Abstract
Transcatheter aortic valve replacement (TAVR) has become the preferred treatment for patients with aortic stenosis (AS) at high surgical risk. However, TAVR is challenging in patients with a pre-existing mitral valve prosthesis, such as a transcatheter mitral valve replacement (TMVR), due to the likelihood of device interference. This study explores the feasibility and safety of performing TAVR in a patient with a pre-existing TMVR procedure using 3D printing, augmented reality (AR) and computational simulations to optimize preprocedural planning. Computational modeling allowed predictions of the spatial relationship between the TAVR and TMVR devices. The simulation output was therefore used as input for augmented visualization of the device interference. The 3D printing of an anatomical replica was used to physically simulate the procedure, ensuring that no significant interference would occur during heart function. The results demonstrated a safe distance of 6.4 mm between the TAVR and TMVR devices, and no functional interference was observed during simulated cardiac cycles. The use of AR in the operating room enhanced the understanding of device positioning, offering a new dimension of precision of the complex cardiovascular intervention. This study concludes that integrating AR, 3D printing, and computational simulations into preprocedural planning for high-risk structural intervention can significantly improve procedural outcomes by enhancing accuracy, safety, and operator confidence.
1. Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as the primary therapeutic approach for patients with aortic stenosis (AS) who are deemed high-risk open chest surgery. The minimally invasive nature of TAVR and the relatively low recovery period have positioned TAVR as the preferred alternative to surgical aortic valve replacement in elderly patients with multiple comorbidities [1]. However, the procedural complexity of TAVR increases significantly in individuals who have previously undergone valve interventions, such as transcatheter mitral valve replacement (TMVR). In such cases, the close anatomical proximity and potential interference between the aortic and mitral valve prostheses present considerable challenges or complications such as left ventricular outflow tract obstruction, valve malpositioning, or compromised function of one or both prosthetic valves.
Although scant data are available on TAVR in the context of pre-existing mitral prosthesis [2], the feasibility of TAVR in such cases relies heavily on pre-operative diagnostic imaging and stringent patient screening. The close anatomical proximity of the aortic and mitral valves suggests a significant risk of physical interference between the prostheses, potentially compromising valve function or leading to procedural failure. While standard imaging modalities, such as echocardiography and computed tomography (CT), provide essential data for preprocedural evaluation, these imaging modalities often lack the predictive precision needed to fully assess potential device interference during TAVR in patients with pre-existing TMVR. Emerging computational technologies offer promising advancements in enhancing preprocedural planning as well as in improving the education and training of interventional healthcare professionals. Recent advancements in computational modeling, augmented reality (AR), and 3D printing technologies present promising solutions to address challenging structural heart interventions [3,4,5,6]. The 3D model was created according to the ISO/ASTM 52900:2015 standard, which defines the principles and guidelines for additive manufacturing processes and specifically for thermoplastic polymers [7]. Computational simulations facilitate the development of patient-specific models derived from imaging data, enabling predictive assessment of the prosthesis positioning with the human host. AR further enhances visualization by providing a detailed view of anatomical structures and the spatial relationships between devices during the procedure. In parallel, 3D-printed replicas of the patient’s anatomy offer a tangible model to support preprocedural planning and surgical rehearsal prior to TAVR.
This study explores the integration of AR, computational simulations, and 3D printing to evaluate the feasibility of performing TAVR in a patient with a pre-existing TMVR. By simulating the procedure both virtually and physically, the study aims to assess the potential for device interference on TAVR in the context of pre-existing TMVR. The findings could have significant implications for the management of high-risk patients with multiple valve prostheses, potentially enhancing the precision and safety of future interventions.
2. Materials and Methods
2.1. Case Presentation
The case of an 84-year-old gentleman was referred to ISMETT IRCCS hospital due to severe aortic valve stenosis. The patient complex medical condition included a previous transcatheter mitral valve-in-valve replacement, which was deemed necessary to treat multiple episodes of mitral valve failure. The TMVR procedure was performed using the 29 mm SAPIEN 3 Ultra (S3) device, Edwards Lifescience, Irwine, CA, USA, which was implanted within a failed Carpentier-Edwards porcine bioprosthesis. Three years after the TMVR procedure, TAVR was also deemed necessary to treat aortic valve stenosis within the context of multiple morbidities and high surgical risk. Due to the presence of the mitral valve prosthesis, careful planning was essential to avoid any interference between the TMVR and TAVR devices. Pre-dilation of the aortic valve was conducted with a 25 mm balloon, which was followed by the successful insertion of a 34 mm self-expandable Evolut FX (EvoFX) prosthesis via transfemoral access. The procedure was completed with minimal paravalvular leak and no signs of functional compromise, but detailed preprocedural planning was critical to ensuring the success of the intervention. Brachial cuff pressure measures and contrast-enhanced angio-CT imaging were performed upon hospital admission, while Doppler echocardiography assessed the function of the stenotic aortic valve.
