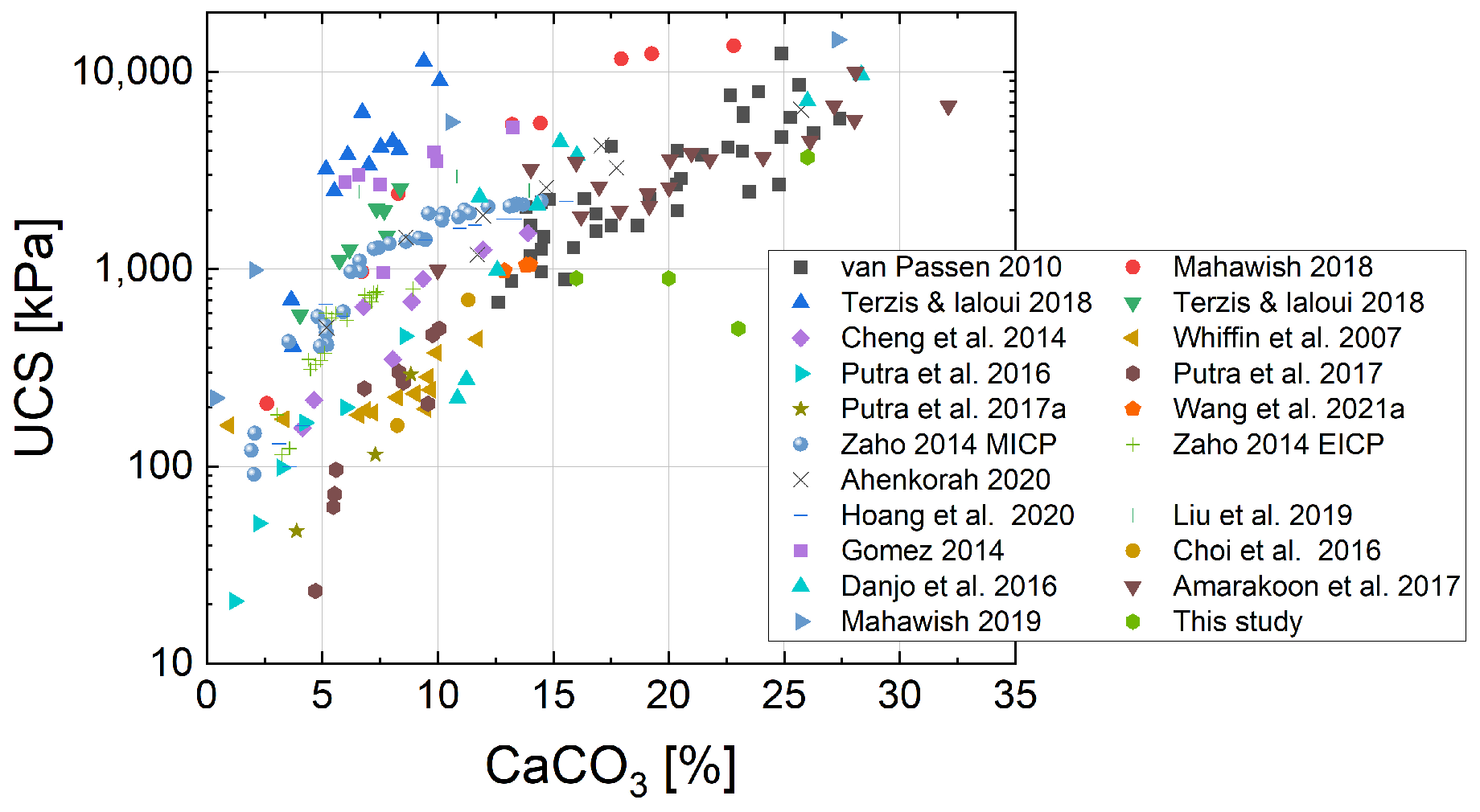
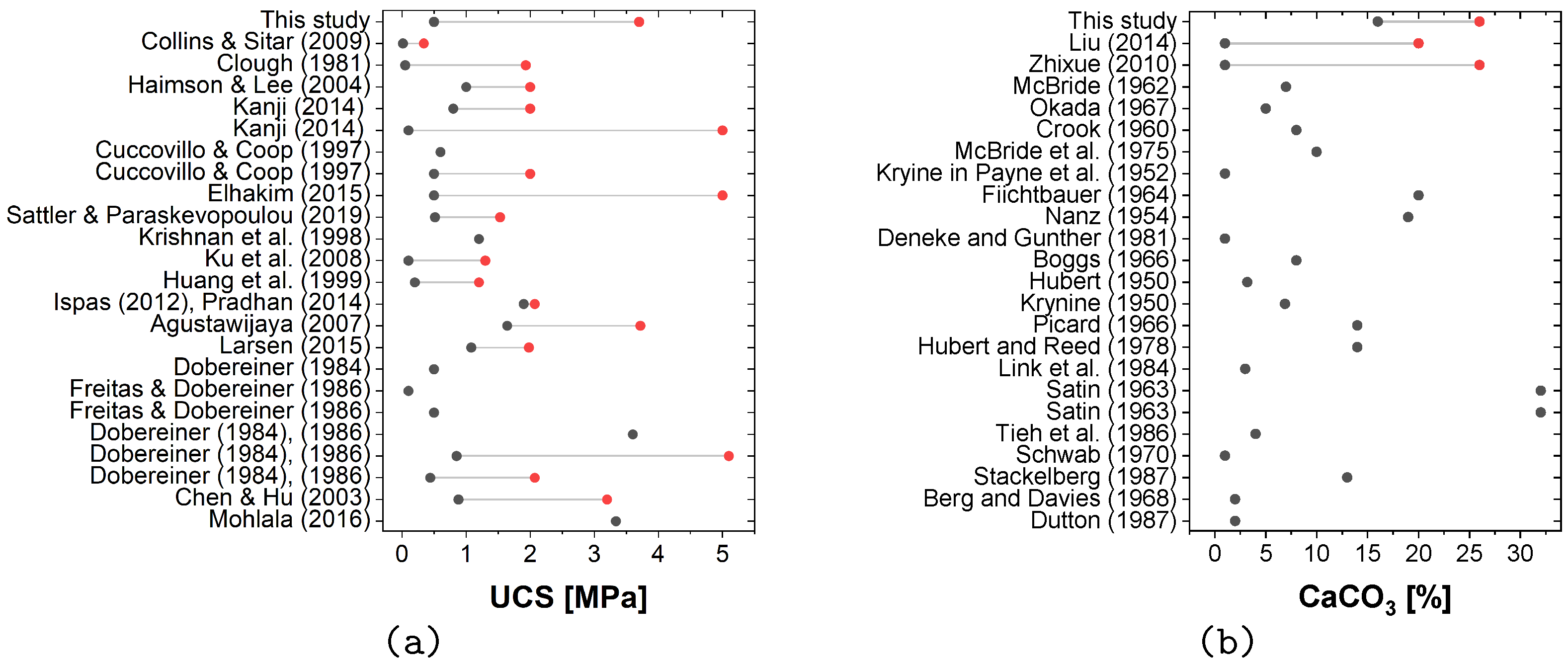
1. Introduction
In the natural environment, the soil matrix undergoes biological, physical, or chemical processes that lead to the formation of natural cementation, resulting in increased soil strength [
1,
2]. Given the prevalence of naturally cemented soil across the Earth’s crust, research into its mechanical properties is crucial for geotechnical engineering applications. However, because of the natural variability, the costs, and the difficulties associated with sampling, conducting parametric studies on the behavior of naturally cemented soil is challenging. Naturally cemented soil has been previously replicated in the lab using chemical cementation to study its behavior and to fulfill the need of replicating various levels of cementation and relative densities [
3]. A realistic way to replicate naturally cemented soil in the lab is microbially induced carbonate precipitation (MICP), which is an emerging technique that combines both economic efficiency and eco-friendly impact to solidify soils. Construction activities contribute to approximately 40% of the emissions of carbon dioxide generated globally each year, which significantly contributes to global warming [
4]. Therefore, it is required to develop eco-friendly alternatives as MICP that reduce the carbon footprint associated with engineering projects. One limitation of MICP is ammonia production, as it is a by-product of the process of urea hydrolysis which may contaminate drinking water and affect health if it seeps into groundwater. However, this can be handled by treating the effluent to remove ammonia, such as by using the response surface method (RSM) [
5] or using it as a fertilizer for plants [
6].
