Hybrid Crown Ether Ligands with Disiloxane Units and Their Complexes with Small s-Block Ions
Abstract
:1. Introduction
2. Materials and Methods
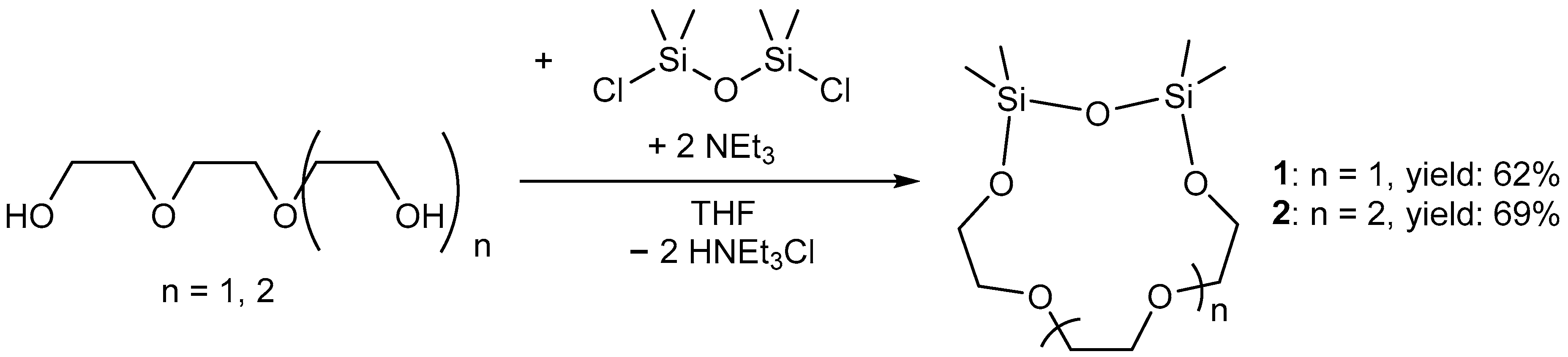
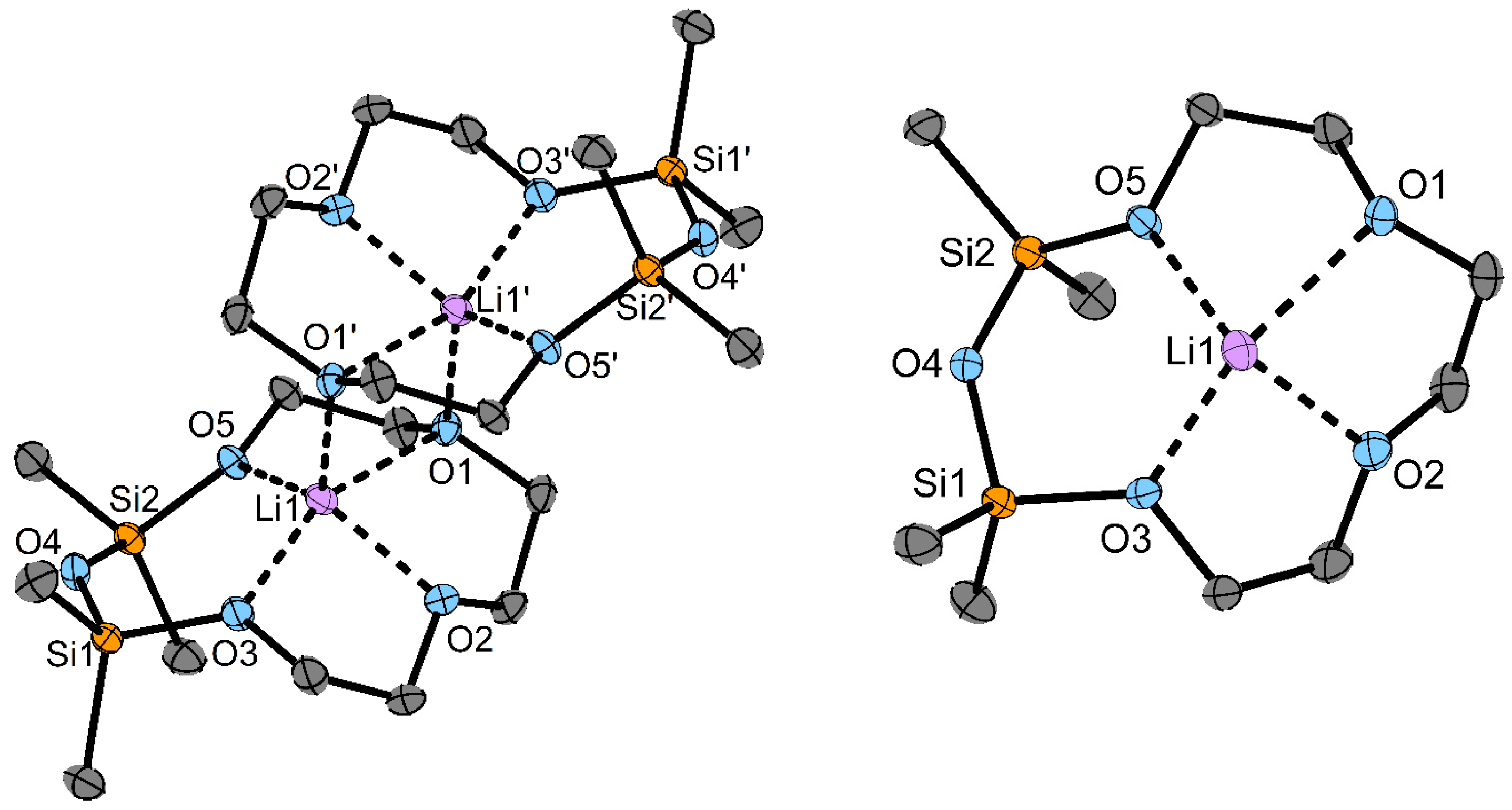
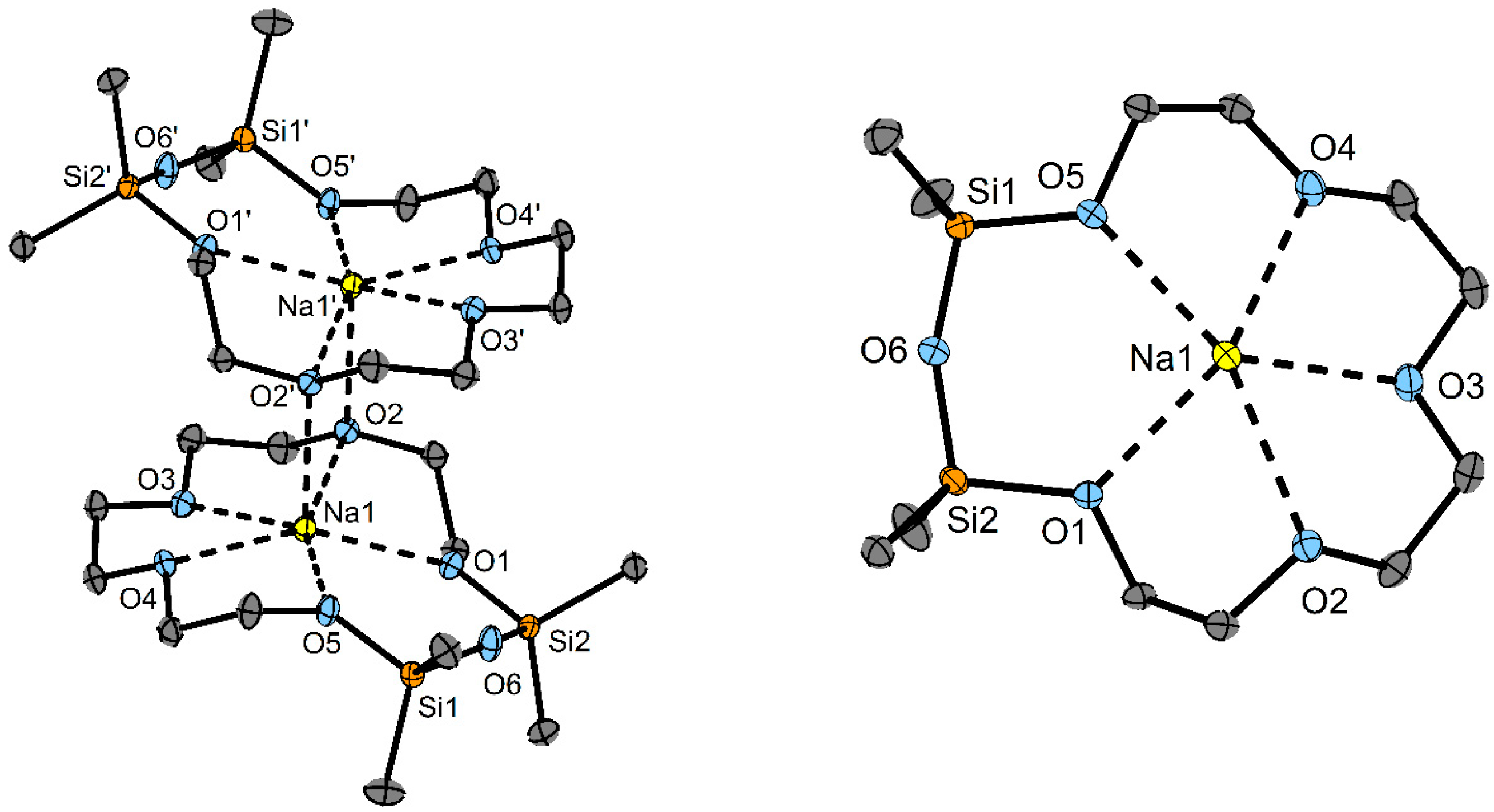
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCM | Dichloromethane |
| Et | Ethyl |
| NMR | Nuclear Magnetic Resonance |
| PhF | Fluorobenzene |
| PTFE | Polytetrafluoroethylene |
| TrEGDS | Triethylenglycoldisiloxane |
| TeEGDS | Tetraethylenglycoldisiloxane |
References
- Decken, A.; Passmore, J.; Wang, X. Cyclic Dimethylsiloxanes as Pseudo Crown Ethers: Syntheses and Characterization of Li (Me2SiO)5[Al{OC(CF3Li(Me2SiO)6[Al{OC(CF3Li(Me2SiO)6[Al{OC(CF3)3}4)3}4)2Ph}4]. Angew. Chem. Int. Ed. 2006, 45, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.D.; Glöckner, A.; Arif, A.M. Crystal structure of hexakis(dimethyl-m-oxosilane)dibromozirconium(IV) bis(nonabromodizirconate(IV)), [Zr{(CH3)2SiO}6Br2][Zr2Br9]2. New Cryst. Struct. 2007, 222, 333–334. [Google Scholar] [CrossRef]
- Cameron, T.S.; Decken, A.; Krossing, I.; Passmore, J.; Rautiainen, J.M.; Wang, X.; Zeng, X. Reactions of a Cyclodimethylsiloxane (Me2SiO)6 with Silver Salts of Weakly Coordinating Anions; Crystal Structures of [Ag(Me2SiO)6][Al] ([Al] = [FAl{OC(CF3)3}3], [Al{OC(CF3)3}4]) and Their Comparison with [Ag(18-Crown-6)]2[SbF6]2. Inorg. Chem. 2013, 52, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Sänger, I.; Gärtner, M.; Bolte, M.; Wagner, M.; Lerner, H.