2.2. Patient-Specific Computational TAVI Modeling
The heart anatomy and failed TMVR bioprosthesis were reconstructed from end-diastole angio-CT scans. Segmentation of the left heart, ascending aorta, and Carpentier valve was performed using semi-automatic thresholding, followed by manual editing and smoothing. The segmented components were subsequently meshed using ICEM meshing software (v2021, ANSYS Inc., Canosburg, PA, United States) following a convergence analysis. The aortic wall was meshed with triangular shell elements (S3R) at a size of 0.9 mm, while the left ventricle and the failed valve were meshed with tetrahedron solid elements (C3D4) at a size of 0.5 mm. The 29 mm S3 Ultra stent frame and 34 mm self-expandable EvoFX were meshed with structured hexahedral elements (C3D8) with a size of 0.1 mm as described in a previous study [8].
A linear–elastic material model was employed to simulate the biomechanical response of the aortic wall with Poisson’s ratio set at ν = 0.475 and a Young’s modulus of 0.15 MPa, as estimated from inverse analysis [9]. The S3 Ultra prosthesis was modeled using chrome–cobalt alloy using bilinear elastoplastic material model whilst the EvoFX prosthesis was modeled using a superelastic Nitinol alloy [8,10]. Table 1 summarizes the material properties and element mesh.

Table 1.
Material parameters used for patient-specific models and bioprosthesis; E = Young modulus; ν = Poisson coefficient; C10 = material constant; D1 = incompressibility factor; σy = yield stress; σult = ultimate tensile stress; εp = plastic strain; D = density.
Structural heart interventions were simulated using the ABAQUS/Explicit finite element solver (v.2021hf7, Dassault Systèmes, Paris, France), as previously described [11]. In brief, the TMVR procedure involved deploying the S3 device within the failed Carpentier-Edwards bioprosthesis. The S3 Ultra was crimped under frictionless contact conditions using a cylindrical surface that was gradually moved radially to achieve a final diameter of 8 mm. Following this, the device was positioned within the mitral annulus and expanded by radially displacing a cylindrical surface representing the balloon wall. This deployment served as the base for the subsequent TAVR simulation, wherein the 34 mm self-expanding EvoFX prosthesis was implanted in the aortic annulus. The self-expandable prosthesis was released gradually by retracting the catheter sleeve toward the distal ascending aorta while entering in contact with the aortic annulus and ascending aorta during the simulation procedure. The general ABAQUS contact algorithm