MICP is capable of adapting numerous functionalities in a variety of engineering tasks. MICP has been applied so far to improve the mechanical properties of soil [
7], decrease its hydraulic conductivity [
8], and increase its thermal conductivity [
9]. In addition, it has been used for the stabilization of geotechnical structures. This includes stabilizing slopes [
10], tunnel walls [
10], dams, and offshore structures [
11]. An example of a field-scale application of MICP is the stabilization of a natural gravel area before the upcoming horizontal directional drilling and the installation of a gas pipeline. A volume of 1000 m
of gravel treated for 7 days showed improved stability during drilling, confirming treatment effectiveness [
12]. Another example is the stabilization of a retaining wall beneath a motorway bridge located in France. Due to access restrictions beneath the bridge and the prohibition of transportation interruption, it was difficult to implement conventional techniques. Thus, the application of MICP treatment was the optimal solution for this project [
12].
MICP is a biologically mediated method that can induce the cementation process, link soil particles together through the formation of calcium carbonate minerals, and ultimately improve the engineering properties of soil. The driving force behind the precipitation phenomena is the presence of urease-producing bacteria such as
Sporosarcina pasteurii, which consume urea as an energy source and form calcium carbonate minerals in a calcium-rich environment. Urea hydrolysis is the mechanism through which urease-producing bacteria form calcium carbonate. Urea is hydrolyzed by urease due to its catalytic effect, resulting in the formation of ammonia and carbonate. The core mechanism of precipitation by urease-producing bacteria can be written as
MICP involves five stages to produce inorganic calcium carbonate minerals: (1) urease enzyme is synthesized by bacteria, (2) urea spreads into the bacterial cell, (3) urea (
) is hydrolyzed, resulting in the formation of ammonia (
) and bicarbonate anions (
) (Equations (1) and (3)), (4) hydroxide ions (
) are formed due to the equilibrium reaction between ammonia and water (Equation (
2)), (5) pH on the bacteria surface increases due to the formation of hydroxide ions, (6) calcium carbonate (
) precipitates upon the supply of calcium ions (
) on the surface of the bacterial cell (Equation (
4)).
The key outputs that characterize MICP treatment are the attained strength and distribution of precipitates. However, MICP is a complex procedure governed by several factors and is applied by different treatment methods. Therefore, a deeper understanding of the key factors which lead to effective MICP outcomes is of vital importance. To tackle this issue, different modifications to the treatment methodology were applied, and these can be categorized into two groups: (1) chemical or physical modification, and (2) procedure modification. As regards the chemical or physical modification, Xu et al. [
13] added magnesium ions to delay calcite precipitation and observed that the addition of 0.5 M magnesium ions to the feeding solution doubled soil strength. In contrast, Abdel Gawwad et al. [
14] concluded that increasing the concentration of MgCl
had a detrimental impact on the strength of the produced bio-mortar. Recently, the issue of homogeneity of calcium carbonate distribution was addressed by one phase injection of a low-pH solution [
15]. This method can avoid the soil clogging phenomena by improving the solution distribution and postponing calcite formation, thus reaching a UCS level of 2.5 MPa [
16]. However, this procedure has to be tested on a larger scale (a meter scale) and examined for other species of ureolytic bacteria than Bacillus sp. Another method to improve soil homogeneity was applied by Xiao et al. [
17], who adjusted the temperature of the cementation solution to 4 °C. In this way, the formation of calcite is delayed by the low temperature, allowing for properly spreading the solution between soil particles. However, this approach requires temperature control equipment, which may limit its usage in field applications. An example of the procedure modification is the premixing method. The bacteria premixing method offers a solution to the problem of random uneven bacterial distribution, resulting from either injecting or percolating bacteria. For example, Yasuhara et al. [
18] premixed urease enzyme with Toyoura sand and the samples exhibited a relatively homogeneous state, indicating UCS values of 400 kPa to 1.6 MPa. Nevertheless, the premixing method did not provide as high UCS values as the other treatment procedures [
19]. Additionally, Centeno Dias et al. [
20] pointed out that the injection through a perforated tube inserted at the center of the sample can improve soil homogeneity and enhance its mechanical properties. However, instability was induced by the effect of the inserted tube along with the case of premixing bacteria with soil, resulting in a low UCS value (60 kPa). Moreover, Terzis et al. [
21] emphasized that direct electric currents can modify the electro-chemical conditions, causing electro-migration of bacteria as well as dissolved solutes in soil voids. This can enhance the spatial distribution of bio-cementation bonding, especially when using calcium acetate and calcium lactate as calcium sources. Nevertheless, the usage of the inexpensive calcium source, calcium chloride, had a negative effect. The soaking or submerging method was established by Zhao et al. [
22] as an improved strategy for supplying reactants to soil, resulting in the enhanced transport of reactants, but the exact distribution of the precipitate along the sample height was not analyzed and the maximum UCS obtained by this method was 2.2 MPa. Wen et al. [
23] developed the submerging method by repeating the treatment in multiple cycles of submerging in the cementation solution. UCS values after four cycles of treatment increased by 5.7 times the strength of one cycle. However, higher soil strength enhancement may be achieved by modifying this method.
