Abstract
Inflammation and thrombosis are implicated in several chronic disorders. Recent studies have outlined the way in which several compounds can offer protection against inflammation. Within this comprehensive review the so-far reported anti-inflammatory health-promoting effects of several metal-based complexes, both in vitro and in vivo, are thoroughly presented. These metal-based compounds usually interfere with various biochemical processes associated with the inflammatory response and thrombus formation and become capable of inhibiting these biochemical pathways with proposed health benefits. Emphasis is given to the multifaceted actions of metal-based complexes that have exhibited potent anti-inflammatory and antithrombotic activities against the inflammatory mediator, platelet-activating factor (PAF), and its thrombo-inflammatory signaling, as well as on their anti-platelet and antitumor health promoting properties. Furthermore, the enhancement of the anti-inflammatory potency of well-established bioactive compounds by their incorporation as ligands in several metal-based complexes is discussed. Metal-based complexes bearing natural anti-inflammatory bioactives are also outlined. Characteristic examples of both free and metal-based porphyrins are explored. These compounds are recognized to have anti-inflammatory and antithrombotic assets, in addition to other pleiotropic advantages including antibacterial or anticancer actions. Additionally, applications of metal complexes in various models of inflammatory and thrombotic complications are demonstrated. The combined results of this study show that further research is required towards the preparation of several metal-based complexes with improved pharmacological profiles. Finally, restrictions on the application of these metal-based compounds are also covered, along with their prospects for the future and the need for additional study in order to improve their efficacy and safety.
1. Introduction
Inflammation is a complex biological response of the body in its attempt to eliminate pathogenic stimuli in order to begin the process of repairing damaged tissue and restore homeostasis [1]. Several agents can cause inflammation, including microorganisms, natural factors, chemicals, inappropriate immune responses and self-anointing responses [1,2]. Inflammation can be acute and/or chronic depending on the duration and the appropriate resolution of the response [3]. Chronic inflammation and thrombo-inflammatory manifestations are implicated in several chronic disorders, including cardiovascular diseases, diabetes, neurodegenerative disorders, renal diseases, pulmonary disorders, autoimmune disorders, persistent infections and cancer [4,5].
As we march in the post-CANTOS era (Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease Trial (CANTOS)) [6], research on the field of anti-inflammatory and antithrombotic compounds that can offer pharmaceutical actions against these disorders has gained much attention. The field of antiplatelet and antithrombotic drugs constitutes an interesting research topic for the treatment of cardiovascular diseases and thrombosis [7,8]. Traditionally targeting platelets, thrombin and other inflammatory mediators is the classic approach by which to prevent acute thromboembolic artery occlusions in cardiovascular diseases and to treat chronic inflammatory diseases [4,5]. Known inhibitor categories against thrombo-inflammatory pathways include (i) cyclooxygenase 1 (COX1) inhibitors of the eicosanoids-related pathways, (ii) proteinase-activated receptor (PAR1) antagonists of the thrombin-related pathways, (iii) inhibitors of the adenosine diphosphate (ADP) P2Y12 receptor of the ADP-related pathways and (iv) glycoprotein GPIIb/IIIa receptor inhibitors of the collagen-related pathways [7,8].
Over recent years a different approach has been reported, one which considers the role of the platelet-activating factor (PAF) and its receptor (PAFR), which is the most potent lipid mediator of inflammation [4,5,9]. In other words, the antiplatelet potency, better expressed as anti-PAF activity, of any substance can be exerted by inhibiting the PAF-induced aggregation in washed rabbit/human platelets (WRPs and hWPs respectively), and rabbit/human platelet reach plasma (rPRP and hPRP, respectively) [8]. Towards this goal, several compounds with antiplatelet potencies and a more general anti-PAF activity have been studied [10,11,12,13], among which some metal-based compounds bear natural bioactive properties as ligands, while others bear some porphyrin derivatives that also pose as attractive bioactive alternative ligand metal complexes [14]. Thus, apart from the classic organic compounds against inflammation and thrombosis, several “metal-based inflammatory mediators,” have also been developed that are able to successfully encounter all of these thrombo-inflammatory pathways [15].
In this respect, a series of chromium(III), manganese(II), iron(II), cobalt(II), nickel(II), copper(II), zinc(II), ruthenium(II) and (III), rhodium(I) and (III), and recently tin(II) and (IV) have been synthesized and structurally characterized and have shown strong anti-inflammatory and antithrombotic properties against the PAF-related pathways and other thrombo-inflammatory pathways, such as those related to thrombin, collagen and ADP [13,16,17,18,19]. The ability of such metal-based compounds to inhibit platelet activation and aggregation by affecting each or more of these thrombo-inflammatory pathways, through specific structure–activity relationships related to their inhibitory binding on the specific receptors of either platelet agonists and/or thrombo-inflammatory mediators, further indicates that these bioactive metal-based antiplatelet compounds can serve as inhibitors of the inflammatory processes induced by such agonists in several other cell types and tissues that express these receptors on their membranes, including leucocytes, endothelial cells, mesangial cells, neural cells, etc. Thus, these metal-based compounds pose as important candidates not only for antiplatelet pharmaceuticals, but also as antithrombotic and anti-inflammatory cardio-protective, anti-tumor and neuroprotective agents.
Moreover, there is another class of metal-based complexes which act as ligands in several natural phenolic bioactive compounds, such as rutin, curcumin, chrysin or vanillin and several of their derivatives. These phenolics of natural origin possess, on their own, antithrombotic and anti-inflammatory properties, but their complexation as ligands in metal-based compounds results in improved biological properties (such as anti-inflammatory or antimicrobial) when compared with the original natural compound in the absence of the metal center [10,11,12,20]. This suggests that the bioactive properties of any of these natural phenolic compounds are improved by their coupling as ligands in metal-based complexes.
In addition, porphyrin-based complexes and their derivatives are another class of interesting metal-based compounds with potential anti-inflammatory properties. Porphyrins are present in many natural systems and have significant biological roles, while they have been extensively utilized in biomedical applications during the last decade. Porphyrins are macrocyclic compounds, consisting of four pyrrole units conjugated through methine bridges [21,22]. They often complex with a metal ion located in the center of the ring system and can be modified with a plethora of substituents at the meso and β positions of the porphyrin ring, which leads to a multitude of different functions. Naturally occurring porphyrins are recognized for their roles in two vital biological processes: (i) oxygen transport in hemoglobin and (ii) photosynthesis in chlorophyll. For example, the porphyrin ring in chlorophyll captures photons of light, initiating the process of converting light energy into chemical energy, which fuels plant growth and sustains life on Earth [23]. Moreover, porphyrins find diverse applications in scientific research, from medical and therapeutic fields to energy-related studies [24,25,26,27,28,29,30]. More specifically, synthetic porphyrins have become of particular interest in medicinal chemistry because they can form chelating compounds with certain toxic heavy metals or radioisotopes that emit gamma rays [22].
Within this article, last decade’s outcomes on the anti-inflammatory and antithrombotic potential of several metal-based complexes (metal-based inflammatory mediators), are thoroughly presented. Emphasis is given to metal-based compounds with antiplatelet properties against the PAF-related thrombo-inflammatory signaling, as well as against several other platelet agonists and thrombotic mediators. Synthetic porphyrins and combination drug therapy that contains porphyrins and metal-based compounds with such ligands are covered. The enhancement of the anti-inflammatory potency of well-established natural bioactives by their incorporation as ligands in metal-based complexes is also discussed. The current challenges and future prospects concerning the exploitation of both porphyrin-based materials and metal-based bioactive complexes in anti-inflammatory and antithrombotic biomedical applications are presented.
2. Metal-Based Complexes with Anti-Inflammatory and Antithrombotic Properties
2.1. Metal-Based Complexes with Anti-Inflammatory and Antithrombotic Potency, Mainly through Their Anti-PAF Effects
Philippopoulos et al. have studied several metal-based complexes of chromium(III), manganese(II), iron(II), cobalt(II), nickel(II), copper(II) and zinc(II) (1–7), containing the 2-(2′-pyridyl)quinoxaline ligand (pqx) as PAFR antagonists (Figure 1). These compounds were biologically evaluated as inhibitors of PAF- and thrombin-induced aggregation in WRPs and their inhibitory effect (IC50 values in μM) were both estimated (Figure 1). These complexes feature different coordination geometries, ranging from tetrahedral to trigonal-pyramidal and octahedral, with the bulkiest, manganese complex 5, identified as the less active of these molecules. Specifically, trigonal-pyramidal iron(II) complex (6) was the most potent inhibitor and all other complexes exhibited an anti-PAF activity in the micromolar range. A synergistic effect was also reported, based on preliminary structure–activity relationship (SAR) data, which further indicates an increase of the anti-PAF (antiplatelet activity) of the relevant compound by such a positive ligand coordination with a metal center [13].
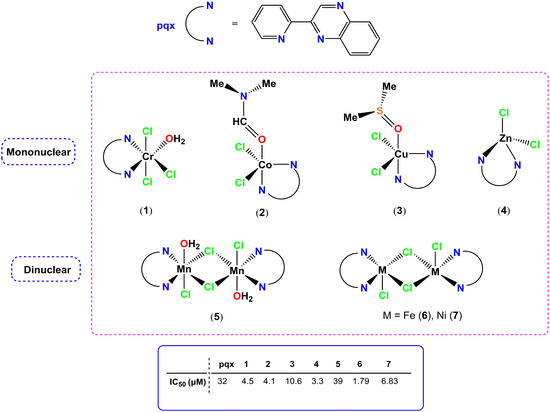
Figure 1.
Molecular structures of metal-based PAF inhibitors 1–7. IC50 values (μM) against the PAF-induced aggregation in WRPs are included.
In line with the SAR studies reported, the overall size of the target molecule (metal-based inhibitors) affects the biological activity expressed. The antithrombotic potency of 1–7 also shows that the iron(II) complex 6 was the most potent, while the pqx ligand was not active towards thrombin-induced aggregation. This feature highlights the effect of metal ions within the overall structure of the formed metal complex. Additionally, complexes (1), (6) and (7) showed activity against PAF-basic biosynthetic and catabolic enzymatic pathways in rabbit leukocytes, underlying their potent anti-inflammatory properties [15]. The inhibitory effects of this series of metal complexes (expressed as IC50 values), against the PAF-induced aggregation in WRPs, are comparable with those of the well-established ginkgolide B series of natural PAF antagonists [29], along with that of a synthetic PAF-antagonist, rupatadine, which is the main component of anti-allergic drugs [31]. Moreover, the pqx ligand precursor was cytotoxic against tumor-derived HeLa cells; however, its complexation to Cr(III) (1), Co(II) (2), Zn(II) (4) Mn(II) (5), Fe(II) (6) and Ni(II) (7) resulted in reduced toxicity in these cellular proliferation studies. The Cu–pqx complex (3) was found to be more effective as a PAF-inhibitor than cisplatin, as it possessed a much lower IC50 value against PAF than that of cisplatin.
