NOx Storage and Reduction (NSR) Performance of Sr-Doped LaCoO3 Perovskite Prepared by Glycine-Assisted Solution Combustion
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalysts Characterization
2.3. Catalytic Activity Measurements
2.3.1. NO Oxidation Experiments
2.3.2. NOx Adsorption–Desorption Measurements
2.3.3. NOx Storage and Reduction Measurements
3. Results and Discussion
3.1. XRD Analysis
3.2. SEM and EDS Mapping Analysis
3.3. N2 Adsorption–Desorption Analysis
3.4. FTIR and H2-TPR Analysis
3.5. O2-TPD
3.6. XPS
3.7. NO Oxidation
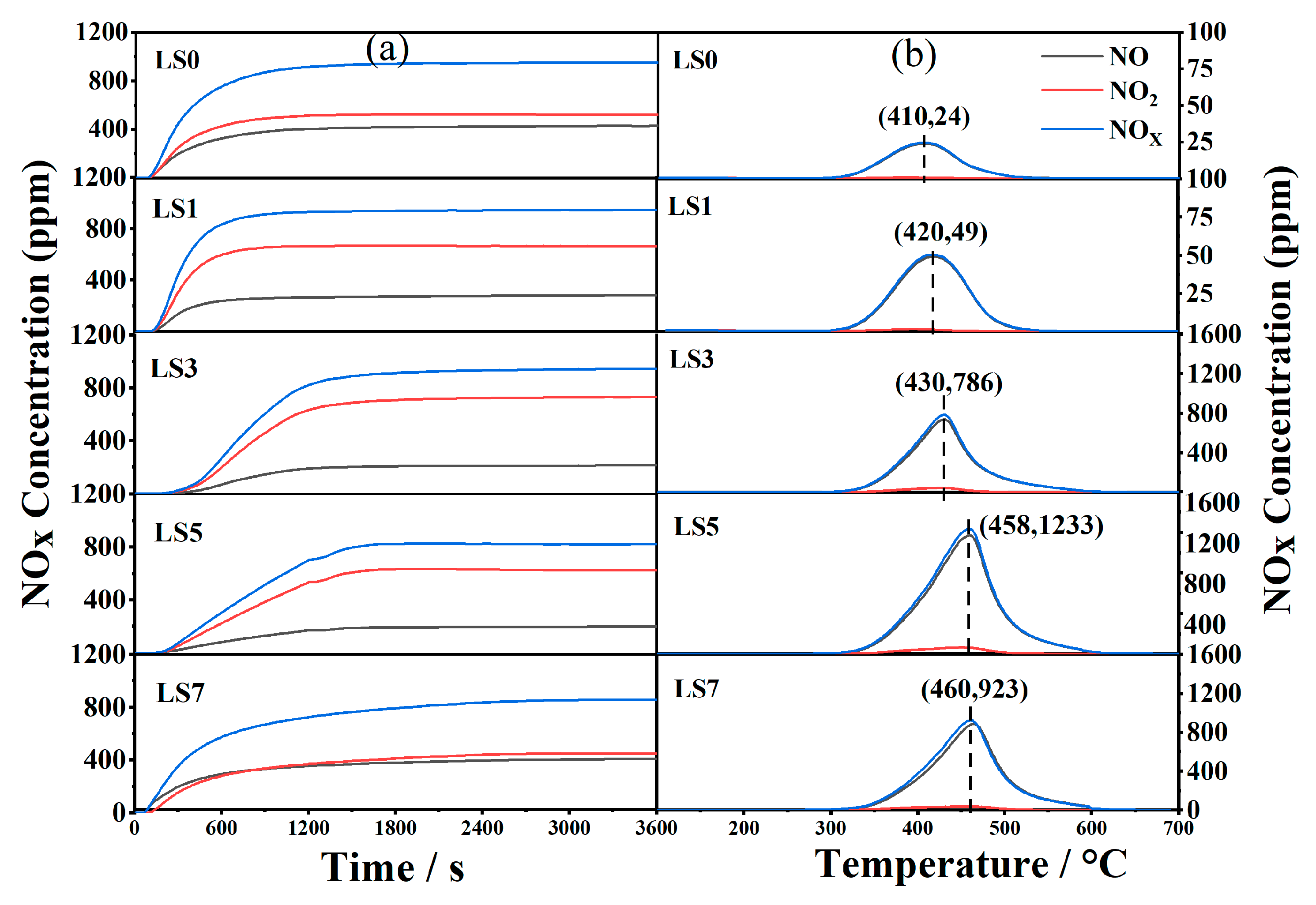
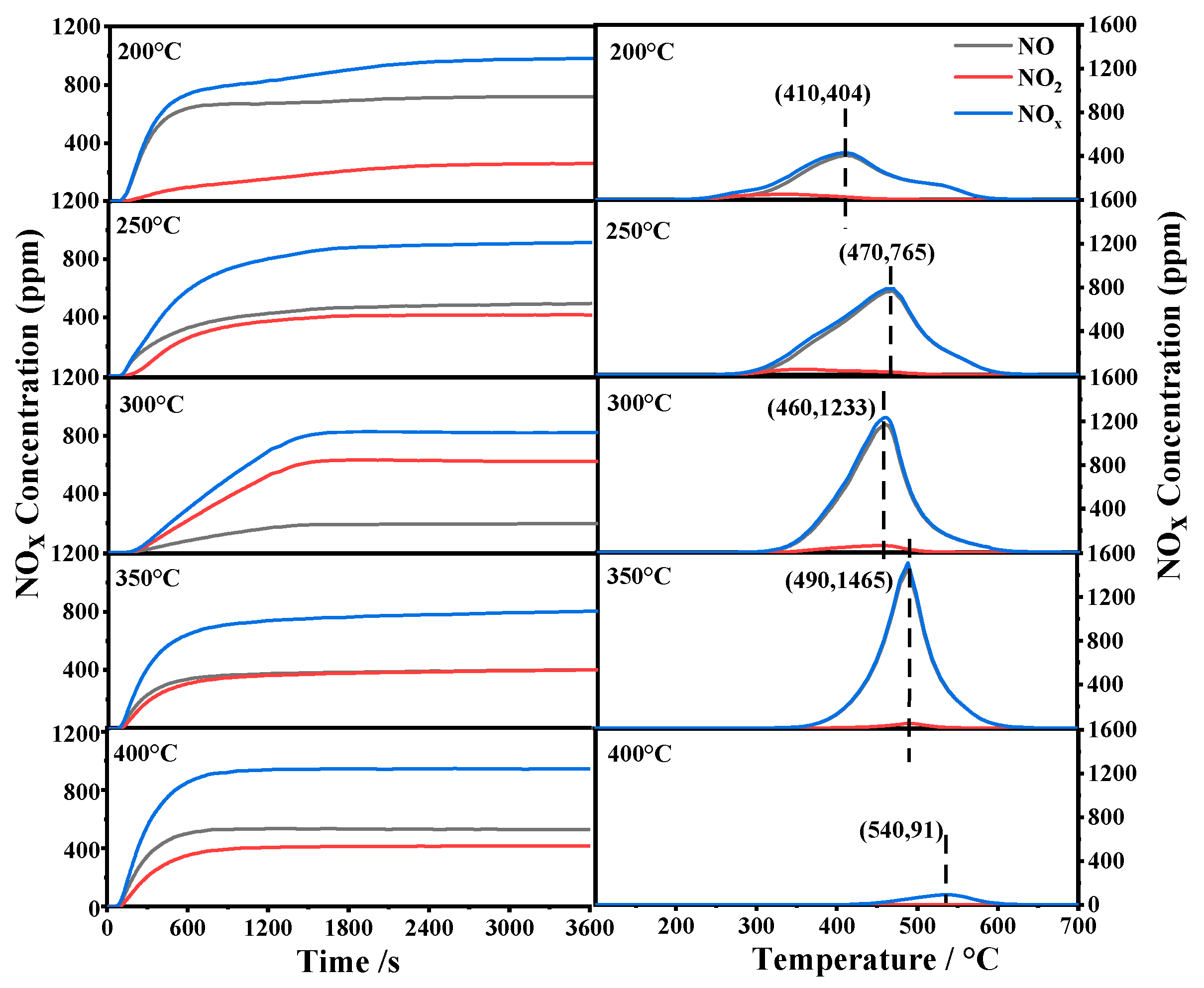
3.8. NOx Adsorption/Storage Capacity
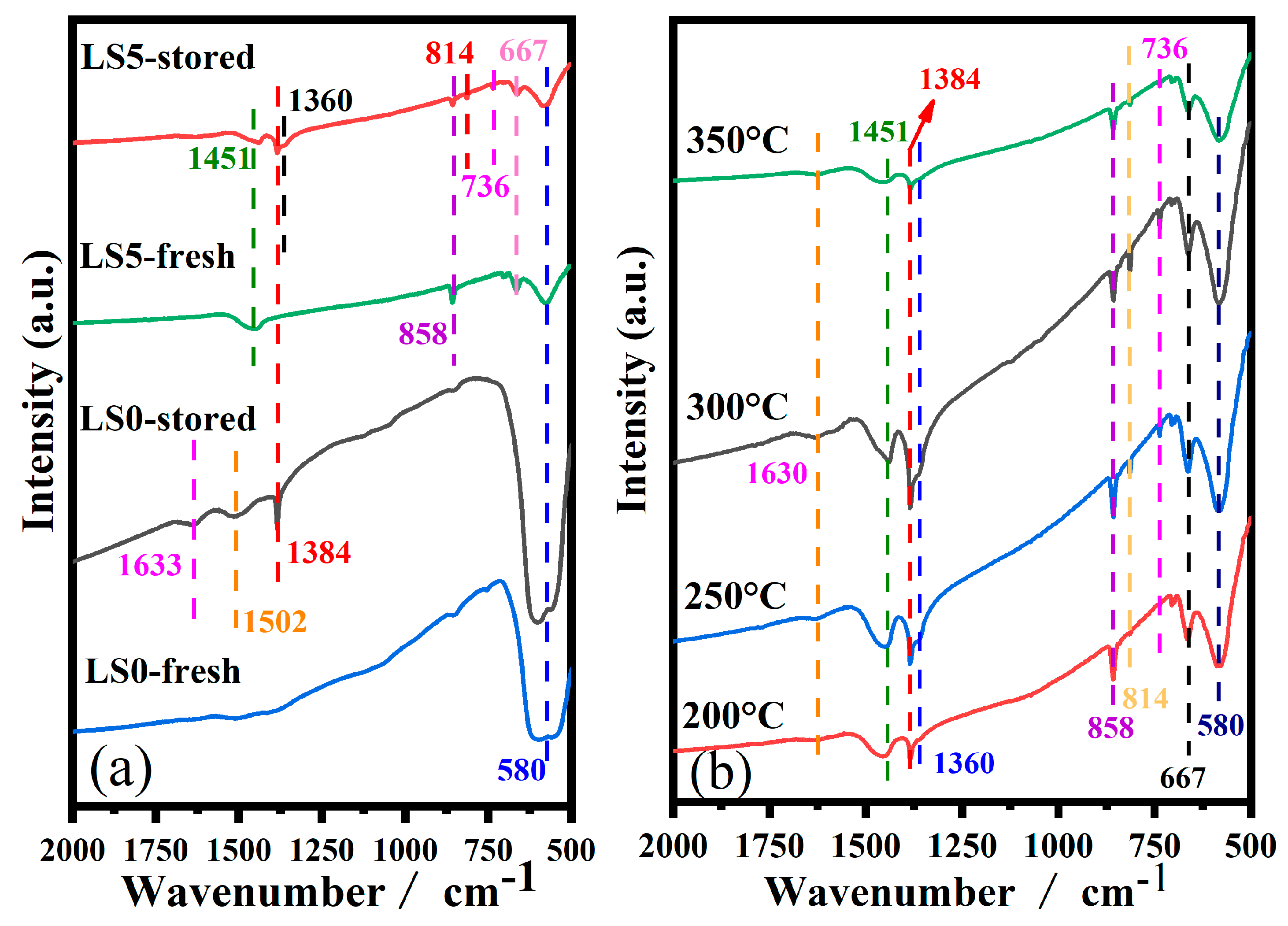
3.9. IR Study of NOx Storage
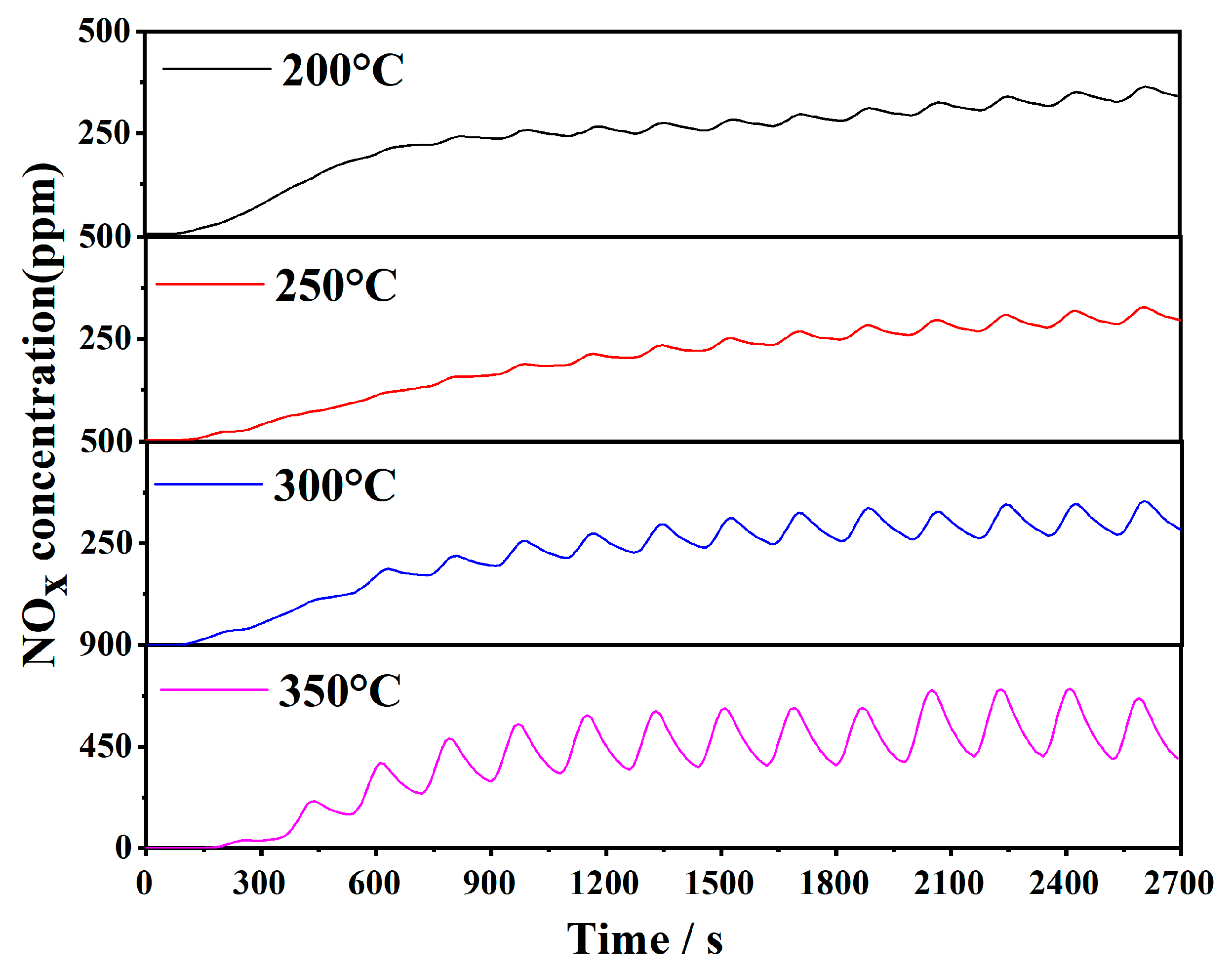
3.10. NOx Storage and Reduction Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Epling, W.S.; Parks, J.E.; Campbell, G.C.; Yezerets, A.; Currier, N.W.; Campbell, L.E. Further evidence of multiple NOx sorption sites on NOx storage/reduction catalysts. Catal. Today 2004, 96, 21–30. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; Illán-Gómez, M.J.; Bueno-López, A.; González-Velasco, J.R. Role of the different copper species on the activity of Cu/zeolite catalysts for SCR of NOx with NH3. Appl. Catal. B Environ. 2014, 147, 420–428. [Google Scholar] [CrossRef]
- Salasc, S.; Skoglundh, M.; Fridell, E. A comparison between Pt and Pd in NOx storage catalysts. Appl. Catal. B Environ. 2002, 36, 145–160. [Google Scholar] [CrossRef]
- Castoldi, L.; Matarrese, R.; Morandi, S.; Righini, L.; Lietti, L. New insights on the adsorption, thermal decomposition and reduction of NOx over Pt- and Ba-based catalysts. Appl. Catal. B Environ. 2018, 224, 249–263. [Google Scholar] [CrossRef]