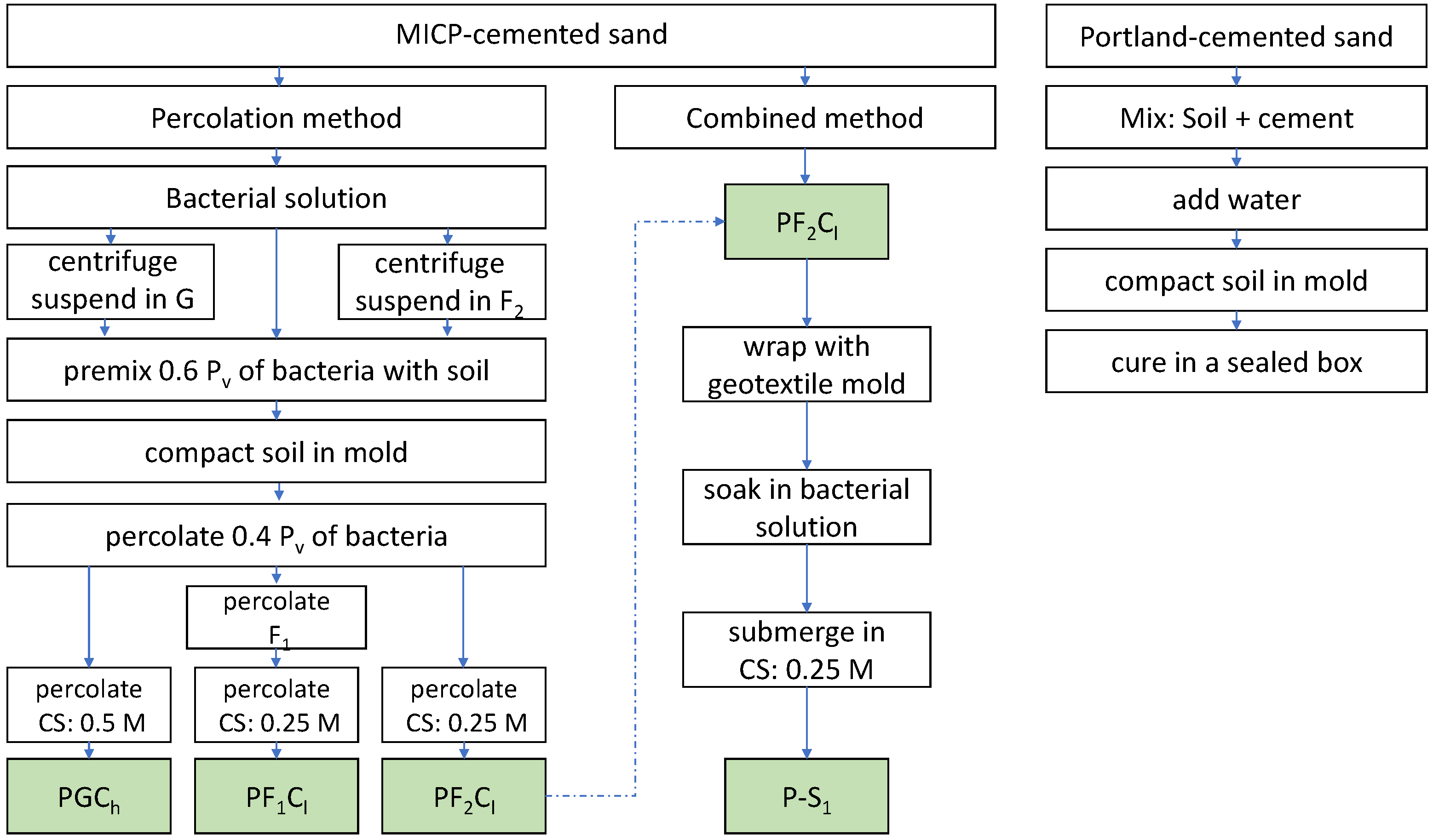
The objective of this paper is to introduce a new MICP treatment approach, the combined method, aimed at enhancing the strength outcome and the homogeneity of the distribution of calcium carbonate, in the context of utilizing this approach to produce artificial sandstones for soil testing. The combined method combines premixing, percolation, and submerging methods, taking advantage of the uniformity of bacteria distribution by the premixing method, the simplicity of the percolation method, and the enhanced transportation of nutrients by the submerging method. To verify the effectiveness of the new combined method, a comparison between the percolation and the combined methods has been performed. Different strategies for the promotion of bacterial growth have been tested for the percolation method. UCS testing has been conducted to evaluate the strength and to investigate the failure mode. The acid washing method has been used to check the distribution of calcium carbonate. SEM analysis has been applied to study the morphology of the calcium carbonate. A comparison has been made between the bio-cemented samples and sand samples that had been treated with Portland cement.
5. Conclusions
Inspired by nature, biological soil treatment (MICP) has recently emerged as a new strategy to solidify soil, improve its mechanical properties, and simulate the behavior of naturally cemented sand. For this aim, different treatment methods have recently been used to improve the homogeneity as well as the strength obtained by MICP. In this paper, a new MICP treatment method was proposed using a combination of the premixing bacteria method, the percolation method, and the submerging method. For comparison, Portland-cemented samples were produced. Based on the results of this investigation, the following conclusions can be drawn:
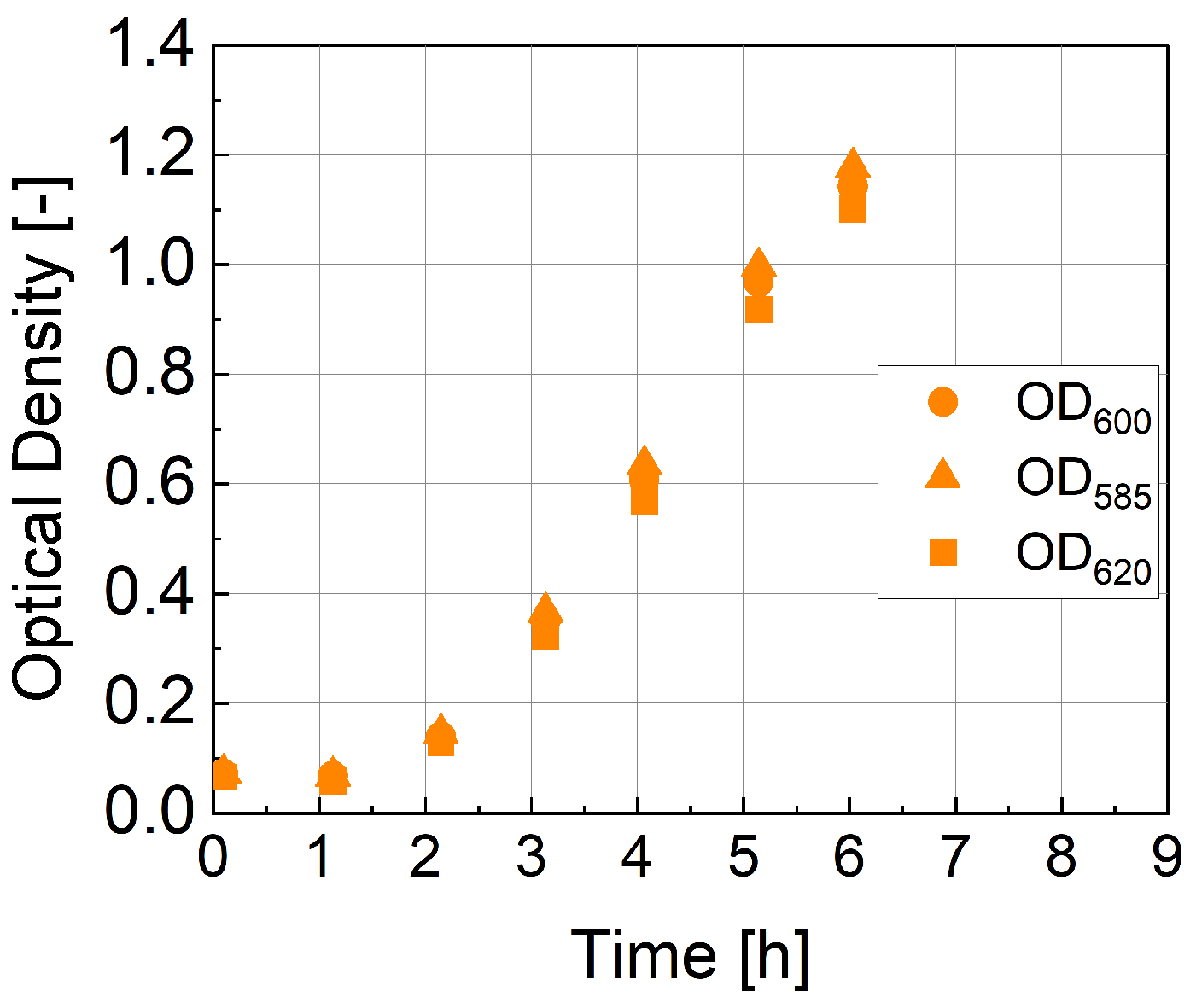
Experimental data revealed that suspending bacterial cells in a calcium-containing solution is more effective than percolating the sample with a urea-containing solution.
