Abstract
Flavylium/Chalcone-based molecular switches comprise features such as pH-gated photochromism and fluorescence properties that make them attractive for many applications, ranging from stimuli-responsive materials to photopharmacology. However, in contrast to other common photoswitches, the application of flavylium compounds in these areas remains largely unexplored. Among other possible reasons, this may be due to the lack of general strategies to attach these molecules to substrates such as polymers, nanoparticles, biomolecules, or surfaces. In this work, we have shown that a copper (I) catalyzed azide-alkyne cycloaddition (CuAAC) can be employed to obtain a chalcone conjugate. We used an isosorbide carbohydrate to demonstrate this strategy and investigated the photochemical properties of the chalcone-isosorbide conjugate. The obtained results show that the photochemical properties of this new compound are similar to other equivalent flavylium/chalcone photoswitches, confirming the feasibility of the conjugation strategy.
1. Introduction
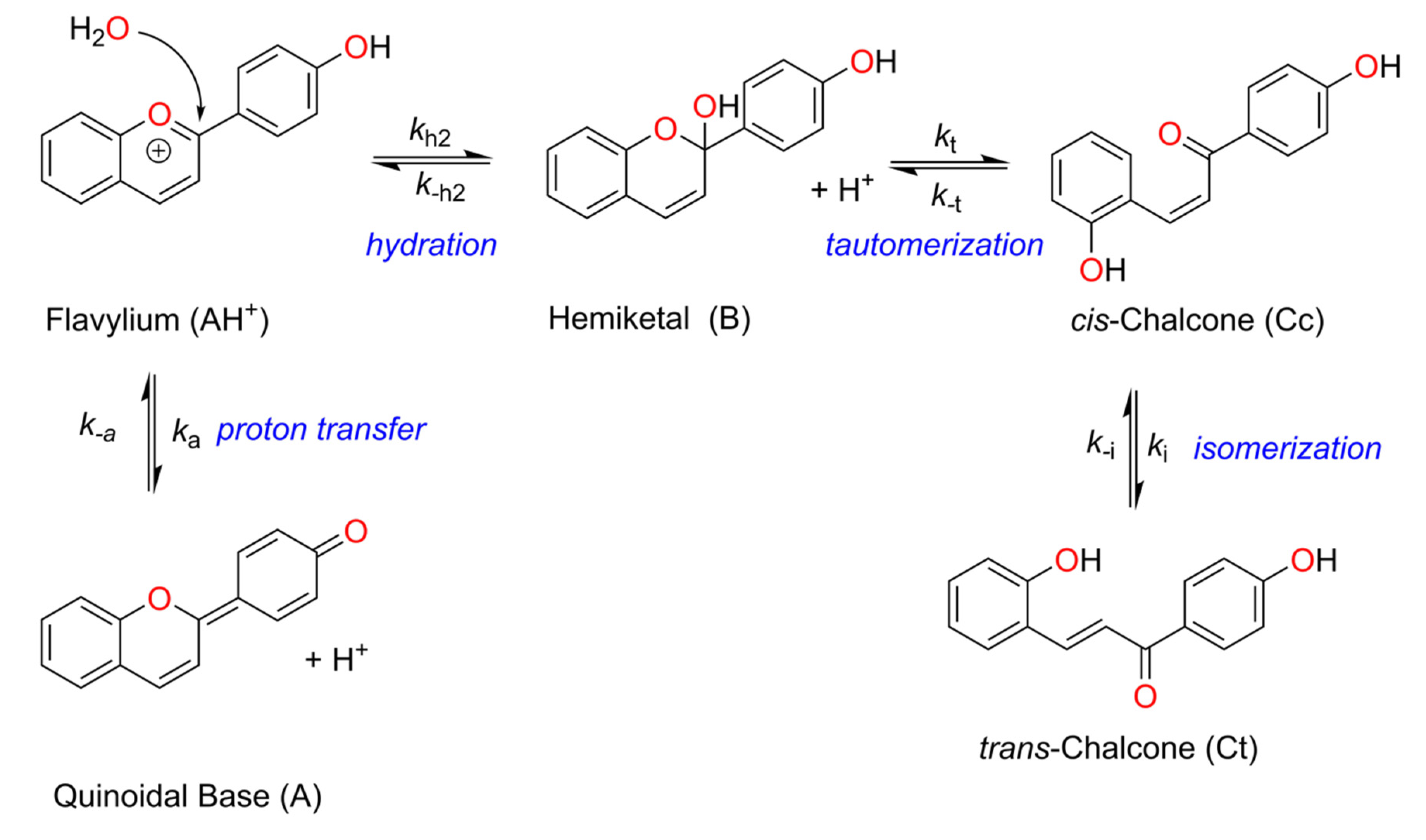
Flavylium salts comprise a large family of dyes that includes both synthetic and natural (e.g., anthocyanins) compounds [1,2]. These dyes are believed to play important roles in plant biology, not only because of their tunable color palette but also due to their antioxidant and photoprotective properties [1,2,3,4,5,6]. Furthermore, synthetic and natural flavylium compounds find several applications that range from fluorescent probes, photoswitches, or dye-sensitive solar cell photosensitizers, to provide some examples [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Despite being generally isolated as (and identified by) the flavylium cation (2-phenylbenzopyrylium) form, this electrophilic species is only stable at very acidic pH values (pH < 1) undergoing a series of reversible reactions (often designated as the flavylium network of chemical reactions) at slightly acidic, neutral, or basic conditions that lead to the disappearance of the flavylium cation to form different species, including the quinoidal base, hemiketal, cis- and trans-chalcone species (see Scheme 1), and their respective ionized species depending on the pH of the solution [23]. Since all these reactions are reversible, the flavylium can be recovered upon reacidification of the solution. Additionally, irradiation of the trans-chalcone species with light of the appropriate wavelength, at carefully selected moderately acidic pH values, leads to the formation of the flavylium cation as a metastable species that revert to the trans-chalcone in the dark, accounting for the well-known photochromic properties of flavylium compounds [24]. Even though at weakly acidic, neutral, or basic pH values, the flavylium cannot be formed upon irradiation of the trans-chalcone, other species such as hemiketal, cis-chalcone, or the quinoidal base can be observed as outputs of the photochemical stimulation. Due to these properties, flavylium compounds are considered multi-stimuli-responsive/multistate molecular switches [25,26,27,28,29].
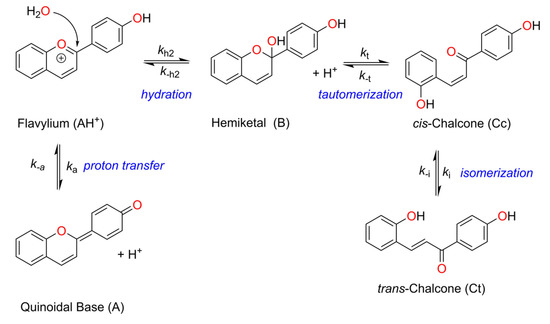
Scheme 1.
General reaction network displayed by flavylium cations under acidic to neutral conditions.
On account of the above-mentioned properties, flavylium compounds hold great potential to be employed as stimuli-responsive/fluorescence-reporting molecular components of functional (supra)molecular materials [30,31,32,33,34,35]. However, their application in these contexts remains underexplored. This can be due, in part, to the fact that flavylium compounds pose some challenges regarding their functionalization: Ideally, the desired functional groups attached to aldehyde, ketone, or phenols precursors must be stable under the very acidic/basic conditions used to obtain the corresponding flavylium salt or chalcone, respectively. Furthermore, quantitative reactant conversions are often desirable, especially when dealing with highly polar/charged functional groups that can be challenging to separate from the desired flavylium product [1,36,37,38,39,40,41]. Post-functionalization of flavylium dyes (without modifying their electronic properties), on the other hand, offers an alternative and potentially attractive strategy to attach them to different molecules and materials such as polymers, biomolecules, surfaces, nanoparticles, etc. However, to the best of our knowledge, post-functionalization strategies aiming at the attachment of flavylium compounds to different molecules and materials remain largely unexplored [42]. In this work, we report the synthesis and spectroscopic characterization of a chalcone-isosorbide conjugate showing that the copper-catalyzed azido-alkyne click reaction can be effectively applied for the post-functionalization of flavylium compounds (via the trans-chalcone species).
