Formulation and Evaluation of Olmesartan Medoxomil Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
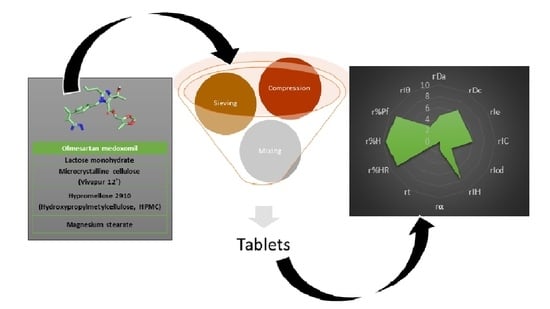
2.2. Methodological Approach
2.2.1. Preparation of Physical Mixtures
2.2.2. Characterization of Physical Mixtures
Scanning Electron Microscopy (SEM) Studies
Differential Scanning Calorimetry (DSC) Analysis
Fourier Transforms Infrared Spectroscopy (FT-IR) Analysis
2.2.3. SeDeM Methodology
Experimental Results for the SeDeM Methodology
- Apparent density (Da): the occupied volume of 10 g of the powder mixture was determined and its density in g/mL was calculated [28].
- Compressibility density (Dc): it is the volume occupied by the same amount of powder after 2500 hits on the sample. The analysis was performed in a powder density tester PT-TD200 (Pharma Test, Hainburg, Germany) and the result was expressed in g/mL [28].
- Inter-particle porosity (Ie): inter-particle porosity is calculated by means of Equation (1), dimensionless.
- Carr’s index (IC): IC is used to calculate the compression capacity of the powder mixture in percent (Equation (2)) [29].
- Cohesiveness’s index (Icd): the hardness (resistance to breakage) was determined in a sample of five 120 mg tablets. It was used the durometer Pharmatest PTB 311 (Hamburg, Germany) and the result was reported in Newtons [30].
- Hausner’s index (IH): the flow and slip capacity of the powder is calculated by means of Equation (3), which is dimensionless [29].
- Angle of repose (α): a funnel 9.5 cm high, 7.2 cm in diameter of the upper mouth and 1.8 cm in diameter of the lower mouth is placed in a support at 20 cm from the surface of the test. The lower mouth of the funnel is covered, and it is filled with the powder mixture. Then the lower mouth is uncovered to allow the powder to exit the funnel. The height of the cone (h), the four radiuses of the base of the cone formed were measured and the average value of the radiuses (r) were calculated. The angle was determined by Equation (4) [29,31].
- Sliding time (t”): the time it takes to pass 10 g of powder mixture through a funnel to the surface is timed. If the powder does not flow, it is rated seconds [32].
- Relative humidity (%HR): humidity was determined by calculating the difference in weight of a 4 g sample of powder before and after oven drying. The analysis was carried out at 105.0 °C ± 2.0 °C during 2 h using the Rayna Liebherr FKS1800 oven type 200041 (Bad Schussenried, Germany). The different in percent is the %HR [33].
- Hygroscopicity (%H): it determines the weight increase of the sample after being kept in a humidifier at 76.0% ± 2.0% relative humidity and 22.0 °C ± 2.0 °C temperature for 24 h, the different in percent is the %H [33].
- Determination of percentage of particles <50 μm (%Pf): it was calculated the % of powder particles passing through a 50 μm mesh size sieve while vibrating for 10 min at level three on a vibrating shaker for cascade of CISA sieves (Biotech, Barcelona, Spain). A 20 g sample of dust was weighed [34].
Incidence Factors for the SeDeM Methodology
- Dimensional impact factor (Fdimens): ability of the powder mixture to compact and the consequences on tablet dimensions (Equation (6)).
- Compressibility impact factor (Fcompressib): ability of the powder mixture to be compacted and maintain its shape (Equation (7)).
- Incidence factor of slippage/fluidity (Fflowability): flowability of the powder mixture (Equation (8)).
- Incidence factor of lubricity/stability (Flub/stability): consequence of residual moisture and hygroscopicity of the powder mixture on sliding and compaction (Equation (9)).
- Incidence factor of lubricity/dosage (Flub/dosage): Consequence of the powder particle size distribution on the sliding and correct filling of the compression matrices (Equation (10)).
Incidence Factors to Determine the Capacity to Be Used in Direct Compression for the Sedem Methodology
- Parametric index (IP) (Equation (11)).
- Parametric profile index (IPP) (Equation (12)).
- Good compression index (IGC) (Equation (13)).