- Ecker, S.I.; Dornseiffer, J.; Werner, J.; Schlenz, H.; Sohn, Y.J.; Sauerwein, F.S.; Baumann, S.; Bouwmeester, H.J.M.; Guillon, O.; Weirich, T.E.; et al. Novel low-temperature lean NOx storage materials based on La0.5Sr0.5Fe1−xMxO3−δ/Al2O3 infiltration composites (M = Ti, Zr, Nb). Appl. Catal. B Environ. 2021, 286, 119919. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Jiang, Z.; Zhang, S.; Huang, Y. A series of ceria supported lean-burn NOx trap catalysts LaCoO3/K2CO3/CeO2 using perovskite as active component. Chem. Eng. J. 2015, 260, 357–367. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; Gonzalez-Velasco, J.R. Strontium doping and impregnation onto alumina improve the NOx storage and reduction capacity of LaCoO3 perovskites. Catal. Today 2019, 333, 208–218. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; Pilar Gonzalez-Marcos, M.; Gonzalez-Velasco, J.R. Influence of ceria loading on the NOx storage and reduction performance of model Pt-Ba/Al2O3 NSR catalyst. Catal. Today 2015, 241, 133–142. [Google Scholar] [CrossRef]
- Mráček, D.; Kočí, P.; Choi, J.-S.; Partridge, W.P. New operation strategy for driving the selectivity of NOx reduction to N2, NH3 or N2O during lean/rich cycling of a lean NOx trap catalyst. Appl. Catal. B Environ. 2016, 182, 109–114. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Scofield, C.F.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. Thermal deactivation of Pt/Rh commercial automotive catalysts. Chem. Eng. J. 2010, 160, 85–92. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Luo, J.; Su, W.; Chang, H.; Li, J.; Hao, J.; Crittenden, J. Surface Tuning of La0.5Sr0.5CoO3 Perovskite Catalysts by Acetic Acid for NOx Storage and Reduction. Environ. Sci. Technol. 2016, 50, 6442–6448. [Google Scholar] [CrossRef]
- Uran, L.; Gallego, J.; Li, W.Y.; Santamaria, A. Effect of catalyst preparation for the simultaneous removal of soot and NOX. Appl. Catal. A Gen. 2019, 569, 157–169. [Google Scholar] [CrossRef]
- Ueda, A.; Yamada, Y.; Katsuki, M.; Kiyobayashi, T.; Xu, Q.; Kuriyama, N. Perovskite catalyst (La, Ba)(Fe, Nb, Pd)O3 applicable to NOx storage and reduction system. Catal. Commun. 2009, 11, 34–37. [Google Scholar] [CrossRef]
