Electromyography-Based Human-in-the-Loop Bayesian Optimization to Assist Free Leg Swinging
Abstract
1. Introduction
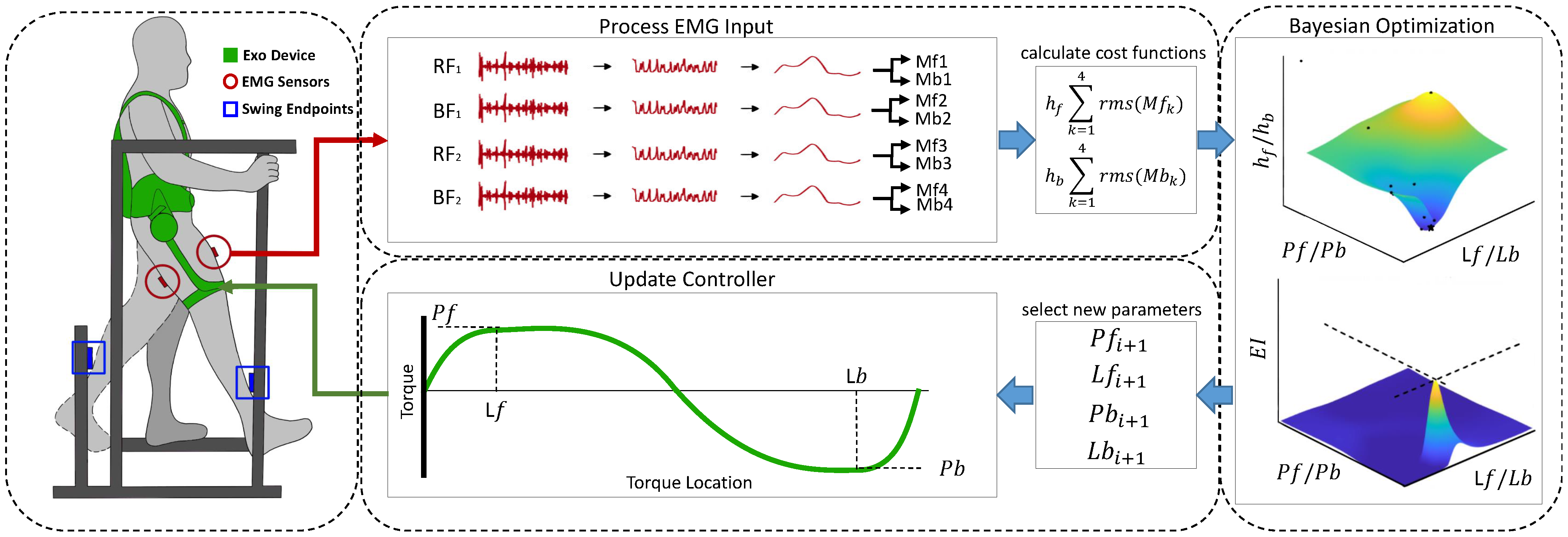
2. Materials and Methods
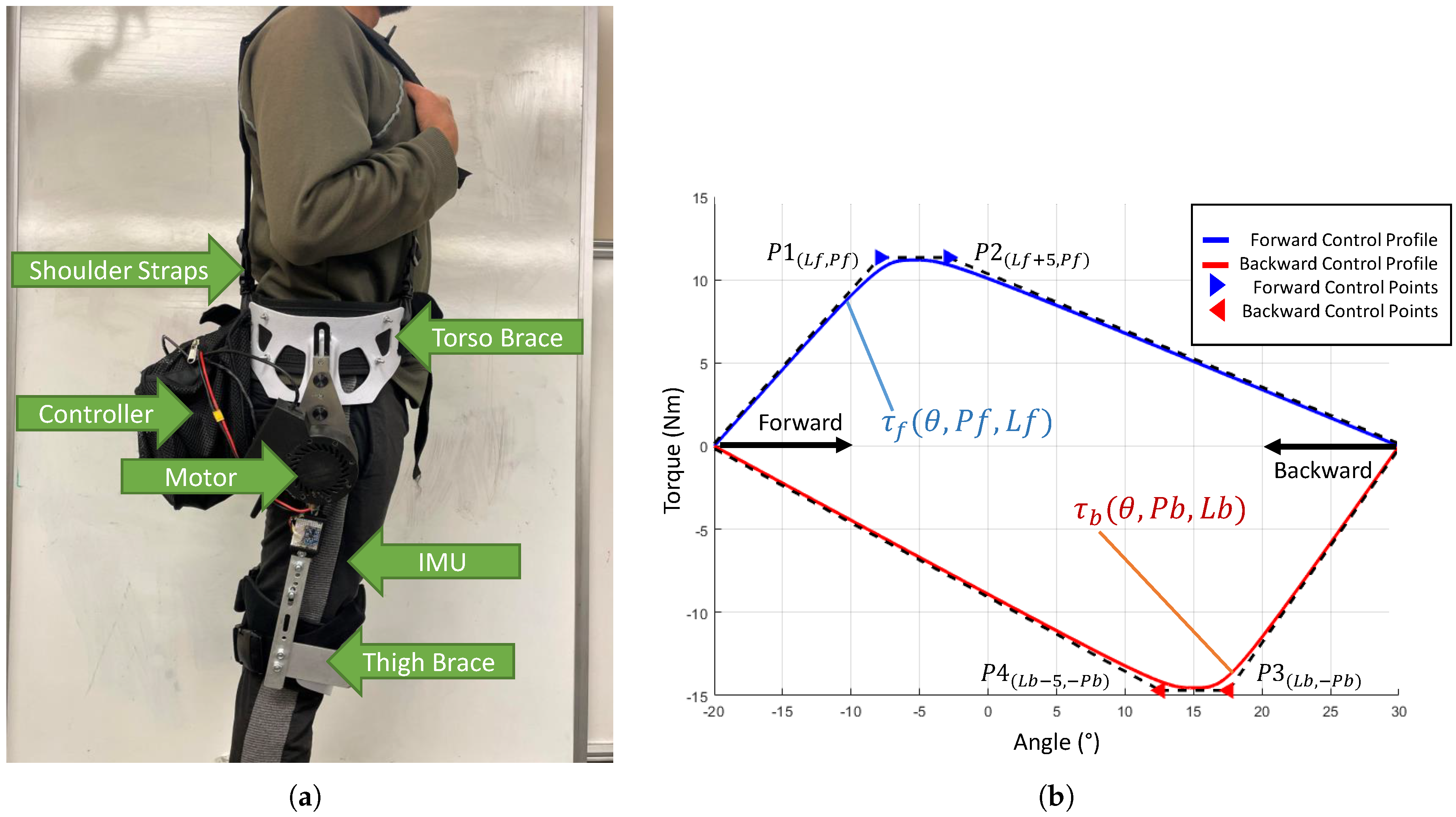
2.1. Hip Exoskeleton Device
2.2. EMG Data
2.2.1. Sensor Placement and MVC Determination
2.2.2. Signal Preprocessing
2.2.3. Signal Rectification and Dynamic Smoothing
- A moving max filter with a window size of up to 50 data points (less than 0.25% of the total dataset length) is used to highlight signal peaks.