2.2.4. Preparation and Characterization of Tablets
Preparation of Tablets Formulation
Tablets Characterization
- Diameter and Thickness
- Content Uniformity
- Hardness
- Friability Test
- Disintegration Time
- Dissolution Rate Study
- (1)
- Linearity: linearity was determined using the least squares method, the absorbance of the standards was related to their concentration. Linearity is acceptable with a coefficient of determination (r2) greater than 0.995.
- (2)
- Accuracy: accuracy (expressed as recovery, %) was assessed by relating the theoretical concentration (A) to the real concentration (B) using the following formula: A/B × 100%. Each sample was evaluated in triplicate.
- (3)
- Sensitivity: sensitivity was determined by calculating the limits of detection (LOD) and quantification (LOQ). The LOD is the lowest concentration of analyte that can be detected with precision and accuracy. The LOQ is the lowest concentration of analyte that can be quantified with precision and accuracy. Thus, it was evaluated the minimum amount of analyte needed to obtain a meaningful result.
- (4)
- Precision: precision was evaluated in triplicate at four concentration levels (8.40; 11.20; 14.00 and 16.00 μg/mL). To determine the precision of the analytical method the coefficient of variation must be less than 2%.
3. Results and Discussion
3.1. Solid-State Characterization
3.1.1. SEM Studies
3.1.2. DSC Studies
3.1.3. FT-IR Studies
3.2. Characterization of Powder Blends
3.3. Critical Quality Attributes of Olmesartan Medoxomil Tablets
3.3.1. Dissolution Rate Study
- Quantification of Olmesartan Medoxomil
3.3.2. Method Validation
- Linearity.
- Accuracy and Sensitivity.
- Precision.
3.3.3. Dissolution Profile of Tablets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adler, A.J.; Prabhakaran, D.; Bovet, P.; Kazi, D.S.; Mancia, G.; Mungal-Singh, V.; Poulter, N. Reducing cardiovascular mortality through prevention and management of raised blood pressure. Glob. Heart 2015, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Titova, N.S. Olmesartan medoxomil is an effective sartan with unique additional effects: A review of the evidence base. Hypertension 2022, 14, 6–16. [Google Scholar] [CrossRef]
- Llabata, P.; Carmona, J. Educación para la salud: Hipertensión arterial. Epidemiología. Enferm. Cardiol. 2005, 12, 28–32. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti, E.; Azizi, M.; Burnier, M. ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [Green Version]
- Alba, A.; Fajardo, G.; Papaqui, J. Farmacoepidemiología de los fármacos antagonistas de los receptores de angiotensina (ARA) II en el tratamiento de la hipertensión arterial esencial. México. Rev. Enferm. Neurológica 2014, 13, 139–146. [Google Scholar] [CrossRef]
- Chang, S.H.; Chang, C.C.; Wang, L.J.; Chen, W.C.; Fan, S.Y.; Zang, C.Z.; Hsu, Y.H.; Lin, M.C.; Tseng, S.H.; Wang, D.Y. A multi-analyte LC-MS/MS method for screening and quantification of nitrosamines in sartans. J. Food Drug Anal. 2020, 28, 98–107. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Kreutz, R. Olmesartan/amlodipine: A review of its use in the management of hypertension. Vasc. Health Risk Manag. 2011, 7, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Porta, B.; Borrás, C.; Jiménez, N.V. Normalización del intercambio terapéutico de antagonistas del receptor de la angiotensina-II para el tratamiento de la hipertensión en el medio hospitalario. Farm. Hosp. 2005, 29, 104–112. [Google Scholar] [CrossRef]
- Abd-El Bary, A.; Louis, D.; Sayed, S. Olmesartan medoxomil surface solid dispersion-based orodispersible tablets: Formulation and in vitro characterization. J. Drug Deliv. Sci. Technol. 2014, 24, 665–672. [Google Scholar] [CrossRef]
