Fructooligosaccharides (FOSs): A Condensed Overview
Abstract
:1. Introduction
2. Structure, Properties, and Health Benefits
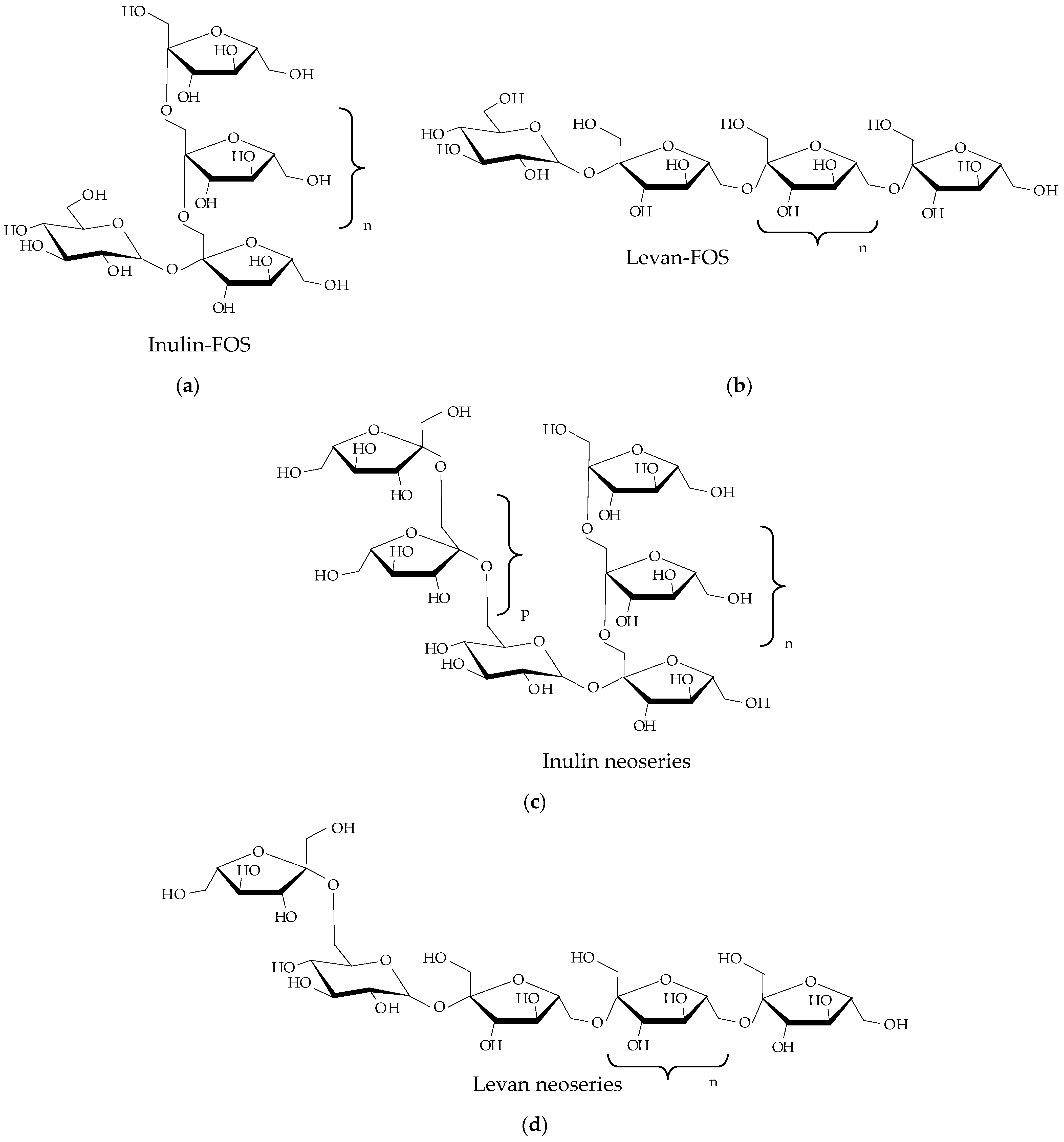
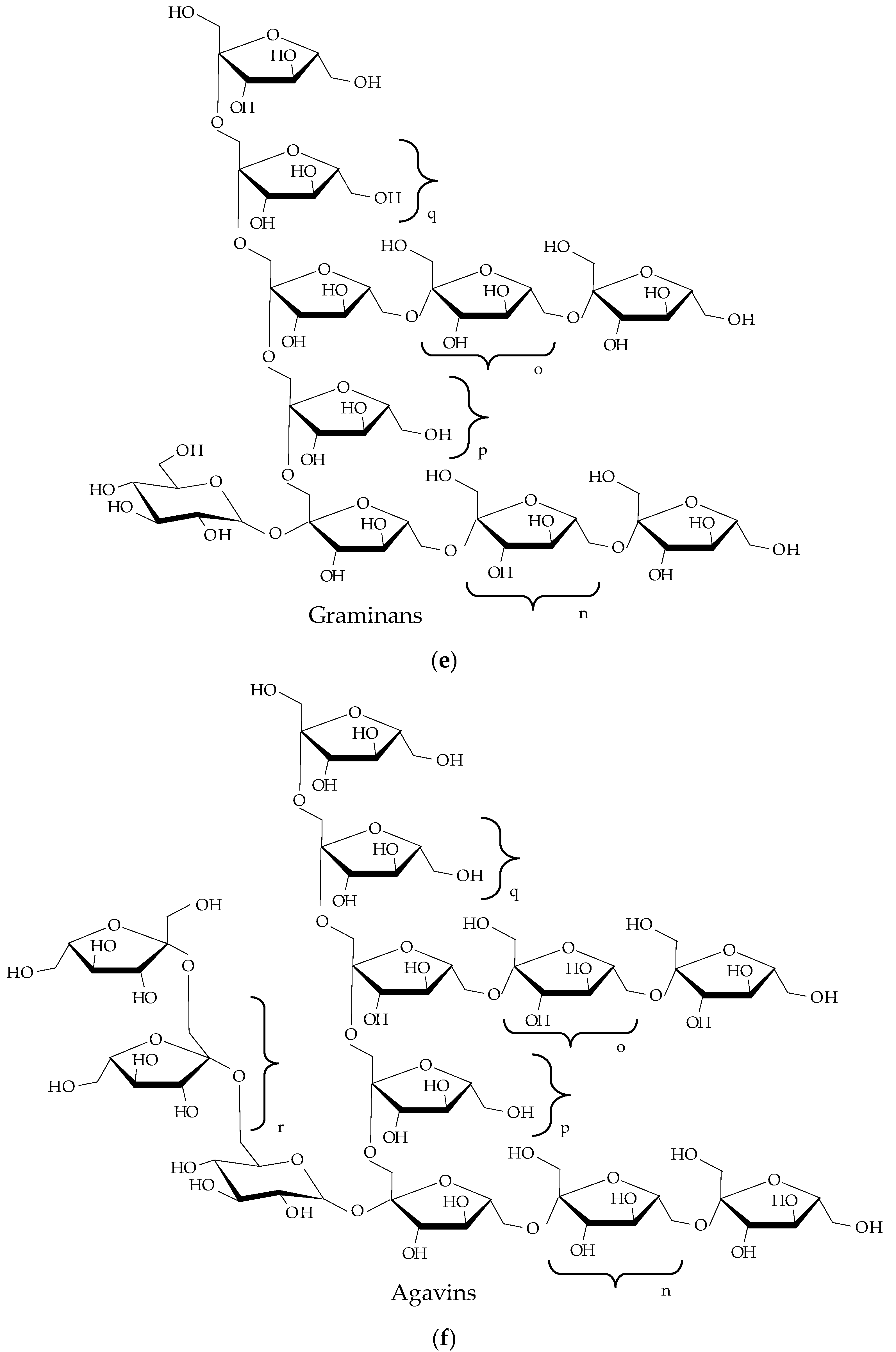
2.1. Chemical Structure
2.2. Properties
2.3. Health Benefits
3. Natural Occurrence and Production Methods
3.1. Natural Occurrence
3.2. Production Methods
3.2.1. Extraction from Natural Sources
3.2.2. Enzymatic Production by Hydrolysis
| Reaction Conditions | Comments |
|---|---|
| Enzyme concentration | Higher enzyme concentrations generally lead to a higher rate of hydrolysis, resulting in shorter FOS chains (lower DP). |
| Reaction time | Longer reaction times can lead to a further breakdown of the FOS, reducing . |
| Temperature and pH | The temperature and pH of the reaction influence enzyme activity and stability. Optimal conditions can maximize enzyme efficiency and control the DP of FOSs. |
| Substrate concentration | Higher substrate concentrations may lead to a higher FOS yield but also to a broader distribution of DP due to varying enzyme–substrate interactions. |
3.2.3. Enzymatic Production by Synthesis
3.2.4. FOS Production by Microbial Fermentation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benkeblia, N. Fructooligosaccharides and Fructans Analysis in Plants and Food Crops. J. Chromatogr. A 2013, 1313, 54–61. [Google Scholar] [CrossRef]
- Guo, M.; Chen, G.; Chen, K. Fructooligosaccharides: Effects, Mechanisms, and Applications. In Research Progress in Oligosaccharins; Springer: New York, NY, USA, 2016; pp. 51–63. [Google Scholar]
- Kamchonemenukool, S.; Buasum, W.; Weerawatanakorn, M.; Thongsook, T. Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder. Foods 2023, 12, 2895. [Google Scholar] [CrossRef] [PubMed]
- Kherade, M.; Solanke, S.; Tawar, M.; Wankhede, S. Fructooligosaccharides: A Comprehensive Review. J. Ayurvedic Herb. Med. 2021, 7, 193–200. [Google Scholar] [CrossRef]
- IUB-IUPAC Joint Commission on Biochemical Nomenclature (JCBN). Abbreviated Terminology of Oligosaccharide Chains Recommendations 1980. J. Biol. Chem. 1982, 257, 3347–3351. [Google Scholar] [CrossRef]
- Rastall, R.A. Functional Oligosaccharides: Application and Manufacture. Annu. Rev. Food Sci. Technol. 2010, 1, 305–339. [Google Scholar] [CrossRef]
- Smith, J.B. Safety of short-chain fructooligosaccharides and GRAS Affirmation by the U.S. FDA. Biosci. Microflora 2002, 21, 27–29. [Google Scholar] [CrossRef]
- Hawrelak, J. Prebiotics, Synbiotics, and Colonic Foods. In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2013; pp. 966–978. [Google Scholar]
- Chelliah, R.; Kim, N.H.; Park, S.; Park, Y.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Oh, D.-H. Revolutionizing Renewable Resources: Cutting-Edge Trends and Future Prospects in the Valorization of Oligosaccharides. Fermentation 2024, 10, 195. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Functional Oligosaccharides: Production, Properties and Applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Chen, Y.; Sui, X.; Wang, Y.; Zhao, Z.; Han, T.; Liu, Y.; Zhang, J.; Zhou, P.; Yang, K.; Ye, Z. Preparation, Structural Characterization, Biological Activity, and Nutritional Applications of Oligosaccharides. Food Chem. X 2024, 22, 101289. [Google Scholar] [CrossRef]
- Insight Ace Analytical. Fructooligosaccharide Market Size, Share & Trends Analysis Report by Source (Sucrose, Insulin), by Form (Solid, Liquid), by Application (Infant Formulations, Food & Beverages, Animal Feed, Dietary Supplements, Pharmaceuticals), by Region, and by Segment Forecasts, 2024–2031; InsightAce Analytic Pvt. Ltd.: Pune, India, 2024. [Google Scholar]
- Khanvilkar, S.S.; Arya, S.S. Fructooligosaccharides: Applications and Health Benefits: A Review. Agro Food Ind. Hi Tech 2015, 26, 8–12. [Google Scholar]
- Valladares-Diestra, K.K.; Souza Vandenberghe, L.P.d.; Carvalho Neto, D.P.d.; Goyzueta-Mamani, L.D.; Soccol, C.R. Microbial Enzymes for Production of Fructooligosaccharides. In Microbial Enzymes in Production of Functional Foods and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2022; pp. 153–172. [Google Scholar]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Sripada, S.; Poornachandra, Y. Status and Future Prospects of Fructooligosaccharides as Nutraceuticals. In Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 451–503. [Google Scholar]
- Rahim, M.A.; Saeed, F.; Khalid, W.; Hussain, M.; Anjum, F.M. Functional and Nutraceutical Properties of Fructo-Oligosaccharides Derivatives: A Review. Int. J. Food Prop. 2021, 24, 1588–1602. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. Int. J. Food Prop. 2014, 17, 949–965. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Woolley, E.; Rahimifard, S. Identification and Analysis of Attributes for Industrial Food Waste Management Modelling. Sustainability 2019, 11, 2445. [Google Scholar] [CrossRef]
- Nobre, C.; Simões, L.S.; Gonçalves, D.A.; Berni, P.; Teixeira, J.A. Fructooligosaccharides Production and the Health Benefits of Prebiotics. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–138. [Google Scholar]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Recent Trends in the Microbial Production, Analysis and Application of Fructooligosaccharides. Trends Food Sci. Technol. 2005, 16, 442–457. [Google Scholar] [CrossRef]
- Wang, T.-H. Synthesis of Neofructooligosaccharides. Org. Chem. Insights 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Nabeshima, E.H.; Tavares, P.E.d.R.; Lemos, A.L.D.S.C.; Moura, S.C.S.R.d. Emerging Ingredients for Clean Label Products and Food Safety. Braz. J. Food Technol. 2024, 27, e2023160. [Google Scholar] [CrossRef]
- Silva, P.I.S.E.; Oriente, S.F.D.; Ramos, N.J.d.S.; Gusmão, T.A.D.S.; Gusmão, R.P.d. Fructooligosaccharide and Application in Dairy Products: A Literature Review. Res. Soc. Dev. 2023, 12, e13812541582. [Google Scholar] [CrossRef]
- Belmonte-Izquierdo, Y.; Salomé-Abarca, L.F.; González-Hernández, J.C.; López, M.G. Fructooligosaccharides (FOS) Production by Microorganisms with Fructosyltransferase Activity. Fermentation 2023, 9, 968. [Google Scholar] [CrossRef]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Respondek, F.; Wagner, A. Benefits of Fructooligosaccharides in Formula Fed Infants. Agro Food Ind. Hi Tech 2013, 24, 39–41. [Google Scholar]
- Jackson, P.P.J.; Wijeyesekera, A.; Rastall, R.A. Inulin-type Fructans and Short-chain Fructooligosaccharides—Their Role within the Food Industry as Fat and Sugar Replacers and Texture Modifiers—What Needs to Be Considered! Food Sci. Nutr. 2023, 11, 17–38. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, X.; Luo, Y.; Chen, B.; Ma, D.; Zhu, J. Effect of Fructooligosaccharides Supplementation on the Gut Microbiota in Human: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3298. [Google Scholar] [CrossRef] [PubMed]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological Production and Application of Fructooligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Visscher, C.; Kaltschmitt, M. Plant-Based Fructans for Increased Animal Welfare: Provision Processes and Remaining Challenges. Biomass Convers. Biorefinery 2023, 13, 2667–2685. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, R.S. Production of Fructooligosaccharides from Inulin by Endoinulinases and Their Prebiotic Potential. Food Technol. Biotechnol. 2010, 48, 435–450. [Google Scholar]
- Rawat, H.K.; Nath, S.; Sharma, I.; Kango, N. Recent Developments in the Production of Prebiotic Fructooligosaccharides Using Fungal Fructosyltransferases. Mycology 2024, 15, 564–584. [Google Scholar] [CrossRef]