Previously, the same research group synthesized some organometallic tin(II) and tin(IV) complexes, featuring the amphiphilic oxygen tripodal Kläui ligands [(η5-C5R5)Co{P(OEt)2O}3]−, {R = H, (NaLOEt), and Me (NaL*OEt) and have been investigated as PAFR antagonists (Figure 2). The rare tin(II) four-coordinate complexes (8a–8b) along with the six-coordinate tin(IV) analogues (9a–9b) have been found to be potent inhibitors of the PAF and thrombin-induced aggregation in WPRPs and in rabbit PRP aggregation assays (rPRPs). The permethylated organotin(II) analogue 8b, and the organotin(IV) complex 9a incorporating the non-methylated LOEt ligand, were the most potent against PAF-induced aggregation in WRPs [17]. Conclusively, the authors suggest that, within this series, the inhibitory activity drops in the following order: 8a > 9b > 9a ≅ 8b > NaLOEt ≅ NaL*OEt. These findings are in accordance with the synergetic effect, a trend which has been verified upon examination of a library of metal-based inhibitors [18].
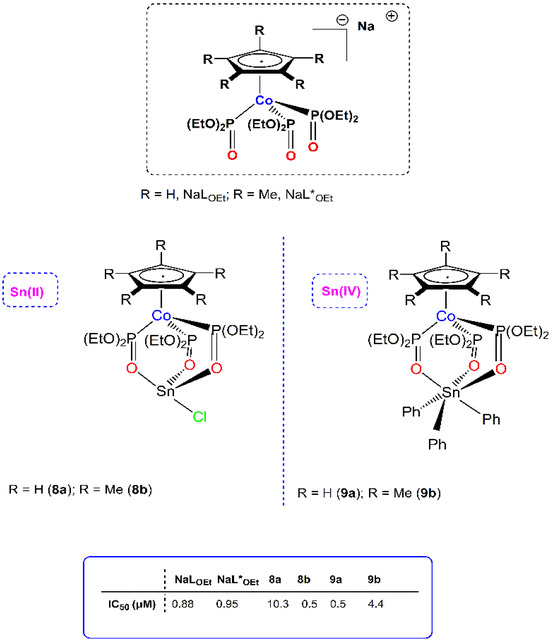
Figure 2.
Molecular structures of metal-based PAF inhibitors 8–9. IC50 values (μM) against the PAF-induced aggregation in WRPs are included.
Interestingly, 8b, 9a and 9b displayed inhibitory effects comparable or even better than ginkgolides, which are a natural and specific inhibitor of PAF. In ex vivo PAF-induced rabbit PRP aggregation assays (rPRPs) that simulate the in vivo conditions, the anti-PAF activity was lowered, clearly due to the presence of plasma proteins and other biomolecules. Complex 8b remained the most potent, followed by 9b and 9a, with 8a being the less active. This was reinforced by cross-desensitization tests suggesting that 8b induces aggregation of WRPs through the PAF/PAFR related pathway of platelet aggregation. This complex can beneficially affect the PAF/PAFR induced inflammation and thrombosis either antagonistically (low concentrations) or agonistically (in higher concentrations). Molecular docking calculations showed that such organotin complexes bearing the bulky oxygen tripodal ligand cannot fit inside the PAF-binding site of the receptor. It has been suggested that they can interact with the extracellular domain of the receptor, probably by blocking the entrance and binding of PAF on its receptor, PAFR, and thus the PAF/PAFR-induced pathways.
The antithrombotic potency of these organotin complexes has been studied using other well-established thrombotic mediators and platelet agonists, such as thrombin. The six-coordinate organotin(IV) analogues 9a and 9b displayed the strongest antithrombotic effect (0.55 μΜ and 0.23 μΜ, respectively). This was attributed to the higher affinity exerted for the PAR-3 receptors of thrombin. Interestingly, 8b and 9a were also evaluated for antitumor activity, on human Jurkat T lymphoblastic tumor cell line, and found to be more effective than cisplatin.
The same group has been reported previously in terms of the biological activities of mer-[Rh(Br-Qpy)Cl3(CH3OH)] (10, Br-Qpy = 6-bromo-4-phenyl-2-pyridin-2-yl-quinoline) and mer-[Rh(OH-Ph-Qpy)Cl3(CH3OH)] (11, OH-Ph-Qpy = 4-(4-phenyl-2-(pyridin-2-yl)quinolin-6-yl)phenol) as inhibitors of PAF-induced aggregation in WRPs in vitro (Figure 3) [32]. Upon coordination of the less sterically encumbered Br-Qpy to afford complex 10, a three-fold increase of the inhibitory activity was reported, while for 11, the inhibitory effect was slightly increased. The inhibitory activity against PAF-induced aggregation decreased in the following order 10 > Br-Qpy > 11 > OH-Ph-Qpy, underlying the role of the final structure adopted by each complex. Interestingly the inhibitory effect of these compounds could be potentially compared with the inhibitory effect of ginkgolide B, a class of natural and specific PAF antagonists extracted from the leaves and roots of the Chinese tree Ginkgo biloba.
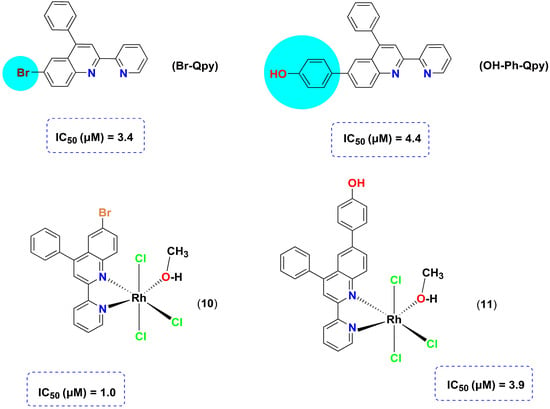
Figure 3.
Molecular structures of metal-based PAF inhibitors 10–11.
Based on the previous results, Kalampalidis et al. have reported on the anti-PAF (antiplatelet) activity of a square planar organometallic rhodium(I) complex of the formula [Rh(cod)Cl(tpc)] (12) (cod = cis-1,5-cyclooctadiene; tpc = methyl 2-amino-4-(diethylamino)-thieno-[2,3-d]-pyrimidine-6-carboxylate) in vitro, both in human and washed rabbit platelets (Figure 4) [16]. In human platelets, and in conditions close to the in vivo conditions, the inhibitory effect was significantly reduced due to the presence of plasma proteins and other biomolecules [33]. Molecular docking calculations illustrate that the rhodium(I) complex 12 can be accommodated within the ligand-binding site of PAF receptor and block the activity of PAF. This is in accordance with the experimental findings rendering this complex a selective and potent inhibitor. Additionally, 12 is an inhibitor of other inflammatory and thrombotic mediators, like thrombin, and well-established platelet agonists, like ADP and collagen. Therefore, 12 can be considered a metal-based PAF inhibitor with promising antiplatelet, antithrombotic, and anti-inflammatory activity.
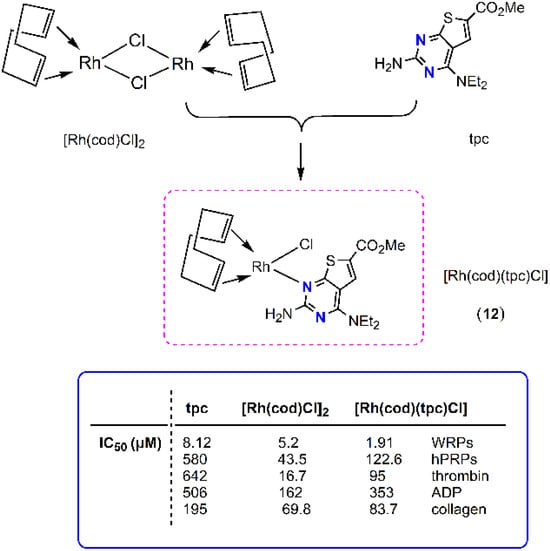
Figure 4.
Molecular structure of metal-based PAF inhibitor 12. IC50 values (μM) against the PAF-induced aggregation in WRPs, hPRPs, thrombin, ADP and collagen are included.
Previously the same research group studied the anti-PAF and antithrombotic activities of a series of rhodium(I) and rhodium(III) complexes with nitrogen containing bidentate ligands (Figure 5) [18,34]. The cationic square planar rhodium (I) complex [Rh(cod)(pqx)]X (cod = cis-1,5-cyclooctadiene; pqx = 2-(2′-pyridyl)quinoxaline; X = Cl− (13), X = NO3− (14)) displayed very strong anti-PAF action at the nanomolar scale. The rhodium(III) analogues 15–18, featuring octahedral coordination geometries, were less active (Figure 5). Theoretical docking studies suggest that 13–14 complexes are selective and potent inhibitors of PAF-induced aggregation, as they fit into the binding site of PAFR. The bulkier octahedral rhodium(III) analogues 15–18, which cannot fit into the ligand binding domain, could potentially exhibit their activity at the extracellular domain of the receptor.
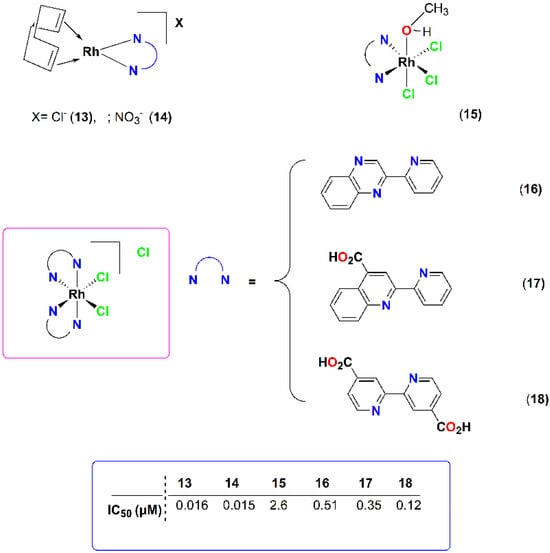
Figure 5.
Molecular structures of metal-based PAF inhibitors 13–18. IC50 values (μM) against the PAF-induced aggregation in WRPs are included.
Another class of Ru-based PAF inhibitors was also synthesized (Figure 6) [19], showing that ruthenium(II) and ruthenium(III) complexes displayed comparable activities. Within the series, ionic complexes were found to be more potent in comparison with their neutral analogous.
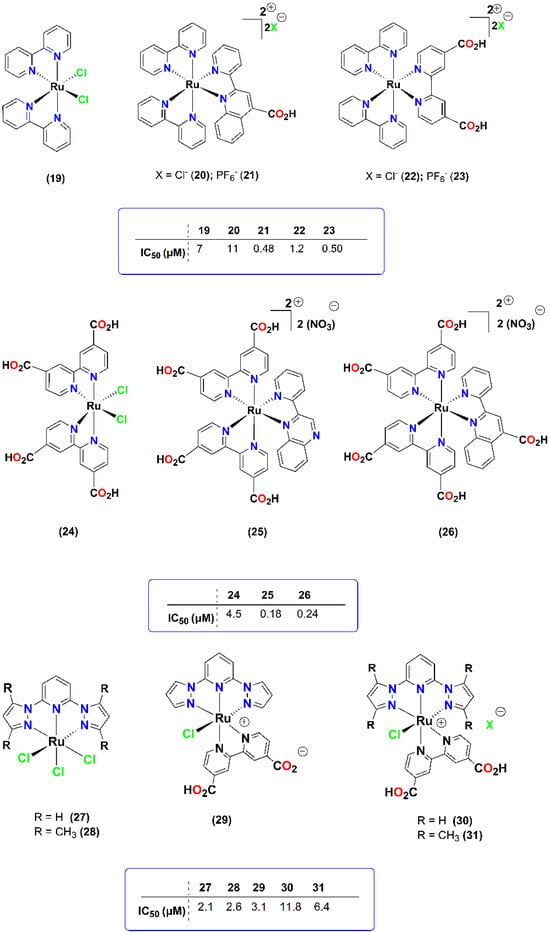
Figure 6.
