3D-Printed Encapsulation of Thin-Film Transducers for Reliable Force Measurement in Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
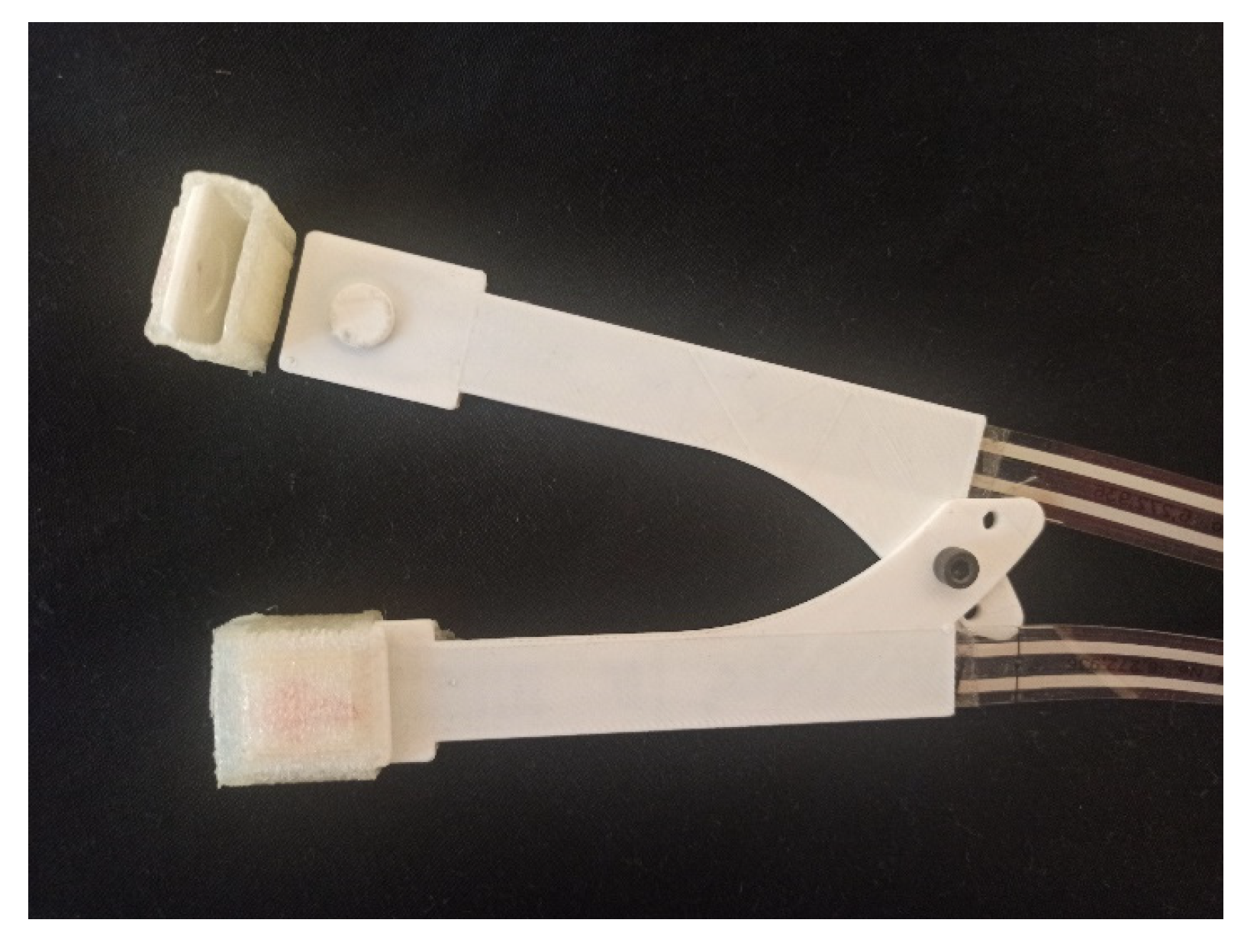
2.1. Thin-Film Sensors
2.2. 3D Printing
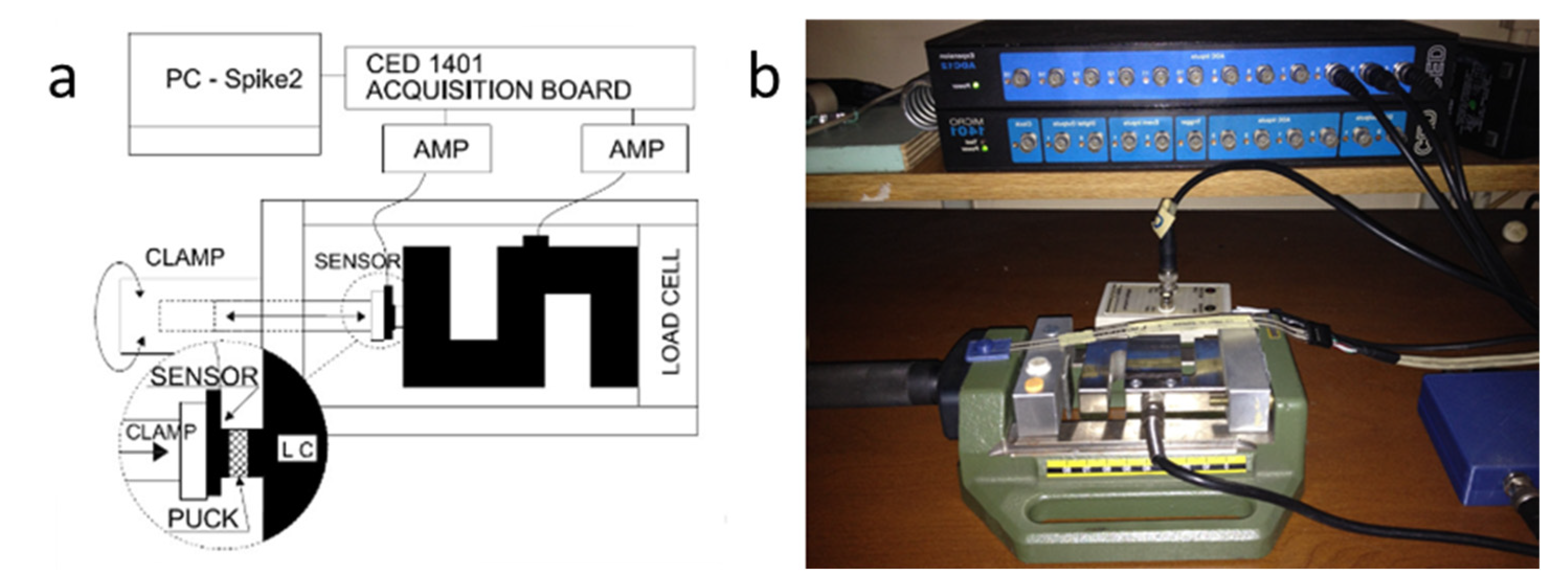
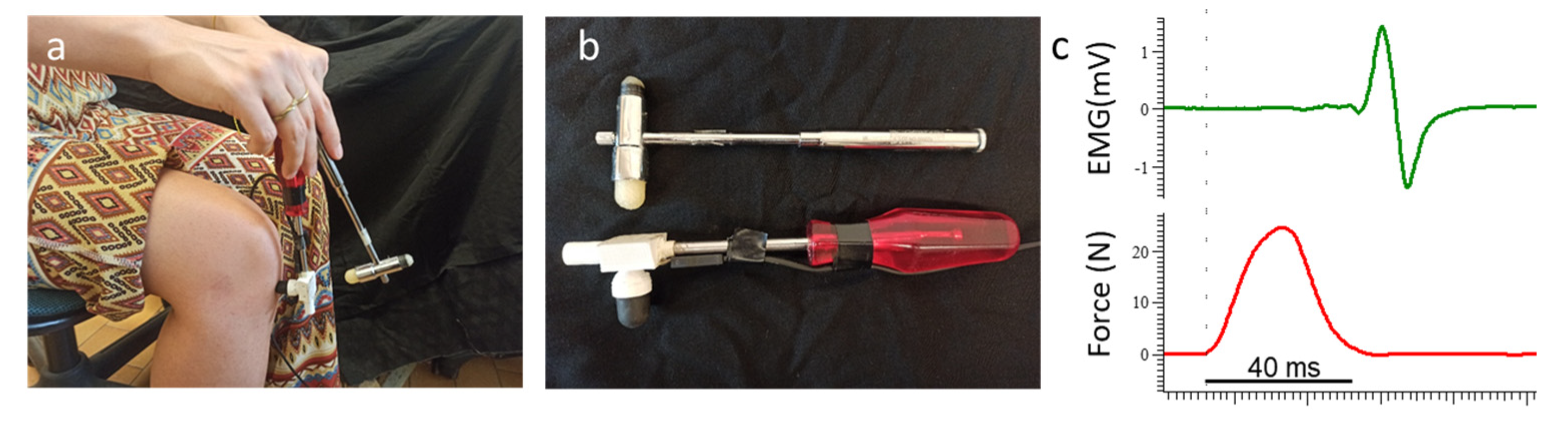
2.3. Experimental Set-Up
2.4. Experimental Protocol
2.5. Analysis and Statistics
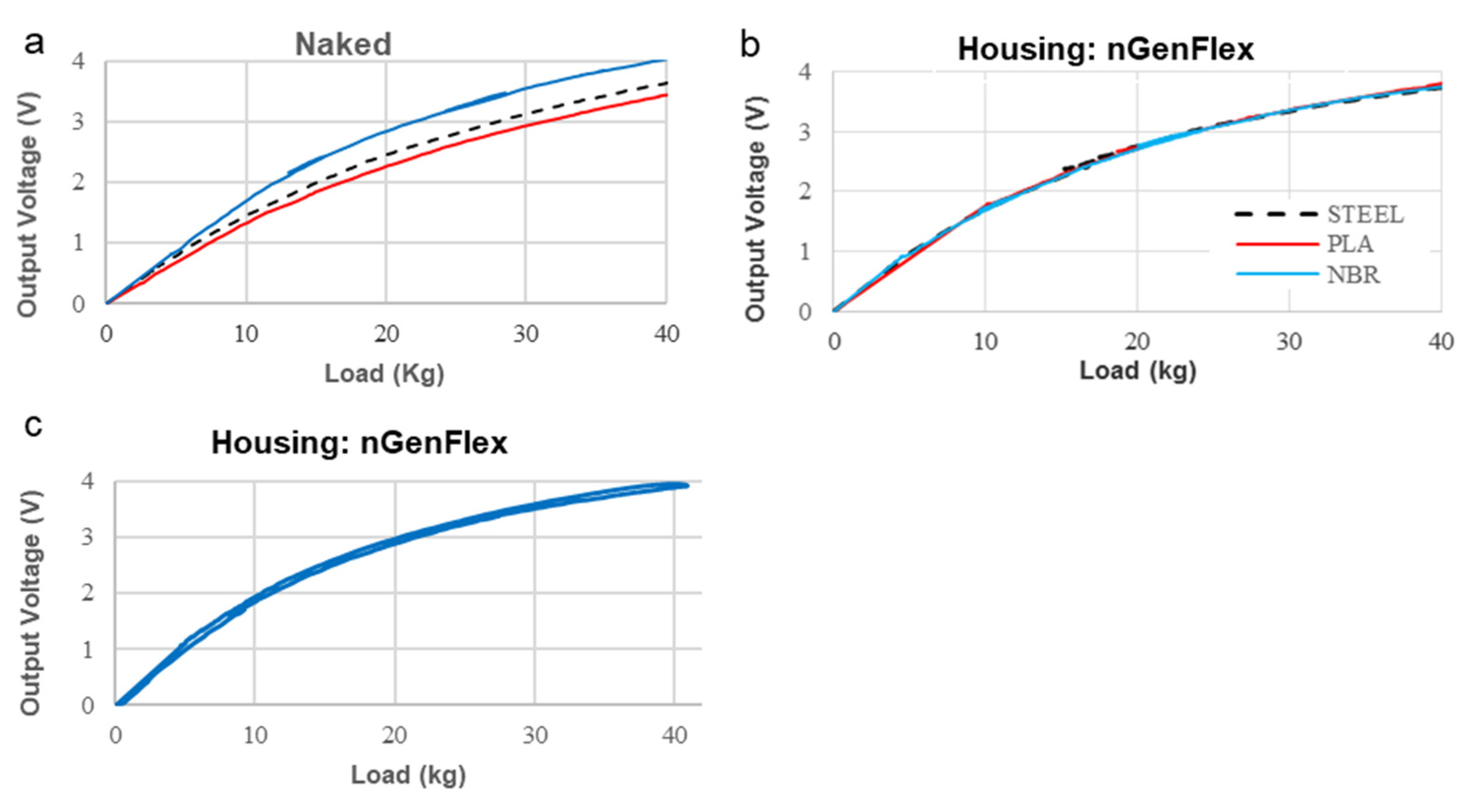
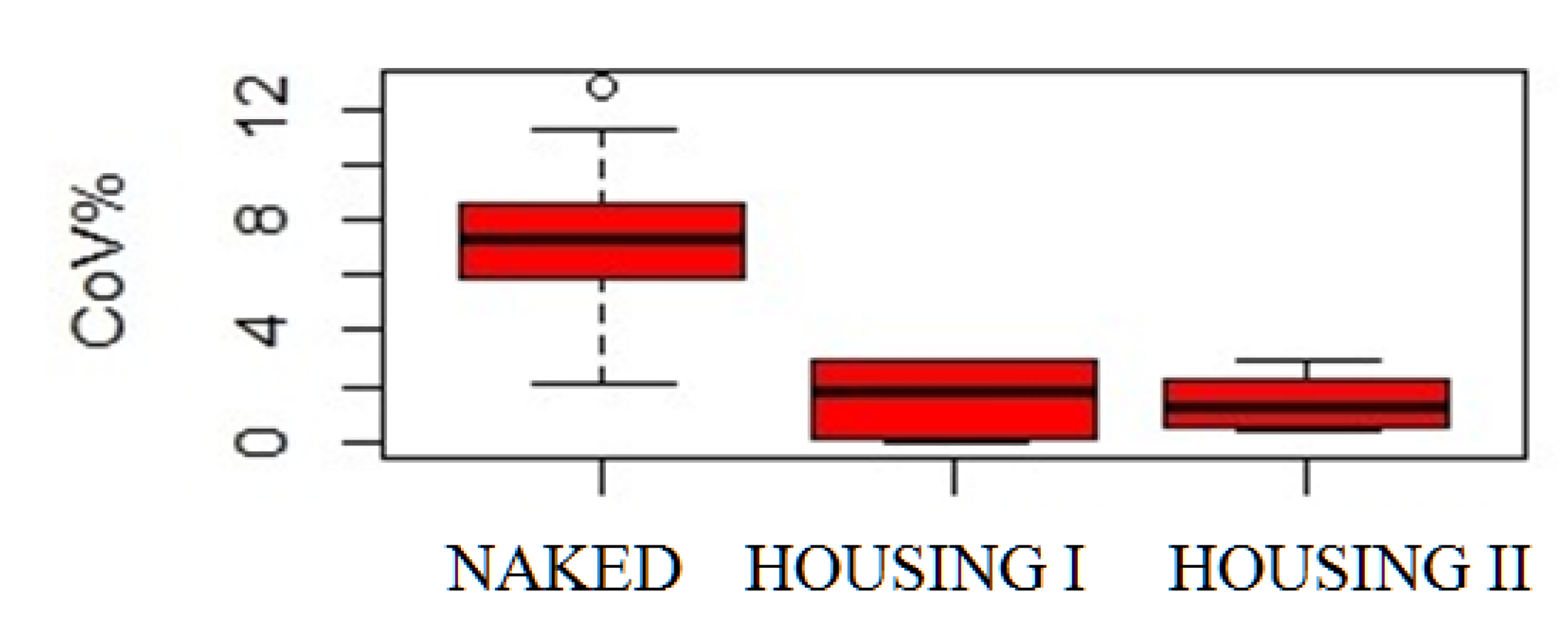
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botter, A.; Vieira, T.M.; Geri, T.; Roatta, S. The peripheral origin of tap-induced muscle contraction revealed by multi-electrode surface electromyography in human vastus medialis. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berwin, J.T.; Burton, T.M.W.; Taylor, J.; McGregor, A.H.; Roche, A. Plantar Loading Forces While Walking in a Below-Knee Cast with an Attached Loadbearing Frame. Foot Ankle Int. 2015, 36, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Kwok, Y.L.P.; Li, Y.P.; Lao, T.T.H.M.; Zhang, X.; Dai, X.Q. Objective Evaluation of Skin Pressure Distribution of Graduated Elastic