was employed to account for interactions between the prostheses and the surrounding anatomical structures, whilst three simulation steps were used to deploy heart valves (26 h using a 60 core AMD EPYC cpu). The distal ends of the aortic wall were constrained in the longitudinal direction using cylindrical coordinate systems whilst tie contact constraints were applied to connect the failed bioprosthesis to the left ventricular wall.
2.3. Augmented Reality
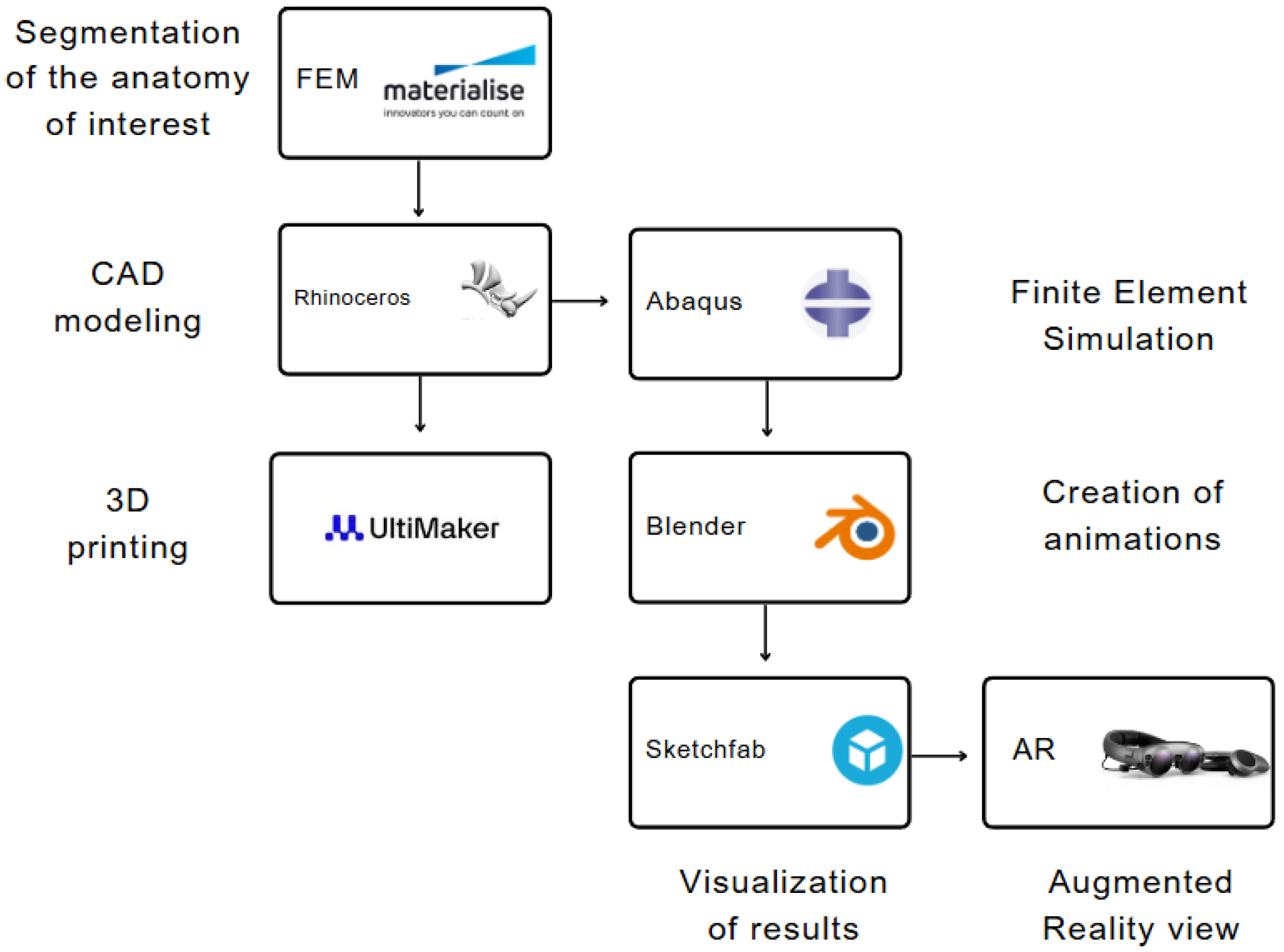
Following the computational simulation, the deformed anatomical structures and prostheses were exported at various simulation time frames and imported into Blender software, V3.5 (Blender Foundation BV, Amsterdam, Netherlands). Blender was utilized to color each component and generate the animation (see Figure 1). Specifically, the heart chambers were merged into a single shape to unify the model color whilst the prostheses remained unchanged to assign specific colors to distinguish them from the other anatomical parts. All geometric components were incorporated into the animation command with each simulation time frame generating five animation frames in Blender to slow down the overall animation. The Sketchfab platform (Sketchfab Inc., New York City, NY, United States) was used to upload the animation file exported from Blender. Finally, the AR content was loaded into MagicLeap AR glasses, enabling the surgical team to explore the spatial positioning of the devices in real time, providing an immersive and interactive experience to better anticipate potential challenges during the actual procedure.
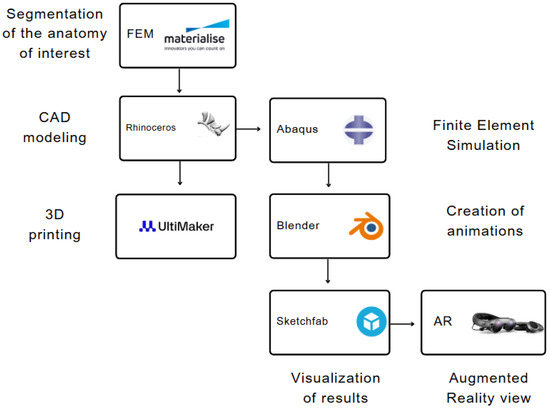
Figure 1.
Design and process workflow diagram to generate 3D model and augmented reality view.
2.4. Three-Dimensional (3D) Printing
Three-dimensional (3D) printing involved the replication of the ascending aorta and left ventricle, including the TMVR prosthesis. The replicas were fabricated using a soft biocompatible material to mimic the heart compliance. Specifically, fused deposition modeling (FDM) with TPU filament was employed to create the phantom model, utilizing a layer thickness of 0.15 mm (see Table 2). Prior to 3D printing, the segmented masks were edited in Rhinoceros CAD software (Rhinoceros v.7, McNeel & Associates, Seattle, WA, USA) to develop an open ventricle model and thus removing the apex to facilitate visual exploration. Following manufacturing, a demonstrative EvoFX device was manually implanted in the phantom model to interpret the device interference.

Table 2.
Control parameters for FDM 3D printing of TPU model.