Molecular structures of metal-based PAF inhibitors 19–31. IC50 values (μM) against the PAF-induced aggregation in WRPs are included.
Additionally, this study revealed the effect of different counter anions (Cl− vs. PF6−), as these complexes, containing the hexafluoridophosphate anion, were more potent. The anti-PAF activities of 25 and 26 were comparable to that of the well-established PAF-antagonist for PAFR (0.26 μM), rupatadine fumarate, which is the main drug component of Rupafin, which is usually prescribed against allergic rhinitis in clinical practice [35]. Philippopoulos et al. have also observed that some of these complexes (i.e., 25 and 26) that possess a strong anti-PAF effect (IC50 value of 25 was 0.18 μM against PAF) were less cytotoxic against the in vitro cellular proliferation of a range of cell lines (HEK-293 and breast cancer cell lines MCF-7 and MDA-MB-468), than the well-known anti-cancer drug, cisplatin, which is less potent against PAF with an IC50 value of 0.55 μM (unpublished results).
Other groups have also worked on the anti-PAF anti-platelet effects of complexes based on other metals. For example, Mitsopoulou et al. have reported on the anti-PAF activity of the Re(I) derivative fac-[Re(phendione)(CO)3Cl] (32) (phendione = 1,10-phenanthroline-5,6-dione) as an inhibitor of PAF-induced aggregation in WRPs [36]. The inhibitory effect of metal precursor [Re(CO)5Cl] was higher than those of both phendione and the Re(I) complex, which is in contrast with the synergetic effect that has been well-established in previous reports [13]. Because molecular docking theoretical calculations have not been reported, we may suggest that complex 32 will probably not fit into the binding site of PAFR, as reported for other similar complexes.
Nolan et al. have reported various aurate(I) salts of the formula [NHC·H][AuCl2] (NHC = N-heterocyclic carbene), and their biological activities against PAF-induced aggregation on human platelets in vitro were investigated [37]. These gold compounds displayed anti-PAF activity in the micromolar range (~2 μM). However, the role of the N-heterocyclic carbene has not been clarified in support of these findings, as some of these complexes consist of anion/cation pairs and others are neutral Au(I) complexes. Additionally, such substances do not contain the same substituents in the nitrogen atoms. Interestingly, based on the IC50 values and standard deviations (SD) reported for the substituent versus the Au(I)-based complex (IPR·HCl = 3.52 ± 1.39 μM vs. [IPR·HCl][AuCl2] = 2.01 ± 0.15 μM), the anti-PAF potencies can be mostly attributed to the free ligand (NHC), rendering the role of Au(I) rather negligible (IC50 values within the experimental error). Finally, no studies with thrombin or other well established platelet agonists (i.e., ADP or collagen) were performed to maintain the suggested antithrombotic activity.
Apart from the currently reported coordination compounds and organometallic complexes, several other metal-based inhibitors of PAF-induced aggregation in WRPs and on human platelets in vitro, at the nanomolar and sub-micromolar ranges, have been investigated, such as several ruthenium(II)/(III) [38], rhodium(I)/(III) [Philippopoulos et al., unpublished], iridium (I)/(III) [39], copper(I) [Philippopoulos et al., unpublished] and Ga(III) [Philippopoulos et al., unpublished]-based PAF-inhibitors (Figure 7). This highlights the increased impact of the vast area of metal-based coordination compounds that could potentially be tested as inhibitors against PAF-induced biological activities.
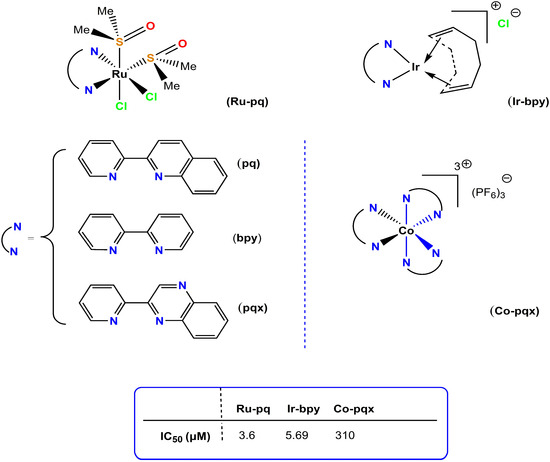
Figure 7.
Molecular structures of Ru(II), Ir(III), and Co(III) anti-PAF inhibitors.
In the studies presented in this section, control experiments that included metal ions and free ligand precursors were always performed. Therefore, the observed “synergetic effect” was not attributed to a cumulative effect of free ligands and metal ions, but to a combination of both to afford the metal-based complexes. Additionally, stabilities of the complexes were reported in dimethyl sulfoxide, which also serves as the appropriate medium for cytotoxicity tests.
To this end, we may report that this research has been focused mainly on the anti-PAF activities of metal-based inhibitors against the PAF-induced aggregation in WRPRs. Additionally, the antithrombotic potency of several complexes was also studied using thrombin. Experiments on hPRPs, in conditions closer to those found in vivo, were also performed for a series of copper(I) and rhodium(I) complexes in other well-established platelet agonists, like ADP and collagen. To support our findings, further theoretical docking calculations are required in order to test our hypothesis of the most potent inhibitor that fits within the binding site of PAFR. Provided that the X-ray crystal structure of PAFR, in complex with the SR27417 antagonist, has been recently elucidated, these results will enable us to better organize and suggest better metal-based inhibitors in the future [40]. In any case, in vivo studies must be performed in order to support the aforementioned in vitro results.
2.2. Other Metal-Based Complexes with a General Anti-Inflammatory and Antithrombotic Potency through Other Pathways, Assessed In Vivo in Animal Models
The in vivo and in vitro anti-inflammatory activity of 4-thio-emicarbazone derivatives of 2,3-dihydroxybenzaldehyde which are coordinated as ligands (L) with nickel metal was studied against leg edema in mice models [41]. From the experiments, it was observed that the complex of the form [Ni(H2L1)(PPh3)]Cl, binds to the active site of the enzyme IKKβ (kinase of IκB Kinase; IκB: inhibitory factor of kappa-B) and probably inactivates the nuclear factor kappa beta (NF-κB)-related pathways of inflammatory gene expressions (Figure 8). Moreover, the complex limited the tumor necrosis factor alpha (TNF-α)-induced expression of the inflammatory enzyme cyclooxygenase-2 (COX-2), always depending on the dose administered, so that the formation of edema in the leg of the mice was also reduced.
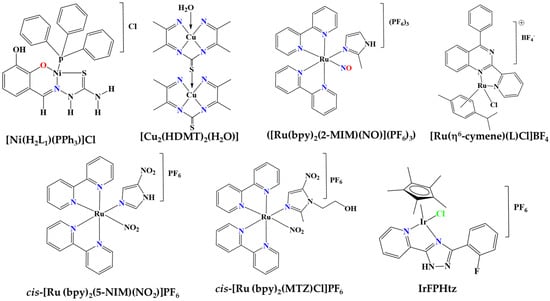
Figure 8.
Molecular structures of Ni, Cu, Ru and Ir metal complexes.
In addition, the anti-inflammatory profile of the copper (II) complex [Cu2(HDMT)2(H2O)] was also examined in case of arthritis due to the effect of collagen in rats (Figure 8). This copper (II)-based complex is obtained by the reaction between the ligand H3DMT (bis-(diacetylmonoxime)-thiacarbohydrazone) and the acetate salts of copper(II), while it showed remarkable anti-inflammatory activity due to its contribution to the suppression of COX-2 [42].
The anti-inflammatory activity of the FOR811A ruthenium-based complex ([Ru(bpy)2(2-MIM)(NO)](PF6)3) (2-MIM = 2-methylimidazole) was studied on a group of mice of a specific genus [43]. Here, it was found that this ruthenium complex inhibited the formation of edema induced by carrageenan in a mouse leg over a period of 4 h at a high rate, one that reached 73% when the optimal suppressive dose was employed with a complex concentration of 10 mg/kg (Figure 8). As a benchmark, the drug indomethacin was used with 45% inhibition. Moreover, this Ru(II) complex interferes with the inflammatory pathways involved in carrageenan-induced edema formation, such as the TNF-α associated pathways, but also with nitric oxide (NO)-related inflammatory pathways. Finally, FOR811A also showed an anti-inflammatory effect against L-arginine-induced foot edema.
TQ5, a Ru(II)-based complex with the form [Ru(η6-cymene)(L)Cl]BF4 (L = 4-phenyl-2-pyridin-2-yl-quinazoline) [44], has been found to inhibit collagen-induced aggregation of washed human platelets in a concentration-dependent way comparable to the similar anti-platelet effect of aspirin (Figure 8). This effect is associated with a suppression of ATP release due to collagen from human platelets and suppression of the increase in Ca2+ ion concentration. Nevertheless, the complex caused concentration-dependent inhibition of the collagen-induced phosphorylation of protein kinase B (Akt) and c-Jun N-terminal kinase (JNK), highlighting its high correlation with platelet accumulation due to collagen and thus counteracting phosphorylation through the antiplatelet behavior of the Ru complex. Nevertheless, TQ5 also had an anti-platelet effect against the thrombin or against the 9,11-dideoxy-11a and 9a-potassium prostaglandin pathways, but not in a concentration dependent manner. In addition, the TQ5 complex was studied in ex vivo conditions, showing that the complex increases occlusion time due to collagen and ADP in blood vessels and more specifically in mouse intestinal venules, again in a concentration-dependent manner. In subsequent in vivo tests, it was determined that the complex significantly increases occlusion time due to clots in blood vessels compared with the control sample, where DMSO was administered.
The antioxidant and anti-inflammatory properties of another two ruthenium-based complexes with 5-nitroimidazole (5-NIM) and metronidazole (MTZ) as ligands, cis-[Ru (bpy)2(5-NIM)(NO2)]PF6 and cis-[Ru (bpy)2(MTZ)Cl]PF6, respectively, have also been reported in cancer cells (Figure 8) [45]. Both complexes presented expressive in vitro antioxidant activity, reducing lipid peroxidation and decreasing intracellular levels of reactive oxygen species (ROS) with comparable effectiveness to the standard steroidal drug dexamethasone or α-tocopherol, although with no noticeable cytotoxicity on the tested cancer cell lines. The first of these complexes also showed strong anti-inflammatory effects in macrophages, as it was able to inhibit IL-6 and TNF- α production as well as IL-1β and COX-2 expression in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages, which have not been reported for other ruthenium-based complexes. Based on these outcomes, it has been suggested that Ru-(5-NIM) can act against COX-2 or IL-1β-related inflammatory conditions.
The anti-inflammatory and antioxidant activity of the Ir-based complex (IrFPHtz; FPHtz = (2-(3-(2-fluorophenyl)-1H-1,2,4-triazol-5-yl)pyridine) has been studied on rats’ spinal cords and hippocampal neurons (Figure 8) [46,47]. Tests of the antioxidant activity of the complex on hippocampal neurons from rats were carried out in newborn rats. The IrFPHtz complex was shown to upregulate the enzyme superoxide dismutase 1 (SOD1), contributing to the inhibition of inflammation and the repair of spinal cord neurons in rats that had suffered spinal cord injury due to oxygen free radicals (due to oxidative stress). The anti-inflammatory properties of the IrFPHtz complex were also supported by its ability to reduce the concentration of the inflammatory factors TNF-α, IL-6 and IL-10, and especially in limiting TNF-α and subsequently the inflammation present in the spinal cord of rats.
