Cyclograms Reveal Alteration of Inter-Joint Coordination during Gait in People with Multiple Sclerosis Minimally Disabled
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Data Acquisition and Processing
- The main spatio-temporal parameters of gait: speed, stride length, cadence, step width, stance, swing, and duration of double support phases.
- The flexion-extension angle for hip and knee joints and the dorsi-plantarflexion angle for the ankle joint for each of the 100 points in which the gait cycle was divided. Such data were employed to quantify the inter-joint coordination (as described in detail later) and to calculate the dynamic range of motion (dynamic ROM) as the difference between the maximum and minimum values assumed by each angle within the gait cycle.
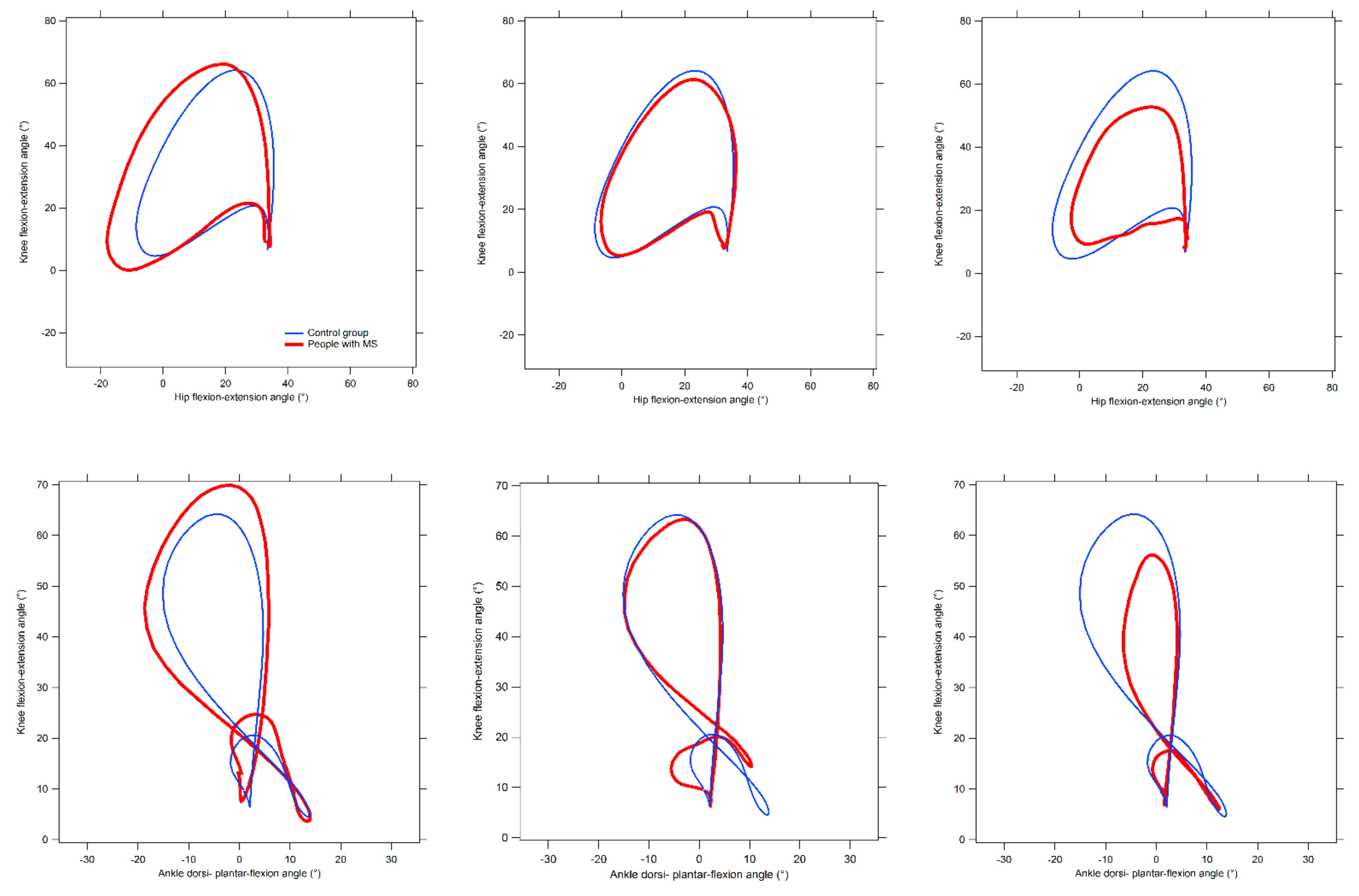
2.3. Quantification of Inter-Joint Coordination by Means of Cyclograms
- Cyclogram area (degrees2): the area of the closed trajectory described by the simultaneous angular variation that occurs at the two joints of interest during the gait cycle [46]. The interpretation of this parameter is quite straightforward, as larger areas are usually representative of higher conjoint range of angular movements experienced at a certain joint pair within a complete gait cycle [46,47].