- Tannebaum, E.J.; Halaby, S. Oral solid dosage facilities. In Good Design Practices for GMP Pharmaceutical Facilities, 2nd ed.; Jacobs, T., Signore, A.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 241–267. [Google Scholar]
- Narang, A.; Mantri, R.M.; Raghavan, K. Excipient compatibility and functionality. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G., Yu, L., Mantri, R.V., Eds.; Elsevier: Cambridge, MA, USA, 2017; pp. 151–179. [Google Scholar]
- Lee, B. Pharmaceutical preformulation: Physicochemical properties of excipients and powders and tablet characterization. Pharm. Sci. Encycl. Drug Discov. Dev. Manuf. 2010, 18, 1–54. [Google Scholar]
- Chadha, R.; Bhandari, S. Drug–excipient compatibility screening—Role of thermoanalytical and spectroscopic techniques. J. Pharm. Biomed. Anal. 2014, 87, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Sachin, T.; Deodhar, M.; Prakya, V. Advances in analytical techniques used in predicting drug-excipient interactions. Int. J. Pharm. Technol. 2014, 6, 6388. [Google Scholar]
- Suñé, J.M.; Roiģ, M.; García, R.F.; Montoya, E.G.; Lozano, P.P.; Aģuilar, J.E.; Miñarro, M.; Ticó, J.R. SeDeM Diagram: An expert system for preformation, characterization and optimization of tablets obtained by direct compression. In Formulation Tools for Pharmaceutical Development; Aguilar, J.E., Ed.; Elsevier: Cambridge, MA, USA, 2013. [Google Scholar]
- Aguilar, J.E.; García, E.; Pérez, P.; Suñé, J.M.; Miñarro, M.; Ticó, J.R. SeDeM expert system a new innovator tool to develop pharmaceutical forms. Drug Dev. Ind. Pharm. 2014, 40, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, Y.; Zhang, G.G.; Yu, L.; Mantri, R.V. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Davies, P. Oral Solid Dosage Forms. In Pharmaceutical Preformulation and Formulation, 2nd ed.; Gibson, M., Ed.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Royce, A.M.; Ruegger, C.; Mecadon, M.; Karnachi, A.; Valazza, S. Scale-up of the compaction and tableting process. In Pharmaceutical Process Scale-Up; Swarbrick, J., Ed.; CRC Press: New York, NY, USA, 2005; pp. 405–442. [Google Scholar]
- González, R.; Peña, M.A.; Torres, N.S.; Torrado, G. Design, development and characterization of amorphous rosuvastatin calcium tablets. PLoS ONE 2022, 3, e0265263. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, J. Caracterización de materiales a través de medidas de microscopía electrónica de barrido (SEM). Elementos 2013, 3, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Gao, W.; Lu, G.; Ding, J.; Wu, X.; Huang, X.; Chen, J.; Liu, M.; Jiang, J.; Wu, H. Preparation, characterization and in vitro release of microparticles based on dextran-rosuvastatin conjugate. Carbohydr. Polym. 2013, 96, 156–162. [Google Scholar] [CrossRef]
- Carrasco, L.; Isabel, M. Estudio de Adiciones de Bismuto en Aleaciones Zinc-Aluminio. Doctoral Thesis, Universidad Complutense of Madrid, Madrid, Spain, 2013. [Google Scholar]
- Gómez, R.; Murillo, R. Espectroscopía Infrarroja. Master’s Thesis, Universidad Nacional Autónoma of Mexico, Mexico City, Mexico, 2006. [Google Scholar]
- Suñé, J.; Pérez, P.; Miñarro, M.; Roig, M.; Fuster, R.; Hernández, C.; Ruhí, R.; García, M.; Ticó, J.R. Application of the SeDeM Diagram and a new mathematical equation in the design of direct compression tablet formulation. Eur. J. Pharm. Biopharm. 2008, 69, 1029–1039. [Google Scholar]
- Council of Europe. European Pharmacopoeia 2.9.15. Apparent Vol. 7.0; Council of Europe: Strasbour, France, 2011; Volume 7a. [Google Scholar]
- Real Farmacopea Española 2.9.36. Flujo de Polvo. 2010. Available online: https://extranet.boe.es/farmacopea/doc.php?id=20936 (accessed on 10 May 2020).
- Real Farmacopea Española 2.9.8. Resistencia de los Comprimidos a la Rotura. 2008. Available online: https://extranet.boe.es/farmacopea/doc.php?id=20908 (accessed on 22 April 2020).