- A discretization filter reduces the resolution of the data using bin sizes between 10 and 30.
- A moving average filter with a window size of up to 400 data points (∼1.6% of the dataset length) smooths the overall signal (Figure 3b).
2.2.4. Angle and Velocity Data Integration
2.2.5. Epoching and Cost Function Calculation
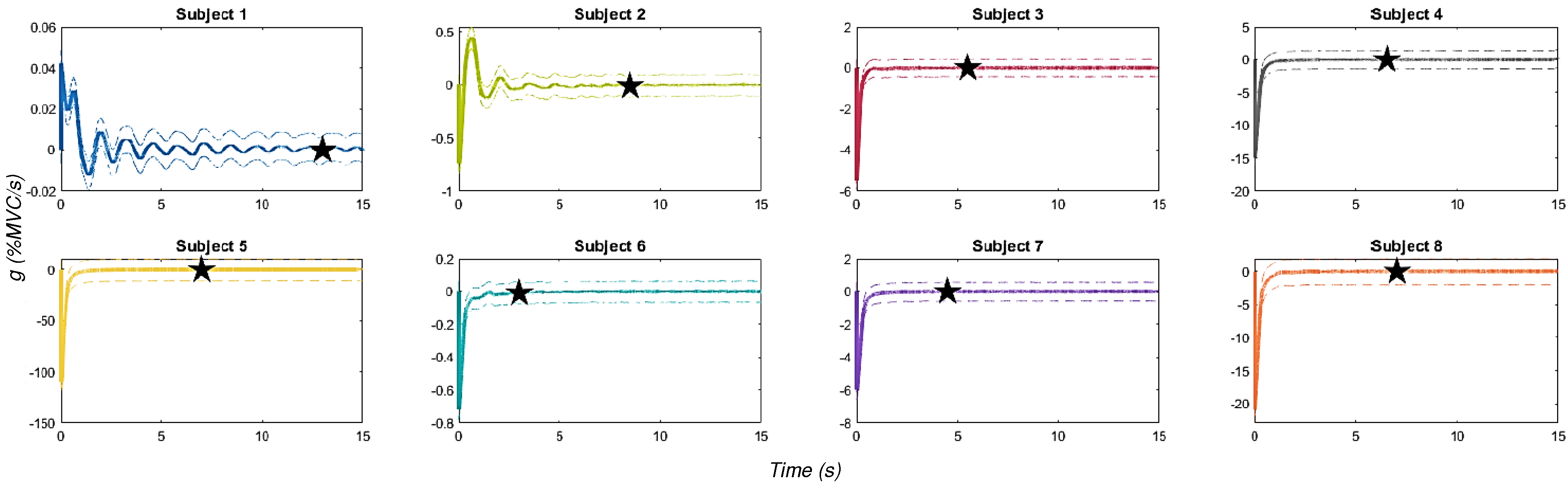
2.2.6. Data Convergence
- A running average () at time i is calculated for each sensor using the formulawhere represents the signal values at sample j.