- Panunzi, A.P.; Duranti, L.; Luisetto, I.; Lisi, N.; Marelli, M.; Di Bartolomeo, E. Triggering electrode multi-catalytic activity for reversible symmetric solid oxide cells by Pt-doping lanthanum strontium ferrite. Chem. Eng. J. 2023, 471, 144448. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Jiang, H.; Zhang, L.; Zhang, H.; Cao, Y. Catalytic removal of toluene over three-dimensionally ordered macroporous Eu1−xSrxFeO3. Chem. Eng. J. 2013, 214, 262–271. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef]
- Say, Z.; Mihai, O.; Kurt, M.; Olsson, L.; Ozensoy, E. Trade-off between NOx storage capacity and sulfur tolerance on Al2O3/ZrO2/TiO2–based DeNOx catalysts. Catal. Today 2019, 320, 152–164. [Google Scholar] [CrossRef]
- Say, Z.; Vovk, E.I.; Bukhtiyarov, V.I.; Ozensoy, E. Enhanced Sulfur Tolerance of Ceria-Promoted NOx Storage Reduction (NSR) Catalysts: Sulfur Uptake, Thermal Regeneration and Reduction with H2(g). Top. Catal. 2013, 56, 950–957. [Google Scholar] [CrossRef]
- Theis, J.R. An assessment of Pt and Pd model catalysts for low temperature NOx adsorption. Catal. Today 2016, 267, 93–109. [Google Scholar] [CrossRef]
- Elbouazzaoui, S.; Corbos, E.C.; Courtois, X.; Marecot, P.; Duprez, D. A study of the deactivation by sulfur and regeneration of a model NSR Pt/Ba/Al2O3 catalyst. Appl. Catal. B Environ. 2005, 61, 236–243. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, H.J.; Park, S.J.; Nam, I.-S.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. Role of cobalt on γ-Al2O3 based NOx storage catalyst. Top. Catal. 2007, 42, 61–64. [Google Scholar] [CrossRef]
- Ao, M.; Pham, G.H.; Sage, V.; Pareek, V. Structure and activity of strontium substituted LaCoO3 perovskite catalysts for syngas conversion. J. Mol. Catal. A Chem. 2016, 416, 96–104. [Google Scholar] [CrossRef]
- Hueso, J.L.; Holgado, J.P.; Pereñíguez, R.; Mun, S.; Salmeron, M.; Caballero, A. Chemical and electronic characterization of cobalt in a lanthanum perovskite. Effects of strontium substitution. J. Solid State Chem. 2010, 183, 27–32. [Google Scholar] [CrossRef]