-W. New Aspects with Regard to Silanide Chemistry in Particular Formation and Structure of the First Disilyl Sodate [K(Me2SiO)7][(tBu3Si)2MeSi-Na-SiMe(SitBu3)2]. Z. Anorg. Allg. Chem. 2018, 644, 925–929. [Google Scholar] [CrossRef]
- Weinhold, F.; West, R. The Nature of the Silicon–Oxygen Bond. Organometallics 2011, 30, 5815–5824. [Google Scholar] [CrossRef]
- Moraru, I.-T.; Petrar, P.M.; Nemeş, G. Bridging a Knowledge Gap from Siloxanes to Germoxanes and Stannoxanes. A Theoretical Natural Bond Orbital Study. J. Phys. Chem. A 2017, 121, 2515–2522. [Google Scholar] [CrossRef]
- Fugel, M.; Hesse, M.F.; Pal, R.; Beckmann, J.; Jayatilaka, D.; Turner, M.J.; Karton, A.; Bultinck, P.; Chandler, G.S.; Grabowsky, S. Covalency and Ionicity Do Not Oppose Each Other-Relationship Between Si-O Bond Character and Basicity of Siloxanes. Chemistry 2018, 24, 15275–15286. [Google Scholar] [CrossRef]
- Grabowsky, S.; Hesse, M.F.; Paulmann, C.; Luger, P.; Beckmann, J. How to make the ionic Si-O bond more covalent and the Si-O-Si linkage a better acceptor for hydrogen bonding. Inorg. Chem. 2009, 48, 4384–4393. [Google Scholar] [CrossRef]
- Grabowsky, S.; Beckmann, J.; Luger, P. The Nature of Hydrogen Bonding Involving the Siloxane Group. Aust. J. Chem. 2012, 65, 785. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Ritch, J.S.; Chivers, T. Silicon Analogues of Crown Ethers and Cryptands: A New Chapter in Host–Guest Chemistry? Angew. Chem. Int. Ed. 2007, 46, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Dankert, F.; von