- Ávila-Fernández, Á.; Galicia-Lagunas, N.; Rodríguez-Alegría, M.E.; Olvera, C.; López-Munguía, A. Production of Functional Oligosaccharides through Limited Acid Hydrolysis of Agave Fructans. Food Chem. 2011, 129, 380–386. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Chemical Synthesis of Oligosaccharides and Their Application in New Drug Research. Eur. J. Med. Chem. 2023, 249, 115164. [Google Scholar] [CrossRef]
- Palcic, M.M. Biocatalytic Synthesis of Oligosaccharides. Curr. Opin. Biotechnol. 1999, 10, 616–624. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Kennedy, J.F. Enzymatic Synthesis of Fructooligosaccharides from Inulin in a Batch System. Carbohydr. Polym. Technol. Appl. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Crawford, C.J.; Seeberger, P.H. Advances in Glycoside and Oligosaccharide Synthesis. Chem. Soc. Rev. 2023, 52, 7773–7801. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Samak, D.; Shehata, A.M.; Allam, A.A.; Abd El-Hack, M.E. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. New Trends and Technological Challenges in the Industrial Production and Purification of Fructo-Oligosaccharides. Crit. Rev. Food Sci. Nutr. 2015, 55, 1444–1455. [Google Scholar] [CrossRef]
- Cangiano, L.R.; Yohe, T.T.; Steele, M.A.; Renaud, D.L. Invited Review: Strategic Use of Microbial-Based Probiotics and Prebiotics in Dairy Calf Rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar] [CrossRef]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128120699. [Google Scholar]
- Ibrahim, O. Technological Aspects of Fructo-Oligosaccharides (FOS), Production Processes, Physiological Properties, Applications and Health Benefits. J. Food Chem. Nanotechnol. 2021, 7, 41–46. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a Flexible Oligosaccharide I: Review of Its Physicochemical Characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef]
- Sánchez-Martínez, M.J.; Soto-Jover, S.; Antolinos, V.; Martínez-Hernández, G.B.; López-Gómez, A. Manufacturing of Short-Chain Fructooligosaccharides: From Laboratory to Industrial Scale. Food Eng. Rev. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; Teixeira, J.A.; Nobre, C. In Situ Enzymatic Synthesis of Prebiotics to Improve Food Functionality. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 253–267. [Google Scholar]
- Hill, A.; Tian, F.; Karboune, S. Synthesis of Levan and Fructooligosaccharides by Levansucrase: Catalytic, Structural and Substrate-Specificity Properties. Curr. Org. Chem. 2016, 21, 149–161. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Functional Effects of Food Components and the Gastrointestinal System: Chicory Fructooligosaccharides. Nutr. Rev. 2009, 54, S38–S42. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Uc-Chuc, M.A.; Loyola-Vargas, V.M.; Santiago-García, P.A.; López, M.G. Fructosyltransferases in Plants: Structure, Function and Application: A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100343. [Google Scholar] [CrossRef]
- Ni, D.; Zhang, S.; Liu, X.; Zhu, Y.; Xu, W.; Zhang, W.; Mu, W. Production, Effects, and Applications of Fructans with Various Molecular Weights. Food Chem. 2024, 437, 137895. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Margalli, N.A.; López, M.G. Water-Soluble Carbohydrates and Fructan Structure Patterns from Agave and Dasylirion Species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef]
- Salomé-Abarca, L.F.; Márquez-López, R.E.; López, M.G. Agave Amica a Potential Model for the Study of Agavins Metabolism. Sci. Rep. 2023, 13, 19888. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, X.; Cao, L.; Ji, J.; Zheng, Q.; Gao, J. Isolation and Structural Identification of a Novel Fructan from Radix codonopsis. J. Carbohydr. Chem. 2020, 39, 163–174. [Google Scholar] [CrossRef]
- Wienberg, F.; Hövels, M.; Deppenmeier, U. High-Yield Production and Purification of Prebiotic Inulin-Type Fructooligosaccharides. AMB Express 2022, 12, 144. [Google Scholar] [CrossRef]
- Sridevi, V.; Sumath, V.; Guru Prasad, M.; Satish Kumar, M. Fructooligosaccharides-Type Prebiotic: A Review. J. Pharm. Res. 2014, 8, 321–330. [Google Scholar]
- Teferra, T.F. Possible Actions of Inulin as Prebiotic Polysaccharide: A Review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Yıldız, S. The Metabolism of Fructooligosaccharides and Fructooligosaccharide-Related Compounds in Plants. Food Rev. Int. 2010, 27, 16–50. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, Y.; Gao, H.; Sun, J.; Ma, H. Fructooligosaccharides Impact on the Hydration and Retro-Gradation of Wheat Starch and Gel. Int. J. Food Prop. 2016, 19, 2682–2692. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Sato, A.C.K. Biopolymer Gels Containing Fructooligosaccharides. Food Res. Int. 2017, 101, 88–95. [Google Scholar] [CrossRef]
- Peñaranda, I.; Garrido, M.D. Viability of Fructooligosaccharides as Substitutes for Methylcellulose Reduction in Plant-Based Burgers. Food Hydrocoll. 2024, 154, 110104. [Google Scholar] [CrossRef]
- Sudha, M.L.; Soumya, C.; Saravanan, M.; Madhushree, P.; Singh, J.; Roy, S.; Prabhasankar, P. Influence of Short Chain Fructo-Oligosaccharide (SC-FOS) on the Dough Rheological, Microstructural Properties and, Bread Quality during Storage. LWT 2022, 158, 113102. [Google Scholar] [CrossRef]
- Pravallika, K.; Shaik, L.; Alzahrani, K.J.; Misra, N.N.; Chakraborty, S. Pulsed Light Processing of Low Sugar, Added Fiber RTD Mango Beverage. J. Food Process. Eng. 2024, 47, e14539. [Google Scholar] [CrossRef]
- Le, H.P.; Hong, D.T.N.; Nguyen, T.T.L.; Le, T.M.H.; Koseki, S.; Ho, T.B.; Ly-Nguyen, B. Thermal Stability of Fructooligosaccharides Extracted from Defatted Rice Bran: A Kinetic Study Using Liquid Chromatography-Tandem Mass Spectrometry. Foods 2022, 11, 2054. [Google Scholar] [CrossRef]
- Matusek, A.; Merész, P.; Le, T.K.D.; Örsi, F. Effect of Temperature and PH on the Degradation of Fructo-Oligosaccharides. Eur. Food Res. Technol. 2009, 228, 355–365. [Google Scholar] [CrossRef]
- Yun, J.W. Fructooligosaccharides—Occurrence, Preparation, and Application. Enzym. Microb. Technol. 1996, 19, 107–117. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Mancilha, I.M. Non-Digestible Oligosaccharides: A Review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Franck, A. Technological Functionality of Inulin and Oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef] [PubMed]
- Kanakupt, K.; Vester Boler, B.M.; Dunsford, B.R.; Fahey, G.C. Effects of Short-Chain Fructooligosaccharides and Galactooligosaccharides, Individually and in Combination, on Nutrient Digestibility, Fecal Fermentative Metabolite Concentrations, and Large Bowel Microbial Ecology of Healthy Adults Cats. J. Anim. Sci. 2011, 89, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The Relationship Between Bound Water and Carbohydrate Reserves in Association with Cellular Integrity in Fragaria Vesca Stored Under Different Conditions. Food Bioprocess Technol. 2015, 8, 875–884. [Google Scholar] [CrossRef]
- Rivero-Urgell, M.; Santamaria-Orleans, A. Oligosaccharides: Application in Infant Food. Early Hum. Dev. 2001, 65, S43–S52. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic Digestion and Fermentation. Am. J. Clin. Nutr. 2001, 73, 415s–420s. [Google Scholar] [CrossRef]
- Bello, F.D.; Walter, J.; Hertel, C.; Hammes, W.P. In Vitro Study of Prebiotic Properties of Levan-Type Exopolysaccharides from Lactobacilli and Non-Digestible Carbohydrates Using Denaturing Gradient Gel Electrophoresis. Syst. Appl. Microbiol. 2001, 24, 232–237. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Alatorre-Santamaría, S.; Cruz-Guerrero, A.; Guzmán-Rodríguez, F. Fructooligosaccharides (FOS). In Handbook of Food Bioactive Ingredients; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–30. [Google Scholar]
- Roberfroid, M.B.; Delzenne, N.M. Dietary Fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef]