- Cyclogram perimeter (degrees): the length of the trajectory previously described, which is typically expected to increase as the area increases. Thus, its interpretation is similar to that of the area. However, there are cases in which repeated abrupted angular variations (due to lack of coordination) originate relevant increases of the perimeter even without correspondent area changes [46].
- Cyclogram dimensionless ratio: this parameter, obtained by the ratio of the perimeter and the square root of the area, represents the shape of the diagram. Lower values indicate cyclograms of regular shape (i.e., not particularly elongated towards a specific direction).
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. General Considerations
4.2. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motl, R.W. Ambulation and multiple sclerosis. Phys. Med. Rehabil Clin. N. Am. 2013, 24, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Bass, B.; Rice, G.P.A.; Noseworthy, J.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study: II predictive value of the early clinical course. Brain 1989, 112, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Minden, S.L.; Hoaglin, D.C.; Hadden, L.; Frankel, D. Quality of life in people with multiple sclerosis: Data from the Sonya Slifka longitudinal multiple sclerosis study. J. Health Hum. Serv. Adm. 2007, 30, 233–267. [Google Scholar] [PubMed]
- LaRocca, N.G. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient 2011, 4, 189–201. [Google Scholar] [CrossRef]
- Gustavsen, S.; Olsson, A.; Søndergaard, H.B.; Andresen, S.R.; Sørensen, P.S.; Sellebjerg, F.; Oturai, A. The association of selected multiple sclerosis symptoms with disability and quality of life: A large Danish self-report survey. BMC Neurol. 2021, 21, 317. [Google Scholar] [CrossRef]
- Heesen, C.; Haase, R.; Melzig, S.; Poettgen, J.; Berghoff, M.; Paul, F.; Zettl, U.; Marziniak, M.; Angstwurm, K.; Kern, R.; et al. Perceptions on the value of bodily functions in multiple sclerosis. Acta Neurol. Scand. 2018, 137, 356–362. [Google Scholar] [CrossRef]
- Riemenschneider, M.; Hvid, L.G.; Stenager, E.; Dalgas, U. Is there an overlooked “window of opportunity” in MS exercise therapy? Perspectives for early MS rehabilitation. Mult. Scler. J. 2018, 24, 886–894. [Google Scholar] [CrossRef]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Coca-Tapia, M.; Cuesta-Gomez, A.; Molina-Rueda, F.; Carratalà-Tejada, M. Gait pattern in people with multiple sclerosis: A systematic review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef]
- Spain, R.I.; St. George, R.J.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Borduette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, L.A.C.; Teixeira, L.; Sabino, P.; Filho, H.A.; Alvarenga, R.M.P.; Thuler, L.C. Gait characteristics of multiple sclerosis patients in the absence of clinical disability. Disabil. Rehabil. 2013, 35, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Caggiari, S.; Mura, A.; Corona, F.; Leban, B.; Coghe, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Mult. Scler. Relat. Disord. 2016, 10, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Mandaresu, S.; Pilloni, G.; Porta, M.; Coghe, G.; Marrosu, M.G.; Cocco, E. Smoothness of gait detects early alterations of walking in persons with multiple sclerosis without disability. Gait Posture 2017, 58, 307–309. [Google Scholar] [CrossRef]
- Morel, E.; Allali, G.; Laidet, M.; Assal, F.; Lalive, P.H.; Armand, S. Gait Profile Score in multiple sclerosis patients with low disability. Gait Posture 2017, 51, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. 1999, 5, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, F.; Galea, M.P. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult. Scler. 2006, 12, 620–628. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Sandroff, B.M.; Motl, R.W. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef]