3. Results
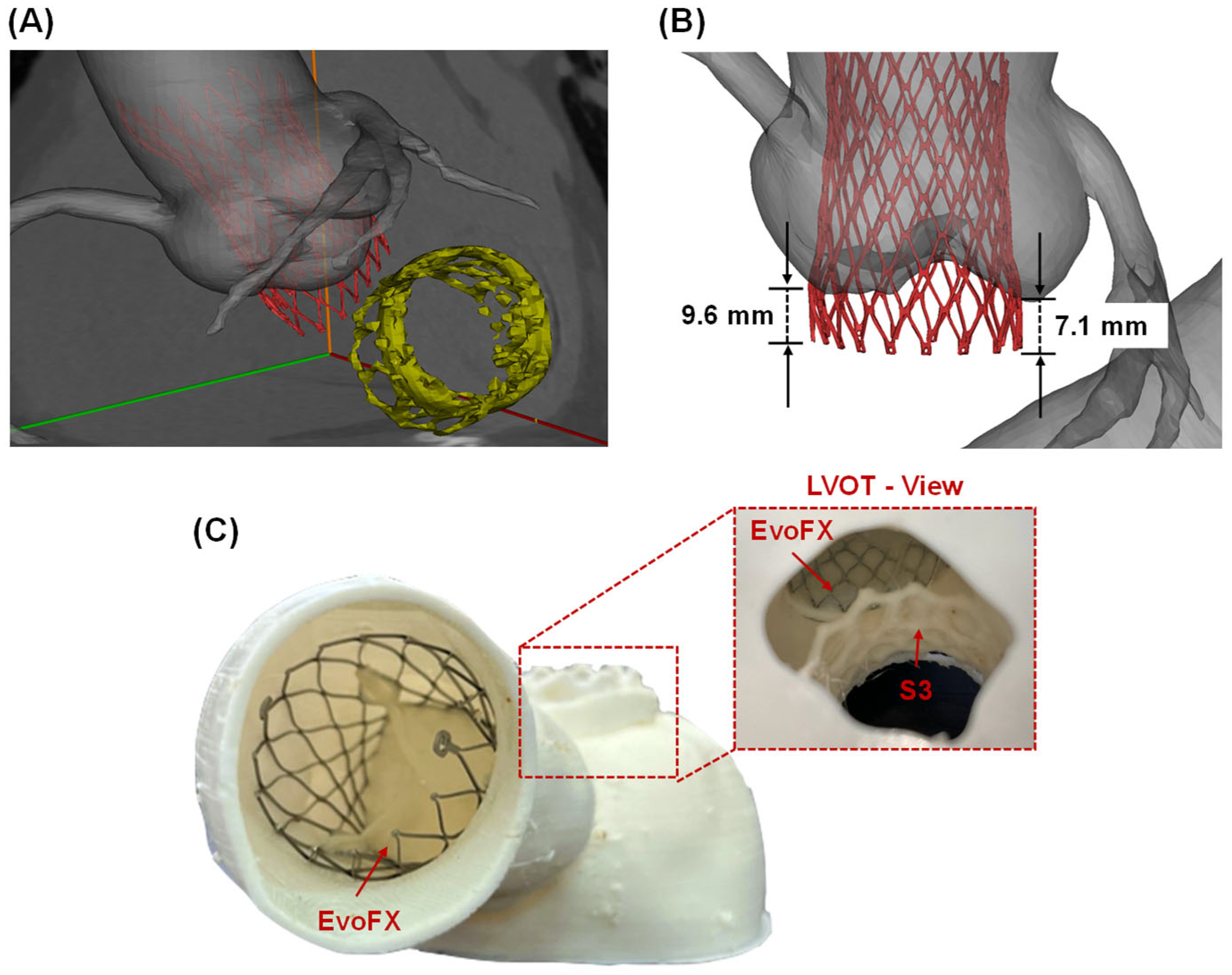
The simulated deployment of the TAVR procedure using the self-expandable EvoFX prosthesis was superimposed on the post-TMVR angio CT scan (Figure 2A). These simulations provided valuable insights into the spatial relationships between the TMVR and TAVR stent frames. The distance from the ventricular edge of the TMVR device to the lower edge of the TAVR stent frame was measured at 6.4 mm, indicating a safe margin to prevent direct interference among prostheses. Furthermore, the extension of the Evolut FX device into the left ventricular outflow tract ranged from 7.1 to 9.6 mm (Figure 2B).
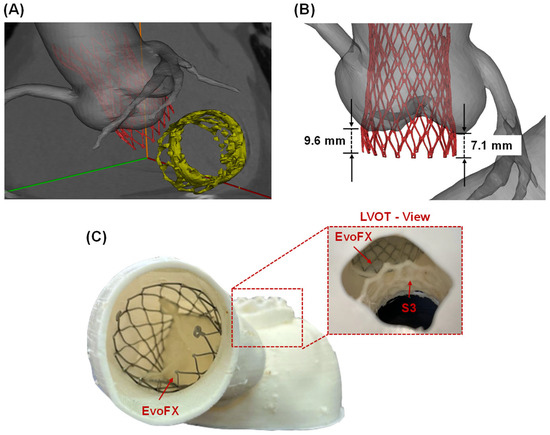
Figure 2.