- Teitelbaum, J.E.; Walker, W.A. Nutritional Impact of Pre- and Probiotics as Protective Gastrointestinal Organism. Annu. Rev. Nutr. 2002, 22, 107–138. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, S.K.; Kundu, A.; Grover, M.; Saha, S. Role of Fructooligosaccharides in Promoting Beneficial Gut Bacteria: A Prebiotic Perspective. Food Biosci. 2025, 63, 105726. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zou, M.; Bei, T.; Zhang, N.; Li, D.; Wang, M.; Li, C.; Tian, H. Effect of Fructooligosaccharides on the Colonization of Lactobacillus rhamnosus AS 1.2466T in the Gut of Mice. Food Sci. Hum. Wellness 2023, 12, 607–613. [Google Scholar] [CrossRef]
- Silva, P.B.; Garcia, S.; Baldo, C.; Celligoi, M.A.P.C. Prebiotic Activity of Fructooligosaccharides Produced by Bacillus subtilis Natto CCT 7712. Acta Aliment. 2017, 46, 145–151. [Google Scholar] [CrossRef]
- Kiran, S.; Sreeja, V.; Patel, H.K. In Vitro Probiotic and Bio-Functional Properties of a Synbiotic Composed of Lactobacillus helveticus MTCC 5463 and Fructo-Oligosaccharide. Food Biosci. 2025, 63, 105747. [Google Scholar] [CrossRef]
- Cheon, S.; Kim, G.; Bae, J.-H.; Lee, D.H.; Seong, H.; Kim, D.H.; Han, J.-S.; Lim, S.-Y.; Han, N.S. Comparative Analysis of Prebiotic Effects of Four Oligosaccharides Using In Vitro Gut Model: Digestibility, Microbiome, and Metabolome Changes. FEMS Microbiol. Ecol. 2023, 99. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zhang, T.; Yu, Y.; Mao, S.; Liu, J. Fructo-Oligosaccharide Supplementation Enhances the Growth of Nursing Dairy Calves While Stimulating the Persistence of Bifidobacterium and Hindgut Microbiome’s Maturation. J. Dairy Sci. 2024, 107, 5626–5638. [Google Scholar] [CrossRef]
- Yin, P.; Yi, S.; Du, T.; Zhang, C.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Dynamic Response of Different Types of Gut Microbiota to Fructooligosaccharides and Inulin. Food Funct. 2024, 15, 1402–1416. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of Dietary Protein and Fructooligosaccharides on Fecal Fermentative End-Products, Fecal Bacterial Populations and Apparent Total Tract Digestibility in Dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Caloric Value of Inulin and Oligofructose. J. Nutr. 1999, 129, 1436S–1437S. [Google Scholar] [CrossRef]
- Cáceres, E.; García, M.L.; Toro, J.; Selgas, M.D. The Effect of Fructooligosaccharides on the Sensory Characteristics of Cooked Sausages. Meat Sci. 2004, 68, 87–96. [Google Scholar] [CrossRef]
- Resconi, V.C.; Keenan, D.F.; Gough, S.; Doran, L.; Allen, P.; Kerry, J.P.; Hamill, R.M. Response Surface Methodology Analysis of Rice Starch and Fructo-Oligosaccharides as Substitutes for Phosphate and Dextrose in Whole Muscle Cooked Hams. LWT-Food Sci. Technol. 2015, 64, 946–958. [Google Scholar] [CrossRef]
- Promsakha na Sakon Nakhon, P.; Aimkaew, M.; Leesuksawat, W.; Tongsai, S. Optimization of Sorbitol, Fructooligosaccharides and Sugar Levels in the Syrup Based on Physicochemical Properties and Sensory Acceptance of Healthy, Sweet Egg Yolk Drop (a Traditional Egg-Based Dessert) Using Response Surface Methodology. Int. J. Food Prop. 2023, 26, 2229–2242. [Google Scholar] [CrossRef]
- Na, Y.; Nam, A.Y.; Park, S.H.; Lee, S.H. Production of Fructooligosaccharide-Containing Bakery and Sweet Paste Products Using Invertase. Food Sci. Biotechnol. 2024, 33, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Handa, C.; Goomer, S.; Siddhu, A. Physicochemical Properties and Sensory Evaluation of Fructoligosaccharide Enriched Cookies. J. Food Sci. Technol. 2012, 49, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, P.; Klewicki, R.; Klewicka, E. Stability of Fructooligosaccharides in Convectively Dried Fruits After Initial Osmoconcentration. Food Bioprocess Tech. 2023, 16, 2511–2520. [Google Scholar] [CrossRef]
- Vega, R.; Zuniga-Hansen, M.E. The Effect of Processing Conditions on the Stability of Fructooligosaccharides in Acidic Food Products. Food Chem. 2015, 173, 784–789. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary Fructooligosaccharides and Potential Benefits on Health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Caetano, B.; De Moura, N.; Almeida, A.; Dias, M.; Sivieri, K.; Barbisan, L. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 436. [Google Scholar] [CrossRef]
- Correa, A.d.C.; Lopes, M.S.; Perna, R.F.; Silva, E.K. Fructan-Type Prebiotic Dietary Fibers: Clinical Studies Reporting Health Impacts and Recent Advances in Their Technological Application in Bakery, Dairy, Meat Products and Beverages. Carbohydr. Polym. 2024, 323, 121396. [Google Scholar] [CrossRef]
- Nadeau, D.A. Intestinal Warfare: The Role of Short-Chain Fructooligosaccharides in Health and Disease. Nutr. Clin. Care 2000, 3, 266–273. [Google Scholar] [CrossRef]
- Bornet, F.R.J.; Brouns, F. Immune-Stimulating and Gut Health-Promoting Properties of Short-Chain Fructo-Oligosaccharides. Nutr. Rev. 2002, 60, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.R.J.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional Aspects of Short-Chain Fructooligosaccharides: Natural Occurrence, Chemistry, Physiology and Health Implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Sousa, S.C.; Silva, S.P.; Pinheiro, A.C.; Coelho, E.; Vicente, A.A.; Gomes, A.M.P.; Coimbra, M.A.; Teixeira, J.A.; Rodrigues, L.R. In Vitro Digestibility and Fermentability of Fructo-Oligosaccharides Produced by Aspergillus ibericus. J. Funct. Foods 2018, 46, 278–287. [Google Scholar] [CrossRef]
- van Trijp, M.P.H.; Rios-Morales, M.; Witteman, B.; Abegaz, F.; Gerding, A.; An, R.; Koehorst, M.; Evers, B.; van Dongen, K.C.V.; Zoetendal, E.G.; et al. Intraintestinal Fermentation of Fructo- and Galacto-Oligosaccharides and the Fate of Short-Chain Fatty Acids in Humans. iScience 2024, 27, 109208. [Google Scholar] [CrossRef]
- Roupar, D.; Coelho, M.C.; Gonçalves, D.A.; Silva, S.P.; Coelho, E.; Silva, S.; Coimbra, M.A.; Pintado, M.; Teixeira, J.A.; Nobre, C. Evaluation of Microbial-Fructo-Oligosaccharides Metabolism by Human Gut Microbiota Fermentation as Compared to Commercial Inulin-Derived Oligosaccharides. Foods 2022, 11, 954. [Google Scholar] [CrossRef]
- Molis, C.; Flourié, B.; Ouarne, F.; Gailing, M.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J. Digestion, Excretion, and Energy Value of Fructooligosaccharides in Healthy Humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef]
- Mao, B.; Li, D.; Zhao, J.; Liu, X.; Gu, Z.; Chen, Y.Q.; Zhang, H.; Chen, W. In Vitro Fermentation of Fructooligosaccharides with Human Gut Bacteria. Food Funct. 2015, 6, 947–954. [Google Scholar] [CrossRef]
- Singh, N.; Malik, S.; Gupta, A.; Srivastava, K.R. Revolutionizing Enzyme Engineering through Artificial Intelligence and Machine Learning. Emerg. Top Life Sci. 2021, 5, 113–125. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Rastall, R.A. Prebiotics: Metabolism, Structure, and Function. Funct. Food Rev. 2011, 3, 93–106. [Google Scholar]
- Mahalak, K.K.; Firrman, J.; Narrowe, A.B.; Hu, W.; Jones, S.M.; Bittinger, K.; Moustafa, A.M.; Liu, L. Fructooligosaccharides (FOS) Differentially Modifies the in Vitro Gut Microbiota in an Age-Dependent Manner. Front. Nutr. 2023, 9, 1058910. [Google Scholar] [CrossRef]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.C.-Y.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in Understanding and Applying Prebiotics in Research and Practice—An ISAPP Conference Paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.; Conlon, M.; Christophersen, C.; Topping, D. Resistant Starch, Large Bowel Fermentation and a Broader Perspective of Prebiotics and Probiotics. Benef. Microbes 2010, 1, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in Healthy Young Population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic Stimulation of Human Colonic Butyrate-Producing Bacteria and Bifidobacteria, In Vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. [Google Scholar] [CrossRef]