- The rate of change () of the running average is computed using a forward difference method:where denotes the running average of the k-th EMG signal at time i.
- These rates of change are aggregated into a single vector g. To analyze the stability of the signal, we segment this vector into windows using a partition method, with a window size w, based on a sampling frequency of 2 Hz. This frequency is chosen to balance refined detection (minimal lag) and an ample search window:
- To identify the steady-state point, the rate of change is analyzed within overlapping time windows. Statistical methods, such as a two-sample z-test, are used to compare the mean rates of change between consecutive windows:where and represent the rate of change in the current and previous windows, respectively.
- A p-value of 0.95 is used to confirm no significant difference in the rates of change between consecutive windows. When this condition is met, the signal is considered to have reached a steady state.
2.3. Experimental Protocol
2.3.1. Acclimation
2.3.2. HIL Optimization
2.3.3. Validation
- No exoskeleton (NE), where the subject performs free swinging.
- Zero actuation (ZA), where the subject wears the device but it remains unpowered.
- General control (GC), where the subject receives assistance from a predefined intuition controller supplying a magnitude of torque equivalent to the mean of the possible torque values at the beginning of each half swing.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMG | Electromyography |
| HIL | Human-in-the-loop |
| MVC | Maximum voluntary contraction |
| FSM | Finite state machine |
| RF | Rectus femoris |
| BF | Bicep femoris |
References
- Chen, B.; Zi, B.; Qin, L.; Pan, Q. State-of-the-art research in robotic hip exoskeletons: A general review. J. Orthop. Transl. 2020, 20, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, W.; Zhang, F.; Li, X.; Chen, J.; Han, J.; Zhang, J. Selection of muscle-activity-based cost function in human-in-the-loop optimization of multi-gait ankle exoskeleton assistance. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Sanz-Morère, C.B.; Livolsi, C.; Pergolini, A.; Arnetoli, G.; Doronzio, S.; Giffone, A.; Conti, R.; Giovacchini, F.; Friðriksson, Þ.; et al. Lower-limb amputees can reduce the energy cost of walking when assisted by an Active Pelvis Orthosis. In Proceedings of the 2020 IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; pp. 809–815. [Google Scholar] [CrossRef]
- Malcolm, P.; Derave, W.; Galle, S.; De Clercq, D. A simple exoskeleton that assists plantarflexion can reduce the metabolic cost of human walking. PLoS ONE 2013, 8, e56137. [Google Scholar] [CrossRef]
- Mooney, L.M.; Rouse, E.J.; Herr, H.M. Autonomous exoskeleton reduces metabolic cost of human walking during load carriage. J. Neuroeng. Rehabil. 2014, 11, 80. [Google Scholar] [CrossRef]
- Collins, S.H.; Wiggin, M.B.; Sawicki, G.S. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature 2015, 522, 212–215. [Google Scholar] [CrossRef]
- Koller, J.R.; Jacobs, D.A.; Ferris, D.P.; Remy, C.D. Learning to walk with an adaptive gain proportional myoelectric controller for a robotic ankle exoskeleton. J. Neuroeng. Rehabil. 2015, 12, 97. [Google Scholar] [CrossRef]
- Zhang, J.; Fiers, P.; Witte, K.A.; Jackson, R.W.; Poggensee, K.L.; Atkeson, C.G.; Collins, S.H. Human-in-the-loop optimization of exoskeleton assistance during walking. Science 2017, 356, 1280–1284. [Google Scholar]
- Zelik, K.E.; Collins, S.H.; Adamczyk, P.G.; Segal, A.D.; Klute, G.K.; Morgenroth, D.C.; Hahn, M.E.; Orendurff, M.S.; Czerniecki, J.M.; Kuo, A.D. Systematic variation of prosthetic foot spring affects center-of-mass mechanics and metabolic cost during walking. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 411–419. [Google Scholar]