(A) Computational modeling predictions of TAVI after TMVR superimposed on the ECG-gated images; (B) measurements of EvoFX stent frame extension on the native left ventricular outflow tract (LVOT); (C) 3D=printed replica with the TAVI device and view from the LVOT to see the device-related proximity.
These findings were further corroborated by visually assessing the space between the TAVR and TMVR stent frames in the 3D-printed phantom (Figure 2C). The physical model confirmed that the devices could coexist without direct interference, and the flexible material used for the 3D print facilitated a realistic simulation of procedural implantation.
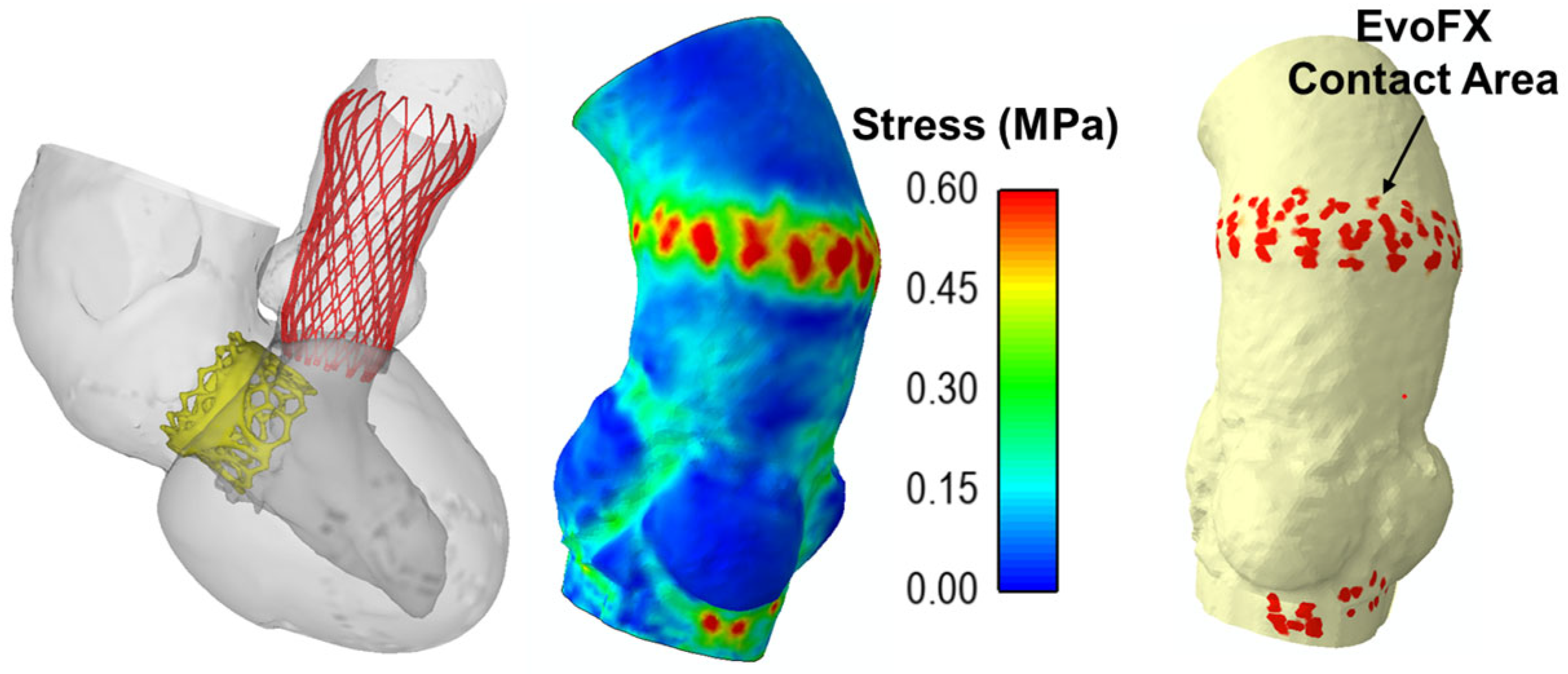
Figure 3 shows the maximum principal stress distribution on the aortic wall and the contact areas between the EvoFX device and the aortic wall. The stress concentrated primarily at the sites of device anchoring, and this may cause tissue damage. The contact area observed at the anchoring points of the EvoFX within the aorta serves as a potential indicator of device migration risk, providing critical insights into the stability and long-term positioning of the implantation procedure. There was no significant mechanical interaction between the TAVR and TMVR prostheses during simulated heartbeats, indicating that functional interference should not occur (Figure 3).