- Kilian, S.; Kritzinger, S.; Rycroft, C.; Gibson, G.; du Preez, J. The Effects of the Novel Bifidogenic Trisaccharide, Neokestose, on the Human Colonic Microbiota. World J. Microbiol. Biotechnol. 2002, 18, 637–644. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C. In Vitro Fermentation Profiles, Gas Production Rates, and Microbiota Modulation as Affected by Certain Fructans, Galactooligosaccharides, and Polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- Kaewarsar, E.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Peerajan, S.; Sirilun, S. Optimization of Mixed Inulin, Fructooligosaccharides, and Galactooligosaccharides as Prebiotics for Stimulation of Probiotics Growth and Function. Foods 2023, 12, 1591. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Bielecka, M. Prebiotic Effectiveness of Fructans of Different Degrees of Polymerization. Trends Food Sci. Technol. 2004, 15, 170–175. [Google Scholar] [CrossRef]
- Bourgot, C.L.; Fantino, M.; Respondek, F. Systematic Review of the Safety and Suitability of Dietary Supplementation with Short-Chain Fructo-Oligosaccharides in Infants and Young Children. Int. J. Food Sci. Nutr. 2020, 5, 90–98. [Google Scholar]
- Kato, T.; Kagawa, M.; Suda, W.; Tsuboi, Y.; Inoue-Suzuki, S.; Kikuchi, J.; Hattori, M.; Ohta, T.; Ohno, H. Integrated Multi-Omics Analysis Reveals Differential Effects of Fructo-Oligosaccharides (FOS) Supplementation on the Human Gut Ecosystem. Int. J. Mol. Sci. 2022, 23, 11728. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An Update on Prebiotics and on Their Health Effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Kulkarni, C.; Singh, P.; Sharma, S.; Singh, P.; Prajapati, G.; Gayen, J.R.; Ampapathi, R.S.; Mullick, A.; et al. A Prebiotic, Short-Chain Fructo-Oligosaccharides Promotes Peak Bone Mass and Maintains Bone Mass in Ovariectomized Rats by an Osteogenic Mechanism. Biomed. Pharmacother. 2020, 129, 110448. [Google Scholar] [CrossRef]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An Overview of the Recent Developments on Fructooligosaccharide Production and Applications. Food Bioprocess Tech. 2014, 7, 324–337. [Google Scholar] [CrossRef]
- Sakuma, K. Molecular Mechanism of the Effect of Fructooligosaccharides on Calcium Absorption. Biosci. Microflora 2002, 21, 13–20. [Google Scholar] [CrossRef]
- Xiao, J.; Metzler-Zebeli, B.; Zebeli, Q. Gut Function-Enhancing Properties and Metabolic Effects of Dietary Indigestible Sugars in Rodents and Rabbits. Nutrients 2015, 7, 8348–8365. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Fukushima, A.; Aizaki, Y.; Sakuma, K. Short-Chain Fatty Acids Increase the Level of Calbindin-D9k Messenger RNA in Caco-2 Cells. J. Nutr. Sci. Vitaminol. 2012, 58, 287–291. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, Y.; Qiu, Y.; Wu, Z.; Zhong, Z.; Zeng, X.; Zeng, Y.; Xiong, L.; Wen, Y.; Liu, R. Fructooligosaccharide Supplementation Alleviated the Pathological Immune Response and Prevented the Impairment of Intestinal Barrier in DSS-Induced Acute Colitis Mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef]
- Costa, G.T.; Vasconcelos, Q.D.J.S.; Aragão, G.F. Fructooligosaccharides on Inflammation, Immunomodulation, Oxidative Stress, and Gut Immune Response: A Systematic Review. Nutr. Rev. 2022, 80, 709–722. [Google Scholar] [CrossRef]
- Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10879. [Google Scholar] [CrossRef]
- Caetano, M.A.F.; Castelucci, P. Role of Short Chain Fatty Acids in Gut Health and Possible Therapeutic Approaches in Inflammatory Bowel Diseases. World J. Clin. Cases 2022, 10, 9985–10003. [Google Scholar] [CrossRef]
- Meksawan, K.; Chaotrakul, C.; Leeaphorn, N.; Gonlchanvit, S.; Eiam-Ong, S.; Kanjanabuch, T. Effects of Fructo-Oligosaccharide Supplementation on Constipation in Elderly Continuous Ambulatory Peritoneal Dialysis Patients. Perit. Dial. Int. 2016, 36, 60–66. [Google Scholar] [CrossRef]
- Pierre, F.; Perrin, P.; Champ, M.; Bornet, F.; Meflah, K.; Menanteau, J. Short-Chain Fructo-Oligosaccharides Reduce the Occurrence of Colon Tumors and Develop Gut-Associated Lymphoid Tissue in Min Mice. Cancer Res. 1997, 57, 225–228. [Google Scholar]
- Alvandi, E.; Wong, W.K.M.; Joglekar, M.V.; Spring, K.J.; Hardikar, A.A. Short-Chain Fatty Acid Concentrations in the Incidence and Risk-Stratification of Colorectal Cancer: A Systematic Review and Meta-Analysis. BMC Med. 2022, 20, 323. [Google Scholar] [CrossRef]
- Liu, G.; Tang, J.; Zhou, J.; Dong, M. Short-Chain Fatty Acids Play a Positive Role in Colorectal Cancer. Discov. Oncol. 2024, 15, 425. [Google Scholar] [CrossRef]
- Costa, G.T.; Guimarães, S.B.; Sampaio, H.A.d.C. Fructo-Oligosaccharide Effects on Blood Glucose: An Overview. Acta Cir. Bras. 2012, 27, 279–282. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Apper, E.; Blat, S.; Respondek, F. Fructo-Oligosaccharides and Glucose Homeostasis: A Systematic Review and Meta-Analysis in Animal Models. Nutr. Metab. 2018, 15, 9. [Google Scholar] [CrossRef]
- García, G.; Martínez, D.; Soto, J.; Rodríguez, L.; Nuez, M. Short-Chain Fructooligosaccharides Improve Gut Microbiota Composition in Patients with Type 2 Diabetes. A Randomized, Open-Label, Controlled Pilot Clinical Trial. J. Biotechnol. Biomed. 2023, 6, 244–258. [Google Scholar] [CrossRef]
- Iatcu, O.C.; Hamamah, S.; Covasa, M. Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes. Nutrients 2024, 16, 3447. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Silva, R.S.d.; Mendonça, I.P.; Paiva, I.H.R.d.; Souza, J.R.B.d.; Peixoto, C.A. Fructooligosaccharides and Galactooligosaccharides Improve Hepatic Steatosis via Gut Microbiota-Brain Axis Modulation. Int. J. Food Sci. Nutr. 2023, 74, 760–780. [Google Scholar] [CrossRef]
- Paiva, I.H.R.d.; Maciel, L.M.; da Silva, R.S.; Mendonça, I.P.; Souza, J.R.B.d.; Peixoto, C.A. Prebiotics Modulate the Microbiota–Gut–Brain Axis and Ameliorate Anxiety and Depression-like Behavior in HFD-Fed Mice. Food Res. Int. 2024, 182, 114153. [Google Scholar] [CrossRef]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose Promotes Satiety in Healthy Human: A Pilot Study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight Loss during Oligofructose Supplementation Is Associated with Decreased Ghrelin and Increased Peptide YY in Overweight and Obese Adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut Hormone Release and Appetite Regulation in Healthy Non-Obese Participants Following Oligofructose Intake. A Dose-Escalation Study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef]
- Hess, J.R.; Birkett, A.M.; Thomas, W.; Slavin, J.L. Effects of Short-Chain Fructooligosaccharides on Satiety Responses in Healthy Men and Women. Appetite 2011, 56, 128–134. [Google Scholar] [CrossRef]
- Ramos, R.A.A.; García, D.M.; Cruz, E.R.P. Inulin-Type Fructans: Effect on Gut Microbiota, Obesity and Satiety. Gac. Méd. Espirit. 2019, 21, 134–145. [Google Scholar]
- Kopczyńska, J.; Kowalczyk, M. The Potential of Short-Chain Fatty Acid Epigenetic Regulation in Chronic Low-Grade Inflammation and Obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef]
- Paredes, L.L.R.; Smiderle, F.R.; Santana-Filho, A.P.; Kimura, A.; Iacomini, M.; Sassaki, G.L. Yacon Fructans (Smallanthus sonchifolius) Extraction, Characterization and Activation of Macrophages to Phagocyte Yeast Cells. Int. J. Biol. Macromol. 2018, 108, 1074–1081. [Google Scholar] [CrossRef]