Finally the anti-inflammatory activity of a series of [η6-areneCr(CO)3] complexes, with a core structure related to anti-inflammatory diterpenes, produced by the sea whip Pseudopterogorgia elisabethaecomplexes, was studied [48]. In particular, a potent inhibitory activity on tumour necrosis factor (TNF), Toll-like receptor (TLR), and nucleotide binding domain, leucine-rich repeat-containing receptor (NLR) pathways were observed by this series of complexes. Moreover, the inhibition of the inflammatory pathway of the NF-κB factor through the inflammatory responses mediated by the NLR inflammatory pathway of the nucleotide-binding oligomerization protein (NOD2), a pivotal innate immune receptor involved in bacterial recognition, was also observed [48,49]. The effect of such chromium complexes on this inflammatory pathway was more potent in comparison with similar anti-inflammatory effects on these pathways that have been observed when using natural anti-inflammatory compounds, such as curcumin and parthenolide (a lactone), which also have the potential to interfere with the biochemical pathway of NOD2, in turn resulting in the inhibition of interleukin IL-8 and NF-κB factor [50].
2.3. Metal-Based Complexes Containing Well-Established Natural Anti-Inflammatory Bioactives
Free curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione, or, more simply, diferuloylmethane), which is isolated from turmeric, exhibits anti-inflammatory properties; however, its general anti-inflammatory efficacy is limited because this natural compound is hydrophobic and practically insoluble in biological hydrophilic environments (Figure 9). Curcumin is a beta-diketone that becomes capable of assembling with a large number of the d-domain transition metals, with the development of structural covalent bonds between the metal and at least one polydentate ligand [10]. Various curcumin–metal complexes, which contribute to its anti-inflammatory effect, have been studied. Metal–curcumin complexes increase the bioavailability, solubility, and thus cellular uptake, and improve the antioxidant, anti-inflammatory, antimicrobial, and antiviral effects of curcumin, as reviewed extensively by Prasad et al. [10]. Here, we emphasize the improved anti-inflammatory potential of curcumin when assembled to metal-based complexes in comparison with free non-assembled curcumin, since such metal–curcumin complexes have also demonstrated efficacy against various inflammation-related chronic diseases, including cancer, arthritis, osteoporosis, and neurological disorders. These biological activities are associated with the modulation of inflammatory mediators, transcription factors, protein kinases, antiapoptotic proteins, lipid peroxidation, and antioxidant enzymes.
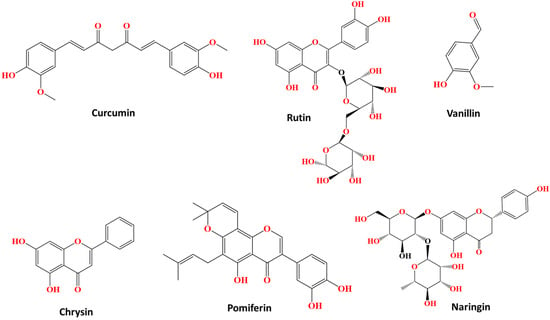
Figure 9.
Chemical structures of bioactive chemical compounds and flavonoids, as free ligands, for which enhanced biological activities after being assembled in several metal-based complexes have been reported.
For example, the curcumin–Zn complex [Zn(Curcumifn)(CH3COO)(H2O)] was studied in a rat model of inflammation, where it was found to contribute to the inhibition of the inflammatory pathways related to NF-kB factor, growth factor beta-1 (TGF-β1) and interleukin-8 (IL-8), while this complex also inhibited the pro-inflammatory cytokines TNF-α and interleukin IL-6 in the same rat model [51,52]. Similarly, a mononuclear Copper (II)–Curcumin (1:1) complex also showed better protection against radiation-induced protein carbonylation and lipid peroxidation and thus higher radioprotection in splenic lymphocytes through a delayed activation of protein kinase delta (PKCδ) and the NF-kB factor, and with less cytotoxic effects compared with free curcumin [53].
Curcumin–Cu(II) and –Zn(II) complex systems also showed neuroprotective effects due to their ability to suppress cell apoptosis via reducing oxidative stress and by downregulating the NF-κB pathway and upregulating the Bcl-2/Bax system of apoptosis regulation [54]. In comparison with non-coordinated curcumin, the antioxidant protective effects of curcumin–Cu(II) complex systems were stronger than those of the curcumin–Zn(II) system. Both curcumin–Cu(II) and –Zn(II) complex systems also significantly enhanced the superoxide dismutase, catalase, and glutathione peroxidase activities and attenuated the increase of malondialdehyde levels and caspase-3 and caspase-9 activities in a dose-dependent manner, with the curcumin–Cu(II) complex system at a 2:1 ratio exhibiting the most significant effect (Figure 10).
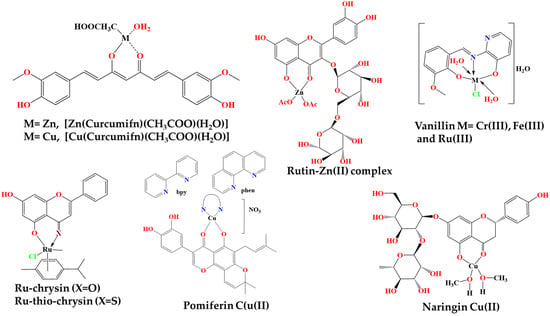
Figure 10.
Chemical complexes of bioactive chemical compounds.
The curcumin–gadolinium complex in PEGylated α-Gd2(MoO4)3 marigold flower-like mesoporous particles, facilitated the conjugation of curcumin in these curcumin nanoparticles and thus the continuous and sustained release of curcumin into the cytoplasm and nucleus of cells. This resulted in an intracellular release of curcumin that inhibited the proliferation in two human pancreatic cancer cell lines, while the particles showed increased inhibition of pIKKα, pIKKα/β and NF-κB-DNA binding activity, as compared with pure curcumin, in these human pancreatic cancer cells, indicating that it also had a suppressive effect on cancer cell proliferation [55].
Rutin is another characteristic bioactive flavonoid found in many plant organisms, even in frequently consumed foods (Figure 9) [11]. In fact, recent studies have highlighted the antithrombotic activity of rutin, as it is able to inhibit protein disulfide isomerase (PDI). However, it is a natural compound with low solubility in biological hydrophilic environments. Several benefits and the improved solubility and thus bioavailability of rutin by its conjugation in Zn(ΙΙ) ions, providing rutin–Zn complexes, have been reported. Natural flavonoid bioactives, like rutin, often acquire improved biological properties by their assembly with metals, as highly active complexes (flavonoid–metal systems) are formed, with potential applications in medicine. For example, it was found that the rutin–Zn(II) complex was more water soluble and more efficiently inhibits the PDI than free rutin, while it also better reduced the thrombosis in mouse arteries that can be induced by an electric current.
Other similar metal complexes bearing vanillin and vanillin-based derivatives have also been examined (Figure 9) [12]. For example, chromium (III), iron (III) and ruthenium (III) complexes were prepared, where the ligand was an imine named 2-[(2-hydroxy-3-methoxy-benzylide)-amino]-pyridine-3-ol, which was synthesized by reaction between ethanol, ortho-vanillin and 2-amino-3-hydroxypyridine as raw materials via reflux (Figure 10). These complexes, at a concentration of 20 mg/kg, showed potent inhibition of edema tumor formation induced by carrageenan (1% w/v) in the leg of Wistar rats, in comparison with the well-established anti-inflammatory drug, sodium diclofenac and DMSO as the control, which were also administered at the same concentration of 20 mg/kg. The drug, as a comparison measure as well as a control, caused the greatest inhibition of tumor formation, and among the three complexes, that of ruthenium showed the greatest percentage of inhibition and hence the greatest anti-inflammatory effect, followed by the iron(III) and chromium(III) complexes, with similar percentage inhibition values.
Chrysin is another naturally occurring flavonoid that exhibits certain biological properties, including antiplatelet and antithrombotic bioactivities (Figure 9). Ruthenium–chrysin and ruthenium–sulfur–chrysin complexes were studied for their potential antiplatelet and antithrombotic activities in comparison with the particular bioactivities of free chrysin (Figure 10) [33]. More specifically, the complexes inhibited platelet aggregation of washed human platelets induced by the peptide, CRP-XL, that activates the collagen pathway. At low concentrations, the ru–sulfur–chrysin complex, as well as free chrysin, were highly inhibitory to platelet aggregation, which was not the case with the ru–chrysin complex. Nevertheless, the antiplatelet activity of all three compounds, chrysin and both ru-based complexes, were less effective, at least at low concentrations, at inhibiting platelet aggregation in human platelet-rich plasma. However, at higher concentrations, the two ru-based complexes of chrysin were more active in comparison with free chrysin.
Pomiferin is also a bioactive flavonoid, belonging to the family of prenylated isoflavones, which exhibits remarkable antioxidant, anti-cancer and anti-inflammatory properties (Figure 9) [56]. Moreover, it is able to associate with various metal ions to form bioactive complexes, resulting in improved properties compared with free pomiferin. Such metal-based complexes, in which pomiferin was assembled with the copper(II) ion, have been synthesized by participating in the reaction of the compound Cu(NO3)2•3H2O, and also of methanol, and each time the nitrogen–nitrogen (N,N′) linker was changed (Figure 10). In the assays performed, the anti-inflammatory properties of these complexes and the free ligand (pomiferin) were evaluated against LPS-induced activation of the transcription factors AP-1 (activator protein 1) and NF-κB. A dose-dependent inhibitory effect against these two factors of inflammatory gene expression was generally found for several of these complexes, which were more effective than the same effect observed for free pomipherin. The activity was therefore inextricably linked to the N,N′ ligand and to the overall structure of the metal complexes, showing an optimal anti-inflammatory profile, even at a low concentration (50 nM). The most active complexes also reduced the inflammatory TNF-α production s in THP-1 monocytes.
The bioactive flavonoid naringin, usually found in citrus fruits, possess anti-inflammatory, antioxidant, antimicrobial and anti-cancer properties (Figure 9) [57]. A naringin–copper(II) complex, namely [Cu-naringin(CH3OH)2], was synthesized through a reaction between this flavonoid, methanol and copper acetate, and its various properties were studied in comparison with free naringin (Figure 10). When naringin was assembled with copper, it exhibited similar inhibitory effects to the anti-inflammatory drug indomethacin in carrageenan-induced inflammation experiments conducted on mice, while these anti-inflammatory properties of the copper-based complex of naringin were better than the anti-inflammatory effects of free naringin. Finally, the copper–naringin complex reduced the NO concentration in experiments carried out on RAW 264.7 macrophage cells, and this reduction was more pronounced as the concentration of the complex increased, always having better effects than free naringin.