2. Materials and Methods
2.1. General
All commercially available reagents were bought from Sigma-Aldrich/Fluka/TCI/Alfa Aesar and were used as received. Spectroscopic experiments were carried out using 1 cm quartz or plastic cells on a Cary100bio or 5000 (Varian, Palo Alto, CA, USA). The solutions (3 mL) were irradiated at 365 nm, under continuous stirring, in a SPEX Fluorolog 1681 0.22 m 150 W Xe-Hg lamp (HORIBA, Kyoto, Japan). The light flux at λirr = 365 nm (I0) was determined using a ferrioxalate actinometer [43]. The photochemical quantum yields were determined from Equation (1), where ΔA/Δt is the slope of line obtained by measuring the absorbance of the product or reactant against the initial irradiation time (linear region), V is the irradiated volume, ε is the molar extinction coefficient of the photoproduct, and Airr is the absorbance at the irradiation wavelength. This equation is only valid when the reactant is the only species absorbing at the irradiation wavelength. Otherwise, a correction for the fraction of absorbed light must be made. In the present case, no corrections are needed because, at the initial irradiation time, the reactant is the only species absorbing light at the irradiation wavelength.
The pH of the solutions was measured using a Crison basic 20+ (Crison Instruments, Barcelona, Spain) or a Radiometer Copenhagen PHM240 pH meter (Copenhagen, Denmark). NMR spectra were acquired using a Bruker Avance III (Billerica, MA, USA) operating at 400 MHz (1H) or 101 MHz (13C). Infrared spectra were acquired using a Perkin Elmer Spectrum Two in ATR mode. The melting points were measured in a Reichert Thermovar microscope (Reichert Technologies, Depew, NY, USA), and the obtained values are uncorrected. ESI-MS analysis was performed on an LTQ OrbitrapTM XL hybrid mass spectrometer (Thermo Fischer Scientific, Bremen, Germany) controlled by LTQ Tune Plus 2.5.5 and Xcalibur 2.1.0.
2.2. Synthesis of -(4-(Prop-2-yn-1-yloxy)phenyl)ethan-1-one (2)
To a solution of 4-hydroxyacetophenone (1) (1.08 g; 7.93 mmol) in dry DMF (17 mL), potassium carbonate (1.5 g; 10.85 mmol) was added, followed by propargyl bromide (1.39 g; 11.68 mmol). The mixture was stirred at room temperature under an inert atmosphere for 16 h. Ice-cold water was added to the reaction mixture, affording a solid that was vacuum filtered and washed with cold water. Compound 2 was obtained as a white solid (1.14 g; 6.54 mmol; 83%).
m.p: 75–76 °C. (lit. 73 °C) [44].
IR (neat) νmax (cm−1): 3221; 3002; 2933; 2875; 2121; 1657; 1600; 1575; 1240; 1015; 824; 591.
1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.8 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 4.76 (d, J = 2.4 Hz, 2H), 2.56 (s, 4H).
13C NMR (101 MHz, CDCl3) δ 196.9, 161.4, 131.2, 130.7, 114.7, 77.9, 76.3, 55.9, 26.5.
The Spectroscopic Data is shown in Figures S1–S3 respectively.
2.3. Synthesis of (E)-3-(2-Hydroxyphenyl)-1-(4-(prop-2-yn-1-yloxy)phenyl)prop-2-en-1-one (3)
To a solution of (2) (0.5 g; 2.87 mmol) in absolute ethanol (5 mL), lithium hydroxide monohydrate (0.35 g; 8.35 mmol) was added. After solubilization of the lithium hydroxide, salicylaldehyde (0.5 mL; 4.69 mmol) was added. The resulting mixture was stirred sheltered from light at 40 °C for 18 h. A solution of HCl 1M was added until a neutral pH was reached, affording a solid that was filtered under vacuum and washed with cold water and cold ethanol. Compound 3 was obtained as a yellow solid (0.5 g; 1.8 mmol; 63%).
m.p: 120 °C (decomposition).
IR (neat) νmax (cm−1): 3237; 2118; 1638; 1601; 1547; 1218; 1012; 835; 749.
1H NMR (400 MHz, DMSO-d6) δ 10.24 (s, 1H), 8.12 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 15.7 Hz, 1H), 7.91–7.81 (m, 2H), 7.27 (t, J = 7.8 Hz, 1H), 7.14 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.2 Hz, 1H), 6.87 (t, J = 7.5 Hz, 1H), 4.94 (s, 2H), 3.65 (s, 1H).
13C NMR (101 MHz, DMSO-d6) δ 187.7, 160.9, 157.2, 138.7, 131.9, 131.3, 130.6, 128.6, 121.5, 120.8, 119.4, 116.2, 114.8, 78.8, 55.7.
The Spectroscopic Data is shown in Figures S4–S9 respectively.
2.4. Synthesis of (3R,3aS,6R,6aR)-6-Hydroxyhexahydrofuro[3,2-b]furan-3-yl 4-methylbenzenesulfonate (5)
To a solution of isomannide (4) (1 g; 6.84 mmol) in dry pyridine (6 mL), tosyl chloride (1.45 g; 7.61 mmol) was added at 0 °C. The resulting mixture was stirred at room temperature under nitrogen for 24 h. A solution of HCl 1M was added until pH = 1, and the mixture was extracted with dichloromethane. The organic phase was washed with a saturated solution of sodium bicarbonate, dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated to dryness. Compound 5 was obtained as a white solid (1.05 g; 3.67 mmol; 54%).
m.p: 105–107 °C (lit 104–105 °C) [45].
IR (neat) νmax (cm−1): 3524; 2984; 2933; 2868; 1596; 1357; 1172; 1098; 816.
1H NMR (400 MHz, DMSO-d6) δ 10.24 (s, 1H), 8.12 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 15.7 Hz, 1H), 7.91–7.81 (m, 2H), 7.27 (t, J = 7.8 Hz, 1H), 7.14 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.2 Hz, 1H), 6.87 (t, J = 7.5 Hz, 1H), 4.94 (s, 2H), 3.65 (s, 1H).
13C NMR (101 MHz, DMSO-d6) δ 187.7, 160.9, 157.2, 138.7, 131.9, 131.3, 130.6, 128.6, 121.5, 120.8, 119.4, 116.2, 114.8, 78.8, 55.7.
The Spectroscopic Data is shown in Figures S10–S12 respectively.
2.5. Synthesis of (3R,3aR,6S,6aR)-6-Azidohexahydrofuro[3,2-b]furan-3-ol (6)
To a solution of 5 (0.94 g; 3.13 mmol) in dry DMF (5 mL), sodium azide (0.66 g; 10.15 mmol) was added. The reaction mixture was stirred at 160 °C under nitrogen for 18 h. The resulting mixture was poured into an ice-cold water mixture and further extracted with dichloromethane. The combined organic phases were washed with water, dried with sodium sulfate, filtered, and evaporated to dryness. The resulting solid was purified using column chromatography (EtOAc/Hex (9:1)). Compound 6 was obtained as a white solid (0.29 g; 1.67 mmol; 53%)
m.p: 47–50 °C.