- Villoria, J.A.; Alvarez-Galvan, M.C.; Al-Zahrani, S.M.; Palmisano, P.; Specchia, S.; Specchia, V.; Fierro, J.L.G.; Navarro, R.M. Oxidative reforming of diesel fuel over LaCoO3 perovskite derived catalysts: Influence of perovskite synthesis method on catalyst properties and performance. Appl. Catal. B Environ. 2011, 105, 276–288. [Google Scholar] [CrossRef]
- Specchia, S.; Galletti, C.; Specchia, V. Solution Combustion Synthesis as intriguing technique to quickly produce performing catalysts for specific applications. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–67. [Google Scholar]
- Feng, N.; Meng, J.; Wu, Y.; Chen, C.; Wang, L.; Gao, L.; Wan, H.; Guan, G. KNO3 supported on three-dimensionally ordered macroporous La0.8Ce0.2Mn1−xFexO3 for soot removal. Catal. Sci. Technol. 2016, 6, 2930–2941. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 181, 227–237. [Google Scholar] [CrossRef]
- Cui, C.; Ma, J.; Wang, Z.; Liu, W.; Liu, W.; Wang, L. High Performance of Mn-Doped MgAlOx Mixed Oxides for Low Temperature NOx Storage and Release. Catalysts 2019, 9, 677. [Google Scholar] [CrossRef]
- Liang, H.; Mou, Y.; Zhang, H.; Li, S.; Yao, C.; Yu, X. Sulfur resistance and soot combustion for La0.8K0.2Co1−yMnyO3 catalyst. Catal. Today 2017, 281, 477–481. [Google Scholar] [CrossRef]
- Montanari, T.; Castoldi, L.; Lietti, L.; Busca, G. Basic catalysis and catalysis assisted by basicity: FT-IR and TPD characterization of potassium-doped alumina. Appl. Catal. A Gen. 2011, 400, 61–69. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Xin, Y.; Zhang, Z.; Zhang, Y.; Hao, C.; Meng, M.; Zheng, L.; Zheng, L. A unified intermediate and mechanism for soot combustion on potassium-supported oxides. Sci. Rep. 2014, 4, 4725. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Segarra, M. Steam reforming and oxidative steam reforming of ethanol over La0.6Sr0.4CoO3-delta perovskite as catalyst precursor for hydrogen production. Appl. Catal. A Gen. 2015, 502, 305–311. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Zha, Y.; Dai, F.; Hu, T.; Xie, Y.; Zhang, J. Highly efficient multifunctional dually-substituted perovskite catalysts La1−xKxCo1−yCuyO3−δ used for soot combustion, NOx storage and simultaneous NOx-soot removal. Appl. Catal. B Environ. 2012, 121–122, 65–74. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.Y.; Ding, Y.; Yu, Z.X. Mesoporous manganese-cobalt oxide spinel catalysts for CO2 hydrogenation to methanol. J. CO2 Util. 2019, 32, 146–154. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, J.; Luo, Y.; Jiang, L. New route to CeO2/LaCoO3 with high oxygen mobility for total benzene oxidation. Appl. Surf. Sci. 2017, 396, 95–101. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Mutoro, E.; Liu, Z.; Grass, M.E.; Biegalski, M.D.; Lee, Y.-L.; Morgan, D.; Christen, H.M.; Bluhm, H.; Shao-Horn, Y. Surface strontium enrichment on highly active perovskites for oxygen electrocatalysis in solid oxide fuel cells. Energy Environ. Sci. 2012, 5, 6081–6088. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142, 677–683. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, C.; Li, Z. Enhancing the Electrochemical Properties of LaCoO(3)by Sr-Doping, rGO-Compounding with Rational Design for Energy Storage Device. Nanoscale Res. Lett. 2020, 15, 184. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Liu, Y.; Bai, B.; Wang, Y.; Li, X.; Xie, S.; Li, J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: Active catalysts for the combustion of methane. J. Catal. 2013, 307, 327–339. [Google Scholar] [CrossRef]
- Akay, G. Plasma Generating-Chemical Looping Catalyst Synthesis by Microwave Plasma Shock for Nitrogen Fixation from Air and Hydrogen Production from Water for Agriculture and Energy Technologies in Global Warming Prevention. Catalysts 2020, 10, 152. [Google Scholar] [CrossRef]