3. Porphyrin-Based Compounds with Anti-Inflammatory and Antithrombotic Bioactivities and Associated Health Promoting Effects
Porphyrin-based compounds demonstrate a wide range of bioactivities, including anti-inflammatory and antithrombotic effects, along with associated health-promoting properties, such as antioxidant, anticancer, and antimicrobial effects, and can be used as molecular magnetic resonance imaging (MRI) contrast agents [14]. Such diverse pharmacological activities underscore the versatility and potential therapeutic utility of porphyrin compounds across various medical conditions. In light of these properties, by elucidating the pharmacological profile of these porphyrins, researchers can gain insights into their mechanisms of action and assess their suitability for the treatment of inflammatory diseases. Herein, examples from the recent literature of free-base and metalated porphyrin complexes are discussed.
3.1. Free-Base Poprhyrins
3.1.1. Tetraphenyl Based Porphyrins
In a recent report four phenylporphyrin derivatives, namely 5,10,15,20-tetraphenylporphyrin (TPP), 5,10,15,20-tetra(4′-fluorophenyl)porphyrin (TpFPP), 5,10,15,20-tetra(4′-chlorophenyl)porphyrin (TpClPP) and 5,10,15,20-tetra(4′-bromophenyl)porphyrin (TpBrPP), were synthesized and assessed for their potential anti-inflammatory and analgesic biological activities (Figure 11) [58]. More specifically, evaluation of heat-induced hemolysis showed that all porphyrin derivatives inhibited hemolysis with a potency comparable to naproxen (NPX). At the same time, none of these porphyrins showed toxic effects in mice; however, chronic toxicity studies need to be conducted in order to validate the safety of such porphyrins for long-term use (months). Three of these porphyrins (TPP, TpFPP and TpBrPP) exhibited anti-inflammatory, antinociceptive and anti-arthritic activities in vivo against inflammatory chronic ear swelling induced by 12-O-tetradecanoylphorbol-13-acetate (TPA). In addition, porphyrins reduced the levels of proinflammatory mediators in carrageenan–caolin-induced arthritis mice (CKIA). Serum was also obtained from mice and the levels of TNF-α, IL-1β and IL-6, PGE2 and NO were measured, the results show that these porphyrins decreased the levels of proinflammatory mediators PGE2 and NO. These results, taken together, further support the potential of porphyrins as anti-inflammatory drug alternatives for the treatment of inflammatory diseases [58].
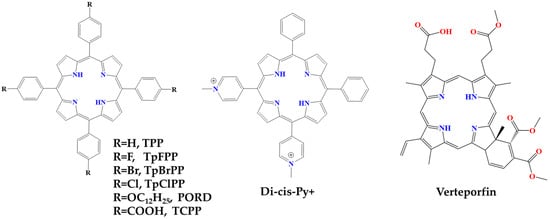
Figure 11.
Chemical structures of free-base porphyrins.
Another synthesized phenyl-porphyrin derivative, namely 5,10,15,20-Tetra(4-dodecyloxyphenyl)porphyrin (POR-D), was also studied for its immunomodulatory activities [22]. Specifically, the study focused on the changes in proinflammatory cytokine production levels under both dark- and light-activated conditions. When activated cells were treated with POR-D under dark conditions, the production of proinflammatory cytokines by macrophages did not change. Whereas when activated cells were treated with photodynamically activated POR-D derivatives, the compound resulted in a substantial and significant reduction in proinflammatory cytokine production. This effect was dose-dependent, as increasing concentration of POR-D generated a more substantial effect than the lower dose. Consequently, the study contributes to a new approach by examining the anti-inflammatory photodynamic effect of porphyrin derivatives at sub-toxic concentrations (Figure 11) [22].
Pan Ran et al., prepared hydrogels, derived from the antimicrobial peptide e-polylysine (ePL), incorporating ePL methacrylate (mPL) and conjugates with tetracycline(4-carboxyphenyl) porphyrin (mPL-TCPP) and phenol red (mPL-Pr) for bacterial elimination and rapid wound healing. After intense illumination, mPL-TCPP generated reactive oxidative species (ROS), initiating free radical cross-linking in PL@Pr-TCPP hydrogels without additional photo-initiators, providing antibacterial photodynamic therapy (PDT) (Figure 11) [59]. The hydrogels capture bacteria, enhancing the PDT effect by reducing ROS lifetime and action distance. In a Staphylococcus aureus-infected abscess model, illumination formed in situ hydrogel dressings, demonstrating synergistic bactericidal effects that reduce inflammation, accelerate collagen deposition, and promote neovascularization. TCPP/Lt treatment reduced cellular necrosis and inflammation and increased viable fibroblasts and epidermal skin layers. The infected site was covered by intact skin after PL@Pr-TCPP/Lt treatment, and epidermal growth and hair follicles in the healed wounds were similar to those of normal skin. The study therefore presented a feasible strategy for the development of therapeutic hydrogel dressings capable of treating wound infections [59].
Meso-substituted cationic porphyrins with N-methylpyridinium, such as 5,10-diphenyl-15,20-di(N-methylpyridinium-4-yl) porphyrin (Di-cis-Py+), showed great potential for use in PDT due to its amphiphilic nature, as it has both charges on the same side of the molecule (Figure 11) [60]. Di-cis-Py+ has been studied in several in vivo models for its photosensitizer effects, specifically targeting psoriasis-like parameters in combination with PDT. For example, photoactivation of Di-cis-Py+ demonstrated anti-inflammatory and antiproliferative actions, evidenced by the reduction of edema and epidermal cell hyperproliferation. Measurements of myeloperoxidase (MPO) and N-acetyl-β-d-glucosaminidase (NAG) enzyme activities, as well as levels of IL-6, IL-1β and TNF-α, which were also involved in the initiation of the psoriatic cycle, showed a decrease in inflammatory markers. PDT using Di-cis-Py+ via topical application effectively reduced psoriasis-like parameters, demonstrating advantages such as minimal photosensitivity. The observed results are comparable to those of dexamethasone, a first-line drug for the treatment of psoriasis, which further suggests the potential of Di-cis-Py+ as a promising therapeutic option for psoriasis, emphasizing its topical application and encouraging further investigation in clinical settings [60].
3.1.2. Verteporfin
Verteporfin is a free-base benzoporphyrin (BPD-MA) derivative monoacid ring A and, furthermore, is a photosensitizing drug for photodynamic therapy (PDT) (Figure 11) [61]. When activated in the presence of oxygen, reactive oxygen radicals are generated and cause local damage to the vascular endothelium. Consequently, damaged vessels activate platelet accumulation and fibrin clot formation through the lipoxygenase and cyclooxygenase pathways that result in vessel occlusion. Verteporfin specifically targets new blood vessels and is therefore an attractive treatment for choroidal neovascularization (CNV). It has been approved by the Food and Drug Administration (FDA) for the treatment of CNV due to age-related macular degeneration (AMD), ocular histoplasmosis (OHS) and pathological myopia [62].
Yuting Wang et al., investigated the anti-inflammatory properties of verteporfin, which is mainly used for age-related macular degeneration [63]. The research focused on its effects on a classical cellular model of lipopolysaccharide (LPS)-induced inflammation using RAW 264.7 cells. The results show that verteporfin effectively suppressed the expression of the inflammatory markers IL-6 and TNF-α at both mRNA and protein levels. This anti-inflammatory effect was attributed to the inhibition of key signaling pathways, namely NF-κB and JAK/STAT. The study further revealed that verteporfin achieved its anti-inflammatory effect through the cross-linking of proteins such as NF-κB p65, JAK1, JAK2, STAT1 and STAT3. These findings suggest that verteporfin may be promising as a therapeutic agent for the treatment of inflammatory diseases [63].
3.2. Metal-Based Porphyrin Complexes
3.2.1. Cobalt Protoporphyrin IX
Increasing evidence shows that pharmacological induction of heme oxygenase-1 (HO-1) with cobalt protoporphyrin (CoPP) improves various inflammatory diseases (Figure 12). Heme metabolism is a key regulator of inflammatory responses. CoPP is an analogue and mimetic of heme that potently activates the transcription NF-E2-related factor 2 (Nrf2)/HO-1 pathway, particularly in monocytes and macrophages. Nrf2 controls the basal and induced expression of an array of antioxidant response element-dependent genes to regulate the physiological and pathophysiological outcomes of oxidant exposure. Like heme, cobalt (II) protoporphyrin IX can reversibly bind oxygen (with much lower affinity) and is similarly metabolized by HO-1. HO-1 affects the production of inflammatory cytokines, while it is also anti-apoptotic and has antioxidant properties partly through the removal of heme and partly through its metabolic products. CoPP-induced HO-1 and its metabolic product, carbon monoxide, improves the killing of bacteria by macrophages and reduces the expression of inflammatory cytokines. CoPP therefore shows multifaceted actions with promising anti-inflammatory therapeutic potential [64,65,66].
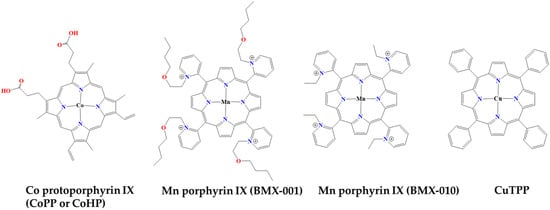
Figure 12.
The structures of characteristic bioactive metal-based porphyrins with anti-inflammatory and antithrombotic health promoting properties.
For example, the effects of CoPP on hyperglycemia and painful diabetic neuropathy were investigated in male db/db mice models of type 2 diabetes, which are a specific strain of laboratory mice that carry a mutation in the gene responsible for encoding the leptin receptor, known as the db gene [65]. More specifically, CoPP treatment in these mice had antihyperglycemic effects and also protective properties, with respect to pain sensitivity. Moreover, CoPP exhibited potent antioxidant properties by modulating oxidative stress-related proteins such as the Nrf2 factor, which is a multifunctional transcription factor that regulates the expression of several antioxidant-encoding genes and of antioxidant, anti-inflammatory and detoxifying enzymes, including heme oxygenase 1 (HO-1), quinone redox NAD(P)H 1 (NQO1), glutathione S-transferase Mu 1 (GSTM1) and superoxide dismutase 1 (SOD1). Under diabetic conditions, the Nrf2 pathway is altered and the expression of the aforementioned enzymes is drastically reduced, leading to an increase in oxidative stress and, consequently, to diabetic neuropathy. CoPP treatment prevents the deregulation of several antioxidant proteins (Nrf2, SOD-1, NQO1) and enhances the levels of HO-1 and GSTM1 in the spinal cord and/or amygdala, suggesting a possible mechanism for the observed anti-degenerative effects. Overall, CoPP has exhibited multifaceted actions in improving hyperglycemia, alleviating neuropathic pain, reducing oxidative stress, suppressing inflammation and preventing apoptosis in the CNS of type 2 diabetic mice [65].