IR (neat) νmax (cm−1): 3425; 2963; 2869; 2862; 2134; 1499; 1417; 1260; 1007.
1H NMR (400 MHz, CDCl3) δ 4.60 (t, J = 4.9 Hz, 1H), 4.45 (d, J = 4.4 Hz, 1H), 4.30 (p, J = 5.8 Hz, 1H), 4.10–4.02 (m, 2H), 3.94 (dd, J = 10.1, 4.0 Hz, 1H), 3.86 (dd, J = 9.6, 5.9 Hz, 1H), 3.58 (dd, J = 9.5, 5.7 Hz, 1H), 2.69 (d, J = 6.4 Hz, 1H).
13C NMR (101 MHz, CDCl3) δ 86.3, 82.1, 73.9, 72.7, 72.2, 66.3.
The Spectroscopic Data is shown in Figures S13–S15 respectively.
2.6. Synthesis of (E)-1-(4-((1-((3S,3aR,6R,6aR)-6-Hydroxyhexahydrofuro[3,2-b]furan-3-yl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(2-hydroxyphenyl)prop-2-en-1-one (7)
A solution of (6) (50 mg; 0.29 mmol) and (3) (89 mg; 0.32 mmol) in a mixture of DMSO:H2O (4:1) (2 mL) was bubbled with N2. After 10 min, Cuprous iodide (3 mg; 0.015 mmol; 5% mol) was added, and the resulting mixture was stirred for 16 h at 50 °C under nitrogen and sheltered from light. Cuprisorb™ resin was added and stirred at room temperature for 2 h. The mixture was filtered and ice-cold water was added, resulting in the formation of a pale-yellow solid. After centrifugation, the solid was washed with cold water, followed by a mixture of EtOAc:Hex (9:1). Compound 7 was obtained as a pale-yellow solid (110 mg; 0.24 mmol; 84%).
m.p: 160 °C (decomposition).
IR (neat) νmax (cm−1): 3430; 3147; 2980; 2947; 1652; 1593; 1453; 1335; 1188; 992; 832; 754.
1H NMR (400 MHz, DMSO-d6) δ 8.31 (s, 1H), 8.12 (d, J = 8.1 Hz, 2H), 8.02 (d, J = 15.7 Hz, 1H), 7.89–7.81 (m, 2H), 7.26 (t, J = 7.7 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 6.93 (d, J = 8.2 Hz, 1H), 6.87 (t, J = 7.6 Hz, 1H), 5.28 (s, 2H), 5.24 (s, 1H), 5.05 (s, 1H), 4.78–4.75 (m, 1H), 4.62 (t, J = 4.5 Hz, 1H), 4.24–4.11 (m, 3H), 3.85–3.77 (m, 1H), 3.55–3.49 (m, 1H).
13C NMR (101 MHz, DMSO-d6) δ 187.7, 161.8, 157.2, 142.5, 138.7, 131.9, 131.0, 130.7, 128.6, 123.9, 121.5, 120.8, 119.4, 116.2, 114.7, 86.4, 82.3, 72.7, 71.9, 71.6, 66.1, 61.3.
ESI-HRMS: 448.15349 [M–H]− (calc. for C24H22N3 448.15141).
The Spectroscopic Data is shown in Figures S16–S21 respectively.
3. Results and Discussion
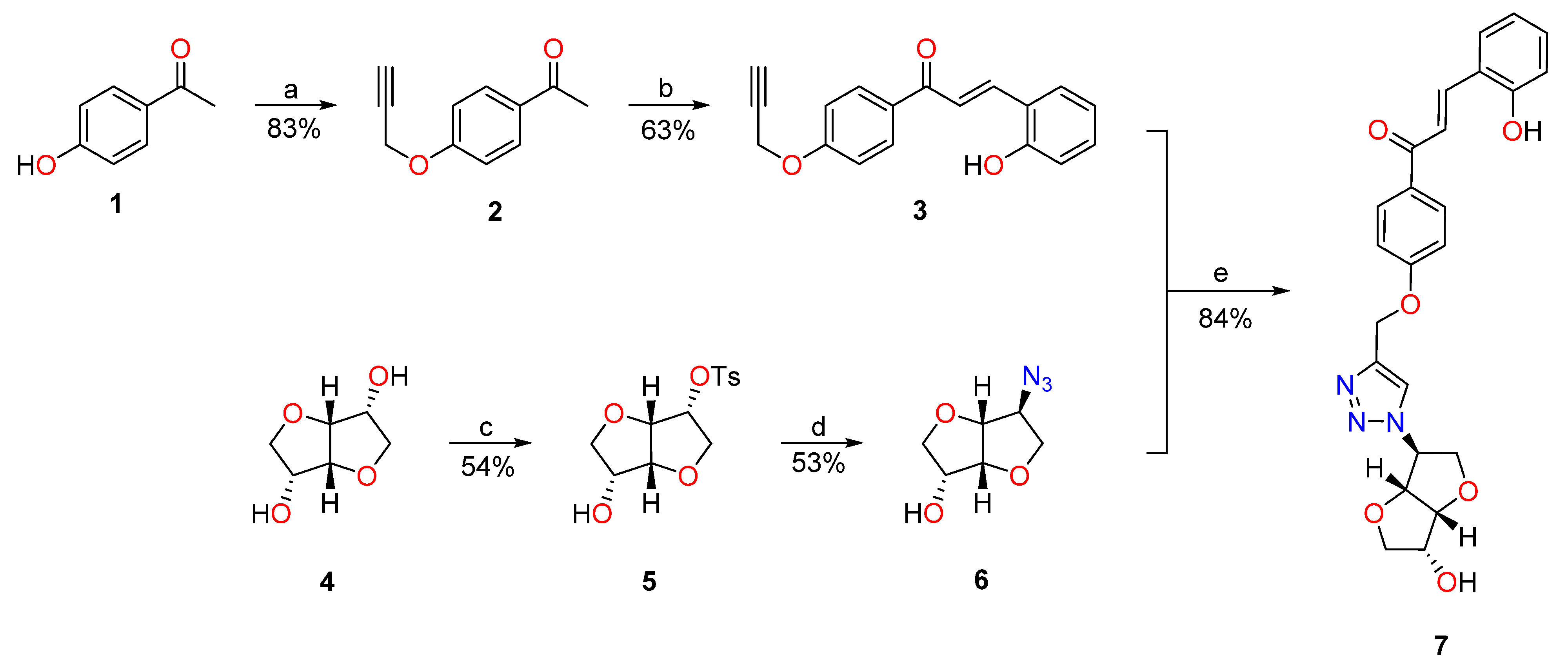
The trans-chalcone- isosorbide conjugate 7 was synthesized according to the strategy depicted in Scheme 2. The acetylenic trans-chalcone 3 was readily obtained in moderate yield from the respective acetophenone precursor 2 [44] through the LiOH catalyzed condensation with salicylaldehyde [46]. The isomannide 4 was treated with 1.1 equiv. of tosyl chloride to obtain the monotosylated derivative 5, which, in the presence of sodium azide, gives the targeted monoazido isosorbide 6, by an SN2 mechanism [45]. The isosorbide derivative 6 was then combined with trans-chalcone 3 in the presence of catalytic amounts of CuI to give the isosorbide-trans-chalcone conjugate 7 through the copper (I) catalyzed azido-alkyne “click” cycloaddition. [47] Usually, in CuAAC protocols, the catalytic Cu(I) species is generated “in situ” by Cu(II) sodium ascorbate reduction. In our studies, the use of catalytic amounts of CuSO4∙5H2O and sodium ascorbate was also tested, leading to lower yields and harder purification methods. [48] Compound 7 was fully characterized and identified by NMR and HRMS. The 1H NMR acquired in DMSO-d6 (see the full assignment in the Supporting Information) shows a singlet at 8.31 ppm corresponding to the triazole proton and a pair of doublets at 8.02/7.86 ppm with a coupling constant of J = 15.7 Hz, confirming that the chalcone 7 is obtained exclusively as the trans isomer.