- Akay, G. Hydrogen, Ammonia and Symbiotic/Smart Fertilizer Production Using Renewable Feedstock and CO2 Utilization through Catalytic Processes and Nonthermal Plasma with Novel Catalysts and In Situ Reactive Separation: A Roadmap for Sustainable and Innovation-Based Technology. Catalysts 2023, 13, 1287. [Google Scholar] [CrossRef]
- Zakaryan, H.A.; Aroutiounian, V.M. Investigation of cobalt doped tin dioxide structure and defects: Density functional theory and empirical force fields. J. Contemp. Phys. Armen. Acad. Sci. 2017, 52, 227–233. [Google Scholar] [CrossRef]
- Catto, A.C.; da Silva, L.F.; Bernardi, M.I.B.; Bernardini, S.; Aguir, K.; Longo, E.; Mastelaro, V.R. Local Structure and Surface Properties of CoxZn1−xO Thin Films for Ozone Gas Sensing. ACS Appl. Mater. Interfaces 2016, 8, 26066–26072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Shan, W.; Lian, Z.; He, H. Effect of support preparation with different concentration precipitant on the NOx storage performance of Pt/BaO/CeO2 catalysts. Catal. Today 2020, 339, 135–147. [Google Scholar] [CrossRef]
- Liu, W.; Cao, H.; Wang, Z.; Cui, C.; Gan, L.; Liu, W.; Wang, L. A novel ceria hollow nanosphere catalyst for low temperature NOx storage. J. Rare Earths 2022, 40, 626–635. [Google Scholar] [CrossRef]
- Fridell, E.; Persson, H.; Westerberg, B.; Olsson, L.; Skoglundh, M. The mechanism for NOx storage. Catal. Lett. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qi, X.X.; Chen, Z.L.; Jiang, L.L.; Wang, R.H.; Wei, K.M. Studies on SO2 Tolerance and Regeneration over Perovskite-Type LaCo1−xPtxO3 in NOx Storage and Reduction. J. Phys. Chem. C 2014, 118, 13743–13751. [Google Scholar] [CrossRef]
- Wen, W.; Wang, X.Y.; Jin, S.; Wang, R.H. LaCoO3 perovskite in Pt/LaCoO3/K/Al2O3 for the improvement of NOx storage and reduction performances. RSC Adv. 2016, 6, 74046–74052. [Google Scholar] [CrossRef]