- Díaz, A.; García, M.A.; Dini, C. Jerusalem Artichoke Flour as Food Ingredient and as Source of Fructooligosaccharides and Inulin. J. Food Compos. Anal. 2022, 114, 104863. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Ellilä, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP Content by Bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Krausová, G.; Carneiro, J.W.P.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. A New Natural Source for Obtainment of Inulin and Fructo-Oligosaccharides from Industrial Waste of Stevia rebaudiana Bertoni. Food Chem. 2017, 225, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Dornez, E.; Van den Ende, W.; Delcour, J.A.; Courtin, C.M. Cereal Grain Fructans: Structure, Variability and Potential Health Effects. Trends Food Sci. Technol. 2015, 43, 32–42. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Isolation and Characterization of Inulin with a High Degree of Polymerization from Roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Aldrete-Herrera, P.I.; López, M.G.; Medina-Torres, L.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Ortiz-Basurto, R.I. Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods 2019, 8, 404. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, Degree of Polymerization Determination and Prebiotic Effect Evaluation of Inulin from Jerusalem Artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef]
- Shi, Y.; Si, D.; Zhang, X.; Chen, D.; Han, Z. Plant Fructans: Recent Advances in Metabolism, Evolution Aspects and Applications for Human Health. Curr. Res. Food Sci. 2023, 7, 100595. [Google Scholar] [CrossRef]
- Gholami, H.; Raouf Fard, F.; Saharkhiz, M.J.; Ghani, A. Yield and Physicochemical Properties of Inulin Obtained from Iranian Chicory Roots under Vermicompost and Humic Acid Treatments. Ind. Crops Prod. 2018, 123, 610–616. [Google Scholar] [CrossRef]
- Petkova, N.T.; Sherova, G.; Denev, P.P. Characterization of Inulin from Dahlia Tubers Isolated by Microwave and Ultrasound-Assisted Extractions. Int. Food Res. J. 2018, 5, 1876–1884. [Google Scholar]
- Ellem Fonseca Contado, W.N.d.; Estela, d.R.Q.; Denise, A.R.; Rodrigo, M.F.; Anderson, A.S.; Lucimara, N.S.B.; Adneia; Mariana Patto de Abreu, A.B.C.M. Extraction, Quantification and Degree of Polymerization of Yacon (Smallanthus sonchifolia) Fructans. Afr. J. Biotechnol. 2015, 14, 1783–1789. [Google Scholar] [CrossRef]
- Mendonça, C.M.N.; Oliveira, R.C.; Freire, R.K.B.; Piazentin, A.C.M.; Pereira, W.A.; Gudiña, E.J.; Evtuguin, D.V.; Converti, A.; Santos, J.H.P.M.; Nunes, C.; et al. Characterization of Levan Produced by a Paenibacillus sp. Isolated from Brazilian Crude Oil. Int. J. Biol. Macromol. 2021, 186, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Matsuhira, H.; Tamura, K.; Tamagake, H.; Sato, Y.; Anzai, H.; Yoshida, M. High Production of Plant Type Levan in Sugar Beet Transformed with Timothy (Phleum pratense) 6-SFT Genes. J. Biotechnol. 2014, 192, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, E.O.; Hayashi, A.H.; Torres, L.M.B.; Figueiredo-Ribeiro, R.C.L.; Shiomi, N.; de Sousa, F.S.; Lago, J.H.G.; Carvalho, M.A.M. Chemical Structure and Localization of Levan, the Predominant Fructan Type in Underground Systems of gomphrena marginata (Amaranthaceae). Front. Plant Sci. 2018, 9, 1745. [Google Scholar] [CrossRef]
- Verspreet, J.; Holmgaard Hansen, A.; Dornez, E.; Delcour, J.A.; Van den Ende, W.; Harrison, S.J.; Courtin, C.M. LC-MS Analysis Reveals the Presence of Graminan- and Neo-Type Fructans in Wheat Grains. J. Cereal Sci. 2015, 61, 133–138. [Google Scholar] [CrossRef]
- He, L.; Yan, B.; Yao, C.; Chen, X.; Li, L.; Wu, Y.; Song, Z.; Song, S.; Zhang, Z.; Luo, P. Oligosaccharides from Polygonatum cyrtonema Hua: Structural Characterization and Treatment of LPS-Induced Peritonitis in Mice. Carbohydr. Polym. 2021, 255, 117392. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, N.; Yamada, J.; Izawa, M. Isolation and Identification of Fructo-Oligosaccharides in Roots of Asparagus (Asparagus officinalis L.). Agric. Biol. Chem. 1976, 40, 567–575. [Google Scholar] [CrossRef]
- Aisara, J.; Wongputtisin, P.; Deejing, S.; Maneewong, C.; Unban, K.; Khanongnuch, C.; Kosma, P.; Blaukopf, M.; Kanpiengjai, A. Potential of Inulin-Fructooligosaccharides Extract Produced from Red Onion (Allium cepa Var. viviparum (Metz) Mansf.) as an Alternative Prebiotic Product. Plants 2021, 10, 2401. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Z.; Li, M.; Cao, B.; Li, J.; Jiang, J.; Liu, H.; Zhang, Q.; Zhang, S. TCP80-1, a New Levan-Neoseries Fructan from Tupistra Chinensis Baker Rhizomes Alleviates Ulcerative Colitis Induced by Dextran Sulfate Sodium in Drosophila Melanogaster Model. Food Res. Int. 2025, 203, 115860. [Google Scholar] [CrossRef]
- Livingston, D.P.; Chatterton, N.J.; Harrison, P.A. Structure and Quantity of Fructan Oligomers in Oat (Avena spp.). New Phytol. 1993, 123, 725–734. [Google Scholar] [CrossRef]
- Pavis, N.; Chatterton, N.J.; Harrison, P.A.; Baumgartner, S.; Praznik, W.; Boucaud, J.; Prud’homme, M.P. Structure of Fructans in Roots and Leaf Tissues of Lolium perenne. New Phytol. 2001, 150, 83–95. [Google Scholar] [CrossRef]
- García-Villalba, W.G.; Rodríguez-Herrera, R.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; López, M.G.; Gallegos-Infante, J.A.; Bermúdez-Quiñones, G.; González-Herrera, S.M. Comparative Study of Four Extraction Methods of Fructans (Agavins) from Agave durangensis: Heat Treatment, Ultrasound, Microwave and Simultaneous Ultrasound-Microwave. Food Chem. 2023, 415, 135767. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Xu, W.; Zhu, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Inulin and Its Enzymatic Production by Inulosucrase: Characteristics, Structural Features, Molecular Modifications and Applications. Biotechnol. Adv. 2019, 37, 306–318. [Google Scholar] [CrossRef]
- López, M.G.; Salomé-Abarca, L.F. The Agavins (Agave carbohydrates) Story. Carbohydr. Polym. 2024, 327, 121671. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Novel Fructan Exohydrolase: Unique Properties and Applications for Human Health. J. Exp. Bot. 2018, 69, 4227–4231. [Google Scholar] [CrossRef]
- Oku, S.; Ueno, K.; Sawazaki, Y.; Maeda, T.; Jitsuyama, Y.; Suzuki, T.; Onodera, S.; Fujino, K.; Shimura, H. Functional Characterization and Vacuolar Localization of Fructan Exohydrolase Derived from Onion (Allium cepa). J. Exp. Bot. 2022, 73, 4908–4922. [Google Scholar] [CrossRef]
- De Roover, J.; Van Laere, A.; Van den Ende, W. Effect of Defoliation on Fructan Pattern and Fructan Metabolizing Enzymes in Young Chicory Plants (Cichorium intybus). Physiol. Plant. 1999, 106, 158–163. [Google Scholar] [CrossRef]
- Degasperi, M.I.; Itaya, N.M.; Buckeridge, M.S.; Figueiredo-Ribeiro, R.D.C.L. Fructan Degradation and Hydrolytic Activity in Tuberous Roots of Viguiera Discolor Baker (Asteraceae), a Herbaceous Species from the Cerrado. Braz. J. Bot. 2003, 26, 11–21. [Google Scholar] [CrossRef]
- Xu, H.; Liang, M.; Xu, L.; Li, H.; Zhang, X.; Kang, J.; Zhao, Q.; Zhao, H. Cloning and Functional Characterization of Two Abiotic Stress-Responsive Jerusalem Artichoke (Helianthus tuberosus) Fructan 1-Exohydrolases (1-FEHs). Plant Mol. Biol. 2015, 87, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; Yoshida, M.; Clerens, S.; Vergauwen, R.; Kawakami, A. Cloning, Characterization and Functional Analysis of Novel 6-kestose Exohydrolases (6-KEHs) from Wheat (Triticum aestivum). New Phytol. 2005, 166, 917–932. [Google Scholar] [CrossRef]
- Valluru, R. Fructan and Hormone Connections. Front. Plant Sci. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Bonnett, G.D. Fructan Exohydrolase from Grasses. New Phytol. 1993, 123, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Krivorotova, T.; Sereikaite, J. Determination of Fructan Exohydrolase Activity in the Crude Extracts of Plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- Banguela, A.; Hernández, L. Fructans: From Natural Sources to Transgenic Plants. Biotecnol. Apl. 2006, 23, 202–210. [Google Scholar]
- UNIPROT Q8W4S6 INV6_ARATH. Available online: https://www.uniprot.org/uniprotkb/Q8W4S6/entry (accessed on 20 March 2025).