- Kalron, A.; Dvir, Z.; Givon, U.; Baransi, H.; Achiron, A. Gait and jogging parameters in people with minimally impaired multiple sclerosis. Gait Posture 2014, 39, 297–302. [Google Scholar] [CrossRef]
- Novotna, K.; Sobisek, L.; Horakova, D.; Havrdova, E.; Lizrova Preiningerova, J. Quantification of Gait Abnormalities in Healthy-Looking Multiple Sclerosis Patients (with Expanded Disability Status Scale 0-1.5). Eur. Neurol. 2016, 76, 99–104. [Google Scholar] [CrossRef]
- Liparoti, M.; Della Corte, M.; Rucco, R.; Sorrentino, P.; Sparaco, M.; Capuano, R.; Minino, R.; Lavorgna, L.; Agosti, V.; Sorrentino, G.; et al. Gait abnormalities in minimally disabled people with Multiple Sclerosis: A 3D-motion analysis study. Mult. Scler. Relat. Disord. 2019, 29, 100–107. [Google Scholar] [CrossRef]
- Müller, R.; Hamacher, D.; Hansen, S.; Oschman, P.; Keune, P.M. Wearable inertial sensors are highly sensitive in the detection of gait disturbances and fatigue at early stages of multiple sclerosis. BMC Neurol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Kaipust, J.P.; Huisinga, J.M.; Filipi, M.; Stergiou, N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Mot. Control 2012, 16, 229–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait variability and disability in multiple sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef]
- Kalron, A. Gait variability across the disability spectrum in people with multiple sclerosis. J. Neurol. Sci. 2016, 361, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huisinga, J.M.; Schmid, K.K.; Filipi, M.L.; Stergiou, N. Gait mechanics are different between healthy controls and patients with multiple sclerosis. J. Appl. Biomech. 2013, 29, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Basaglia, N. Evaluation of Clinical Gait Analysis parameters in patients affected by Multiple Sclerosis: Analysis of kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Khan, F.; Lee, P.S.V.; Galea, M.P. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult. Scler. 2016, 22, 1768–1776. [Google Scholar] [CrossRef]
- Filli, F.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling walking dysfunction in multiple sclerosis: Characterisation, classification and progression over time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef]
- Grieve, D.W. Gait patterns and the speed of walking. Bio-Med. Eng. 1968, 3, 119–122. [Google Scholar]
- Krasovsky, T.; Levin, M.F. Review: Toward a better understanding of coordination in healthy and poststroke gait. Neurorehabil. Neural. Repair 2010, 24, 213–224. [Google Scholar] [CrossRef]
- Longworth, L.A.; Chlosta, S.; Foucher, K.C. Inter-joint coordination of kinematics and kinetics before and after total hip arthroplasty compared to asymptomatic subjects. J. Biomech. 2018, 72, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.H.; Cho, J.; Kim, I.; Lee, J.; Jang, S.H. Effects of knee osteoarthritis severity on inter-joint coordination and gait variability as measured by hip-knee cyclograms. Sci. Rep. 2021, 11, 1789. [Google Scholar] [CrossRef] [PubMed]
- Field-Fote, E.C.; Tepavac, D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys. Ther. 2002, 82, 707–715. [Google Scholar] [CrossRef]
- Awai, L.; Curt, A. Intralimb coordination as a sensitive indicator of motor-control impairment after spinal cord injury. Front. Hum. Neurosci. 2014, 8, 148. [Google Scholar] [CrossRef]
- Lee, H.S.; Ryu, H.; Lee, S.; Cho, J.; You, S.; Park, J.H.; Jang, S. Analysis of gait characteristics using hip-knee cyclograms in patients with hemiplegic stroke. Sensors 2021, 21, 7685. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, M.; Watson, P.J.; Mohammadi, B. Intra-limb coordination in gait pattern in healthy people and multiple sclerosis patients. Clin. Kinesiol. 2013, 67, 32–38. [Google Scholar]