The anti-inflammatory effects of CoPP were also investigated against inflammatory responses in a mouse model of colitis [66]. CoPP administration led to significant changes in myeloid populations in the blood and colon during inflammation. CCR2+Ly6Chi inflammatory monocytes were recruited to the colonic mucosa in the context of dextran sodium sulfate (DSS) injury and differentiated into inflammatory activator cells and antigen-presenting cells. Recruited monocytes are an important source of proinflammatory cytokines, including IL-1β, TNF, IL-12 and IL-6. CCL2, also known as monocyte chemoselective protein-1 (MCP-1), is a chemokine that regulates the migration and infiltration of immune system cells and its expression can be induced by several mediators, including cytokines such as IL-1, IL-4 and TNF-α. CCR2 is a chemokine receptor that serves as the primary receptor for CCL2. Thus, upon binding of CCL2 to CCR2, several intracellular signaling pathways are activated, leading to the expression of genes involved in inflammation, cytokine production, cell growth and differentiation [67]. It was found that CoPP treatment results in dual protective mechanisms in colitis. Specifically, it was revealed that CoPP inhibits CCR2+Ly6Chi monocyte trafficking in the colon, secondary to disruption of the normal CCL2 chemoattractant gradient, culminating in a remarkable suppression of monocyte CCR2 expression by its CCL2 ligand. Consequently, the effects of CoPP on myeloid populations, systemic CCL2 induction and the shift in macrophage phenotype highlight immunosuppressive mechanisms that remained previously unreported, providing insights into the complex dynamics of heme metabolism in the context of inflammation [66].
The potential therapeutic role of CoPP and HO-1 was also examined in a mice model of cholestatic liver disease [68]. Specifically, mice exhibited increased mRNA expression of tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) in the liver. CoPP administration significantly inhibited the activation of factors associated with these inflammatory cytokines’ NF-κB signaling cascades. Subsequently, a decrease in cytokine production, inflammatory cell infiltration and extracellular matrix deposition were also observed by this administration. Moreover, CoPP treatment reduced the expression of fibrosis-related genes and inhibited the activation of the transforming growth factor-β (TGF-β) pathway. These findings collectively suggest that the pharmacological induction of HO-1 by CoPP has a protective effect against cholestatic liver diseases by mitigating oxidative stress, hepatocyte apoptosis, inflammation and fibrosis [68].
CoPP has also demonstrated antithrombotic activities by affecting the protective effects of HO-1 induction when using CoPP on endothelial damage in arterial thrombosis in mice [69]. Specifically, pretreatment with CoPP significantly reduced the maximal clot area and area under the curve after laser injury in crestal arterioles, indicating that pharmacological regulation of HO-1 mRNA is sufficient to suppress microvascular thrombosis without the need for excess heme substrate. Possible mechanisms behind the protective effects of HO-1 regulation include the production of heme catabolites such as carbon monoxide (CO) and biliverdin/bilirubin, although further investigation is needed. It seems that substrate-independent small molecule regulation of HO-1 expression could be a promising approach by which to mitigate endothelial dysfunction in cardiovascular conditions [69].
3.2.2. Porphyrin-Related Nanoparticles and Heme–Albumin Complexes
Multifunctional microspheres (PMT), using poly (lactic co-glycolic acid) (PLGA) to encapsulate a phenylporphyrin derivative, namely tetrakis (4-carboxyphenyl) porphyrin (TCPP), along with magnesium oxide (MgO) nanoparticles have also been produced and assessed for the treatment of periodontitis (Figure 11). PMT exhibited a dual function: an antibacterial photodynamic effect against periodontal pathogens (F. nucleatum and P. gingivalis) through the production of reactive oxygen species and a fibroblast activation effect, crucial for the reconstruction of damaged periodontal structures [70]. PMT enhanced viability, adhesion and activation of fibroblasts, as evidenced by increased gene expressions of fibronectin 1, Col1a1 and vinculin. In vivo experiments using a rat periodontitis model demonstrated the superior therapeutic effects of PMT with laser illumination. Microcomputed tomography analysis and histological staining revealed reduced inflammatory cells, increased collagen production and higher alveolar bone levels in the PMT group. Therefore, PMT, with its combined photodynamic therapy (PDT) and photobiomodulation (PBM)-assisted fibroblast activation modes, holds promise as an effective strategy by which to combat periodontitis and its inflammatory manifestations [70].
Polydopamine nanoparticles (PDA NPs), which are an easily synthesized and mucoadhesive drug delivery system, were also utilized for facilitating the delivery of a human serum albumin protein complex (heme–albumin), designed for its anti-inflammatory properties in the treatment of age-related macular degeneration (AMD) [71]. The heme–albumin complex, by inducing HO-1 in retinal epithelial cells, was intended to provide anti-inflammatory protection through the production of CO and biliverdin during heme catabolism. The results demonstrate the negligible cytotoxicity of heme–albumin to retinal melanocytic epithelial cells (ARPE-19), a reduction in oxidative stress in both inflammatory and ROS models, and a statistically significant increase in HO-1 protein expression, suggesting possible anti-inflammatory protection. Heme–albumin-loaded PDA NPs react with ROS, leading to a remarkable reduction in oxidative stress in vitro, indicating their potential therapeutic efficacy. Therefore, it has been shown that heme–albumin-loaded NP PDAs have the potential to reduce oxidative stress in vitro and provide a stable therapeutic delivery system for the treatment of AMD [71].
Pfefferlé et al., examined whether acute hemolysis and heme exposure induce anti-inflammatory signals in hepatic macrophages [72]. Consequently, a model of sterile liver inflammation with phenylhydrazine (PHZ)-mediated acute erythrophagocytosis revealed that this process blocked the macrophage activation pathway induced by anti-CD40 antibody. This attenuation of macrophage activation results in a reduction in inflammatory cytokine release syndrome and necrotizing hepatitis induced by anti-CD40 antibody treatment in mice. In addition, administration of heme–albumin complexes specifically provides heme to hepatic macrophages, replicating the anti-inflammatory effect observed in hemolysis. Taken together, these results suggest that heme–albumin complexes can be used as a formulation by which to provide heme to hepatic macrophages with minimal adverse toxicity. Moreover, both erythrophagocytosis and heme–albumin complex administration dramatically attenuated the disease pathology, suggesting that the anti-inflammatory effect in the experiments was achieved at the level of macrophage activation [72].
3.2.3. Manganese–Porphyrin Derivatives
Kelsey Stover et al., studied the topical administration of two manganese porphyrins, BMX-001 and BMX-010, as antioxidant compounds (Figure 12). Both derivatives were investigated in a mouse model of allergic dermatitis [73]. More specifically, several formulations of BMX-001 and BMX-010 were administered topically during the induction phase of TH2-dominant toluene-2,4-disocyanate (TDI)-induced allergic dermatitis and the efficacy of BMX-001 and BMX-010 was evaluated by comparing the antipruritic and anti-inflammatory responses. Both BMX compounds demonstrated significant inhibition of cytokine production in keratinocytes, BMDCs and T-cell proliferation. Mice treated with BMX-001 and BMX-010 showed a moderate, dose-dependent reduction in ear thickness. Concentrations of IL-1β and IL-4 cytokines in the inflamed skin were reduced by 80–90% with all treatment options. Therefore, the in vitro results suggest the promising potential of BMX-001 and BMX-010 creams for the treatment of allergic–inflammatory skin diseases, highlighting their anti-inflammatory properties [73].
3.2.4. Copper Tetraphenyl Porphyrin/Titanium Dioxide Nanoparticles
Copper tetraphenyl porphyrin (CuTPP) is a biomolecule that possesses catalytic functions of multiple enzymes, such as eNOS and SOD/CAT (Figure 12). Luying Liu et al. have hypothesized that Cu chelated ion in the porphyrin ring of CuTPP could viably produce NO in vivo by catalyzing the endogenous NO donor nitroso-glutathione (GSNO) [74]. By exploiting the electron transfer capabilities of copper tetraphenyl porphyrin/titanium dioxide nanoparticles (CuTPP/TiO2-NPs), a dual catalytic surface with both nitric oxide (NO) production and reactive oxygen species (ROS) removal activities was established. Unlike single catalytic surfaces, the CuTPP/TiO2 dual catalytic film effectively regulates the microenvironment surrounding the implanted devices. This was achieved by releasing signaling NO molecules and scavenging harmful ROS. The dual catalytic membrane demonstrated excellent biosafety and biocompatibility. It exhibited antithrombotic and anti-inflammatory properties, modulation of vascular wall cells (endothelial cells and smooth muscle cells). The novel dual catalytic and endothelial bionic strategy presents a promising solution with which to address the clinical challenges associated with blood contact devices [74].
3.2.5. Cobalt Haematoporphyrin
Nagaharu Tsukiji et al., identified platelet-activating receptor C-type lectin-like receptor 2 (CLEC-2) as a critical factor in tumor metastasis and clot formation [75]. The interaction between CLEC-2 and podoplanin in some tumor cells facilitates metastasis. Screening of 6770 compounds revealed protoporphyrin IX (H2-PP) as an inhibitor of CLEC-2-podoplanin binding. Further modification of H2-PP led to cobalt hematoporphyrin (Co-HP), which exhibited significantly stronger inhibitory potency (Figure 12). Co-HP was found to bind directly to CLEC-2 at previously unknown podoplanin binding sites, as confirmed by molecular binding studies and plasmon surface resonance analysis. Co-HP, administered intravenously, effectively inhibited hematogenous metastasis of podoplanin-expressing cells to the lung and prevented arterial and venous thrombosis in mice in vivo. Notably, Co-HP did not cause a significant increase in bleeding time, making it a promising candidate for antimetastatic and antiplatelet therapy without causing bleeding tendencies. Thus, Co-HP is a promising compound for anti-CLEC-2 drugs, which may contribute to the inhibition of both hematogenous tumor metastasis and arterial/venous thrombosis [75].
4. Conclusions, Limitations, and Future Perspectives
All studied metal-based complexes, either synthetic ones with strong antiplatelet activities and anti-PAF potential, as well as those containing either natural and synthetic porphyrins or phenolic bioactives like curcumin, rutin, vanillin, chrysin, naringin and pomypherin, were shown to efficiently and beneficially modify several thrombo-inflammatory pathways in several biological systems, in vitro in cell-models and/or in vivo in animal models, thus providing a novel class of anti-inflammatory and antithrombotic drug candidates.
Natural compounds, like curcumin, rutin, vanillin, chrysin, naringin and pomypherin, exhibit notable biological properties; however, their potency is limited for several reasons, such as their reduced bioavailability due to their low hydrophilicity in biological systems. The metal complexes of these natural compounds exhibit improved anti-inflammatory properties over the corresponding free natural compound because the metal imparts new properties to the original molecule or improves the pre-existing ones, while it can also facilitate the delivery and action of these natural bioactives at the site of the cell and at the biomolecules involved in the inflammatory signaling.
Moreover, increasing evidence suggests that porphyrin derivatives, like cobalt protoporphyrin IX and the heme–albumin complex, are heme oxygenase-1 inducers, while pharmacological induction of HO-1 can lead to a reduction in inflammation. Other porphyrin derivatives, like CoPP, have shown multifaceted actions in various inflammatory diseases and antithrombotic activities. Thus, it is a promising therapeutic agent that needs further research and could be clinically tested in humans. Porphyrins and their derivatives, such as the porphyrins POR-D and Di-cis-Py+, the nanoparticles tetracycline(4-carboxyphenyl)porphyrin (TCPP) and magnesium oxide (MgO), the hydrogel PL@Pr-TCPP, and verteporphin (FDA approved drug), also have the potential to act as photosensitizing drug agents used for photodynamic therapy, which is a two-stage treatment that combines light energy with a drug (photosensitizer) designed to destroy cancerous and precancerous cells after light activation. Nevertheless, the efficacy of verteporfin can be further investigated in the treatment of inflammation by targeting specific molecular pathways and its broader application beyond its established use in age-related macular degeneration. It is also notable that only synthetic porphyrins with anti-inflammatory and antithrombotic activity have been reported thus far, no natural porphyrins. This further supports the investigation of new synthetic metal-based porphyrin derivatives.