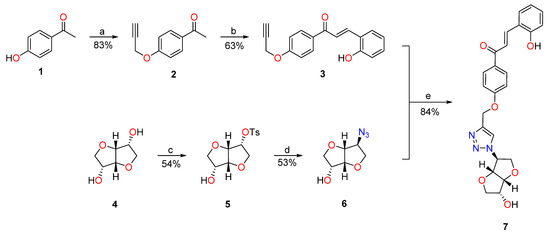
Scheme 2.
Adopted synthetic approach to obtain the isosorbide-chalcone conjugate 7. Reagents and conditions: (a) 1, K2CO3 (1.4 equiv.), propargyl bromide (1.5 equiv.), dry DMF, N2 atmosphere, RT, 16h. (b) 2, LiOH.H2O (2.9 equiv.), salicylaldehyde (1.6 equiv.), absolute ethanol, 40 °C, 18 h. (c) 4, tosyl chloride (1.1 equiv.), dry pyridine, N2 atmosphere, RT, 24 h. (d), NaN3 (3 equiv.), dry DMF, N2 atmosphere, 160 °C, 16 h. (e) 6, 3 (1.1 equiv.), CuI (5% mol), DMSO/H2O (4:1), N2 atmosphere, RT, 18 h.
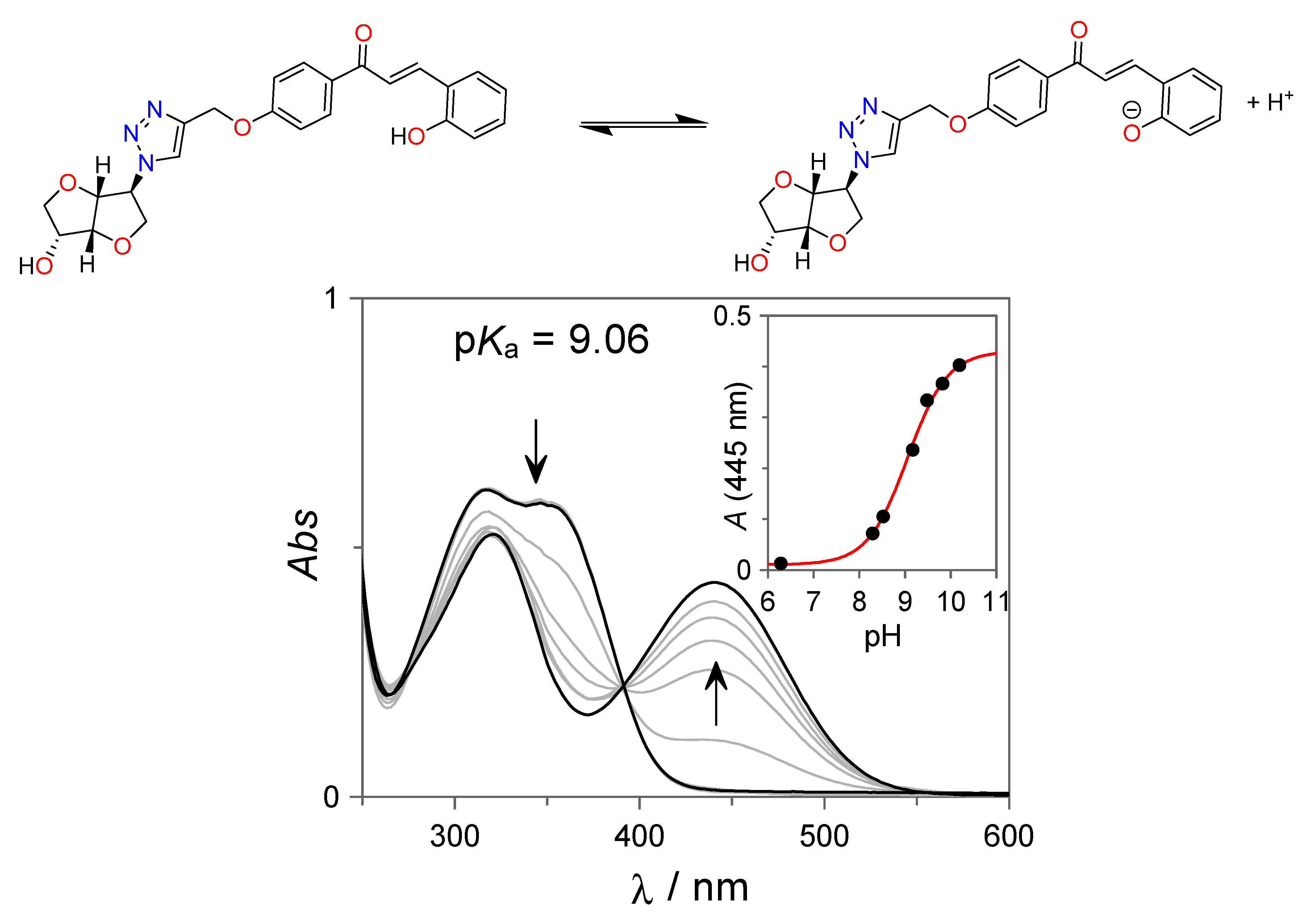
The acid–base and photoresponsive properties of the isosorbide-trans-chalcone conjugate 7 were investigated in H2O:DMSO solutions (85:15 v:v). The acid–base dissociation of the phenolic hydroxyl group of 7 in a basic medium was investigated by UV-Vis spectroscopy (see Figure 1). The neutral trans-chalcone 7 displays the typical absorption spectrum observed for this class of compounds with two overlapping bands centered at approximately 317 nm and 350 nm. As the pH increases, a new red-shifted absorption band, centered at 440 nm, starts to appear concomitantly with the disappearance of the former band. This new absorption spectrum can be assigned to the ionized form of 7. From the spectral variations shown in Figure 1, pKa = 9.06 for the formation of the ionized trans-chalcone 7 was obtained.
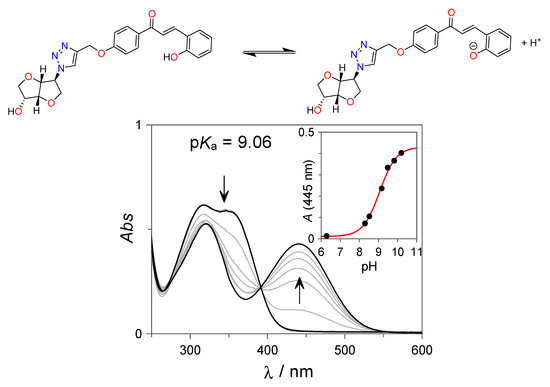
Figure 1.
UV-Vis spectral variations observed for the isosorbide-trans-chalcone conjugate 7 (27 µM) as a function of the pH. All spectra were acquired in H2O:DMSO (85:15 v:v) borate buffer (10 mM) solutions at room temperature.