- Jackson, R.W.; Collins, S.H. An experimental comparison of the relative benefits of work and torque assistance in ankle exoskeletons. J. Appl. Physiol. 2015, 119, 541–557. [Google Scholar]
- Quesada, R.E.; Caputo, J.M.; Collins, S.H. Increasing ankle push-off work with a powered prosthesis does not necessarily reduce metabolic rate for transtibial amputees. J. Biomech. 2016, 49, 3452–3459. [Google Scholar] [CrossRef]
- Handford, M.L.; Srinivasan, M. Robotic lower limb prosthesis design through simultaneous computer optimizations of human and prosthesis costs. Sci. Rep. 2016, 6, 19983. [Google Scholar] [CrossRef] [PubMed]
- Farris, D.J.; Robertson, B.D.; Sawicki, G.S. Elastic ankle exoskeletons reduce soleus muscle force but not work in human hopping. J. Appl. Physiol. 2013, 115, 579–585. [Google Scholar] [CrossRef]
- Ferris, D.P.; Sawicki, G.S.; Daley, M.A. A physiologist’s perspective on robotic exoskeletons for human locomotion. Int. J. Humanoid Robot. 2007, 4, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Allen, T.T.; Notz, W.I.; Zeng, N. Global Optimization of Stochastic Black-Box Systems via Sequential Kriging Meta-Models. J. Glob. Optim. 2006, 34, 441–466. [Google Scholar] [CrossRef]
- Lorenz, R.; Monti, R.P.; Violante, I.R.; Anagnostopoulos, C.; Faisal, A.A.; Montana, G.; Leech, R. The Automatic Neuroscientist: A framework for optimizing experimental design with closed-loop real-time fMRI. NeuroImage 2016, 129, 320–334. [Google Scholar] [CrossRef]
- Ren, P.; Wang, W.; Jing, Z.; Chen, J.; Zhang, J. Improving the Time Efficiency of sEMG-based Human-in-the-Loop Optimization. In Proceedings of the 2019 Chinese Control Conference (CCC), Guangzhou, China, 27–30 July 2019; pp. 4626–4631. [Google Scholar]
- Kim, M.; Ding, Y.; Malcolm, P.; Speeckaert, J.; Siviy, C.J.; Walsh, C.J.; Kuindersma, S. Human-in-the-loop Bayesian optimization of wearable device parameters. PLoS ONE 2017, 12, e0184054. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.F.N.; McGreavy, C.; Christou, A.; Vijayakumar, S. Human-in-the-loop optimization of exoskeleton assistance via online simulation of metabolic cost. IEEE Trans. Robot. 2022, 38, 1410–1429. [Google Scholar] [CrossRef]
- Selinger, J.C.; Donelan, J.M. Estimating instantaneous energetic cost during non-steady-state gait. J. Appl. Physiol. 2014, 117, 1406–1415. [Google Scholar] [CrossRef]
- Makin, T.R.; de Vignemont, F.; Faisal, A.A. Neurocognitive barriers to the embodiment of technology. Nat. Biomed. Eng. 2017, 1, 0014. [Google Scholar]
- Gordon, K.E.; Ferris, D.P. Learning to walk with a robotic ankle exoskeleton. J. Biomech. 2007, 40, 2636–2644. [Google Scholar] [CrossRef]
- Selinger, J.C.; O’Connor, S.M.; Wong, J.D.; Donelan, J.M. Humans Can Continuously Optimize Energetic Cost during Walking. Curr. Biol. 2015, 25, 2452–2456. [Google Scholar] [PubMed]
- Rosen, J.; Brand, M.; Fuchs, M.B.; Arcan, M. A myosignal-based powered exoskeleton system. IEEE Trans. Syst. Man Cybern. Part A Syst. Humans 2001, 31, 210–222. [Google Scholar]
- Cimolato, A.; Driessen, J.J.M.; Mattos, L.S.; De Momi, E.; Laffranchi, M.; De Michieli, L. EMG-driven control in lower limb prostheses: A topic-based systematic review. J. Neuroeng. Rehabil. 2022, 19, 43. [Google Scholar]
- Huang, H.; Zhang, F.; Hargrove, L.J.; Dou, Z.; Rogers, D.R.; Englehart, K.B. Continuous locomotion-mode identification for prosthetic legs based on neuromuscular-mechanical fusion. IEEE Trans. Biomed. Eng. 2011, 58, 2867–2875. [Google Scholar]