- Salehi, R.; Mofateh, R.; Mehravar, M.; Negahban, H.; Tajali, S.; Monjezi, S. Comparison of the lower limb inter-segmental coordination during walking between healthy controls and people with multiple sclerosis with and without fall history. Mult. Scler. Relat. Disord. 2020, 41, 102053. [Google Scholar] [CrossRef]
- Pau, M.; Leban, B.; Massa, D.; Porta, M.; Frau, J.; Coghe, G.; Cocco, E. Inter-joint coordination during gait in people with multiple sclerosis: A focus on the effect of disability. Mult. Scler. Relat. Disord. 2022, 60, 103741. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, L.; Kappos, L.; Lublin, F.D.; Metz, L.H.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the McDonald criteria. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Pau, M.; Coghe, G.; Atzeni, C.; Corona, F.; Pilloni, G.; Marrosu, M.G.; Cocco, E. Novel characterization of gait impairments in people with multiple sclerosis by means of the gait profile score. J. Neurol. Sci. 2014, 345, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Effect of spasticity on kinematics of gait and muscular activation in people with multiple sclerosis. J. Neurol. Sci. 2015, 358, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Quantitative assessment of the effects of 6 months of adapted physical activity on gait in people with multiple sclerosis: A randomized controlled trial. Disabil. Rehabil. 2018, 40, 144–151. [Google Scholar] [CrossRef]
- Pau, M.; Leban, B.; Deidda, M.; Putzolu, F.; Porta, M.; Coghe, G.; Cocco, E. Kinematic analysis of lower limb joint asymmetry during gait in people with multiple sclerosis. Symmetry 2021, 13, 598. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Hershler, C.; Milner, M. Angle-angle diagrams in the assessment of locomotion. Am. J. Phys. Med. 1980, 59, 109–125. [Google Scholar] [PubMed]
- Goswami, A. A new gait parameterization technique by means of cyclogram moments: Application to human slope walking. Gait Posture 1998, 8, 15–36. [Google Scholar] [CrossRef]
- Horak, F.B. Clinical assessment of balance disorders. Gait Posture 1997, 6, 76–84. [Google Scholar] [CrossRef]
- Preiningerova, J.L.; Novotna, K.; Rusz, J.; Sucha, L.; Ruzicka, E.; Havrdova, E. Spatial and temporal characteristics of Gait as outcome measures in multiple sclerosis (EDSS 0 to 6.5). J. NeuroEng. Rehabil. 2015, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Flegel, M.; Knox, K.; Nickel, D. Step-length variability in minimally disabled women with multiple sclerosis or clinically isolated syndrome. Int. J. MS Care 2012, 14, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Massot, C.; Guyot, M.; Donze, C.; Simoneau, E.; Gillet, C.; Leteneur, S. Ankle dysfunction in multiple sclerosis and the effects on walking. Disabil. Rehabil. 2021, 43, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Checchia, G.A.; Giannone, F.; Miccoli, B.; Cantafora, N.; Gazzi, A. Isokinetic testing of muscular function and fatigue in patients with multiple sclerosis. Isokinet. Exerc. Sci. 1993, 3, 101–110. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Krekels, M.; Grevendonk, L.; Eijnde, B.O. Multiple sclerosis affects skeletal muscle characteristics. PLoS ONE 2014, 9, e108158. [Google Scholar] [CrossRef] [PubMed]
- Sieljacks, P.S.; Søberg, C.A.; Michelsen, A.; Dalgas, U.; Hvid, L.G. Lower extremity muscle strength across the adult lifespan in multiple sclerosis: Implications for walking and stair climbing capacity. Exp. Gerontol. 2020, 139, 111025. [Google Scholar] [CrossRef] [PubMed]