Compression Stockings. Dermatol. Surg. 2005, 31, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.P.; Mendes, J.; Cerqueira, J.; Moreira, A.; Vasconcelos, M.; Ferreira, A.P.; Amarante, J.M. Integrating piezoresistive sensors on the embouchure analysis of the lower lip in single reed instrumentalists: Implementation of the lip pressure appliance (LPA). Clin. Exp. Dent. Res. 2019, 5, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, S.; Hotaling, J.M.; Kathrins, M.; Baftiri, A.P.; Freels, S.; Niederberger, C.S. A novel method to determine perineal artery occlusion among male bicyclists. PeerJ 2015, 3, e1477. [Google Scholar] [CrossRef] [PubMed]
- Testa, M.; Geri, T.; Signori, A.; Roatta, S. Visual Feedback of Bilateral Bite Force to Assess Motor Control of the Mandible in Isometric Condition. Mot. Control. 2015, 19, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Testa, M.; Rolando, M.; Roatta, S. Control of jaw-clenching forces in dentate subjects. J. Orofac. Pain 2011, 25, 250–260. [Google Scholar] [PubMed]
- Flanagan, D.; Ilies, H.; O’Brien, B.; McManus, A.; Larrow, B. Jaw Bite Force Measurement Device. J. Oral Implant. 2012, 38, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Weisskircher, H.W. Maximum bilateral masticatory forces in patients with and without pain. Zeitschrift für Kraniomandibuläre Funktion 2016, 5, 19–39. [Google Scholar]
- Testa, M.; Di Marco, A.; Pertusio, R.; Van Roy, P.; Cattrysse, E.; Roatta, S. A validation study of a new instrument for low cost bite force measurement. J. Electromyogr. Kinesiol. 2016, 30, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Likitlersuang, J.; Leineweber, M.J.; Andrysek, J. Evaluating and improving the performance of thin film force sensors within body and device interfaces. Med. Eng. Phys. 2017, 48, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Tekscan. FlexiForce® Sensors User Manual. Tekscan Inc., 2016; pp. 1–15. Available online: https://www.tekscan.com/sites/default/files/resources/FlexiForce%20Sensors%20RevK.pdf (accessed on 25 February 2023).
- Tekscan. Best Practices in Electrical Integration of the Flexi Force TM Sensor. Available online: https://www.tekscan.com/sites/default/files/BP%20-%20Electrical_Integration_FINAL_081817.pdf (accessed on 25 February 2023).