- Ueno, K.; Sonoda, T.; Yoshida, M.; Shiomi, N.; Onodera, S. Purification, Characterization, and Functional Analysis of a Novel 6G&1-FEH Mainly Hydrolyzing Neokestose from Asparagus. J. Exp. Bot. 2018, 69, 4295–4308. [Google Scholar] [CrossRef]
- van den Ende, W.; van Laere, A. Fructan Synthesizing and Degrading Activities in Chicory Roots (Cichorium intybus L.) during Field-Growth, Storage and Forcing. J. Plant Physiol. 1996, 149, 43–50. [Google Scholar] [CrossRef]
- Tamura, K.; Sanada, Y.; Tase, K.; Yoshida, M. Fructan Metabolism and Expression of Genes Coding Fructan Metabolic Enzymes during Cold Acclimation and Overwintering in Timothy (Phleum pratense). J. Plant Physiol. 2014, 171, 951–958. [Google Scholar] [CrossRef]
- Meguro-Maoka, A.; Yoshida, M. Analysis of Seasonal Expression Levels of Wheat Fructan Exohydrolase (FEH) Genes Regulating Fructan Metabolism Involved in Wintering Ability. J. Plant Physiol. 2016, 191, 54–62. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Correlation between Fructan Exohydrolase Activity and the Quality of Helianthus tuberosus L. Tubers. Agronomy 2018, 8, 184. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Eça, K.S.; Vieira, G.S.; Menegalli, F.C.; Martínez, J.; Hubinger, M.D. Prebiotic Oligosaccharides from Artichoke Industrial Waste: Evaluation of Different Extraction Methods. Ind. Crops Prod. 2015, 76, 141–148. [Google Scholar] [CrossRef]
- Tian, H.; Li, N.; Zhang, W.; Zhang, L. Method for Extracting and Refining Inulin. Patent CN102146144B, 4 July 2012. [Google Scholar]
- Pourfarzad, A.; Habibi Najafi, M.B.; Haddad Khodaparast, M.H.; Hassanzadeh Khayyat, M. Characterization of Fructan Extracted from Eremurus spectabilis Tubers: A Comparative Study on Different Technical Conditions. J. Food Sci. Technol. 2015, 52, 2657–2667. [Google Scholar] [CrossRef]
- de Marins, A.R.; Ribeiro, S.T.C.; de Oliveira, M.C.; Cardozo Filho, L.; de Oliveira, A.J.B.; Gonçalves, R.A.C.; Gomes, R.G.; Feihrmann, A.C. Effect of Extraction Methods on the Chemical, Structural, and Rheological Attributes of Fructan Derived from Arctium lappa L. Roots. Carbohydr. Polym. 2024, 324, 121525. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. Biomed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of Bioactive Carbohydrates from Artichoke (Cynara scolymus L.) External Bracts Using Microwave Assisted Extraction and Pressurized Liquid Extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef]
- Rivera, A.; Pozo, M.; Sánchez-Moreno, V.E.; Vera, E.; Jaramillo, L.I. Pulsed Electric Field-Assisted Extraction of Inulin from Ecuadorian Cabuya (Agave americana). Molecules 2024, 29, 3428. [Google Scholar] [CrossRef]
- Sánchez-Madrigal, M.Á.; Viesca-Nevárez, S.L.; Quintero-Ramos, A.; Amaya-Guerra, C.A.; Meléndez-Pizarro, C.O.; Contreras-Esquivel, J.C.; Talamás-Abbud, R. Optimization of the Enzyme-Assisted Extraction of Fructans from the Wild Sotol Plant (Dasylirion wheeleri). Food Biosci. 2018, 22, 59–68. [Google Scholar] [CrossRef]
- Demirci, K.; Zungur-Bastıoğlu, A.; Görgüç, A.; Bayraktar, B.; Yılmaz, S.; Yılmaz, F.M. Microwave Irradiation, Evolutionary Algorithm and Ultrafiltration Can Be Exploited in Process Intensification for High-Purity and Advanced Inulin Powder Production. Chem. Eng. Process.-Process Intensif. 2023, 194, 109565. [Google Scholar] [CrossRef]
- Shalini, R.; Krishna, J.; Sankaranarayanan, M.; Antony, U. Enhancement of Fructan Extraction from Garlic and Fructooligosaccharide Purification Using an Activated Charcoal Column. LWT 2021, 148, 111703. [Google Scholar] [CrossRef]
- Castro, C.C.; Nobre, C.; De Weireld, G.; Hantson, A.-L. Microbial Co-Culturing Strategies for Fructo-Oligosaccharide Production. New Biotechnol. 2019, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Fard, E.M.; Hosseininezhad, M.; Shoorideh, H. Identification and Characterization of Inulinases by Bioinformatics Analysis of Bacterial Glycoside Hydrolases Family 32 (GH32). Eng. Life Sci. 2023, 23, e2300003. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Chettri, D.; Verma, A.K. Microbes in Fructooligosaccharides Production. Bioresour. Technol. Rep. 2022, 20, 101159. [Google Scholar] [CrossRef]
- Won Yun, J.; Hyun Kim, D.; Woo Kim, B.; Koo Song, S. Comparison of Sugar Compositions between Inulo- and Fructo-Oligosaccharides Produced by Different Enzyme Forms. Biotechnol. Lett. 1997, 19, 553–556. [Google Scholar] [CrossRef]
- Van der Meulen, R.; Avonts, L.; De Vuyst, L. Short Fractions of Oligofructose Are Preferentially Metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 2004, 70, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Durieux, A.; Fougnies, C.; Jacobs, H.; Simon, J.-P. Metabolism of Chicory Fructooligosaccharides by Bifidobacteria. Biotechnol. Lett. 2001, 23, 1523–1527. [Google Scholar] [CrossRef]
- de la Rosa, O.; Pérez, A.M.; Paz, J.E.W.; Muñiz-Márquez, D.B.; Flores-Gallegos, A.C.; Aguilar, C.N. Microbial Production of Fructooligosaccharides. In Microbial Production of Food Bioactive Compounds; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–27. [Google Scholar]
- Chikkerur, J.; Samanta, A.K.; Kolte, A.P.; Dhali, A.; Roy, S. Production of Short Chain Fructo-Oligosaccharides from Inulin of Chicory Root Using Fungal Endoinulinase. Appl. Biochem. Biotechnol. 2020, 191, 695–715. [Google Scholar] [CrossRef]
- Huazano-García, A.; López, M.G. Enzymatic Hydrolysis of Agavins to Generate Branched Fructooligosaccharides (a-FOS). Appl. Biochem. Biotechnol. 2018, 184, 25–34. [Google Scholar] [CrossRef]
- Lekakarn, H.; Bunterngsook, B.; Jaikaew, P.; Kuantum, T.; Wansuksri, R.; Champreda, V. Functional Characterization of Recombinant Endo-Levanase (LevBk) from Bacillus koreensis HL12 on Short-Chain Levan-Type Fructooligosaccharides Production. Protein J. 2022, 41, 477–488. [Google Scholar] [CrossRef]
- Dahech, I.; Ayed, H.B.; Belghith, K.S.; Belghith, H.; Mejdoub, H. Microbial Production of Levanase for Specific Hydrolysis of Levan. Int. J. Biol. Macromol. 2013, 60, 128–133. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Ni, D.; Dai, Q.; Guang, C.; Zhang, T.; Mu, W. An Overview of Levan-Degrading Enzyme from Microbes. Appl. Microbiol. Biotechnol. 2019, 103, 7891–7902. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Charoenwongphaibun, C.; Wangpaiboon, K.; Panpetch, P.; Wanichacheva, N.; Pichyangkura, R. Endo- and Exo-Levanases from Bacillus Subtilis HM7: Catalytic Components, Synergistic Cooperation, and Application in Fructooligosaccharide Synthesis. Int. J. Biol. Macromol. 2024, 271, 132508. [Google Scholar] [CrossRef]
- Hang, H. Recent Advances on the Difructose Anhydride IV Preparation from Levan Conversion. Appl. Microbiol. Biotechnol. 2017, 101, 7477–7486. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, D.C.; Palai, T.; Agarwal, A.; Bhattacharya, P.K. Kinetics of Sucrose Conversion to Fructo-Oligosaccharides Using Enzyme (Invertase) under Free Condition. Bioprocess Biosyst. Eng. 2014, 37, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Levan versus Fructooligosaccharide Synthesis Using the Levansucrase from Zymomonas mobilis: Effect of Reaction Conditions. J. Mol. Catal. B Enzym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Park, H.-E.; Park, N.H.; Kim, M.-J.; Lee, T.H.; Lee, H.G.; Yang, J.-Y.; Cha, J. Enzymatic Synthesis of Fructosyl Oligosaccharides by Levansucrase from Microbacterium laevaniformans ATCC 15953. Enzyme Microb. Technol. 2003, 32, 820–827. [Google Scholar] [CrossRef]
- Bekers, M.; Laukevics, J.; Upite, D.; Kaminska, E.; Vigants, A.; Viesturs, U.; Pankova, L.; Danilevics, A. Fructooligosaccharide and Levan Producing Activity of Zymomonas mobilis Extracellular Levansucrase. Process Biochem. 2002, 38, 701–706. [Google Scholar] [CrossRef]
- Olarte-Avellaneda, S.; Rodríguez-López, A.; Patiño, J.D.; Alméciga-Díaz, C.J.; Sánchez, O.F. In Silico Analysis of the Structure of Fungal Fructooligosaccharides-Synthesizing Enzymes. Interdiscip. Sci. 2018, 10, 53–67. [Google Scholar] [CrossRef]
- Kanjanatanin, P.; Pichyangkura, R.; Sitthiyotha, T.; Charoenwongpaiboon, T.; Wangpaiboon, K.; Chunsrivirot, S. Computational Design of Bacillus Licheniformis RN-01 Levansucrase for Control of the Chain Length of Levan-Type Fructooligosaccharides. Int. J. Biol. Macromol. 2019, 140, 1239–1248. [Google Scholar] [CrossRef]
- Chu, J.; Tian, Y.; Li, Q.; Liu, G.; Yu, Q.; Jiang, T.; He, B. Engineering the β-Fructofuranosidase Fru6 with Promoted Transfructosylating Capacity for Fructooligosaccharide Production. J. Agric. Food Chem. 2022, 70, 9694–9702. [Google Scholar] [CrossRef]
- Nobre, C.; Alves Filho, E.G.; Fernandes, F.A.N.; Brito, E.S.; Rodrigues, S.; Teixeira, J.A.; Rodrigues, L.R. Production of Fructo-Oligosaccharides by Aspergillus ibericus and Their Chemical Characterization. LWT 2018, 89, 58–64. [Google Scholar] [CrossRef]