- Xie, W.; Xu, G.; Zhang, Y.; Yu, Y.; He, H. Mesoporous LaCoO3 perovskite oxide with high catalytic performance for NOx storage and reduction. J. Hazard. Mater. 2022, 431, 128528. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.; Li, X.; Li, L.; Zou, H.; Meng, M.; Li, Q.; Tan, Y.; Tsubaki, N. BaFeO3−x Perovskite: An Efficient NOx Absorber with a High Sulfur Tolerance. J. Phys. Chem. C 2010, 114, 11844–11852. [Google Scholar] [CrossRef]
- You, R.; Zhang, Y.; Liu, D.; Meng, M.; Zheng, L.; Zhang, J.; Hu, T. YCeZrO Ternary Oxide Solid Solution Supported Nonplatinic Lean-Burn NOx Trap Catalysts Using LaCoO3 Perovskite as Active Phase. J. Phys. Chem. C 2014, 118, 25403–25420. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal Rev. Sci. Eng. 2000, 42, 144–171. [Google Scholar] [CrossRef]
- Li, X.-G.; Dong, Y.-H.; Xian, H.; Hernandez, W.Y.; Meng, M.; Zou, H.-H.; Ma, A.-J.; Zhang, T.-Y.; Jiang, Z.; Tsubaki, N.; et al. De-NOx in alternative lean/rich atmospheres on La1−xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar] [CrossRef]

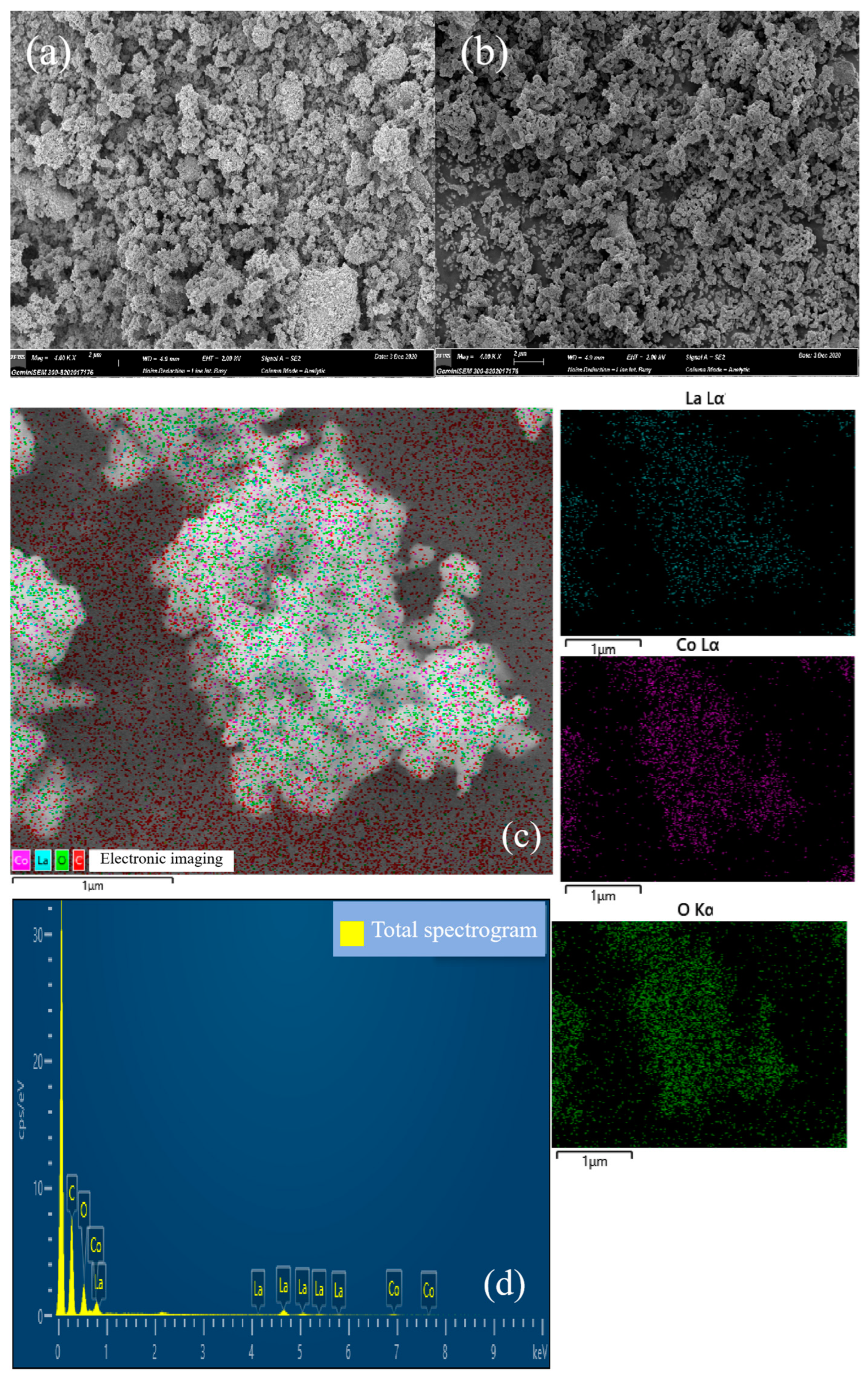

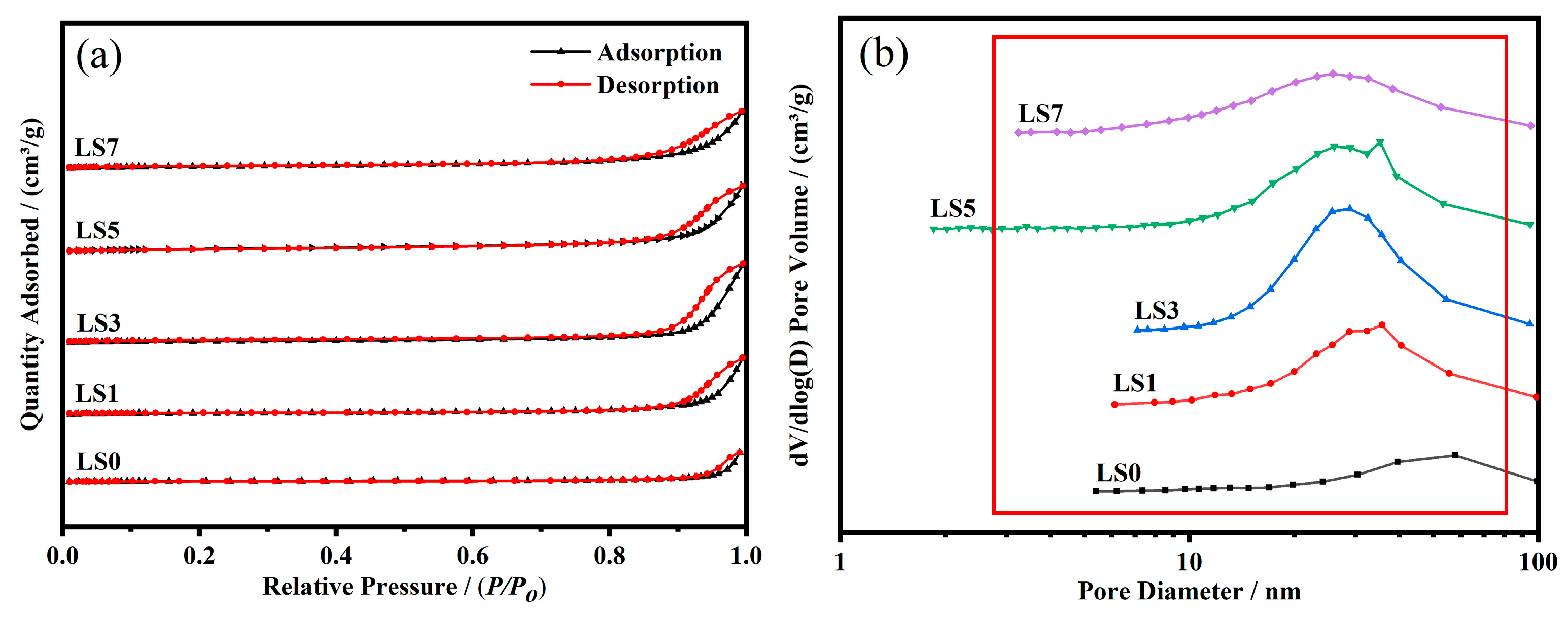
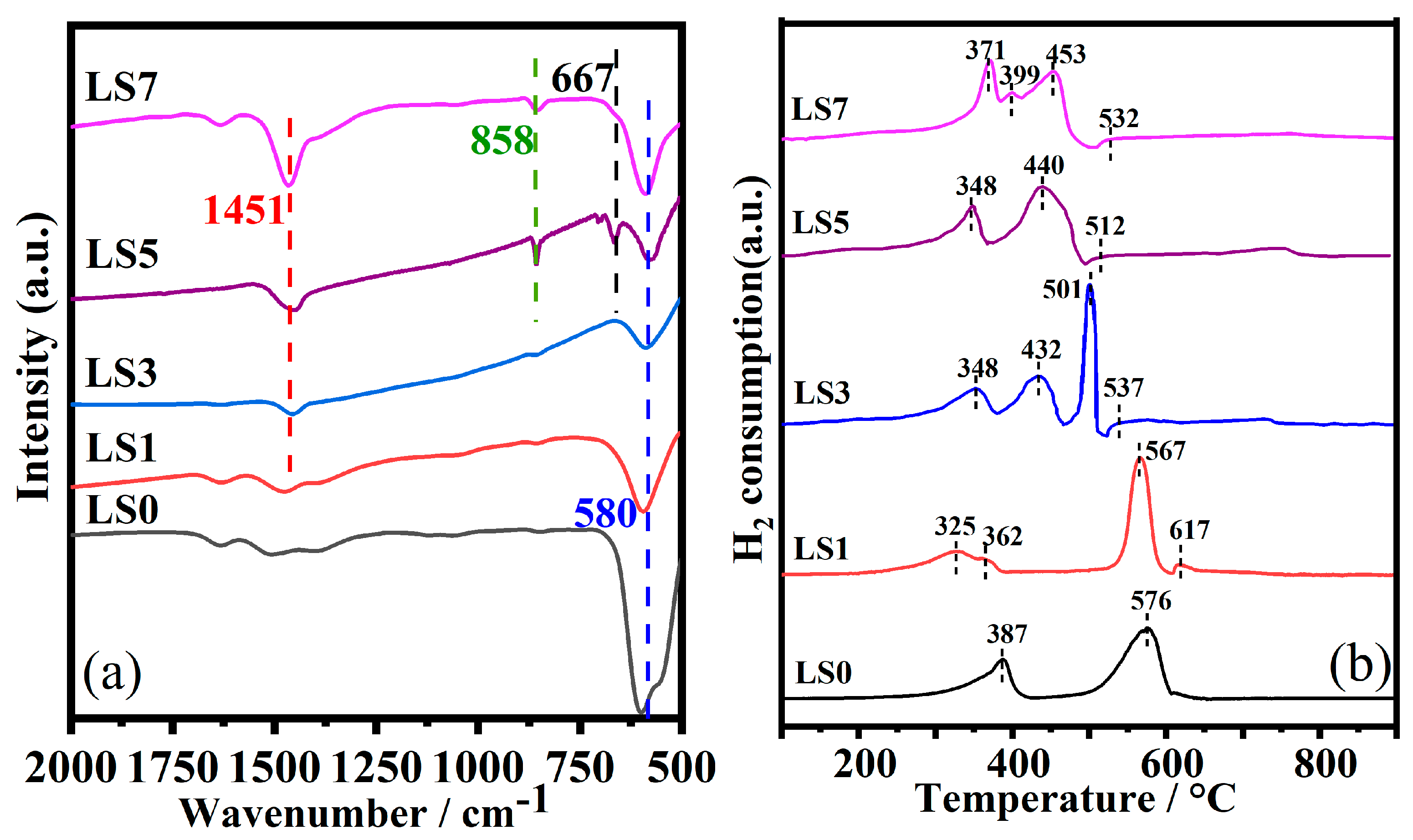


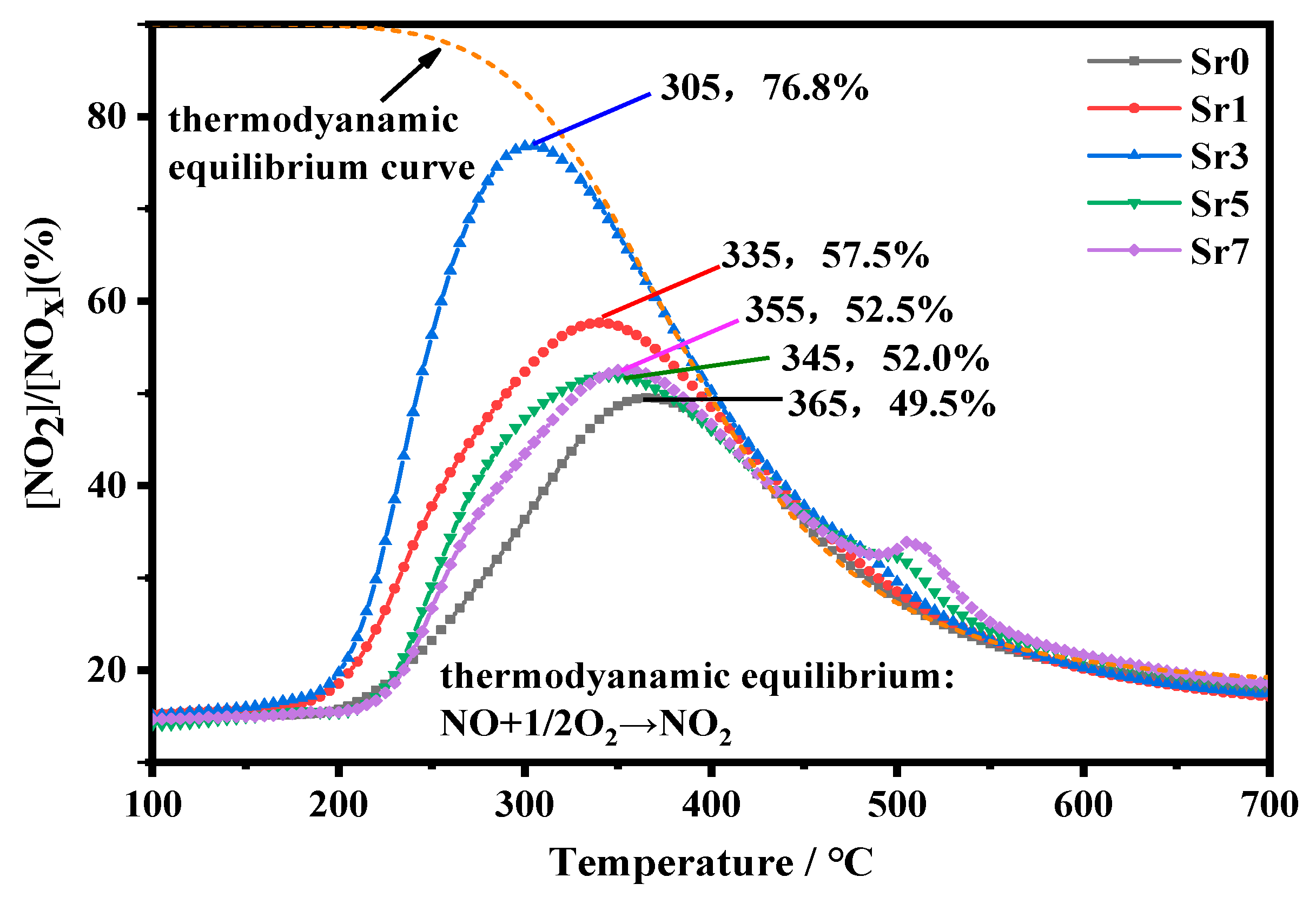
| Catalyst | SBET (m2/g) | Vp (cm3/g) | Dp (nm) | Mesopore Fraction | (La + Sr)/Co |
|---|---|---|---|---|---|
| LS0 | 5.58 | 0.05 | 41 | 0.36 | 1.20 |
| LS1 | 12.38 | 0.08 | 32 | 0.66 | - |
| LS3 | 13.44 | 0.13 | 29 | 0.76 | - |
| LS5 | 17.71 | 0.11 | 22 | 0.75 | 1.09 |
| LS7 | 15.96 | 0.09 | 23 | 0.70 | - |
| Catalyst | H2 Consumption (mmol/g) | Peak Area | |
|---|---|---|---|
| α-Peak | β-Peak | ||
| LS0 | 5.7 | 100 | - |
| LS1 | 6.2 | 177 | 39 |
| LS3 | 8.1 | 194 | 369 |
| LS5 | 7.5 | 219 | 578 |
| LS7 | 6.9 | 172 | 668 |
| Catalyst | OII/(OI + OII + OIII) (%) | Co2+/Co3+ | (La + Sr)/Co | Srsurf/Srlatt |
|---|---|---|---|---|
| LS0 | 57.6 | 1.13 | 2.33 | - |
| LS3 | 68.6 | 1.41 | 1.28 | 1.23 |
| LS5 | 65.8 | 1.43 | 1.05 | 1.6 |
| Catalysts | NAC (μmol/g) | NSC (μmol/g) |
|---|---|---|
| LS0 | 831 | 23 |
| LS1 | 865 | 48 |
| LS3 | 1485 | 635 |
| LS5 | 1889 | 1084 |