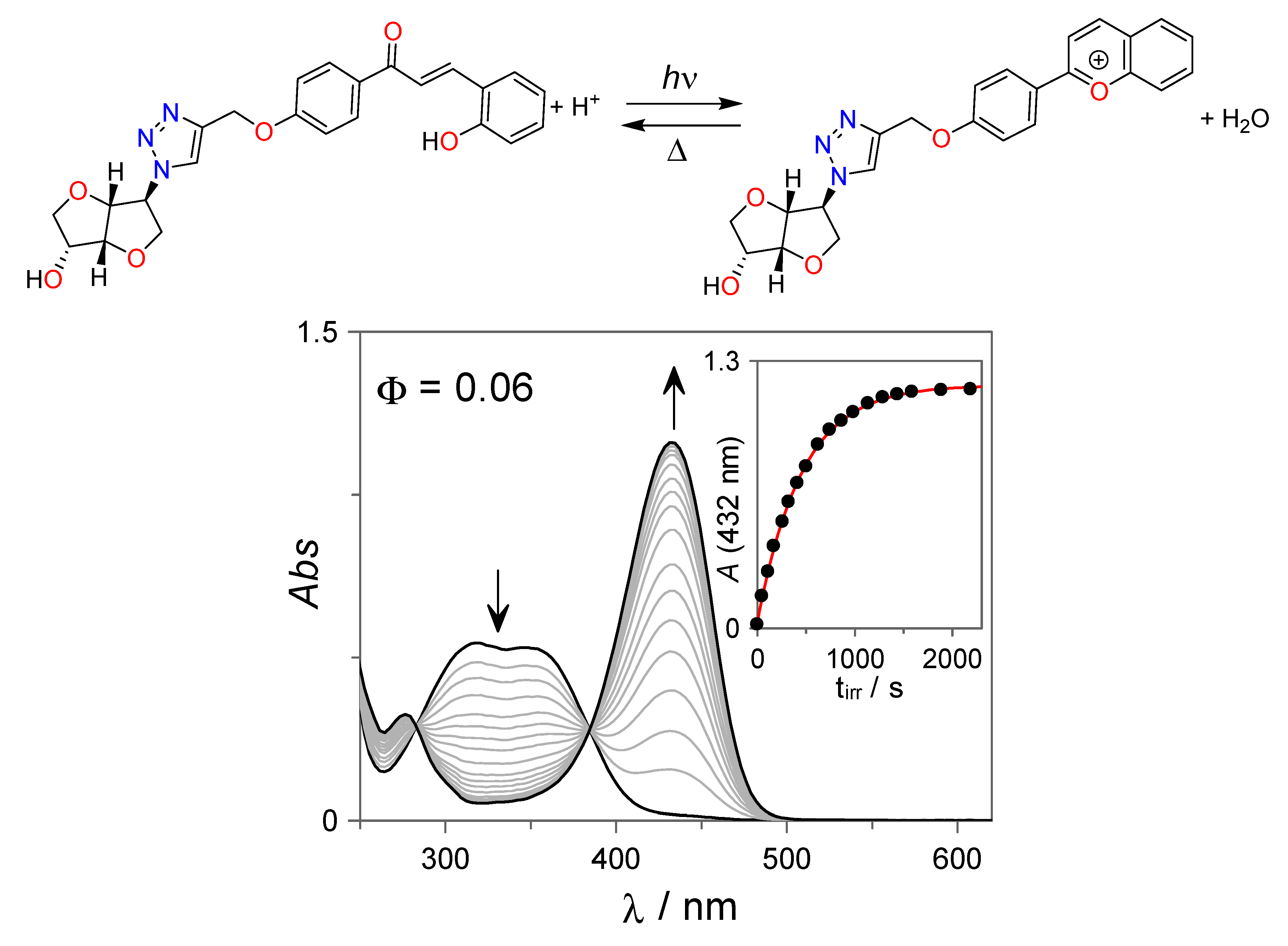
The photochemical properties of the trans-chalcone 7 were initially investigated at acidic conditions (pH = 2, [HCl] = 0.01 M). As can be observed from Figure 2, continuous irradiation of 7 with UV light (λirr = 365 nm) leads to the appearance of a new absorption band centered at 432 nm that can be assigned to the photoinduced formation of the corresponding flavylium cation with an apparent quantum yield of Φ = 0.06, in line with electronically similar flavylium photoswitches [28,33,34,49].
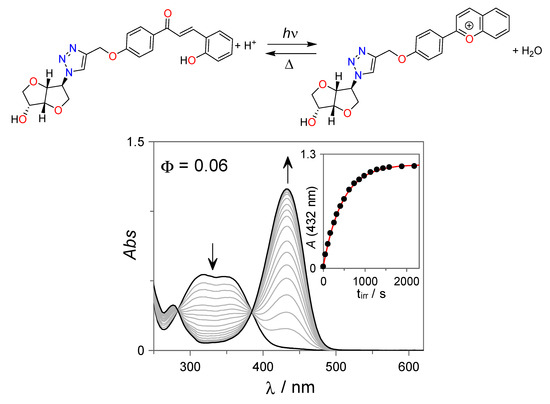
Figure 2.
UV-Vis spectral variations observed for the isosorbide-trans-chalcone conjugate 7 (27 µM) upon irradiation at λirr = 365 nm at pH = 2 (HCl = 0.01 M) in H2O:DMSO (85:15 v:v).
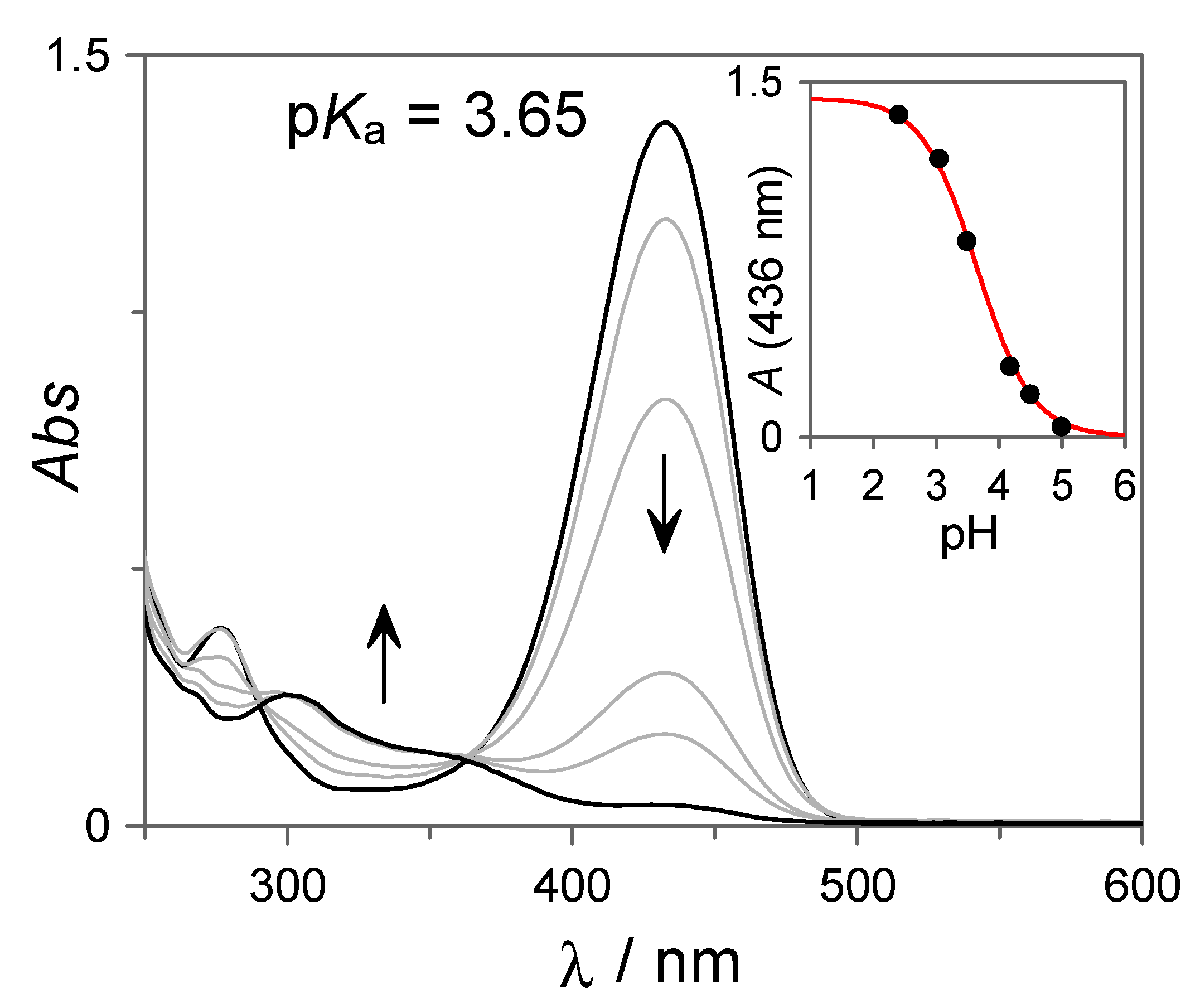
The photoinduced formation of the flavylium cation is pH-dependent, leading to quantitative but irreversible conversion at very acidic conditions and weak or no flavylium formation at higher pH values. To investigate the pH-dependent photochemical properties of 7 in more detail, irradiation experiments such as those shown in Figure 2 were conducted at different pH values. Figure 3 shows the final spectra obtained at the photostationary state as a function of the pH of the different solutions. As can be observed, at pH ~5, the flavylium cation is not formed upon irradiation, but as the pH decreases, its formation becomes more efficient. This behavior is in line with the pH-gated photochromism of flavylium compounds. In particular, for compounds displaying high cis-trans chalcone isomerization barriers, irradiation of the trans-species at higher pH values leads to the formation of an equilibrated mixture of cis-chalcone and hemiketal. At lower pH values, the hemiketal is thermally converted into the flavylium cation through the dehydration of this last species, driving the equilibria towards the formation of the colored cation. From the pH-dependent UV-Vis spectral variations shown in Figure 3, an apparent pKa = 3.65 can be obtained for the pH-dependent photoinduced formation of the flavylium cation.