Hänisch, C. Siloxane Coordination Revisited: Si−O Bond Character, Reactivity and Magnificent Molecular Shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Dankert, F.; Donsbach, C.; Rienmüller, J.; Richter, R.M.; von Hänisch, C. Alkaline Earth Metal Template (Cross-)Coupling Reactions with Hybrid Disila-Crown Ether Analogues. Chem. Eur. J. 2019, 25, 15934–15943. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.; Elsen, H.; Friedrich, A.; Harder, S. Unsupported metal silyl ether coordination. Chem. Commun. 2018, 54, 7846–7849. [Google Scholar] [CrossRef]
- Thum, K.; Friedrich, A.; Pahl, J.; Elsen, H.; Langer, J.; Harder, S. Unsupported Mg-Alkene Bonding. Chem.—Eur. J. 2021, 27, 2513–2522. [Google Scholar] [CrossRef]
- Haynes, M.D.; O’Reilly, A.; Poole, A.J.M.; Roper, A.F.; Thum, S.; Morris, L.J.; Coles, M.P.; Fulton, J.R.; Harder, S.; Turner, Z.R.; et al. Heavier alkaline earth and heterobimetallic s-block “ate” complexes of a di(amido)siloxane ligand: Solid-state structure and dynamic solution-phase behavior. Dalton Trans. 2025, 54, 4542–4555. [Google Scholar] [CrossRef]
- Chai, C.L.L.; Armarego, W.L.F. Purification of Laboratory Chemicals, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Troyanov, S.I.; Krahl, T.; Kemnitz, E. Crystal structures of GaX3 (X = Cl, Br, I) and AlI3. Z. Kristallogr. 2004, 219, 88–92. [Google Scholar] [CrossRef]
- Richter, R.-M.; von Hänisch, C. Complexes of Alkali Metal and the Ammonium Ion with the Cyclodimethylsiloxane Ligands D7 and D8 (D = Me2SiO). Z. Anorg. Allg. Chem. 2024, 650, e202400036. [Google Scholar] [CrossRef]