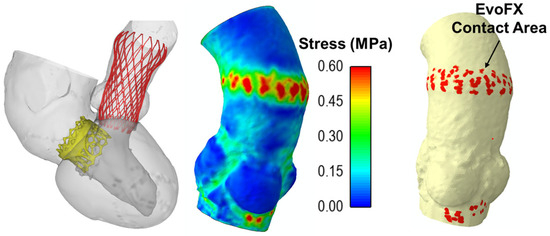
Figure 3.
Final deformed shape from the simulation including the stress map on the ascending aorta and contact area of 34 mm EvoFX device.
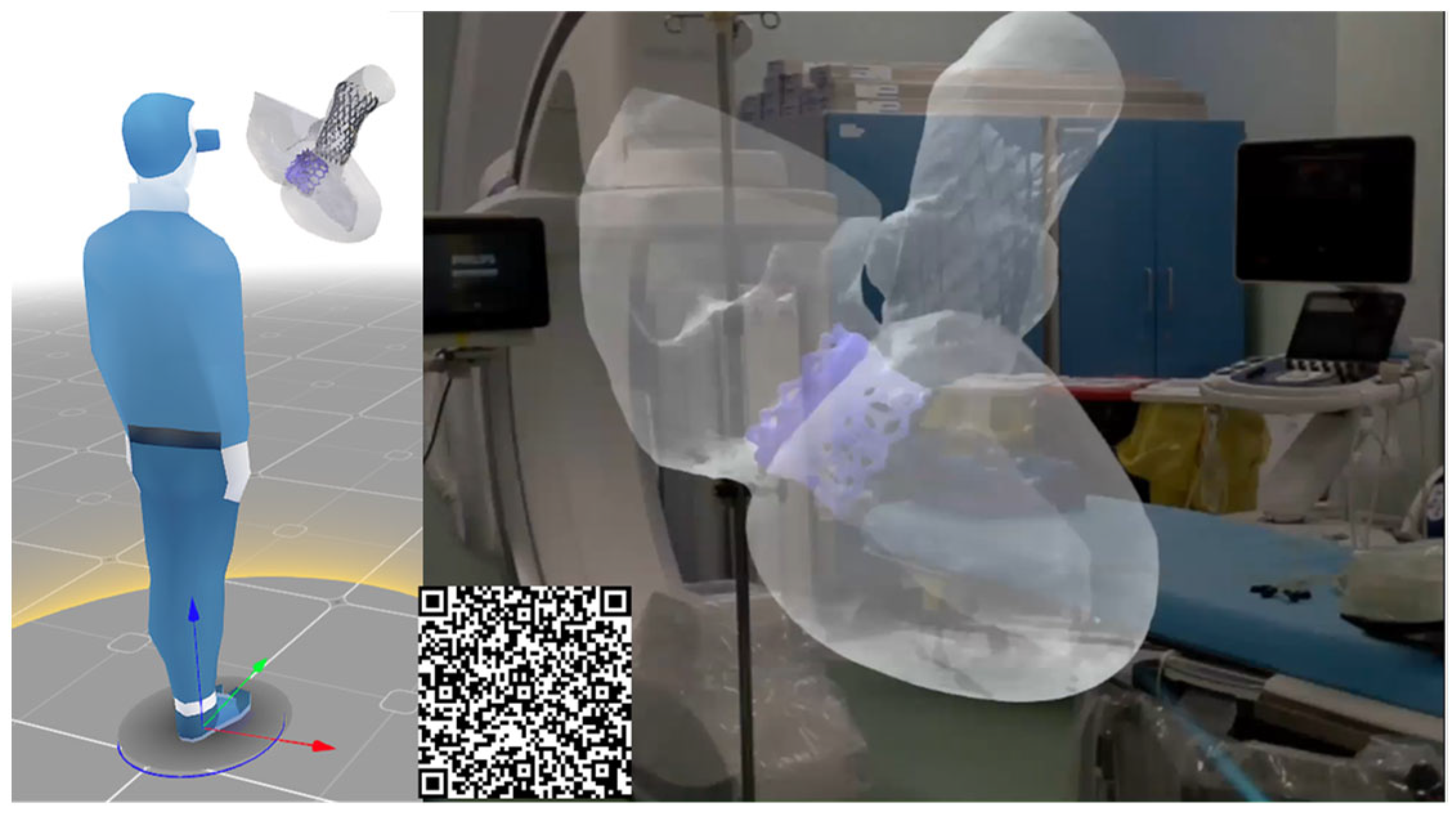
Once the feasibility of TAVR in the context of pre-existing TMVR was assessed, the surgical team utilized AR glasses to visualize the TAVR simulation procedure in a more intuitive manner, enhancing confidence in this complex structural heart intervention (Figure 4). The integration of computational modeling and AR can improve the post-processing of computational results in an immersive environment by offering improved spatial understanding, intuitive interaction, and mobility, which are not achievable with traditional 2D screen-based visualizations.