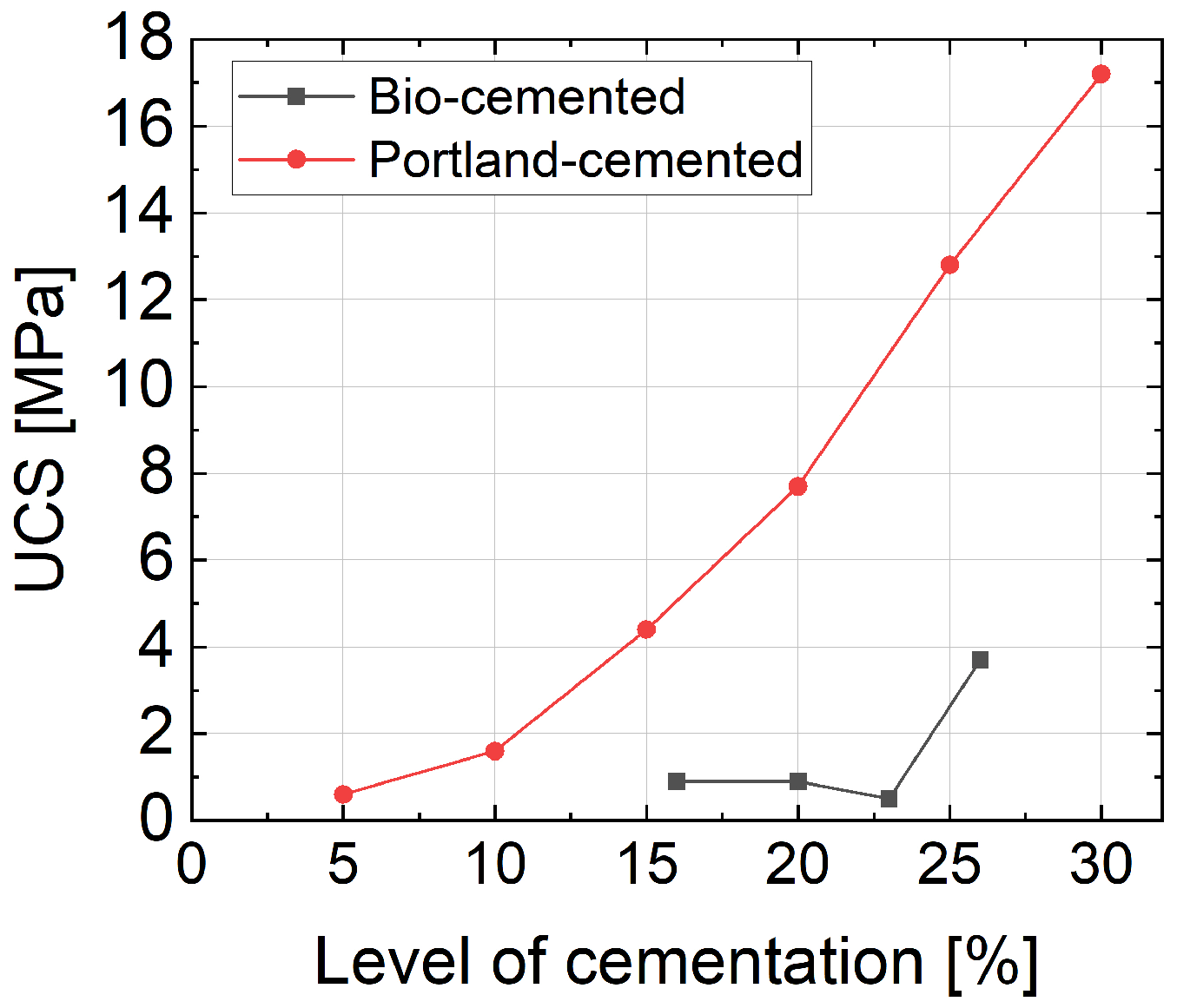
The combined method lead to higher strength than the percolation method by almost 3.7 times, with an increment in strength of around 2.7 MPa, indicating the ability to achieve a higher strength in a shorter time than the submerging method.