Most of the promising metal-based complexes with well-established in vitro and ex vitro anti-inflammatory and antithrombotic potencies against the PAF-associated thrombo-inflammatory pathways, especially in rabbit and human platelets, have yet to be assessed in in vivo settings, either in animal models of inflammation-related disorders or in targeted clinical trials. Currently the aim of this research is to develop new drug candidates with reduced side effects for patients, in contrast with classic metal complexes like cisplatin with well-known important side-effects. This would open up possibilities for more effective and targeted treatment strategies and requires further research into the synthesis and clinical application of new metal-based complexes and porphyrins.
Author Contributions
Conceptualization, A.T., C.A.D. and A.P.; writing—original draft preparation, A.T., A.P., S.P. and C.S.; writing—review and editing, A.T., A.P., K.L. and C.A.D.; visualization, A.T. and A.P.; supervision, A.T. and K.L.; project administration, A.T., A.P. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Vilar Barbosa, N.M.M.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical Aspects of the Inflammatory Process: A Narrative Review. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.L.; Basit, H.; Burns, B. Pathology, Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Iatrou, C.; Frangia, C.; Demopoulos, C. The Implication of Platelet Activating Factor in Cancer Growth and Metastasis: Potent Beneficial Role of PAF-Inhibitors and Antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and Future Antiplatelet Therapies: Emphasis on Preserving Haemostasis. Nat. Rev. Cardiol. 2018, 15, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet Activation and Prothrombotic Mediators at the Nexus of Inflammation and Atherosclerosis: Potential Role of Antiplatelet Agents. Blood Rev. 2021, 45, 100694. [Google Scholar] [CrossRef] [PubMed]
- Hyland, I.K.; O’Toole, R.F.; Smith, J.A.; Bissember, A.C. Progress in the Development of Platelet-Activating Factor Receptor (PAFr) Antagonists and Applications in the Treatment of Inflammatory Diseases. ChemMedChem 2018, 13, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Liao, X.; Ji, P.; Chi, K.; Chen, X.; Zhou, Y.; Chen, S.; Cheng, Y.; Flaumenhaft, R.; Yuan, C.; Huang, M. Enhanced Inhibition of Protein Disulfide Isomerase and Anti-Thrombotic Activity of a Rutin Derivative: Rutin: Zn Complex. RSC Adv. 2023, 13, 11464–11471. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; El-Khatib, R.M.; Abdel-Fatah, S.M.; Moustafa, H.; Alsalme, A.M.; Nafady, A. Novel Cr (III), Fe (III) and Ru (III) Vanillin Based Metallo-Pharmaceuticals for Cancer and Inflammation Treatment: Experimental and Theoretical Studies. Appl. Organomet. Chem. 2019, 33, e5177. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on Platelet Activating Factor Inhibitors: Could a New Class of Potent Metal-Based Anti-Inflammatory Drugs Induce Anticancer Properties? Bioinorg. Chem. Appl. 2017, 2017, 6947034. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Nikoloudakis, E.; Ladomenou, K.; Charalambidis, G.; Coutsolelos, A.G. Porphyrins—Valuable Pigments of Life. Front. Chem. Biol. 2024, 2, 1346465. [Google Scholar] [CrossRef]
- Margariti, A.; Papakonstantinou, V.D.; Stamatakis, G.M.; Demopoulos, C.A.; Machalia, C.; Emmanouilidou, E.; Schnakenburg, G.; Nika, M.-C.; Thomaidis, N.S.; Philippopoulos, A.I. First-Row Transition Metal Complexes Incorporating the 2-(2′-pyridyl)quinoxaline Ligand (pqx), as Potent Inflammatory Mediators: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules 2023, 28, 6899. [Google Scholar] [CrossRef] [PubMed]
- Kalampalidis, A.; Peppas, A.; Schnakenburg, G.; Papakyriakou, A.; Tsoupras, A.; Zabetakis, I.; Philippopoulos, A.I. Antithrombotic and Antiplatelet Activity of an Organometallic Rhodium(I) Complex Incorporating a Substituted Thieno-[2,3-d]-pyrimidine Ligand: Synthesis, Structural Characterization, and Molecular Docking Calculations. Appl. Organomet. Chem. 2021, 35, e6210. [Google Scholar] [CrossRef]
- Kalampalidis, A.; Damati, A.; Matthopoulos, D.; Tsoupras, A.B.; Demopoulos, C.A.; Schnakenburg, G.; Philippopoulos, A.I. Tin(II) and Tin(IV) Complexes Incorporating the Oxygen Tripodal Ligands [(η5-C5R5)Co{P(OEt)2O}3]−, (R = H, Me; Et = -C2H5) as Potent Inflammatory Mediator Inhibitors: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin. Molecules 2023, 28, 1859. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Papakyriakou, A.; Demopoulos, C.A.; Philippopoulos, A.I. Synthesis, Biochemical Evaluation and Molecular Modeling Studies of Novel Rhodium Complexes with Nanomolar Activity against Platelet Activating Factor. J. Inorg. Biochem. 2013, 120, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lett, S.; Tsoupras, A.; Papakonstantinou, V.; Stamatakis, G.; Demopoulos, C.; Falaras, P.; Philippopoulos, A. Biochemical evaluation of ruthenium-based complexes towards PAF (Platelet Activating Factor) and thrombin. Potent anti-inflammatory agents. Sci. Lett. J. 2015, 4, 208. [Google Scholar]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Bryden, F.; Boyle, R.W. Chapter Four–Metalloporphyrins for Medical Imaging Applications. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Insights from Imaging in Bioinorganic Chemistry; Academic Press: Cambridge, MA, USA, 2016; Volume 68, pp. 141–221. [Google Scholar]
- Hakli, Ö.; Yarali, S.; Öner Usta, E.; Ayaz, F. Photodynamic Anti-Inflammatory Activity of Meso-Aryl Substituted Porphyrin Derivative on Mammalian Macrophages. Photodiagnosis Photodyn. Ther. 2024, 45, 103922. [Google Scholar] [CrossRef]
- Tahoun, M.; Gee, C.T.; McCoy, V.E.; Sander, P.M.; Müller, C.E. Chemistry of Porphyrins in Fossil Plants and Animals. RSC Adv. 2021, 11, 7552–7563. [Google Scholar] [CrossRef] [PubMed]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Panda, M.K.; Ladomenou, K.; Coutsolelos, A.G. Porphyrins in Bio-Inspired Transformations: Light-Harvesting to Solar Cell. Coord. Chem. Rev. 2012, 256, 2601–2627. [Google Scholar] [CrossRef]
- Ladomenou, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical Hydrogen Generation with Porphyrin-Based Systems. Coord. Chem. Rev. 2015, 304–305, 38–54. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; López-Duarte, I.; Charalambidis, G.; Ladomenou, K.; Ince, M.; Coutsolelos, A.G. Porphyrins and Phthalocyanines as Biomimetic Tools for Photocatalytic H2 Production and CO2 Reduction. Chem. Soc. Rev. 2022, 51, 6965–7045. [Google Scholar] [CrossRef]
- Gkika, D.A.; Ladomenou, K.; Bououdina, M.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption and Photocatalytic Applications of Porphyrin-Based Materials for Environmental Separation Processes: A Review. Sci. Total Environ. 2024, 908, 168293. [Google Scholar] [CrossRef]
- Fletcher, J.R.; DiSimone, A.G.; Earnest, M.A. Platelet Activating Factor Receptor Antagonist Improves Survival and Attenuates Eicosanoid Release in Severe Endotoxemia. Ann. Surg. 1990, 211, 312–316. [Google Scholar]
- Zhang, J.; Yuan, X.; Li, H.; Yu, L.; Zhang, Y.; Pang, K.; Sun, C.; Liu, Z.; Li, J.; Ma, L.; et al. Novel Porphyrin Derivative Containing Cations as New Photodynamic Antimicrobial Agent with High Efficiency. RSC Adv. 2024, 14, 3122–3134. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Merlos, M.; García-Rafanell, J. Rupatadine: A New Selective Histamine H1 Receptor and Platelet-Activating Factor (PAF) Antagonist. A Review of Pharmacological Profile and Clinical Management of Allergic Rhinitis. Drugs Today 2003, 39, 451–468. [Google Scholar] [CrossRef]
- Margariti, A.; Papakonstantinou, V.D.; Stamatakis, G.M.; Demopoulos, C.A.; Schnakenburg, G.; Andreopoulou, A.K.; Giannopoulos, P.; Kallitsis, J.K.; Philippopoulos, A.I. Substituted Pyridine-Quinoline Ligands as Building Blocks for Neutral Rhodium(III) Complexes. Synthesis, Structural Characterization Studies and Anti-Platelet Activity towards the Platelet-Activating Factor (PAF). Polyhedron 2020, 178, 114336. [Google Scholar] [CrossRef]
- Ravishankar, D.; Salamah, M.; Attina, A.; Pothi, R.; Vallance, T.M.; Javed, M.; Williams, H.F.; Alzahrani, E.M.S.; Kabova, E.; Vaiyapuri, R.; et al. Ruthenium-Conjugated Chrysin Analogues Modulate Platelet Activity, Thrombus Formation and Haemostasis with Enhanced Efficacy. Sci. Rep. 2017, 7, 5738. [Google Scholar] [CrossRef] [PubMed]
- Peppas, A.; Kalabalidis, A.; Papakonstantinou, V.; Demopoulos, C.; Schnakenburg, G.; Philippopoulos, A. Rhodium-Based Inhibitors of the Platelet Activating Factor (PAF): A New Class of Potent Anti-Inflammatory Drugs. In Chemical Elements (Fluorine, Rhodium and Rubidium): Properties, Synthesis and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 127–169. [Google Scholar]
- Merlos, M.; Giral, M.; Balsa, D.; Ferrando, R.; Queralt, M.; Puigdemont, A.; García-Rafanell, J.; Forn, J. Rupatadine, a New Potent, Orally Active Dual Antagonist of Histamine and Platelet-Activating Factor (PAF). J. Pharmacol. Exp. Ther. 1997, 280, 114–121. [Google Scholar] [PubMed]
- Kaplanis, M.; Stamatakis, G.; Papakonstantinou, V.D.; Paravatou-Petsotas, M.; Demopoulos, C.A.; Mitsopoulou, C.A. Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, Anti-Inflammatory and Anti-Coagulant Effects towards Platelet Activating Factor. J. Inorg. Biochem. 2014, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sioriki, E.; Lordan, R.; Nahra, F.; van Hecke, K.; Zabetakis, I.; Nolan, S.P. In vitro Anti-atherogenic Properties of N-Heterocyclic Carbene Aurate(I) Compounds. ChemMedChem 2018, 13, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Siabali, S.A. Ru(II) Complexes and Their Biological Activity towards Platelet Activating Factor. Master’s Thesis, National and Kapodistrian University Athens, Athens, Greece, 2015. [Google Scholar]