- Mao, W.; Han, Y.; Wang, X.; Zhao, X.; Chi, Z.; Chi, Z.; Liu, G. A New Engineered Endo-Inulinase with Improved Activity and Thermostability: Application in the Production of Prebiotic Fructo-Oligosaccharides from Inulin. Food Chem. 2019, 294, 293–301. [Google Scholar] [CrossRef]
- Wongsanittayarak, J.; Leangnim, N.; Unban, K.; Khanongnuch, C.; Lumyong, S.; Wongputtisin, P.; Kanpiengjai, A. Integrated Enzymatic Hydrolysis of Crude Red Onion Extract and Yeast Treatment for Production and Purification of Short-Chain Inulin and Inulin Neoseries Oligosaccharides. J. Agric. Food Res. 2024, 18, 101353. [Google Scholar] [CrossRef]
- Jaswal, A.S.; Elangovan, R.; Mishra, S. Fructooligosaccharides: Production by Recombinant Fructosyltransferase from Festuca arundinacea in a Continuous Reactor and Kinetic Modeling Profile. Carbohydr. Polym. Technol. Appl. 2024, 7, 100511. [Google Scholar] [CrossRef]
- Khatun, M.S.; Hassanpour, M.; Harrison, M.D.; Speight, R.E.; O’Hara, I.M.; Zhang, Z. Highly Efficient Production of Transfructosylating Enzymes Using Low-Cost Sugarcane Molasses by A. Pullulans FRR 5284. Bioresour. Bioprocess. 2021, 8, 48. [Google Scholar] [CrossRef]
- Elzairy, N.H.; Mostafa, F.A.; Abdel Wahab, W.A.; Ragab, Y.M.; Hashem, A.M.; Abdel-Naby, M.A. Enzymatic Synthesis of Biologically Active Fructose-Based Saccharides by Aspergillus niger MK788296 Levansucrase. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100408. [Google Scholar] [CrossRef]
- Polanía Melo, D.; Hernández Bravo, A.; Cruz, J.C.; Reyes, L.H. Invertase Immobilization on Magnetite Nanoparticles for Efficient Fructooligosaccharide Generation: A Comprehensive Kinetic Analysis and Reactor Design Strategy. ChemEngineering 2023, 7, 55. [Google Scholar] [CrossRef]
- Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Marszałek, K. The Application of Fructosyltransferase in Model Solutions to Reduce Sucrose Content and Synthesize Short-Chain Fructooligosaccharides. Food Biosci. 2024, 62, 105471. [Google Scholar] [CrossRef]
- Mao, S.; Liu, Y.; Yang, J.; Ma, X.; Zeng, F.; Zhang, Z.; Wang, S.; Han, H.; Qin, H.-M.; Lu, F. Cloning, Expression and Characterization of a Novel Fructosyltransferase from Aspergillus niger and Its Application in the Synthesis of Fructooligosaccharides. RSC Adv. 2019, 9, 23856–23863. [Google Scholar] [CrossRef]
- Coetzee, G.; van Rensburg, E.; Görgens, J.F. Evaluation of the Performance of an Engineered β-Fructofuranosidase from Aspergillus fijiensis to Produce Short-Chain Fructooligosaccharides from Industrial Sugar Streams. Biocatal. Agric. Biotechnol. 2020, 23, 101484. [Google Scholar] [CrossRef]
- Wan, X.; Wang, L.; Chang, J.; Zhang, J.; Zhang, Z.; Li, K.; Sun, G.; Liu, C.; Zhong, Y. Effective Synthesis of High-Content Fructooligosaccharides in Engineered Aspergillus niger. Microb. Cell Factories 2024, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zhao, N.; Wang, J.; Mchunu, N.P.; Permaul, K.; Singh, S.; Wang, Z. Boosting Fructosyl Transferase’s Thermostability and Catalytic Performance for Highly Efficient Fructooligosaccharides (FOS) Production. Foods 2024, 13, 2997. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Stefanov, S.; Armenova, N.; Petrova, P.; Petrov, K. Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept. Fermentation 2024, 10, 640. [Google Scholar] [CrossRef]
- Ko, H.; Bae, J.-H.; Sung, B.H.; Kim, M.-J.; Park, H.-J.; Sohn, J.-H. Microbial Production of Medium Chain Fructooligosaccharides by Recombinant Yeast Secreting Bacterial Inulosucrase. Enzym. Microb. Technol. 2019, 130, 109364. [Google Scholar] [CrossRef]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Márquez, D.; Contreras-Esquivel, J.C.; Teixeira, J.A.; Nobre, C.; Aguilar, C.N. Successive Fermentation of Aguamiel and Molasses by Aspergillus oryzae and Saccharomyces cerevisiae to Obtain High Purity Fructooligosaccharides. Foods 2022, 11, 1786. [Google Scholar] [CrossRef]
- Pérez, E.R.; Martínez, D.; Menéndez, C.; Alfonso, D.; Rodríguez, I.; Trujillo, L.E.; Sobrino, A.; Ramírez, R.; Pimentel, E.; Hernández, L. Fructooligosaccharides Production by Immobilized Pichia pastoris Cells Expressing Schedonorus arundinaceus Sucrose:Sucrose 1-Fructosyltransferase. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab036. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Maltos, D.A.F.; Aguilar, C.N.; Teixeira, J.A. Maximization of Fructooligosaccharides and β-Fructofuranosidase Production by Aspergillus japonicus under Solid-State Fermentation Conditions. Food Bioprocess Tech. 2013, 6, 2128–2134. [Google Scholar] [CrossRef]
- Ogué-Bon, E.; Khoo, C.; McCartney, A.L.; Gibson, G.R.; Rastall, R.A. In Vitro Effects of Synbiotic Fermentation on the Canine Faecal Microbiota. FEMS Microbiol. Ecol. 2010, 73, 587–600. [Google Scholar] [CrossRef]
| Property | Comments | Reference |
|---|---|---|
| Physicochemical | Gelling/binding features: These features relate to compounds that promote cohesion/cross-linking of liquids/small particles, leading to the formation of a solid-like structure. Although not major gelling agents, FOSs can interact with strong gelling agents, e.g., alginate, gelatin, starch, and methylcellulose, contributing to gel formation, and/or act as a binding agent, impacting structure/shape and texture. | [60,61,62] |
| Rheology: The incorporation of FOSs can impact the flow and deformation of materials under applied forces. Thus, due to its higher molecular weight, FOSs exhibit greater viscosity than sucrose, contributing to textural modifications of food products, such as bread and fruit juices. | [4,28,63,64] | |
| Stability: FOSs are stable under various temperatures and pH values, namely, within the normal pH range for foods (4.0 to 7.0). This feature enables FOSs to retain their probiotic features, enhance their shelf-life, and withstand acidic environments for targeted drug delivery. | [4,65,66,67] | |
| Solubility: FOSs are water soluble, around 80% at room temperature, which facilitates their incorporation in food products. | [4,30,68,69] | |
| Water binding: FOSs help to retain moisture, which can contribute to a softer texture of food products or other materials. | [63,70,71] | |
| Physiological | Non-cariogenic: This relates to substances that do not cause tooth decay (cavities). FOSs are not metabolized in the mouth by bacteria such as Streptococcus mutans, one of the primary microorganisms accountable for tooth decay. Such bacteria metabolize sugars from food, yielding acids that erode the enamel on teeth, leading to decay over time. Replacing sugars by FOSs thus prevents the production of cariogenic compounds, resulting in healthier foods. | [4,72] |
| Non-digestibility: the β-(2→1) osidic links of FOSs endure hydrolysis by human salivary and pancreatic enzymes of the host (specific for α-glycosidic bonds), hence reaching the colon undisturbed. | [7,49,73,74,75,76,77,78] | |
| Prebiotic effect: FOSs have been shown to modulate the gut microbiota, fostering the growth of beneficial microbial species, e.g., Bifidobacterium spp., Lactobacillus spp., and Faecalibacterium prausnitzii. | [29,33,43,49,75,79,80,81,82,83,84,85,86,87] | |
| Nutritional: This is related to the caloric value of foods, or the amount of energy produced when food is metabolized. The caloric value of FOSs, 1.0 to 1.7 kcal/g (4.2 to 7.1 kJ/g), compares favorably with that of dietary carbohydrates such as sucrose, glucose, and fructose, which have a caloric value of 3.9 kcal/g (16.3 kJ/g). Consuming such high caloric compounds without burning off excess calories ultimately results in fat accumulation. The incorporation of FOSs in food products contributes to a low caloric diet, hence to fat reduction. | [4,16,28,88,89,90] | |
| Sensory | Organoleptic: This is related to sensory-apprehended food properties. FOSs are mild sweeteners, with a sweetness intensity of 30 to 60% that of sucrose, and FOSs are used to enhance the taste and sweetness of foods and other products, while reducing the amount of sugar. | [4,17,28,63,91,92,93] |
| Health Advantage | Comments |
|---|---|
| Mineral absorption | The intake of FOSs is intended to improve mineral (e.g., Ca, Zn, Mg, and Zn) absorption, thus contributing to bone health and reducing the risk of fractures and osteoporosis [96,124,125]. The mechanisms underlying the role of FOSs include the following: enhanced solubility and absorption, given the lower pH in the colon due to SCFAs [77,126]. SCFAs can stimulate the intestinal epithelium and increase its absorptive capacity [127,128] and contribute to the increased expression of calbindin-D9k, a calcium binding protein [126,129]. However, a recent review found conflicting clinical study results, as not all works suggest that FOS intake significantly increases calcium absorption [79]. |