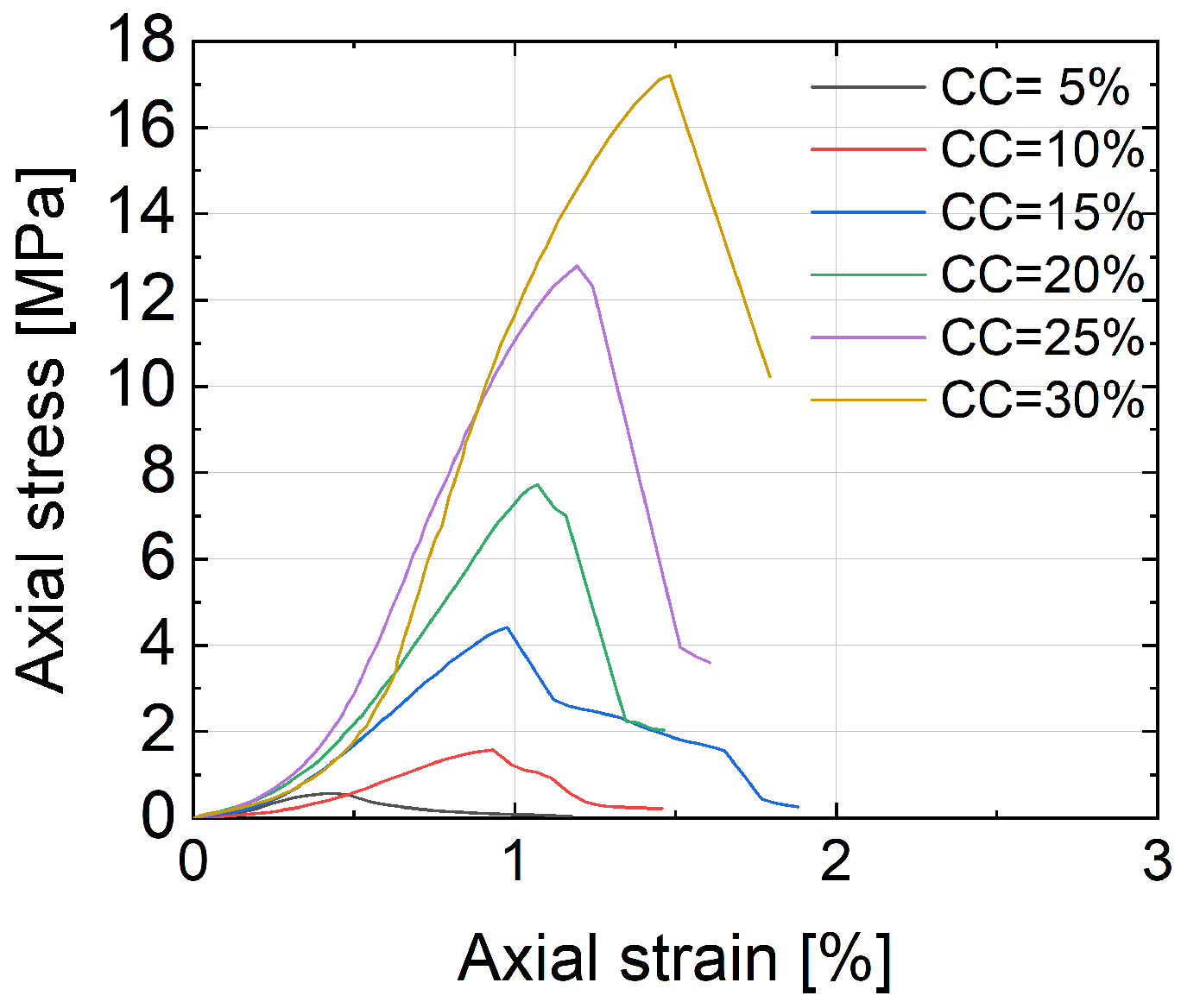
UCS for sandy soil solidified by bio-cementation, ranging from 0.5 to 3.7 MPa, was found lower than that of samples solidified by Portland cement, ranging from 0.6 to 17.2 MPa.
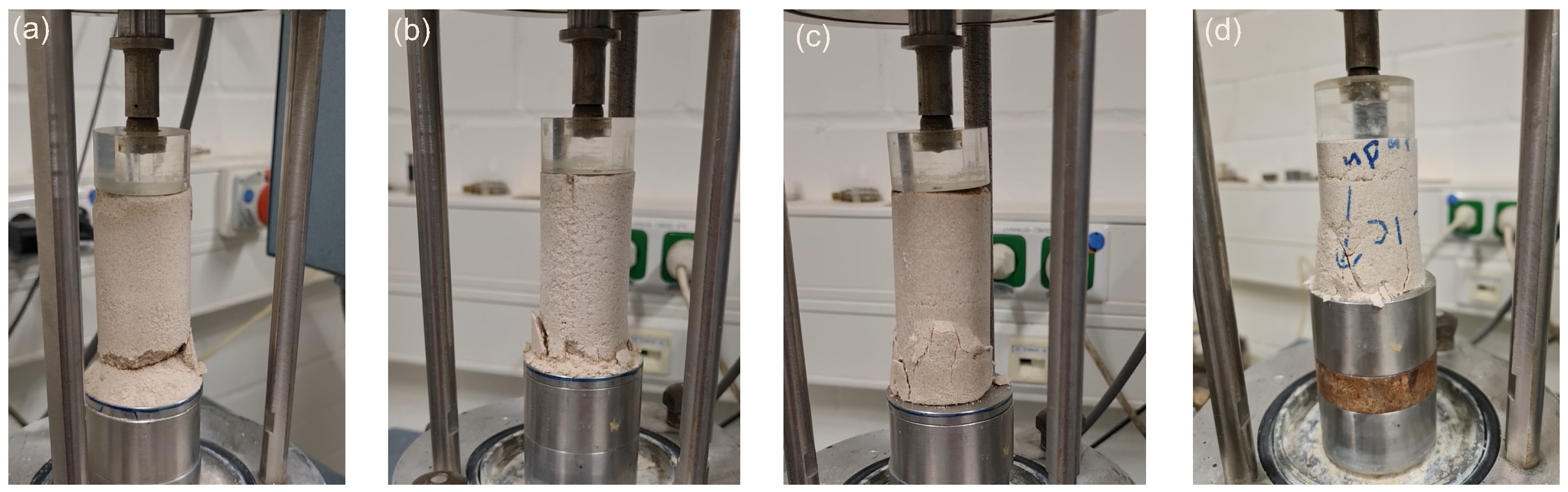
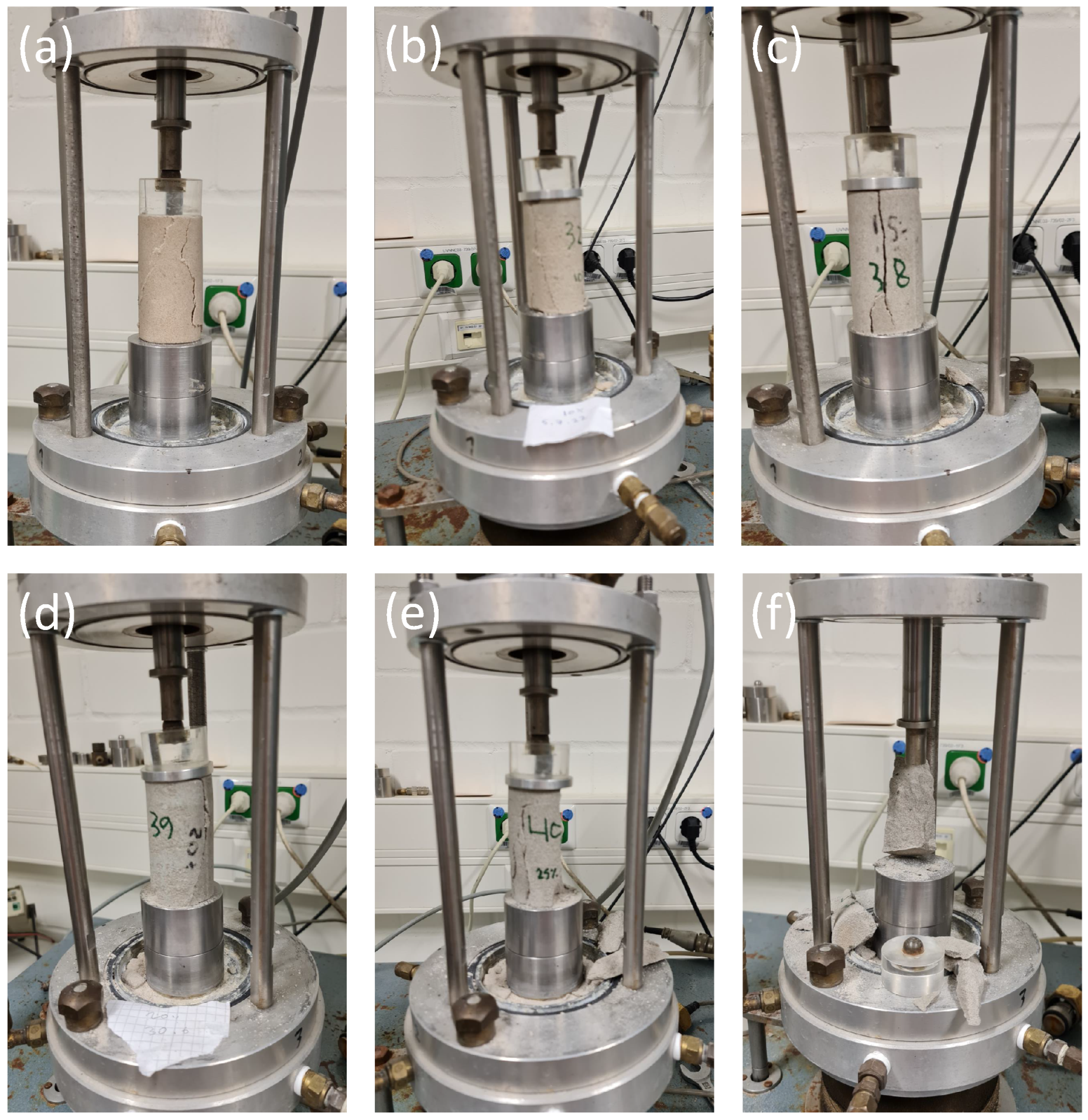
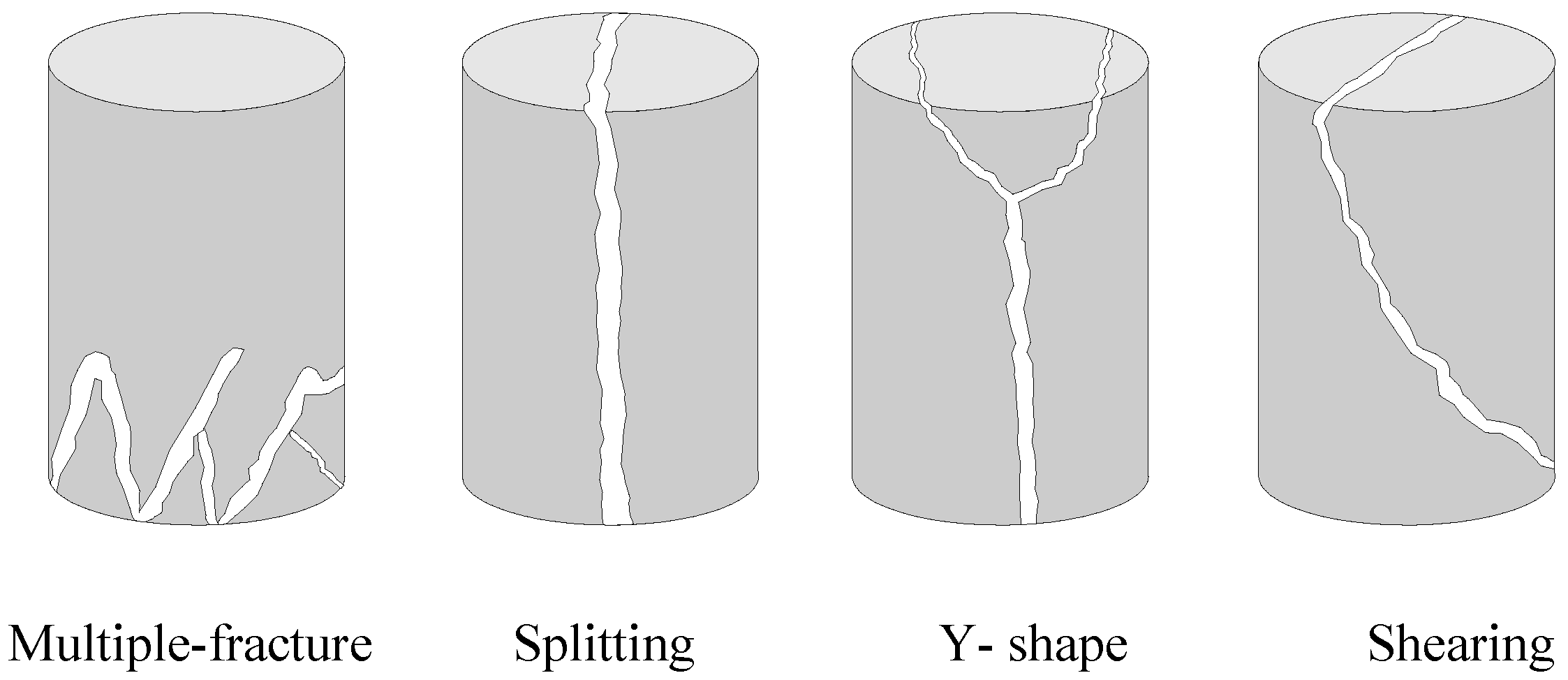
In bio-cemented sand, the failure mode was characterized by the presence of multiple fractures at the bottom of the specimen, while in Portland-cemented sand, the failure mode predominantly exhibited a y-shape pattern.
The combined procedure yielded samples that were about 13 times stiffer than those prepared by the percolation method.