- Aguirre-Ollinger, G. Learning muscle activation patterns via nonlinear oscillators: Application to lower-limb assistance. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 1182–1189. [Google Scholar]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Fleischer, C.; Reinicke, C.; Hommel, G. Predicting the intended motion with EMG signals for an exoskeleton orthosis controller. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; pp. 2029–2034. [Google Scholar]
- Gordon, D.F.N.; Matsubara, T.; Noda, T.; Teramae, T.; Morimoto, J.; Vijayakumar, S. Bayesian optimization of exoskeleton design parameters. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 653–658. [Google Scholar]
- Brochu, E.; Cora, V.M.; de Freitas, N. A Tutorial on Bayesian Optimization of Expensive Cost Functions, with Application to Active User Modeling and Hierarchical Reinforcement Learning. arXiv 2010, arXiv:1012.2599. [Google Scholar]
- Yandell, M.B.; Quinlivan, B.T.; Popov, D.; Walsh, C.; Zelik, K.E. Physical interface dynamics alter how robotic exosuits augment human movement: Implications for optimizing wearable assistive devices. J. Neuroeng. Rehabil. 2017, 14, 40. [Google Scholar] [PubMed]
- Echeveste, S.; Hernandez Hinojosa, E. Optimal Swing Assistance Using a Hip Exoskeleton: Comparing Simulations With Hardware Implementation. In Proceedings of the International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Volume 8: 47th Mechanisms and Robotics Conference (MR), Boston, MA, USA, 20–23 August 2023; p. V008T08A074. [Google Scholar]
- SENIAM, Surface EMG for Non-Invasive Assessment of Muscles. Available online: http://seniam.org/sensor_location.htm (accessed on 13 January 2025).
- Akima, H. A New Method of Interpolation and Smooth Curve Fitting Based on Local Procedures. J. Assoc. Comput. Mach. 1970, 17, 589–602. [Google Scholar]
- Straczkiewicz, M.; Huang, E.J.; Onnela, J.-P. Large-scale study of free-living walking cadence using mobile phone accelerometers. NPJ Digit. Med. 2023, 6, 29. [Google Scholar]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar]
- Miller, L.E.; Zimmermann, A.K.; Herbert, W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices Evid. Res. 2016, 9, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ba, X.; Xu, F.; Leng, Y.; Fu, C. EMG-based Human-in-the-loop Optimization of Ankle Plantar-flexion Assistance with a Soft Exoskeleton. In Proceedings of the 2022 International Conference on Advanced Robotics and Mechatronics (ICARM), Guilin, China, 9–11 July 2022; pp. 453–458. [Google Scholar]




| Baseline | Opt. Slow (% Change) | Opt. Fast (% Change) |
|---|---|---|
| No Device | −26.0% | −14.4% |
| Zero Assistance | −39.5% | −26.8% |
| General Control | −18.5% | 4.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeveste, S.; Bhounsule, P.A. Electromyography-Based Human-in-the-Loop Bayesian Optimization to Assist Free Leg Swinging. Biomechanics 2025, 5, 21. https://doi.org/10.3390/biomechanics5020021
Echeveste S, Bhounsule PA. Electromyography-Based Human-in-the-Loop Bayesian Optimization to Assist Free Leg Swinging. Biomechanics. 2025; 5(2):21. https://doi.org/10.3390/biomechanics5020021
Chicago/Turabian StyleEcheveste, Salvador, and Pranav A. Bhounsule. 2025. "Electromyography-Based Human-in-the-Loop Bayesian Optimization to Assist Free Leg Swinging" Biomechanics 5, no. 2: 21. https://doi.org/10.3390/biomechanics5020021
APA StyleEcheveste, S., & Bhounsule, P. A. (2025). Electromyography-Based Human-in-the-Loop Bayesian Optimization to Assist Free Leg Swinging. Biomechanics, 5(2), 21. https://doi.org/10.3390/biomechanics5020021







