollution prevention and resource recovery. Sep. Purif. Technol. 2023, 314, 123652. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Naz, M.Y.; Shukrullah, S.; Sulaiman, S.A.; Ghaffar, A.; AbdEl-Salam, N.M. Controlling nitrogen pollution via encapsulation of urea fertilizer in cross-linked corn starch. BioResources 2019, 14, 7775–7789. [Google Scholar] [CrossRef]
- Finlay, K.; Patoine, A.; Donald, D.B.; Bogard, M.J.; Leavitt, P.R. Experimental evidence that pollution with urea can degrade water quality in phosphorus-rich lakes of the Northern Great Plains. Limnol. Oceanogr. 2010, 55, 1213–1230. [Google Scholar] [CrossRef]
- Lechevallier, P.; Villez, K.; Felsheim, C.; Rieckermann, J. Towards non-contact pollution monitoring in sewers with hyperspectral imaging. Environ. Sci. Water Res. Technol. 2024, 10, 1160–1170. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClelland, L.; Belak, E.; Curtis, J.; Krekeler, E.; Sanders, A.; Krekeler, M.P.S. A Reflective Spectroscopy Proof-of-Concept Study of Urea for Supporting Investigations of Human Waste in Multiple Forensic Contexts. Forensic Sci. 2024, 4, 463-474. https://doi.org/10.3390/forensicsci4030030
McClelland L, Belak E, Curtis J, Krekeler E, Sanders A, Krekeler MPS. A Reflective Spectroscopy Proof-of-Concept Study of Urea for Supporting Investigations of Human Waste in Multiple Forensic Contexts. Forensic Sciences. 2024; 4(3):463-474. https://doi.org/10.3390/forensicsci4030030
Chicago/Turabian StyleMcClelland, Lilly, Ethan Belak, Juliana Curtis, Ethan Krekeler, April Sanders, and Mark P. S. Krekeler. 2024. "A Reflective Spectroscopy Proof-of-Concept Study of Urea for Supporting Investigations of Human Waste in Multiple Forensic Contexts" Forensic Sciences 4, no. 3: 463-474. https://doi.org/10.3390/forensicsci4030030