- Richter, R.-M.; Heinrichs, J.; Weiss, P.; Subrati, Z.; von Hänisch, C. Complexes of Alkaline Earth Metal Ions with the Cyclodimethylsiloxane ligands D7, D8 and D9 (D = Me2SiO). Z. Anorg. Allg. Chem. 2024, 650, e202400178. [Google Scholar] [CrossRef]
- Dankert, F.; Weigend, F.; von Hänisch, C. Not Non-Coordinating at All: Coordination Compounds of the Cyclodimethylsiloxanes Dn (D = Me2SiO; n = 6, 7) and Group 2 Metal Cations. Inorg. Chem. 2019, 58, 15417–15422. [Google Scholar] [CrossRef]
- Dankert, F.; Erlemeier, L.; Ritter, C.; von Hänisch, C. On the molecular architectures of siloxane coordination compounds: (re-)investigating the coordination of the cyclodimethylsiloxanes Dn (n =5–8) towards alkali metal ions. Inorg. Chem. Front. 2020, 7, 2138–2153. [Google Scholar] [CrossRef]
- Groth, P.; Møllendal, H.; Seip, R.; Weidlein, J.; Spahiu, K. On the Crystal Structure of the (1:1) Complex between Lithium Thiocyanate and 1,4,7,10-Tetraoxacyclododecane at Room Temperature. Acta Chem. Scand. 1981, 35a, 463–465. [Google Scholar] [CrossRef]
- Nöth, H.; Warchhold, M. Sodium Hydro(isothiocyanato)borates: Synthesis and Structures. Eur. J. Inorg. Chem. 2004, 2004, 1115–1124. [Google Scholar] [CrossRef]
- Paparo, A.; Silvia, J.S.; Spaniol, T.P.; Okuda, J.; Cummins, C.C. Countercation Effect on CO2 Binding to Oxo Titanate with Bulky Anilide Ligands. Chem. Eur. J. 2018, 24, 17072–17079. [Google Scholar] [CrossRef]