| LS7 | 1556 | 810 |
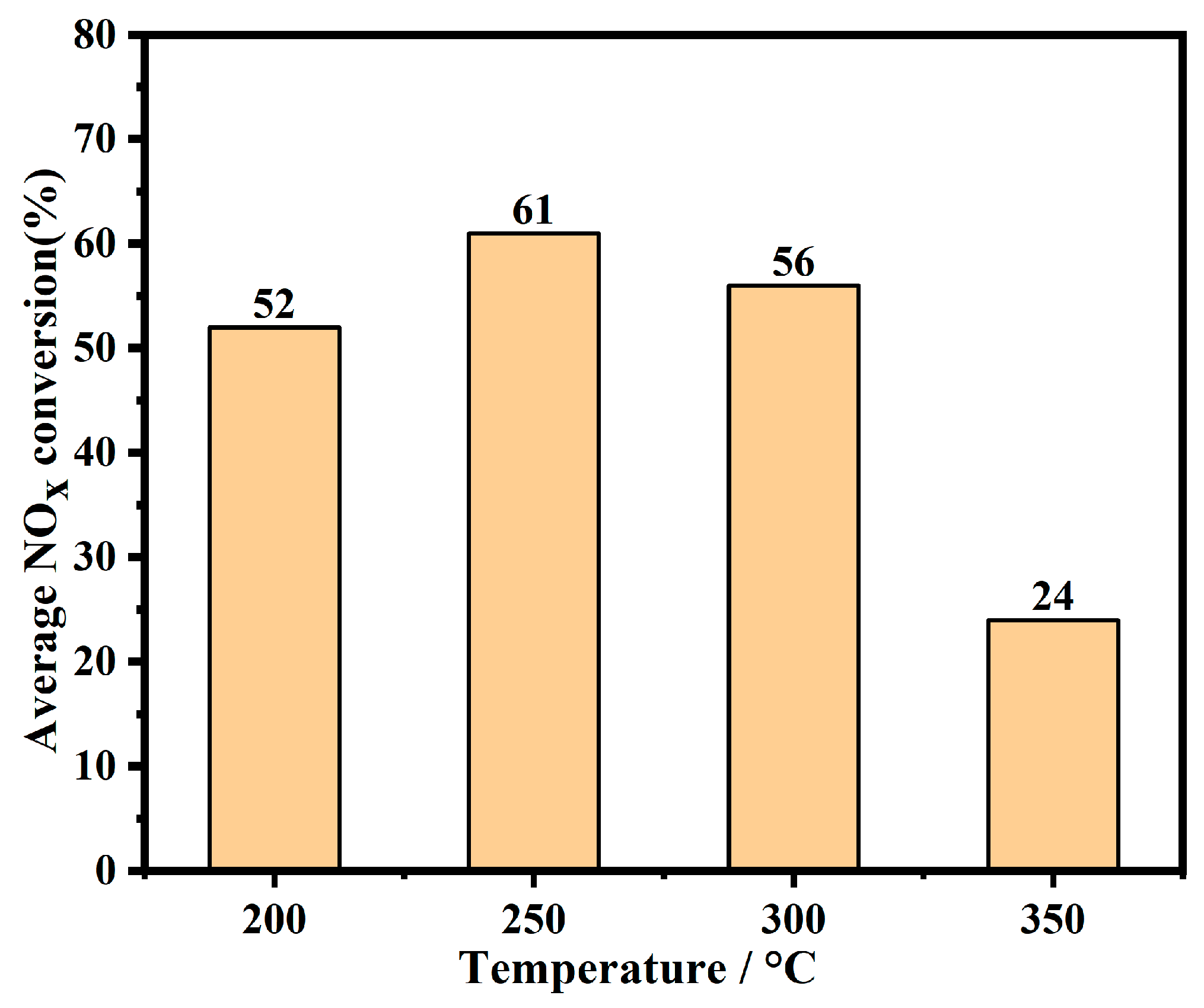
| Adsorption Temperature (°C) | NAC (μmol/g) | NSC (μmol/g) | RNO2 (%) |
|---|---|---|---|
| 200 | 937 | 609 | 30 |
| 250 | 1297 | 984 | 42 |
| 300 | 1889 | 1084 | 63 |
| 350 | 1633 | 1007 | 42 |
| 400 | 750 | 80 | 42 |
| Catalyst | NAC (μmol/g) | RNO2 | The Decline Rate of NAC | ||
|---|---|---|---|---|---|
| Fresh | Hydrothermal Aging | Fresh | Hydrothermal Aging | ||
| LS0 | 831 | 461 | 52 | 33 | 44.5 |
| LS5 | 1889 | 1262 | 63 | 38 | 33.2 |
| Catalyst | NAC (μmol/g) | RNO2 | The Decline Rate of NAC | ||
|---|---|---|---|---|---|
| Fresh | Sulfur Poisoning | Fresh | Sulfur Poisoning | ||
| LS0 | 831 | 554 | 52 | 19 | 33.3 |
| LS5 | 1889 | 1434 | 63 | 28 | 24.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, X.; Wang, X.; Zhang, T.; Gan, L.; Liu, J.; Zhai, Y.; Liu, W.; Wang, L.; Wang, Z. NOx Storage and Reduction (NSR) Performance of Sr-Doped LaCoO3 Perovskite Prepared by Glycine-Assisted Solution Combustion. Compounds 2024, 4, 268-287. https://doi.org/10.3390/compounds4020014
Luan X, Wang X, Zhang T, Gan L, Liu J, Zhai Y, Liu W, Wang L, Wang Z. NOx Storage and Reduction (NSR) Performance of Sr-Doped LaCoO3 Perovskite Prepared by Glycine-Assisted Solution Combustion. Compounds. 2024; 4(2):268-287. https://doi.org/10.3390/compounds4020014
Chicago/Turabian StyleLuan, Xinru, Xudong Wang, Tianfei Zhang, Liangran Gan, Jianxun Liu, Yujia Zhai, Wei Liu, Liguo Wang, and Zhongpeng Wang. 2024. "NOx Storage and Reduction (NSR) Performance of Sr-Doped LaCoO3 Perovskite Prepared by Glycine-Assisted Solution Combustion" Compounds 4, no. 2: 268-287. https://doi.org/10.3390/compounds4020014
APA StyleLuan, X., Wang, X., Zhang, T., Gan, L., Liu, J., Zhai, Y., Liu, W., Wang, L., & Wang, Z. (2024). NOx Storage and Reduction (NSR) Performance of Sr-Doped LaCoO3 Perovskite Prepared by Glycine-Assisted Solution Combustion. Compounds, 4(2), 268-287. https://doi.org/10.3390/compounds4020014