- Prokopi, P.M. Complexes of Ir(I) and Ir(III) and Evaluation of Their Biological Activity against Platelet Activating Factor. Master’s Thesis, National and Kapodistrian University Athens, Athens, Greece, 2015. [Google Scholar]
- Cao, C.; Tan, Q.; Xu, C.; He, L.; Yang, L.; Zhou, Y.; Zhou, Y.; Qiao, A.; Lu, M.; Yi, C.; et al. Structural Basis for Signal Recognition and Transduction by Platelet-Activating-Factor Receptor. Nat. Struct. Mol. Biol. 2018, 25, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kanso, F.; Khalil, A.; Noureddine, H.; El-Makhour, Y. Therapeutic Perspective of Thiosemicarbazones Derivatives in Inflammatory Pathologies: A Summary of in vitro/in vivo studies. Int. Immunopharmacol. 2021, 96, 107778. [Google Scholar] [CrossRef]
- El-Asmy, A.A.; Sherif, Y.E.; Gabr, S.A.; Al-Hazmi, G.A. Synthesis and Antiinflamation Activity of Bis(Diacetylmonoxime) Thiocarbohydrazone and Its Cu(II) Complex. Biochem. Indian J. 2007, 1, 53–62. [Google Scholar]
- Carvalho, I.O.; Queiroz, C.V.G.; Marques, G.F.O.; Craveiro, R.M.C.B.; Xavier Júnior, F.A.F.; Gouveia Júnior, F.S.; Lopes, L.G. de F.; Chaves, E.M.C.; Monteiro, H.S.A.; Assreuy, A.M.S.; et al. The Nitric Oxide Pathway Is Involved in the Anti-Inflammatory Effect of the Rutheniumcomplex [Ru(bpy)2(2-MIM)(NO)](PF6)3. Eur. J. Pharmacol. 2022, 921, 174869. [Google Scholar] [CrossRef] [PubMed]
- Khamrang, T.; Hung, K.-C.; Hsia, C.-H.; Hsieh, C.-Y.; Velusamy, M.; Jayakumar, T.; Sheu, J.-R. Antiplatelet Activity of a Newly Synthesized Novel Ruthenium (II): A Potential Role for Akt/JNK Signaling. Int. J. Mol. Sci. 2017, 18, 916. [Google Scholar] [CrossRef]
- Sasahara, G.L.; Júnior, F.S.G.; de Oliveira Rodrigues, R.; Zampieri, D.S.; da Cruz Fonseca, S.G.; Gonçalves, R.D.C.R.; Athaydes, B.R.; Kitagawa, R.R.; Santos, F.A.; Sousa, E.H.S.; et al. Nitro-Imidazole-Based Ruthenium Complexes with Antioxidant and Anti-Inflammatory Activities. J. Inorg. Biochem. 2020, 206, 111048. [Google Scholar] [CrossRef]
- Chen, H.; Sun, T.; Yan, Y.; Ji, X.; Sun, Y.; Zhao, X.; Qi, J.; Cui, W.; Deng, L.; Zhang, H. Cartilage Matrix-Inspired Biomimetic Superlubricated Nanospheres for Treatment of Osteoarthritis. Biomaterials 2020, 242, 119931. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.-S.; Gao, G.-B.; Ma, Y.-M.; Luo, J.-X.; Zhang, G.-W.; Yang, H.; Li, N.; He, Q.-Y.; Lin, H.-S. Highly Bioactive Iridium Metal-Complex Alleviates Spinal Cord Injury via ROS Scavenging and Inflammation Reduction. Biomaterials 2022, 284, 121481. [Google Scholar] [CrossRef] [PubMed]
- Bielig, H.; Velder, J.; Saiai, A.; Menning, M.; Meemboor, S.; Kalka-Moll, W.; Krönke, M.; Schmalz, H.; Kufer, T.A. Anti-inflammatory Arene—Chromium Complexes Acting as Specific Inhibitors of NOD2 Signalling. ChemMedChem 2010, 5, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and Inflammation: Current Insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, L.; Kim, K.; Lee, D.S.; Hwang, D.H. Inhibition of Nod2 Signaling and Target Gene Expression by Curcumin. Mol. Pharmacol. 2008, 74, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Luo, X.; Xu, S.; Xu, D.; Zheng, Y.; Xu, S.; Lv, J. Gastroprotective Effects of a New Zinc(II)–Curcumin Complex against Pylorus-Ligature-Induced Gastric Ulcer in Rats. Chem. Biol. Interact. 2009, 181, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel role of Zn(II)–Curcumin in Enhancing Cell Proliferation and Adjusting Proinflammatory Cytokine-Mediated Oxidative Damage of Ethanol-Induced Acute Gastric Ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Narang, H.; Priyadarsini, K.I.; Krishna, M.; Pandey, R.; Sainis, K.B. Delayed Activation of PKCδ and NFκB and Higher Radioprotection in Splenic Lymphocytes by Copper (II)–Curcumin (1:1) Complex as Compared to Curcumin. J. Cell. Biochem. 2007, 102, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.-S.; Sun, J.-L.; Xie, W.-H.; Shen, L.; Ji, H.-F. Neuroprotective Effects and Mechanisms of Curcumin-Cu(II) and -Zn(II) Complexes Systems and Their Pharmacological Implications. Nutrients 2017, 10, 28. [Google Scholar] [CrossRef]
- Seeta Rama Raju, G.; Pavitra, E.; Purnachandra Nagaraju, G.; Ramesh, K.; El-Rayes, B.F.; Yu, J.S. Imaging and Curcumin Delivery in Pancreatic Cancer Cell Lines Using PEGylated α-Gd2(MoO4)3 Mesoporous Particles. Dalton Trans. 2014, 43, 3330–3338. [Google Scholar] [CrossRef]
- Vančo, J.; Trávníček, Z.; Hošek, J.; Malina, T.; Dvořák, Z. Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-Inflammatory Effects. Int. J. Mol. Sci. 2021, 22, 7626. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Andrades, N.; Paulino, N.; Sawaya, A.; Eberlin, M.; Marcucci, M.; Favero, G.; Novak, E.; Bydlowski, S. Synthesis and Characterization of a Metal Complex Containing Naringin and Cu, and its Antioxidant, Antimicrobial, Antiinflammatory and Tumor Cell Cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Zapata-Morales, J.R.; Hernández-Munive, A.; Campos-Xolalpa, N.; Pérez-Gutiérrez, S.; Pérez-González, C. Synthesis, Antinociceptive and Anti-Inflammatory Effects of Porphyrins. Bioorg. Med. Chem. 2015, 23, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Ran, P.; Xia, T.; Zheng, H.; Lei, F.; Zhang, Z.; Wei, J.; Li, X. Light-Triggered Theranostic Hydrogels for Real-Time Imaging and on-Demand Photodynamic Therapy of Skin Abscesses. Acta Biomater. 2023, 155, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Carrenho, L.Z.B.; Moreira, C.G.; Vandresen, C.C.; Gomes Junior, R.; Gonçalves, A.G.; Barreira, S.M.W.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R.B.; Dietrich, M.; et al. Investigation of Anti-Inflammatory and Anti-Proliferative Activities Promoted by Photoactivated Cationic Porphyrin. Photodiagnosis Photodyn. Ther. 2015, 12, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Furlan, C.; Berenbeim, J.A.; Dessent, C.E.H. Photoproducts of the Photodynamic Therapy Agent Verteporfin Identified via Laser Interfaced Mass Spectrometry. Molecules 2020, 25, 5280. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M. Verteporfin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2009; pp. 1–7. ISBN 978-0-08-055232-3. [Google Scholar]
- Wang, Y.; Wang, L.; Wise, J.T.F.; Shi, X.; Chen, Z. Verteporfin Inhibits Lipopolysaccharide-Induced Inflammation by Multiple Functions in RAW 264.7 cells. Toxicol. Appl. Pharmacol. 2020, 387, 114852. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Pouso-Vázquez, E.; Bai, X.; Batallé, G.; Roch, G.; Pol, O. Effects of Heme Oxygenase 1 in the Molecular Changes and Neuropathy Associated with type 2 Diabetes in Mice. Biochem. Pharmacol. 2022, 199, 114987. [Google Scholar] [CrossRef]
- Schaefer, R.E.M.; Callahan, R.C.; Atif, S.M.; Orlicky, D.J.; Cartwright, I.M.; Fontenot, A.P.; Colgan, S.P.; Onyiah, J.C. Disruption of MONOCYTE-Macrophage Differentiation and Trafficking by a Heme Analog during Active Inflammation. Mucosal Immunol. 2022, 15, 244–256. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Ni, J.; Hua, Y.; He, F.; Li, L. Role of the CCL2-CCR2 Axis in Cardiovascular Disease: Pathogenesis and Clinical Implications. Front. Immunol. 2022, 13, 975367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, Y.; Leem, J.; Song, J.E. Heme Oxygenase-1 Induction by Cobalt Protoporphyrin Ameliorates Cholestatic Liver Disease in a Xenobiotic-Induced Murine Model. Int. J. Mol. Sci. 2021, 22, 8253. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.G.; Zelent, D.; Ao, Z.; Bradley, B.T.; Cooke, A.; Contino, L.; Hu, E.; Douglas, S.A.; Jaye, M.C. Heme-Oxygenase Induction Inhibits Arteriolar Thrombosis In Vivo: Effect of The non-Substrate Inducer Cobalt Protoporphyrin. Eur. J. Pharmacol. 2009, 606, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, Y.; Tian, Y.; Chen, B.; Liang, Y.; Liang, Y.; Li, C.; Li, Y. TCPP/MgO-loaded PLGA Microspheres Combining Photodynamic Antibacterial Therapy with PBM-Assisted Fibroblast Activation to Treat Periodontitis. Biomater. Sci. 2023, 11, 2828–2844. [Google Scholar] [CrossRef] [PubMed]
- Allyn, M.M.; Rincon-Benavides, M.A.; Chandler, H.L.; Higuita-Castro, N.; Palmer, A.F.; Swindle-Reilly, K.E. Sustained Release of Heme-Albumin as a Potential Novel Therapeutic Approach for Age-Related Macular Degeneration. Biomater. Sci. 2022, 10, 7004–7014. [Google Scholar] [CrossRef] [PubMed]
- Pfefferlé, M.; Ingoglia, G.; Schaer, C.A.; Hansen, K.; Schulthess, N.; Humar, R.; Schaer, D.J.; Vallelian, F. Acute Hemolysis and Heme Suppress Anti-CD40 Antibody-Induced Necro-Inflammatory Liver Disease. Front. Immunol. 2021, 12, 680855. [Google Scholar] [CrossRef] [PubMed]
- Stover, K.; Fukuyama, T.; Young, A.T.; Daniele, M.A.; Oberley, R.; Crapo, J.D.; Bäumer, W. Topically Applied Manganese-Porphyrins BMX-001 and BMX-010 Display a Significant Anti-Inflammatory Response in a Mouse Model of Allergic Dermatitis. Arch. Dermatol. Res. 2016, 308, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, P.; Yang, Y.; Dai, S.; Wang, Z.; Zhao, A.; Huang, N.; Chen, J.; Yang, P. Dual-catalytic CuTPP/TiO2 Nanoparticles for Surface Catalysis Engineering of Cardiovascular Materials. Mater. Today Bio 2022, 17, 100494. [Google Scholar] [CrossRef]
- Tsukiji, N.; Osada, M.; Sasaki, T.; Shirai, T.; Satoh, K.; Inoue, O.; Umetani, N.; Mochizuki, C.; Saito, T.; Kojima, S.; et al. Cobalt hematoporphyrin Inhibits CLEC-2-Podoplanin Interaction, Tumor Metastasis, and Arterial/Venous Thrombosis in Mice. Blood Adv. 2018, 2, 2214–2225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).