- Pochekutova, T.S.; Khamylov, V.K.; Fukin, G.K.; Kurskii, Y.A.; Petrov, B.I.; Shavyrin, A.S.; Arapova, A.V. New calcium β-diketonate complexes: The monomeric complexes [Ca(PhCOCHCOCF3)2(15-crown-5)], [Ca(AdCOCHCOCF3)2(15-crown-5)] and the binuclear hydrated complex [{Ca(adtfa)(18-crown-6)(H2O)}{Ca(adtfa)3(H2O)}(EtOH)]. Synthesis, characterization and crystal structure. Polyhedron 2011, 30, 1945–1952. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, R.-M.; Stauf, D.J.; Rauchel, A.L.; Ruppach, L.; Bania, K.; Duncker, A.; Hänisch, C.v. Hybrid Crown Ether Ligands with Disiloxane Units and Their Complexes with Small s-Block Ions. Compounds 2025, 5, 11. https://doi.org/10.3390/compounds5020011
Richter R-M, Stauf DJ, Rauchel AL, Ruppach L, Bania K, Duncker A, Hänisch Cv. Hybrid Crown Ether Ligands with Disiloxane Units and Their Complexes with Small s-Block Ions. Compounds. 2025; 5(2):11. https://doi.org/10.3390/compounds5020011
Chicago/Turabian StyleRichter, Roman-Malte, Daniel James Stauf, Anna Lena Rauchel, Lutz Ruppach, Kevin Bania, Annalena Duncker, and Carsten von Hänisch. 2025. "Hybrid Crown Ether Ligands with Disiloxane Units and Their Complexes with Small s-Block Ions" Compounds 5, no. 2: 11. https://doi.org/10.3390/compounds5020011
APA StyleRichter, R.-M., Stauf, D. J., Rauchel, A. L., Ruppach, L., Bania, K., Duncker, A., & Hänisch, C. v. (2025). Hybrid Crown Ether Ligands with Disiloxane Units and Their Complexes with Small s-Block Ions. Compounds, 5(2), 11. https://doi.org/10.3390/compounds5020011