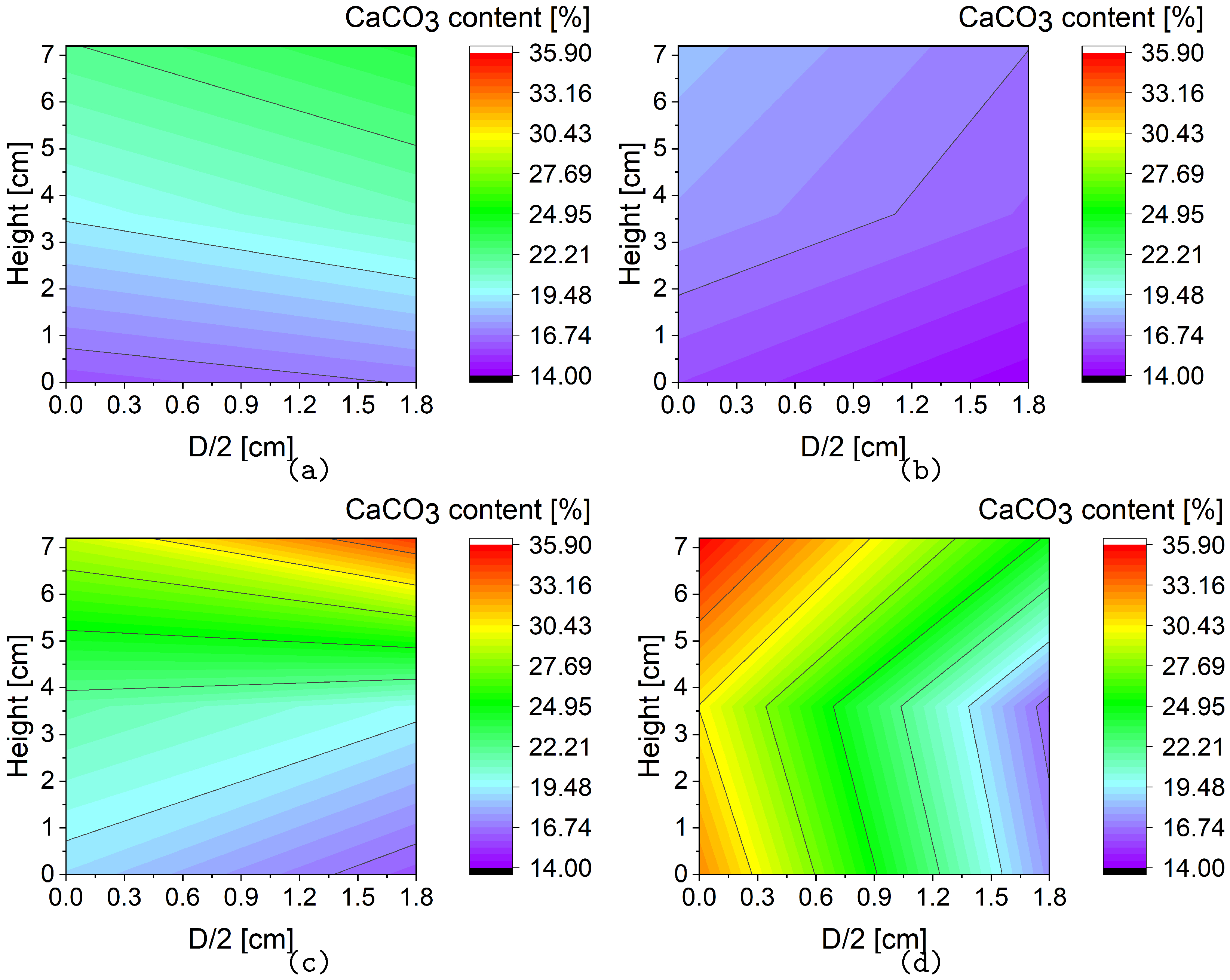
Applying the combined method, calcium carbonate was well-distributed throughout the sample, with the exception of a lower concentration at the center of the lower section of the sample. In samples prepared by the