- Council of Europe. European Pharmacopoeia 2.9.36. Angle of Repose. 7a. Vol. 7.0; Council of Europe: Strasbour, France, 2011. [Google Scholar]
- Council of Europe. European Pharmacopoeia 2.9.16. Flowability. 7a. Vol. 7.0; Council of Europe: Strasbour, France, 2011. [Google Scholar]
- Council of Europe. European Pharmacopoeia 2.2.32. Loss Son Drying. 7a. Vol. 7.0; Council of Europe: Strasbour, France, 2011. [Google Scholar]
- Council of Europe. European Pharmacopoeia 2.9.12. Sieve Test. 7a. Vol. 7.0; Council of Europe: Strasbour, France, 2011. [Google Scholar]
- Council of Europe. European Pharmacopoeia 2.9.38. Particle-Size Distribution Estimation by Analytical Sieving. 7a. Vol. 7.0; Council of Europe: Strasbour, France, 2011. [Google Scholar]
- Nofrerias, I.; Nardi, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; García-Montoya, E.; Pérez, P.; Ticó, J.R.; Miñarro, M. Comparison between microcrystalline celluloses of different grades made by four manufacturers using the SeDeM diagram expert system as a pharmaceutical characterization tool. Powder Technol. 2019, 342, 780–788. [Google Scholar] [CrossRef]
- Oh, G.H.; Park, J.H.; Shin, H.W.; Kim, J.E.; Park, Y.J. Quality-by-design approach for the development of telmisartan potassium tablets. Drug Dev. Ind. Pharm. 2018, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, Y.J.; Qb, D. Consideration for developing a double-layered tablet into a single-layered tablet with telmisartan and amlodipine. Pharmaceutics 2022, 14, 377. [Google Scholar] [CrossRef]
- European Pharmacopoeia 2.9.40. Uniformity of Dosage Units. Content Uniformity. 2005. Available online: http://www.uspbpep.com/ep50/2.9.40%20Uniformity%20of%20dosage%20units.pdf (accessed on 28 June 2022).
- Real Farmacopea Española 2.9.7. Friabilidad de los Comprimidos no Recubiertos. 2010. Available online: https://extranet.boe.es/farmacopea/doc.php?id=20907 (accessed on 22 April 2020).
- Real Farmacopea Española 2.9.1. Disgregación de Comprimidos y Cápsulas. 2011. Available online: https://extranet.boe.es/farmacopea/doc.php?id=20901 (accessed on 24 April 2020).
- Swartz, M.E.; Krull, I.S. (Eds.) Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018; Available online: https://www.taylorfrancis.com/books/9781482229776 (accessed on 22 July 2022).
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chromatogr. B. 2009, 877, 2224–2234. [Google Scholar] [CrossRef]
- Real Farmacopea Española 2.9.3. Ensayo de Disolución de las Formas Farmacéuticas Sólidas. 2012. Available online: https://extranet.boe.es/farmacopea/doc.php?id=20903 (accessed on 24 April 2020).
- Bolhuis, G.; Zuurman, K. Tableting properties of experimental and commercially available lactose granulations for direct compression. Drug Dev. Ind. Pharm. 1995, 21, 2057–2071. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: Washington, DC, USA, 2009. [Google Scholar]
- Sánchez, R.; Damas, R.; Domínguez, P.; Cerezo, P.; Salcedo, I.; Aguzzi, C.; Viseras, C. Uso de la hidroxipropilmetilcelulosa (HPMC) en liberación modificada de fármacos. Farmaespaña Ind. 2010, 10, 48–51. [Google Scholar]
- Miller, T.; York, P. Pharmaceutical tablet lubrication. Int. J. Pharm. 1988, 41, 1–19. [Google Scholar] [CrossRef]
- Jain, S.; Patel, K.; Arora, S.; Reddy, V.A.; Dora, C.P. Formulation, optimization, and in vitro–in vivo evaluation of olmesartan medoxomil nanocrystals. Drug Deliv. Transl. Res. 2017, 7, 292–303. [Google Scholar] [CrossRef]
- Pharma, J. JRS Pharma Family. 2020. Available online: https://www.jrspharma.com/pharma_en/products-services/excipients/binders/vivapur.php (accessed on 4 May 2022).