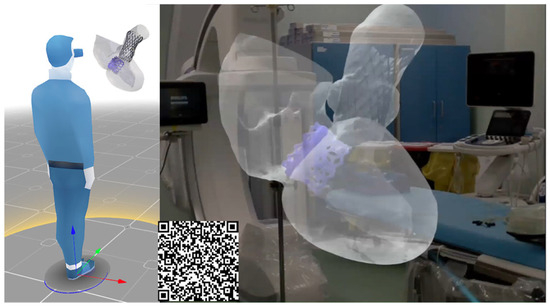
Figure 4.
AR images of the digital models during the TAVI procedure; (D) final deformed shape from the simulation including the stress map on the ascending aorta and contact area of 34 mm EvoFX device.
4. Discussion
This study demonstrates the value of utilizing advanced computational tools, augmented visualization and 3D printing in preprocedural planning for high-risk cardiac interventions, particularly in cases involving multiple prosthetic device implantation. The case presented insight into the complexities of performing TAVR in the context of a pre-existing TMVR, where the risk of device interference is substantial. When implanting a TAVR device in the presence of a pre-existing TMVR, evaluating procedural factors such as device implantation depth, device size, and controlled valve release is crucial for the success of the intervention. Simulations allowed for quantification of the distance between the TAVR and TMVR prostheses, ensuring that the 34 mm EvoFX stent frame achieved optimal implantation within the range of 7.1 to 9.6 mm without interference from the pre-existing TMVR prosthesis. The surgical team reported that the 3D-printed replica provided invaluable tactile feedback, significantly enhancing their confidence in the success of the planned procedure. The AR system effectively mirrored the positioning and deployment of the devices, reinforcing the confidence and precision of the intervention.
The combination of dynamic and morphological risk factors, along with the anatomical complexity of the landing zone adjacent to a pre-existing device in the mitral position, indicates the need for careful preprocedural imaging to ensure successful outcomes in patients undergoing multiple structural heart interventions [12,13]. This typically involves echocardiographic evaluation in conjunction with cardiac CT imaging. However, these imaging techniques may not provide the spatial resolution or predictive capabilities required to ensure safe and effective device placement in such challenging cases. The integration of computational modeling, 3D printing and augmented reality may prove indispensable for enhancing the management of high-risk patients undergoing TAVR in a pre-existing TMVR procedure. Computational simulations enabled not only the prediction of no direct or functional interference among prostheses but also quantified the stress distribution and dynamic implication of two devices during heart beating. The incorporation of AR in this study provided an additional layer of precision and confidence, allowing the surgical team to explore the spatial relationships between the devices prior to the TAVR procedure. Moreover, the utilization of AR enhanced the post-processing of computational results within an immersive environment by offering improved spatial understanding, intuitive interaction, and mobility, which are not achievable with traditional 2D screen-based visualization. The 3D-printed replica allowed for the accurate development of a model representing the patient’s left ventricle, aorta and implanted devices in the aortic valve, providing the physician with a direct interpretation of the case.
Critical considerations such as the space between the mitral and candidate TAVR prostheses, risk of deformation or distortion of the device, device embolization, and interaction among devices are paramount when performing TAVR in the setting of a pre-existing mitral valve prosthesis. While limited studies have documented the feasibility of TAVR in previous mitral valve prostheses [2,12,13], imaging remains an essential tool for preprocedural screening. For instance, Vavuranakis et al. [14] suggested a distance of 4 mm from the device ventricular edge when using the CoreValve to ensure safe deployment without compromising the function of the mitral bioprosthesis. In the modern era of structural heart diseases, the collaboration between interventional cardiologists and biomedical engineers has the potential to improve the management of cardiovascular interventions. As in silico and augmented reality continues to evolve, these technologies hold promise for further enhancing procedural precision, patient safety, and overall treatment efficacy in managing complex cardiac anatomies. This study serves as a preliminary case so that it is not yet feasible to quantify the collective impact of these technologies. Future investigations should prioritize rigorous evaluation to quantify their potential to improve clinical outcomes and practices. Such studies should employ standardized metrics and a large patient cohort to robustly assess the efficacy of these approaches.
5. Conclusions
This study demonstrates the feasibility and safety of integrating augmented reality, computational simulations and 3D printing in the preprocedural planning of TAVR in a patient with pre-existing TMVR. These technologies provide enhanced visualization, accurate prediction of device interference, and improved procedural confidence prior to the structural heart intervention. These findings highlight the potential for multidisciplinary approaches to enhance the precision and safety of complex cardiovascular interventions, paving the way for improved patient outcomes in high-risk cases.