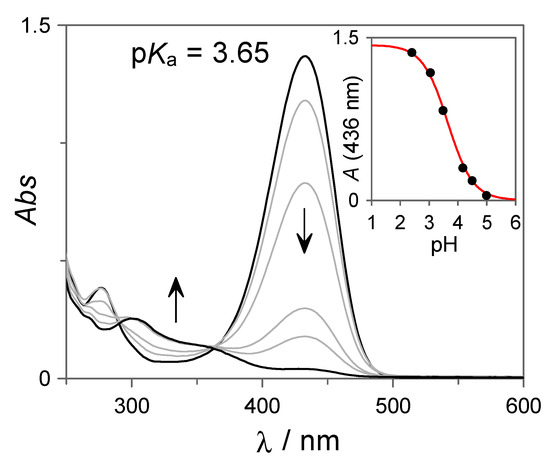
Figure 3.
UV-Vis spectral variations recorded at the photostationary state upon irradiation of 7 (27 µM in H2O:DMSO 85:15 v:v) at different pH values in 10 mM citrate buffer.
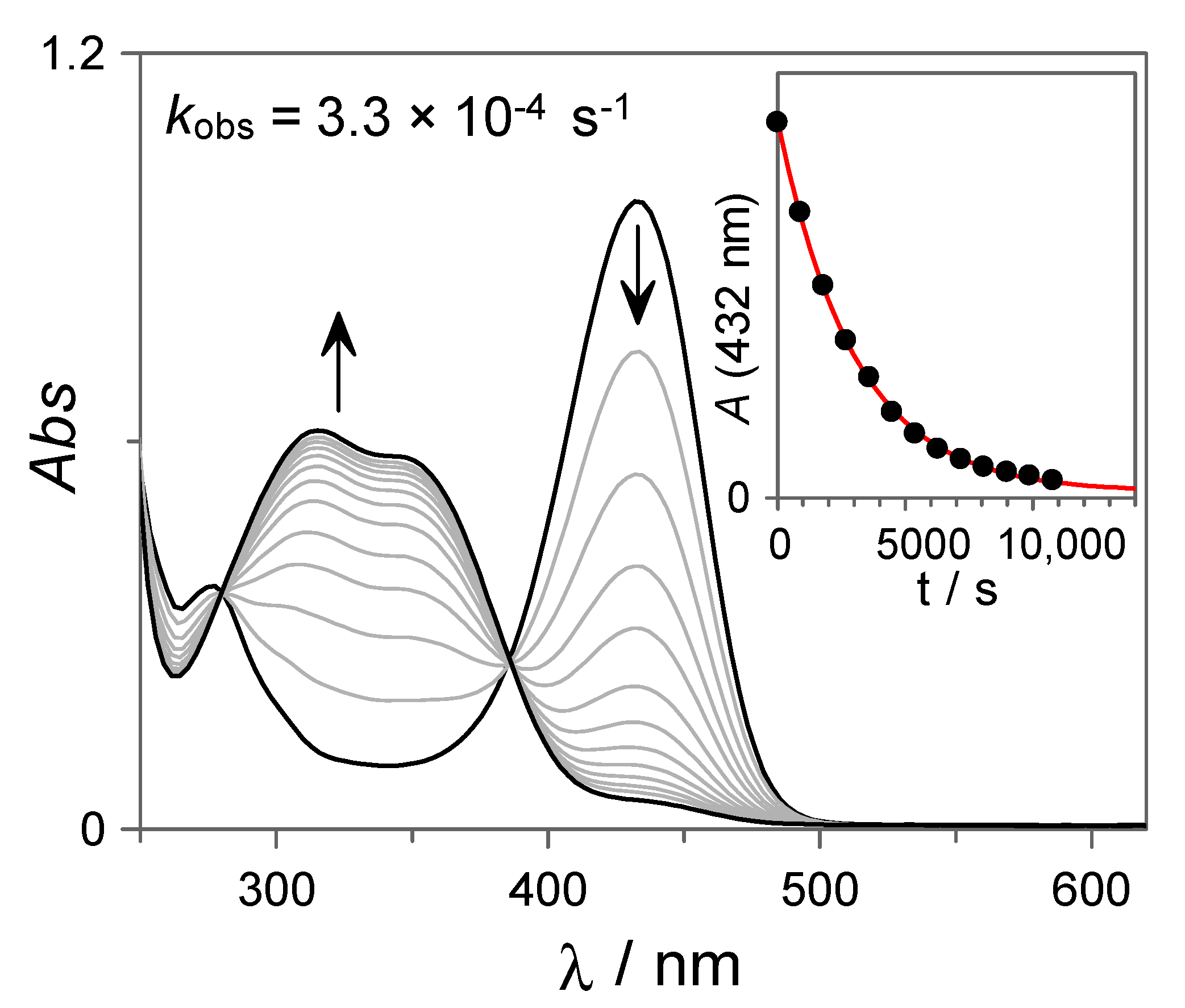
In order to investigate the reversibility of the photochromism observed for 7, the recovery of the trans-chalcone was monitored at T = 60 °C by UV-Vis spectroscopy. As can be observed from Figure 4, the absorption band assigned to the flavylium cation disappears according to first-order kinetics (kobs = 3.3 × 10−4 s−1) leading to the concomitant formation of the trans-chalcone, demonstrating the reversibility of the system at this pH value.
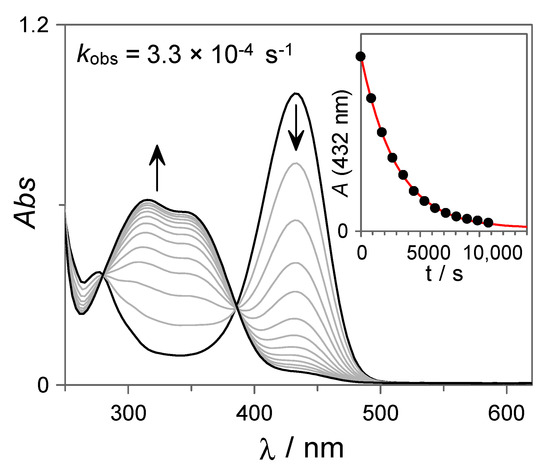
Figure 4.