- Bregman, D.J.J.; Van Der Krogt, V.; De Groot, V.; Harlaar, J.; Wisse, M.M.; Collins, S.H. The effect of ankle foot orthosis stiffness on the energy cost of walking: A simulation study. Clin. Biomech. 2011, 26, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Kempen, J.C.E.; Doorenbosch, C.A.M.; Knol, D.L.; de Groot, V.; Beckerman, H. Newly identified gait patterns in patients with multiple sclerosis may be related to push-off quality. Phys. Ther. 2016, 96, 1744–1752. [Google Scholar] [CrossRef] [Green Version]
- Lôbo Corrêa, P.L.; Sisterolli Diniz, D.S.; Diniz Carneiro, M.A. Biomechanical analysis of gait disturbances in just diagnosed multiple sclerosis:A case series. Mult. Scler. 2016, 22, 84. [Google Scholar] [CrossRef]
- Cofré Lizama, L.E.; Bastani, A.; van der Walt, A.; Kilpatrick, T.; Khan, F.; Galea, M.P. Increased ankle muscle coactivation in the early stages of multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320905870. [Google Scholar] [CrossRef] [Green Version]
- Ohle, L.; Ellenberger, D.; Flachenecker, P.; Friede, T.; Haas, J.; Hellwig, K. Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS registry. Sci. Rep. 2021, 11, 13340. [Google Scholar] [CrossRef]
- Lukšys, D.; Jatužis, D.; Jonaitis, G.; Griškevičius, J. Application of continuous relative phase analysis for differentiation of gait in neurodegenerative disease. Biomed. Signal Process. Control 2021, 67, 102558. [Google Scholar] [CrossRef]

| Healthy Controls | Multiple Sclerosis | |
|---|---|---|
| Participants (M, F) | 27 (17 F, 10 M) | 27 (17 F, 10 M) |
| Age (years) | 41.6 (10.9) | 40.5 (7.0) |
| Body mass (kg) | 63.3 (12.0) | 66.2 (11.8) |
| Height (cm) | 166.4 (8.9) | 167.3 (8.7) |
| Type of MS | - | 27 RR |
| Time since diagnosis (years) | - | 6.8 (6.1) |
| EDSS | - | 1.4 (0.5) |
| Healthy Controls | Multiple Sclerosis | |
|---|---|---|
| Gait speed (m s−1) | 1.17 (0.15) | 1.17 (0.16) |
| Stride length (m) | 1.25 (0.08) | 1.20 (0.13) |
| Cadence (steps min−1) | 111.7 (10.1) | 114.2 (7.2) |
| Step width (m) | 0.19 (0.03) | 0.19 (0.03) |
| Stance phase (% of the gait cycle) | 59.19 (2.29) | 59.60 (2.51) |
| Swing phase (% of the gait cycle) | 39.78 (1.85) | 40.10 (2.81) |
| Double support (% of the gait cycle) | 20.85 (3.89) | 20.48 (3.49) |
| Healthy Controls | Multiple Sclerosis | |
|---|---|---|
| Hip ROM (degrees) | 45.2 (3.7) | 44.0 (4.9) |
| Knee ROM (degrees) | 60.7 (3.4) | 57.9 (5.1) a |
| Ankle ROM (degrees) | 31.1 (5.0) | 27.8 (6.9) a |
| Joint Couple | Parameter | Healthy Controls | Multiple Sclerosis |
|---|---|---|---|
| Hip–Knee | Cyclogram Area | 1746.94 (246.21) | 1568.20 (293.73) a |
| Cyclogram Perimeter | 192.85 (12.16) | 185.42 (17.18) a | |
| Dimensionless Ratio | 4.64 (0.38) | 4.72 (0.43) | |
| Knee–Ankle | Cyclogram Area | 789.29 (260.09) | 647.95 (260.23) |
| Cyclogram Perimeter | 186.76 (14.58) | 170.46 (19.22) a | |
| Dimensionless Ratio | 6.83 (0.80) | 7.97 (5.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, M.; Leban, B.; Porta, M.; Frau, J.; Coghe, G.; Cocco, E. Cyclograms Reveal Alteration of Inter-Joint Coordination during Gait in People with Multiple Sclerosis Minimally Disabled. Biomechanics 2022, 2, 331-341. https://doi.org/10.3390/biomechanics2030026
Pau M, Leban B, Porta M, Frau J, Coghe G, Cocco E. Cyclograms Reveal Alteration of Inter-Joint Coordination during Gait in People with Multiple Sclerosis Minimally Disabled. Biomechanics. 2022; 2(3):331-341. https://doi.org/10.3390/biomechanics2030026
Chicago/Turabian StylePau, Massimiliano, Bruno Leban, Micaela Porta, Jessica Frau, Giancarlo Coghe, and Eleonora Cocco. 2022. "Cyclograms Reveal Alteration of Inter-Joint Coordination during Gait in People with Multiple Sclerosis Minimally Disabled" Biomechanics 2, no. 3: 331-341. https://doi.org/10.3390/biomechanics2030026
APA StylePau, M., Leban, B., Porta, M., Frau, J., Coghe, G., & Cocco, E. (2022). Cyclograms Reveal Alteration of Inter-Joint Coordination during Gait in People with Multiple Sclerosis Minimally Disabled. Biomechanics, 2(3), 331-341. https://doi.org/10.3390/biomechanics2030026