Author Contributions
Conceptualization, C.G.; methodology, R.S., C.C. and E.C.; writing—original draft preparation, S.P. and S.C.; writing—review and editing, S.P. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Edwards Lifescience, grant number THV-I20-532.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of IRCCS ISMETT (protocol code IRRB/04/04 and 01.02.2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Beller, C.J.; Bekeredjian, R.; Krumsdorf, U.; Leipold, R.; Katus, H.A.; Karck, M.; Rottbauer, W.; Kallenbach, K. Transcatheter aortic valve implantation after previous mechanical mitral valve replacement: Expanding indications? Heart Surg. Forum 2011, 14, E166–E170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samant, S.; Bakhos, J.J.; Wu, W.; Zhao, S.; Kassab, G.S.; Khan, B.; Panagopoulos, A.; Makadia, J.; Oguz, U.M.; Banga, A.; et al. Artificial Intelligence, Computational Simulations, and Extended Reality in Cardiovascular Interventions. Cardiovasc. Interv. 2023, 16, 2479–2497. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Onuma, Y.; Zlahoda-Huzior, A.; Kageyama, S.; Dudek, D.; Wang, Q.; Lim, R.P.; Garg, S.; Poon, E.K.W.; Puskas, J.; et al. Merging virtual and physical experiences: Extended realities in cardiovascular medicine. Eur. Heart J. 2023, 44, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cervantes, I.; Morales, M.A.; Agustín-Serrano, R.; Cardenas-García, M.; Pérez-Luna, P.V.; Arroyo-Reyes, B.L.; Maldonado-García, A. Polylactic acid/sodium alginate/hydroxyapatite composite scaffolds with trabecular tissue morphology designed by a bone remodeling model using 3D printing. J. Mater. Sci. 2019, 54, 9478–9496. [Google Scholar] [CrossRef]
- Wu, D.; Spanou, A.; Diez-Escudero, A.; Persson, C. 3D-printed PLA/HA composite structures as synthetic trabecular bone: A feasibility study using fused deposition modeling. J. Mech. Behav. Biomed. Mater. 2020, 103, 103608. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Agustín, F.; Ruiz-Salgado, S.; Zenteno-Mateo, B.; Rubio, E.; Morales, M.A. 3D pattern formation from coupled Cahn-Hilliard and Swift-Hohenberg equations: Morphological phases transitions of polymers, bock and diblock copolymers. Comp. Mater. Sci. 2022, 210, 111431. [Google Scholar] [CrossRef]
- Pasta, S.; Gandolfo, C. Computational Analysis of Self-Expanding and Balloon-Expandable Transcatheter Heart Valves. Biomechanics 2021, 1, 43–52. [Google Scholar] [CrossRef]
- Catalano, C.; Turgut, T.; Zahalka, O.; Gotzen, N.; Cannata, S.; Gentile, G.; Agnese, V.; Gandolfo, C.; Pasta, S. On the Material Constitutive Behavior of the Aortic Root in Patients with Transcatheter Aortic Valve Implantation. Cardiovasc. Eng. Technol. 2024, 15, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Morganti, S.; Conti, M.; Aiello, M.; Valentini, A.; Mazzola, A.; Reali, A.; Auricchio, F. Simulation of transcatheter aortic valve implantation through patient-specific finite element analysis: Two clinical cases. J. Biomech. 2014, 47, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; Cannata, S.; Agnese, V.; Gentile, G.; Gandolfo, C.; Pasta, S. On the spectrum of transcatheter mitral valve replacement: In silico and in vitro assessment of neo-LVOT area in ViR, ViV and ViMAC. Bioprinting 2023, 32, e00285. [Google Scholar] [CrossRef]
- Kushimo, O.A.; Yadav, M.S.; Pandey, P.; Singh, S.; Kumar, V. Transcatheter aortic valve replacement in patients with a pre-existing prosthetic mitral valve: A single center experience with two cases. Egypt. Heart J. 2024, 76, 3. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Webb, J.; Schaefer, U.; Moss, R.; Deuschl, F.G.; Conradi, L.; Denti, P.; Latib, A.; Kiaii, B.; Bagur, R.; et al. Transcatheter Mitral Valve Replacement in Patients With Previous Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2018, 11, e006412. [Google Scholar] [CrossRef] [PubMed]
- Vavuranakis, M.; Vrachatis, D.A.; Kariori, M.G.; Moldovan, C.; Kalogeras, K.; Lavda, M.; Aznaouridis, K.; Stefanadis, C. TAVI in the case of preexisting mitral prosthesis: Tips & tricks and literature review. J. Invasive Cardiol. 2014, 26, 609–613. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).