| Intestinal diseases | FOS supplementation has been found to alleviate the pathological immune response and prevent the impairment of the intestinal barrier in dextran sulfate sodium-induced acute colitis mice. This suggests that FOSs can help manage symptoms of inflammatory bowel disease by modulating gut microbiota and reducing inflammation [130]. FOSs can modulate inflammatory, oxidative, and immune activity in the gut, leading to a systemic response that improves overall health. Studies have shown that FOS supplementation can increase the number of Bifidobacterium spp. colonies, stimulate IgA secretion, and decrease proinflammatory cytokines [131]. SCFAs produced by the fermentation of FOSs play a critical role in regulating intestinal inflammation. These SCFAs, particularly butyrate, have immunomodulatory effects that can be used as a therapeutic approach in managing inflammatory bowel disease [132]. More specific details on the role of SCFAs in managing intestinal diseases can be obtained elsewhere [133]. |
| Constipation | In elderly continuous ambulatory peritoneal dialysis (CAPD) patients, a 20 g/day FOS supplementation for 30 days significantly increased bowel frequency, softened stools, and accelerated the colonic transit time. FOSs were well tolerated, with only mild side effects like bloating and flatulence. Unlike traditional laxatives, FOSs can be easily integrated into one’s diet. Although further studies were suggested, given the small sample dimension, FOSs appeared to present a promising, well-tolerated alternative for managing constipation in CAPD patients [134]. |
| Cancer | FOSs have been shown to induce a decrease in or even suppress colon tumor in animal models, which was tentatively ascribed to the stimulation of gut-associated lymphoid tissue due to the modulation of the colon microbiome [135]. A recent meta-analysis, which ultimately considered 17 case-control and 6 cross-sectional studies, established that the fecal concentrations of SCFAs, namely, acetic, propionic, and butyric acids, correlated inversely with both the risk and incidence of colorectal cancer [136]. Detailed insights into the positive impact of SCFAs in colorectal cancer can be found elsewhere [137]. |
| Diabetes | FOSs are safe for individuals with diabetes given their scarce digestibility [79,125]. A meta-analysis by Costa and co-workers in 2012 suggested that the consumption of FOSs has a beneficial influence on glucose metabolism. Controversies related to this are associated with unsatisfactory methodologies/a small number of individuals enrolled in studies [138]. This outcome was somehow corroborated by a meta-analysis using animal models that evidenced the beneficial contribution of FOSs in the reduction in circulating postprandial glucose and insulin concentrations [139]. Garcia and co-workers reported the positive impact of controlled FOS administration in the composition of gut microbiota of type 2 diabetes patients, namely, the increased growth of Bifidobacterium spp. and Lactobacillus spp. [140]. Still, in a more recent review, Iatcu and co-workers again highlighted inconsistent findings—FOSs either had no effect or a positive effect on glucose metabolism and insulin levels—and a lack of the reproducibility of positive results in animal models and in vitro studies and in vivo studies in humans [141]. Besides individual variability, discrepancies may be related to different dosages, durations of administration, and combinations with other prebiotics. |
| Neurodegenerative conditions | Studies in animal models showed that FOS supplementation ameliorated cognitive deficits and pathological changes caused by Alzheimer’s disease by regulating the gut microbiota–GLP-1/GLP-1R pathway [142]. |
| Liver diseases | Combined with galactooligosaccharides (GOSs), FOS supplementation proved effective in the treatment of individuals with steatotic liver disease associated with metabolic dysfunction and associated complications. Thus, insulin resistance, hyperglycemia, triglyceridemia, cholesterolemia, and IL-1β serum levels were reduced. Additionally, FOSs and GOSs modulated the lipogenic (SREBP-1c, ACC, and FAS) and lipolytic (ATGL) signaling pathways, reduced inflammatory markers, and enhanced the number of acetate-producing bacteria. Overall, the authors estimate that FOSs and GOSs mitigated this health condition by reducing the hepatic lipogenic pathways and the intestinal permeability through the gut microbiome–brain axis [143]. |
| Anxiety and depression | Using animal models, FOSs and GOSs were shown to reverse symptoms of anxiety and depression. This improvement resulted from an increase in acetate-producing bacteria and intestinal permeability, which lowered chronic peripheral and central inflammation. Moreover, FOSs and GOSs fostered a decrease in proinflammatory cytokines [144]. |
| Obesity | FOSs have been associated with the treatment and prevention of obesity [77,79,80]. This effect has been partly associated with their role in promoting satiety, thereby reducing meals and energy intake [145]; fostering weight loss and adjusting glucose regulation in overweight adults [146]; and suppressing hunger, albeit not significantly altering energy intake [147]. Despite some contradictory results, as Hess and co-workers reported a limited impact of FOS supplementation on acute satiety and energy intake [148], a systematic review by Ramos and co-workers suggests that FOSs influence satiety and lipid metabolism, namely through SCFAs produced during fermentation [149]. Inconsistencies may stem from individual variability, methodological details, dosages, and FOS compositions. Additionally, a recent review suggested that SCFAs exert epigenetic effects, potentially reversing metabolic and immune dysfunction caused by metabolic endotoxemia, thereby disrupting the cycle of obesity and inflammation [150]. |
| Plant | Titer (Average % in w/w) |
|---|---|
| Chicory roots | 22.9 |
| Candy leaf (Stevia rebaudiana) | 15.0 |
| Jerusalem artichoke tubers | 13.6 |
| Yacon | 13.2 |
| Garlic | 5.0 |
| Onions | 4.3 |
| Asparagus | 2.5 |
| Bananas | 2.5 |
| Wheat | 2.4 |
| Tomatoes | 1.8 |
| Barley | 0.2 |
| Fructan Type | Source and Degree of Polymerization (DP) |
|---|---|
| Inulin | Candy leaf, Stevia rubidian (Bert.) Bertoni (root): 1 = 28 [156] |
| Candy leaf, Stevia rebaudiana (Bert.) Bertoni (stem, two extracts): = 12 (inulin-rich extract); DP < 6 (FOS-rich extract), = 4.5 [154] | |
| Chicory, Cichorium intybus L.: = 28.67 [160] | |
| Dahlia, Dahlia decorative: 19 < < 23 [161] | |
| Jerusalem artichoke tubers, Helianthus tuberosus L.: ≈ 10 (0 days of flowering); ≈ 18 and DPmax 2 = 19 (50 days after flowering); ≈ 8 (80 days after flowering) [158] | |
| Yacon, Smallanthus sonchifolius: 3 < DP < 7 [162]; 2 < DP < 10 [151] | |
| Levan | Paenibacillus sp.: = 18 [163] 3 |
| Transformed sugar beet, Beta vulgaris L: PpFT1 transformants, DP > 40; PpFT2 transformants 3 < DP < 40; transformants were obtained with timothy (Phleum pratense) 6-SFT genes [164] | |
| Gomphrena marginata: DP ≈ 40 [165] | |
| Graminans | Wheat grains, Triticum aestivum L. var. Homeros: DP < 5 [166] |
| Polygonatum cyrtonema Hua: 5 < DP < 10 [167] | |
| Neo-inulin | Asparagus (Asparagus officinalis L.) [168], red onion (Allium cepa var. viviparum (Metz) Mansf.) [169]: DP = 3 (neokestose) |
| Neo-levan | Tupistra chinensis Baker rhizome; DP = 20 [170] |
| Oat {Avena spp.): DP = 4 (6G,6-kestotetraose) [171] | |
| Lolium perenne: DP = 8 [172] | |
| Agavin | Agave durangensis: DP > 10 [173] |
| Agave spp.: DP ≤ 9 (two- to four-year-old plants), DP ≤ 70 (10- to 12-year-old plants); A. salmiana spp. crassipina: DP ≤ 50; Agave tequilana variety cenizo: DP ≤ 70 [157] | |
| Unnamed, novel α-D-fructofuranosyl-(2→3)-β-d-fructofura- nosyl linkage | Dangshen, Codonopsis pilosula (roots, Radix Codonopsis): DP ≈ 9.4 × 103 (estimated) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, P. Fructooligosaccharides (FOSs): A Condensed Overview. Compounds 2025, 5, 8. https://doi.org/10.3390/compounds5020008
Fernandes P. Fructooligosaccharides (FOSs): A Condensed Overview. Compounds. 2025; 5(2):8. https://doi.org/10.3390/compounds5020008
Chicago/Turabian StyleFernandes, Pedro. 2025. "Fructooligosaccharides (FOSs): A Condensed Overview" Compounds 5, no. 2: 8. https://doi.org/10.3390/compounds5020008
APA StyleFernandes, P. (2025). Fructooligosaccharides (FOSs): A Condensed Overview. Compounds, 5(2), 8. https://doi.org/10.3390/compounds5020008