- Listiohadi, Y.; Hourigan, J.A.; Sleigh, R.W.; Steele, R.J. Thermal analysis of amorphous lactose and α-lactose monohydrate. Dairy Sci. Technol. 2009, 89, 43–67. [Google Scholar] [CrossRef]
- Aprilia, N.S.; Davoudpour, Y.; Zulqarnain, W.; Khalil, H.A.; Hazwan, C.C.M.; Hossain, M.; Dungani, R.; Fizree, H.M.; Zaidon, A.; Haafiz, M. Physicochemical characterization of microcrystalline cellulose extracted from kenaf bast. BioResources 2016, 1, 3875–3889. [Google Scholar] [CrossRef] [Green Version]
- Gómez, A.; Alvarez, C.; Gómez, J.; Concheiro, A. Chemical structure and glass transition temperature of non-ionic cellulose ethers. J. Therm. Anal. Calorim. 2003, 73, 587–596. [Google Scholar] [CrossRef]
- Ali, F.; Kumar, R.; Sahu, P.L.; Singh, G.N. Physicochemical characterization and compatibility study of roflumilast with various pharmaceutical excipients. J. Therm. Anal. Calorim. 2017, 130, 1627–1641. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Silva, M.A.S. Estudo termoanalítico de comprimidos revestidos contendo captopril através de termogravimetria (TG) e calorimetria exploratória diferencial (DSC). Rev. Bras. Ciênc Farm. 2005, 41, 315–322. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Stulzer, H.K.; Tagliari, M.P.; Cruz, A.P.; Silva, M.A.S.; Laranjeira, M.C.M. Compatibility studies between piroxicam and pharmaceutical excipients used in solid dosage forms. Pharm. Chem. J. 2008, 42, 215–219. [Google Scholar] [CrossRef]
- Audibert, J.Y.; Catoni, O. Robust Linear Least Squares Regression. Ann Stat. 2011. Available online: https://projecteuclid.org/journals/annals-of-statistics/volume-39/issue-5/Robust-linear-least-squares-regression/10.1214/11-AOS918.full (accessed on 19 July 2022).










| Parameter | Conversion Factor | Parameter (r) |
|---|---|---|
| Apparent density (Da) | 10 × Da | rDa |
| Compressibility density (Dc) | 10 × Dc | rDc |
| Interparticle porosity (Ie) | (10 × Ie)/1.2 | rIe |
| Carr’s index (IC) | IC/5 | rIC |
| Cohesiveness’s index (Icd) | Icd/20 | rIcd |
| Hausner’s index (IH) | 5 × (3 − IH) | rIH |
| Angle of repose (α) | 10 − (α/5) | rα |
| Slidding time (t”) | 10 − (t”/2) | rt” |
| Relative humidity (%HR) | 10 − %HR | r%HR |
| Higroscopicity (%H) | 10 − (%H/2) | r%H |
| Determination of percentage of particles <50 μm (%Pf) | 10 − (%Pf/5) | r%pf |
| Homogeneity index (Iθ) | 500 × Iθ | rIθ |
| Olmesartan Medoxomil | Drug | 20.0 mg |
|---|---|---|
| Lactose monohydrate | Diluent | 79.0 mg |
| Microcrystalline cellulose (Vivapur 12®) | Diluent, binding agent, disintegrant | 11.0 mg |
| Hypromellose 2910 (Hydroxypropylmetylcellulose, HPMC) | Binding agent | 6.0 mg |
| Magnesium stearate | Lubricant | 4.0 mg |
| TOTAL TABLET | 120.0 mg | |
| Olmesartan (cm−1) | Olmesartan: Hypromellose (cm−1) | Olmesartan: Lactose Monohydrate (cm−1) |
|---|---|---|
| - | - | 3382.54 |
| - | 3421.32 | 3342.96 |
| 3293.04 | - | - |
| 3041.22 | - | - |
| 3005.49 | - | 3006.05 |
| 2973.52 | 2972.71 | 2975.95 |
| Experimental Results | |||||||||||
| Da (g/mL) | Dc (g/mL) | Ie | IC (%) | Icd (N) | IH | α (°) | t” | %HR | %H | %Pf | Iθ |
| 0.45 | 0.65 | 0.66 | 30 | 49.03 | 1.43 | 42.46˚ | ∞ | 1.32 | 0.92 | 5.21 | 0.006 |
| Parameters (r) | |||||||||||
| rDa | rDc | rIe | rIC | rIcd | rIH | rα | rt | r%HR | r%H | r%Pf | rIθ |
| 4.55 | 6.50 | 5.50 | 6.00 | 2.45 | 7.85 | 1.51 | 0.00 | 8.68 | 9.54 | 8.96 | 2.95 |
| Impact Factor | |||||||||||