UV-Vis spectral variations corresponding to the thermal recovery (T = 60 °C) of the trans-chalcone 7 (27 µM in H2O:DMSO 85:15 v:v) from the photostationary state at pH = 3.06 (10 mM citrate buffer). The red line in the inset shows the fit to a first-order integrated rate equation.
4. Conclusions
In conclusion, a photochromic trans-chalcone-isosorbide conjugate was successfully synthesized using copper (I) catalyzed azide-alkyne cycloaddition, and its photo- and pH-responsive properties were analyzed. The results showed that its physicochemical properties are similar to other equivalent compounds (in terms of substituents directly attached to the flavylium skeleton), suggesting this synthetic approach can be safely explored to functionalize more complex materials, such as nanoparticles, polymers, or biomolecules. We are currently exploring some of these possibilities to develop stimuli-responsive materials in the frame of our interests in supramolecular systems and nanotechnology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/compounds2020008/s1, Figure S1: 1H NMR of Compound 2 in CDCl3; Figure S2: 13C NMR of compound 2 in CDCl3; Figure S3: FTIR of compound 2; Figure S4: 1H NMR of compound 3 in DMSO-d6; Figure S5: 13C NMR of compound 3 in DMSO-d6; Figure S6: COSY of compound 3 in DMSO-d6; Figure S7: HSQC of compound 3 in DMSO-d6; Figure S8: HMBC of compound 3 in DMSO-d6; Figure S9: FTIR of compound 3; Figure S10: 1H NMR of compound 5 in CDCl3; Figure S11: 13C NMR of compound 5 in CDCl3; Figure S12: FTIR of compound 5; Figure S13: 1H NMR of compound 6 in CDCl3; Figure S14: 1H NMR of compound 6 in CDCl3; Figure S15: FTIR of compound 6; Figure S16: 1H NMR of compound 7 in DMSO-d6; Figure S17: 13C NMR of compound 7 in DMSO-d6; Figure S18: COSY of compound 7 in DMSO-d6; Figure S19: HSQC of compound 7 in DMSO-d6; Figure S20: HMBC of compound 7 in DMSO-d6; Figure S21: FTIR of Compound 7.
Author Contributions
Conceptualization, M.M.A.P. and N.B.; investigation, M.P.; writing—original draft preparation, N.B.; writing—review and editing, M.M.A.P., N.B. and M.P.; supervision, M.M.A.P. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Associate Laboratory for Green Chemistry—LAQV (UIDB/50006/2020), financed by FCT/MCTES. FCT/MCTES is also acknowledged for supporting the National Portuguese NMR Network (ROTEIRO/0031/2013-PINFRA/22161/2016, cofinanced by FEDER through COMPETE 2020, POCI, PORL, and FCT through PIDDAC) and for the grants PTDC/QUI-COL/32351/2017 and CEECIND/00466/2017 (N.B.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cruz, L.; Basílio, N.; Mateus, N.; De Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2021, 122, 1416–1481. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.O.; Freitas, A.A.; Maçanita, A.L.; Quina, F.H. Chemistry and photochemistry of natural plant pigments: The anthocyanins. J. Phys. Org. Chem. 2016, 29, 594–599. [Google Scholar] [CrossRef]
- Pina, F.; Alejo-Armijo, A.; Clemente, A.; Mendoza, J.; Seco, A.; Basílio, N.; Parola, A.J. Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us. Int. J. Mol. Sci. 2021, 22, 3833. [Google Scholar] [CrossRef]
- Crnolatac, I.; Giestas, L.; Horvat, G.; Parola, A.J.; Piantanida, I. Flavylium Dye as pH-Tunable Fluorescent and CD Probe for Double-Stranded DNA and RNA. Chemosensors 2020, 8, 129. [Google Scholar] [CrossRef]
- Czerney, P.; Graneß, G.; Birckner, E.; Vollmer, F.; Rettig, W. Molecular engineering of cyanine-type fluorescent and laser dyes. J. Photochem. Photobiol. A Chem. 1995, 89, 31–36. [Google Scholar] [CrossRef]
- Costa, D.; Galvao, A.M.; Di Paolo, R.E.; Freitas, A.A.; Lima, J.C.; Quina, F.H.; Macanita, A.L. Photochemistry of the hemiketal form of anthocyanins and its potential role in plant protection from UV-B radiation. Tetrahedron 2015, 71, 3157–3162. [Google Scholar] [CrossRef]
- Matsushima, R.; Fujimoto, S.; Tokumura, K. Dual photochromic properties of 4-dialkylamino-2-hydroxychalcones. Bull. Chem. Soc. Jpn. 2001, 74, 827–832. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Basílio, N.; Freitas, A.A.; Maçanita, A.L.; Lima, J.C.; Parola, A.J.; Pina, F. Ground and excited state properties of furanoflavylium derivatives. Phys. Chem. Chem. Phys. 2019, 21, 21651–21662. [Google Scholar] [CrossRef] [PubMed]
- Calogero, G.; Sinopoli, A.; Citro, I.; Di Marco, G.; Petrov, V.; Diniz, A.M.; Parola, A.J.; Pina, F. Synthetic analogues of anthocyanins as sensitizers for dye-sensitized solar cells. Photochem. Photobiol. Sci. 2013, 12, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.L.; Oliveira, J.; Araujo, P.; Calogero, G.; de Freitas, V.; Pina, F.; Parola, A.J.; Lima, J.C. Study of the multi-equilibria of red wine colorants pyranoanthocyanins and evaluation of their potential in dye-sensitized solar cells. Sol. Energy 2019, 191, 100–108. [Google Scholar] [CrossRef]
- Lopes-Costa, T.; Basílio, N.; Pedrosa, J.M.; Pina, F. Photochromism of the natural dye 7, 4′-dihydroxy-5-methoxyflavylium (dracoflavylium) in the presence of (2-hydroxypropyl)-β-cyclodextrin. Photochem. Photobiol. Sci. 2014, 13, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Czerney, P.; Grummt, U.-W. New near infrared absorbing acidochromic dyes and their application in sensor techniques. Sens. Actuators B Chem. 1997, 39, 395–400. [Google Scholar] [CrossRef]
- Ferreira da Silva, P.; Lima, J.C.; Quina, F.H.; Maçanita, A.L. Excited-state electron transfer in anthocyanins and related flavylium salts. J. Phys. Chem. A 2004, 108, 10133–10140. [Google Scholar] [CrossRef]
- Cosco, E.D.; Arús, B.A.; Spearman, A.L.; Atallah, T.L.; Lim, I.; Leland, O.S.; Caram, J.R.; Bischof, T.S.; Bruns, O.T.; Sletten, E.M. Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection. J. Am. Chem. Soc. 2021, 143, 6836–6846. [Google Scholar] [CrossRef]
- Cosco, E.D.; Spearman, A.L.; Ramakrishnan, S.; Lingg, J.G.P.; Saccomano, M.; Pengshung, M.; Arús, B.A.; Wong, K.C.Y.; Glasl, S.; Ntziachristos, V.; et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020, 12, 1123–1130. [Google Scholar] [CrossRef]
- Galindo, F.; Lima, J.C.; Luis, S.V.; Parola, A.J.; Pina, F. Write-Read-Erase Molecular-Switching System Trapped in a Polymer Hydrogel Matrix. Adv. Funct. Mater. 2005, 15, 541–545. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lima, J.C.; Santos, H.; Wigand, M.C.; Brouillard, R.; Macanita, A.L.; Pina, F. Photochromism of the synthetic 4′, 7-dihydroxyflavylium chloride. J. Am. Chem. Soc. 1994, 116, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Pina, F.; Melo, M.J.; Parola, A.J.; Maestri, M.; Balzani, V. pH-Controlled Photochromism of Hydroxyflavylium Ions. Chem. Eur. J. 1998, 4, 2001–2007. [Google Scholar] [CrossRef]