- Tekscan. Best Practices in Mechanical Integration of the Flexi Force TM Sensor. Available online: https://www.tekscan.com/sites/default/files/FLX-Best-Practice-Mechanical-Integration-RevB.pdf (accessed on 25 February 2023).
- Nilam Ram Multilevel Model with Heterogeneous Variance. Pennsylvania State University. Available online: https://quantdev.ssri.psu.edu/tutorials/analysis-experience-sampling-ema-data-chapter-6-multilevel-model-heterogeneous-variance (accessed on 25 February 2023).
- Valentim, A.F.; Furlan, R.M.M.M.; Perilo, T.V.D.C.; Berbert, M.C.B.; Motta, A.R.; Casas, E.L. Evaluation of the force applied by the tongue and lip on the maxillary central incisor tooth. Codas 2014, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Prusa Face Shield Disinfection. Available online: https://help.prusa3d.com/en/article/prusa-face-shield-disinfection_125457 (accessed on 25 February 2023).


| Acronym | Material | Used for | Young Modulus (MPa) |
|---|---|---|---|
| PLA | Polylactic Acid | 3D Housing/Puck | 2500–3500 |
| nGen_Flex | Polymer Amphora Flex FL6000 | 3D Housing | 100–150 |
| ABS | Acrylonitrile–Butadiene–Styrene | 3D Housing | 1500–2500 |
| Steel | Steel | Puck | 200,000 |
| NBR | Nitrile Butadiene Rubber | Puck | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertusio, R.; Roatta, S. 3D-Printed Encapsulation of Thin-Film Transducers for Reliable Force Measurement in Biomedical Applications. Biomechanics 2023, 3, 115-123. https://doi.org/10.3390/biomechanics3010011
Pertusio R, Roatta S. 3D-Printed Encapsulation of Thin-Film Transducers for Reliable Force Measurement in Biomedical Applications. Biomechanics. 2023; 3(1):115-123. https://doi.org/10.3390/biomechanics3010011
Chicago/Turabian StylePertusio, Raffaele, and Silvestro Roatta. 2023. "3D-Printed Encapsulation of Thin-Film Transducers for Reliable Force Measurement in Biomedical Applications" Biomechanics 3, no. 1: 115-123. https://doi.org/10.3390/biomechanics3010011
APA StylePertusio, R., & Roatta, S. (2023). 3D-Printed Encapsulation of Thin-Film Transducers for Reliable Force Measurement in Biomedical Applications. Biomechanics, 3(1), 115-123. https://doi.org/10.3390/biomechanics3010011