| Dimensional | Compressibility | Flowability | Lubricity/Stability | Lubricity/Dosage | |||||||
| 5.53 | 4.65 | 3.12 | 9.11 | 5.95 | |||||||
| Index | |||||||||||
| IP | IPP | IGC | |||||||||
| 0.58 | 5.37 | 5.12 | |||||||||
| Critical Quality Attributes | Olmesartan Medoxomil Tablets 20 mg |
|---|---|
| Physicals characteristics, dimension, thickness | Bright White Ø = 6.00 mm T = 3.50 mm (1) |
| Content uniformity | AV = 4.56 (2) |
| Hardness | 49.03 N |
| Friability | W0 = 2.58 g Wf = 2.57 g D = 0.27% (3) |
| Disintegration Time | Between 10.00–15.00 s |
| Dissolution Rate | Nearly 100% |
| Linear Range (μg/mL) | Slope | Intercept (Absorbance) | Coefficient of Determination (r2) |
|---|---|---|---|
| 2.80–22.40 | 0.043 | 0.0045 | 0.997 |
| Theorical Concentration (μg/mL) | Average Abs. | Real Concentration (μg/mL) | Accuracy a | Sensitivity |
|---|---|---|---|---|
| 2.80 | 0.128 | 2.86 | 102 | 0.046 |
| 5.60 | 0.246 | 5.58 | 100 | 0.044 |
| 7.00 | 0.302 | 6.88 | 98 | 0.043 |
| 8.40 | 0.359 | 8.19 | 97 | 0.043 |
| 11.20 | 0.486 | 11.13 | 99 | 0.043 |
| 14.00 | 0.600 | 13.75 | 98 | 0.043 |
| 16.00 | 0.736 | 16.90 | 106 | 0.046 |
| 19.00 | 0.841 | 19.33 | 102 | 0.044 |
| 21.00 | 0.899 | 20.65 | 98 | 0.043 |
| 22.40 | 0.959 | 22.05 | 98 | 0.043 |
| %SD | 0.001 | |||
| LOD (μg/mL) | 0.64 | |||
| LOQ (μg/mL) | 2.14 |
| Concentration (μg/mL) | Absorbances | Average Abs. | % RSD | CV (%) | ||
|---|---|---|---|---|---|---|
| 8.40 | 0.364 | 0.355 | 0.358 | 0.359 | 0.005 | 1 |
| 11.20 | 0.491 | 0.492 | 0.476 | 0.486 | 0.009 | 2 |
| 14.00 | 0.607 | 0.596 | 0.597 | 0.600 | 0.006 | 1 |
| 16.00 | 0.743 | 0.745 | 0.721 | 0.736 | 0.013 | 2 |
| CV average | 1 | |||||
| Time (min) | Absorbances | Average Abs. | Concentration (μg/mL) | Standard Deviation | CV (%) | % Dissolved | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tablet 1 | Tablet 2 | Tablet 3 | Tablet 4 | Tablet 5 | Tablet 6 | ||||||
| 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | 0.000 | 0 | 0% |
| 5 | 0.248 | 0.205 | 0.177 | 0.267 | 0.265 | 0.293 | 0.243 | 5.52 | 0.043 | 18 | 25% |
| 10 | 0.489 | 0.384 | 0.343 | 0.458 | 0.501 | 0.504 | 0.447 | 10.22 | 0.068 | 15 | 46% |
| 15 | 0.629 | 0.556 | 0.497 | 0.602 | 0.646 | 0.637 | 0.595 | 13.63 | 0.058 | 10 | 62% |
| 20 | 0.695 | 0.650 | 0.590 | 0.673 | 0.703 | 0.729 | 0.673 | 15.45 | 0.049 | 7 | 71% |
| 30 | 0.772 | 0.770 | 0.708 | 0.769 | 0.816 | 0.805 | 0.773 | 17.75 | 0.038 | 5 | 81% |
| 40 | 0.998 | 0.847 | 0.784 | 0.814 | 0.868 | 0.844 | 0.859 | 19.73 | 0.074 | 9 | 91% |
| 60 | 0.861 | 0.877 | 0.827 | 0.880 | 0.952 | 0.891 | 0.881 | 20.24 | 0.041 | 5 | 94% |
 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, R.; Peña, M.Á.; Torrado, G. Formulation and Evaluation of Olmesartan Medoxomil Tablets. Compounds 2022, 2, 334-352. https://doi.org/10.3390/compounds2040028
González R, Peña MÁ, Torrado G. Formulation and Evaluation of Olmesartan Medoxomil Tablets. Compounds. 2022; 2(4):334-352. https://doi.org/10.3390/compounds2040028
Chicago/Turabian StyleGonzález, Rocío, María Ángeles Peña, and Guillermo Torrado. 2022. "Formulation and Evaluation of Olmesartan Medoxomil Tablets" Compounds 2, no. 4: 334-352. https://doi.org/10.3390/compounds2040028
APA StyleGonzález, R., Peña, M. Á., & Torrado, G. (2022). Formulation and Evaluation of Olmesartan Medoxomil Tablets. Compounds, 2(4), 334-352. https://doi.org/10.3390/compounds2040028