- Gago, S.; Basílio, N.; Moro, A.J.; Pina, F. Flavylium based dual photochromism: Addressing cis-trans isomerization and ring opening-closure by different light inputs. Chem. Commun. 2015, 51, 7349–7351. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Basílio, N.; de Freitas, V.; Pina, F. New procedure to calculate all equilibrium constants in flavylium compounds: Application to the copigmentation of anthocyanins. ACS Omega 2019, 4, 12058–12070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, F.; Petrov, V.; Laia, C.A. Photochromism of flavylium systems. An overview of a versatile multistate system. Dye. Pigment. 2012, 92, 877–889. [Google Scholar] [CrossRef]
- Pina, F.; Roque, A.; Melo, M.J.; Maestri, M.; Belladelli, L.; Balzani, V. Multistate/multifunctional molecular-level systems: Light and pH switching between the various forms of a synthetic flavylium salt. Chem. Eur. J. 1998, 4, 1184–1191. [Google Scholar] [CrossRef]
- Basílio, N.; Cruz, L.; De Freitas, V.; Pina, F. A Multistate Molecular Switch Based on the 6,8-Rearrangement in Bromo-apigeninidin Operated with pH and Host-Guest Inputs. J. Phys. Chem. B 2016, 120, 7053–7061. [Google Scholar] [CrossRef]
- Castet, F.; Champagne, B.; Pina, F.; Rodriguez, V. A Multistate pH-Triggered Nonlinear Optical Switch. ChemPhysChem 2014, 15, 2221–2224. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Maestri, M.; Ballardini, R.; Balzani, V. Photochromism of 4′-Methoxyflavylium Perchlorate. A “Write-Lock-Read-Unlock-Erase” Molecular Switching System. J. Am. Chem. Soc. 1997, 119, 5556–5561. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Maestri, M.; Passaniti, P.; Balzani, V. Artificial chemical systems capable of mimicking some elementary properties of neurons. J. Am. Chem. Soc. 2000, 122, 4496–4498. [Google Scholar] [CrossRef]
- Seco, A.; Yu, S.; Tron, A.; McClenaghan, N.D.; Pina, F.; Jorge Parola, A.; Basílio, N. Light-and pH-regulated Water-soluble Pseudorotaxanes Comprising a Cucurbit [7] uril and a Flavylium-based Axle. Chem. Eur. J. 2021, 27, 16512–16522. [Google Scholar] [CrossRef]
- Seco, A.; Diniz, A.M.; Sarrato, J.; Mourão, H.; Cruz, H.; Parola, A.J.; Basílio, N. A pseudorotaxane formed from a cucurbit[7] uril wheel and a bioinspired molecular axle with pH, light and redox-responsive properties. Pure Appl. Chem. 2020, 92, 301–313. [Google Scholar] [CrossRef]
- Romero, M.A.; Mateus, P.; Matos, B.; Acuña, A.; García-Río, L.; Arteaga, J.F.; Pischel, U.; Basílio, N. Binding of flavylium ions to sulfonatocalix [4] arene and implication in the photorelease of biologically relevant guests in water. J. Org. Chem. 2019, 84, 10852–10859. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, A.; Ferreira, P.; Parola, A.J.; Gago, S.; Basílio, N. pH-Gated photoresponsive shuttling in a water-soluble pseudorotaxane. Chem. Commun. 2018, 54, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Basílio, N.; Pischel, U. Drug delivery by controlling a supramolecular host–guest assembly with a reversible photoswitch. Chem. Eur. J. 2016, 22, 15208–15211. [Google Scholar] [CrossRef]
- Romero, M.A.; Fernandes, R.J.; Moro, A.J.; Basílio, N.; Pischel, U. Light-induced cargo release from a cucurbit[8]uril host by means of a sequential logic operation. Chem. Commun. 2018, 54, 13335–13338. [Google Scholar] [CrossRef]
- Al Bittar, S.; Mora, N.; Loonis, M.; Dangles, O. A simple synthesis of 3-deoxyanthocyanidins and their O-glucosides. Tetrahedron 2016, 72, 4294–4302. [Google Scholar] [CrossRef]
- Mora-Soumille, N.; Al Bittar, S.; Rosa, M.; Dangles, O. Analogs of anthocyanins with a 3′, 4′-dihydroxy substitution: Synthesis and investigation of their acid-base, hydration, metal binding and hydrogen-donating properties in aqueous solution. Dye. Pigment. 2013, 96, 7–15. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Czerney, P.; Levell, J.R. Benzotriazole-mediated conversions of para-H-substituted pyrylium, benzo [b] pyrylium, and xanthylium salts into para-position functionalized derivatives (an indirect electrophilic substitution of electron-deficient heteroaromatics). J. Org. Chem. 1997, 62, 8198–8200. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Czerney, P.; Levell, J.R.; Du, W. Molecular engineering of benzo [b] pyrylium salts by indirect electrophilic substitution. Eur. J. Org. Chem. 1998, 1998, 2623–2629. [Google Scholar] [CrossRef]
- Chassaing, S.; Kueny-Stotz, M.; Isorez, G.; Brouillard, R. Rapid preparation of 3-deoxyanthocyanidins and novel dicationic derivatives: New insight into an old procedure. Eur. J. Org. Chem. 2007, 2007, 2438–2448. [Google Scholar] [CrossRef]
- Basílio, N.; Garnier, T.; Avó, J.; Danel, M.; Chassaing, S.; Pina, F. Synthesis and multistate characterization of bis-flavylium dications–symmetric resorcinol-and phloroglucinol-type derivatives as stochastic systems. RSC Adv. 2016, 6, 69698–69707. [Google Scholar] [CrossRef]
- Cruz, L.; Guimarães, M.; Araujo, P.; Evora, A.; de Freitas, V.; Mateus, N. Malvidin 3-glucoside–fatty acid conjugates: From hydrophilic toward novel lipophilic derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef]
- Hatchard, C.G.; Parker, C.A. A new sensitive chemical actinometer-II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. A Math. Phys. Eng. Sci. 1956, 235, 518–536. [Google Scholar]
- Hans, R.H.; Guantai, E.M.; Lategan, C.; Smith, P.J.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg. Med. Chem. Lett. 2010, 20, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ramachandran, U. The synthesis and applications of asymmetric phase-transfer catalysts derived from isomannide and isosorbide. Tetrahedron 2005, 61, 4141–4148. [Google Scholar] [CrossRef]
- Bhagat, S.; Sharma, R.; Sawant, D.M.; Sharma, L.; Chakraborti, A.K. LiOH·H2O as a novel dual activation catalyst for highly efficient and easy synthesis of 1, 3-diaryl-2-propenones by Claisen–Schmidt condensation under mild conditions. J. Mol. Catal. A Chem. 2006, 244, 20–24. [Google Scholar] [CrossRef]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Rodionov, V.O.; Fokin, V.V.; Finn, M.G. Mechanism of the ligand-free CuI-catalyzed azide-alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 2005, 44, 2210–2215. [Google Scholar] [CrossRef]
- Anastácio, R.; Seco, A.; Mateus, P.; Parola, A.J.; Basílio, N. Exploring the pH-dependent kinetics, thermodynamics and photochemistry of a flavylium-based pseudorotaxane. Pure Appl. Chem. 2021, 93, 1313–1325. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).