percolation procedure, the content of calcium carbonate gradually decreased along the direction away from the sample’s surface.
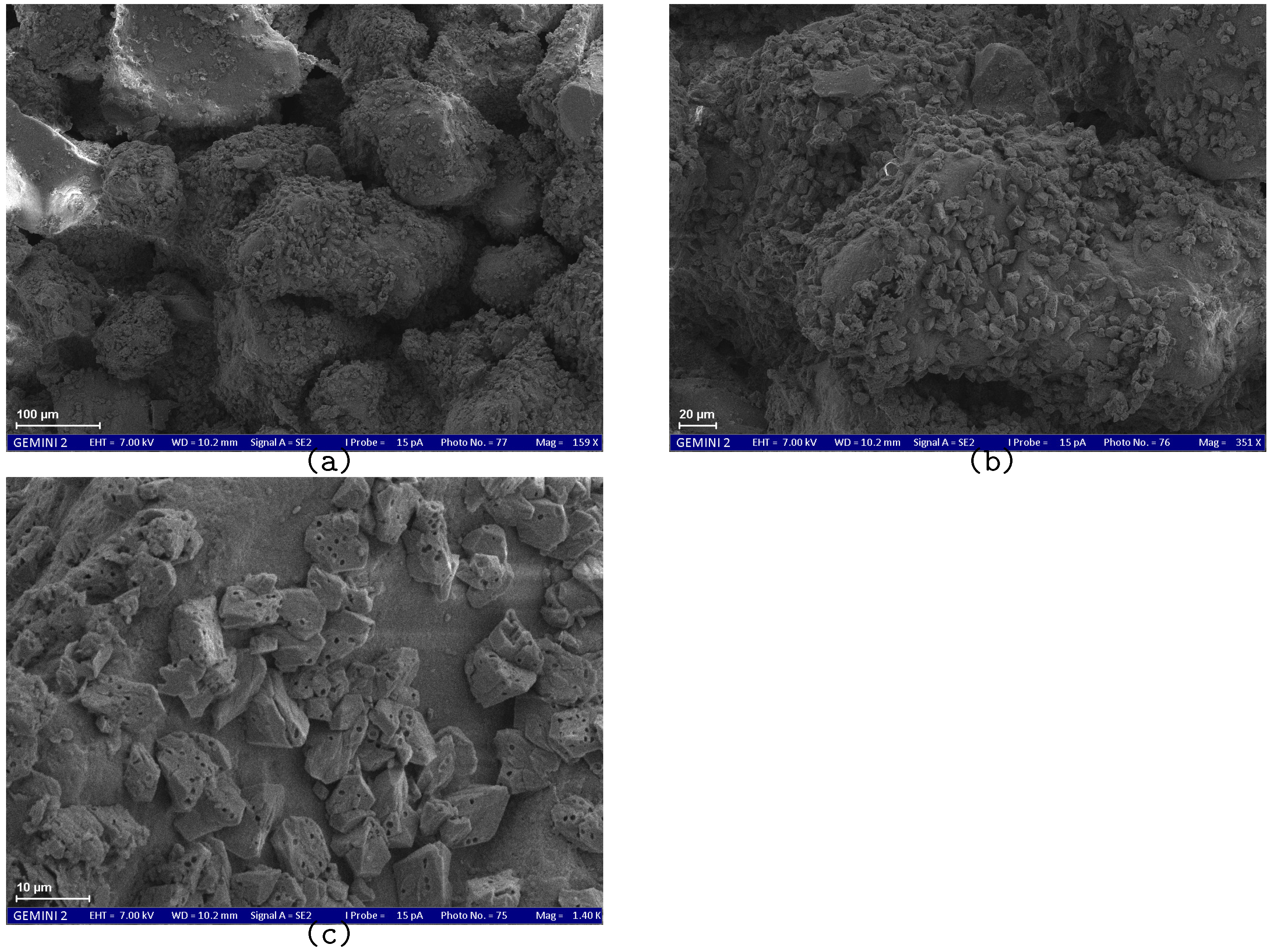
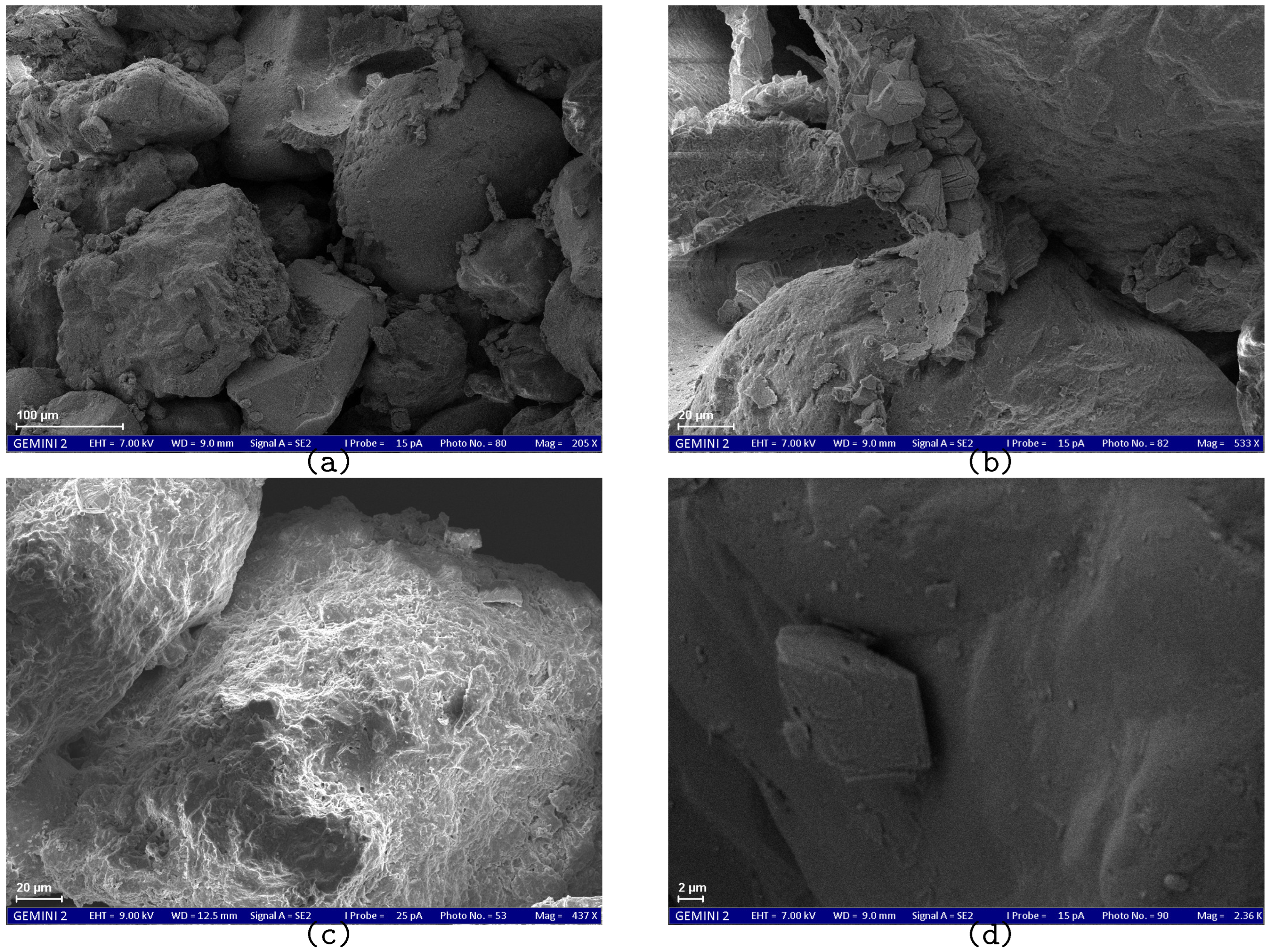
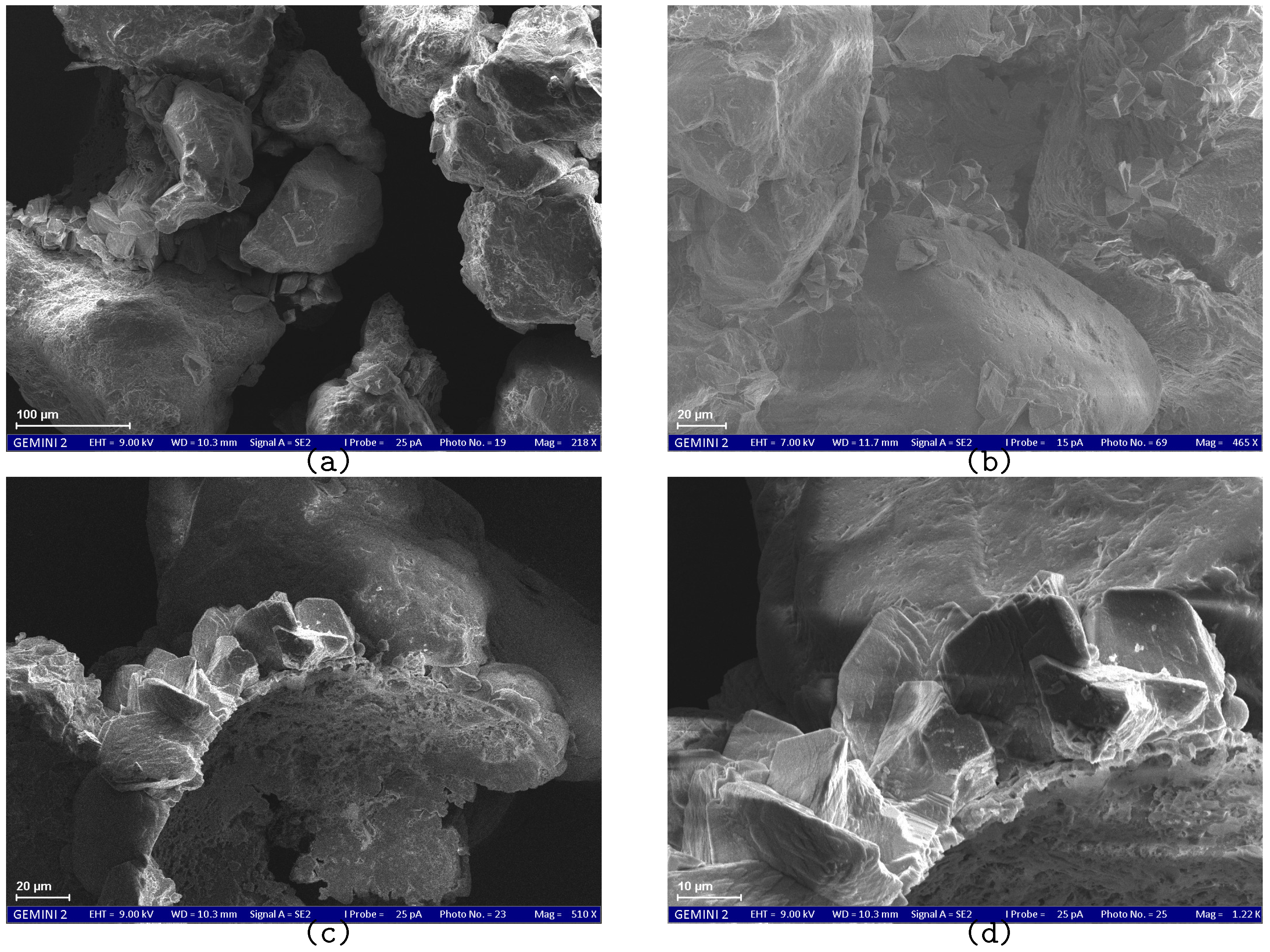
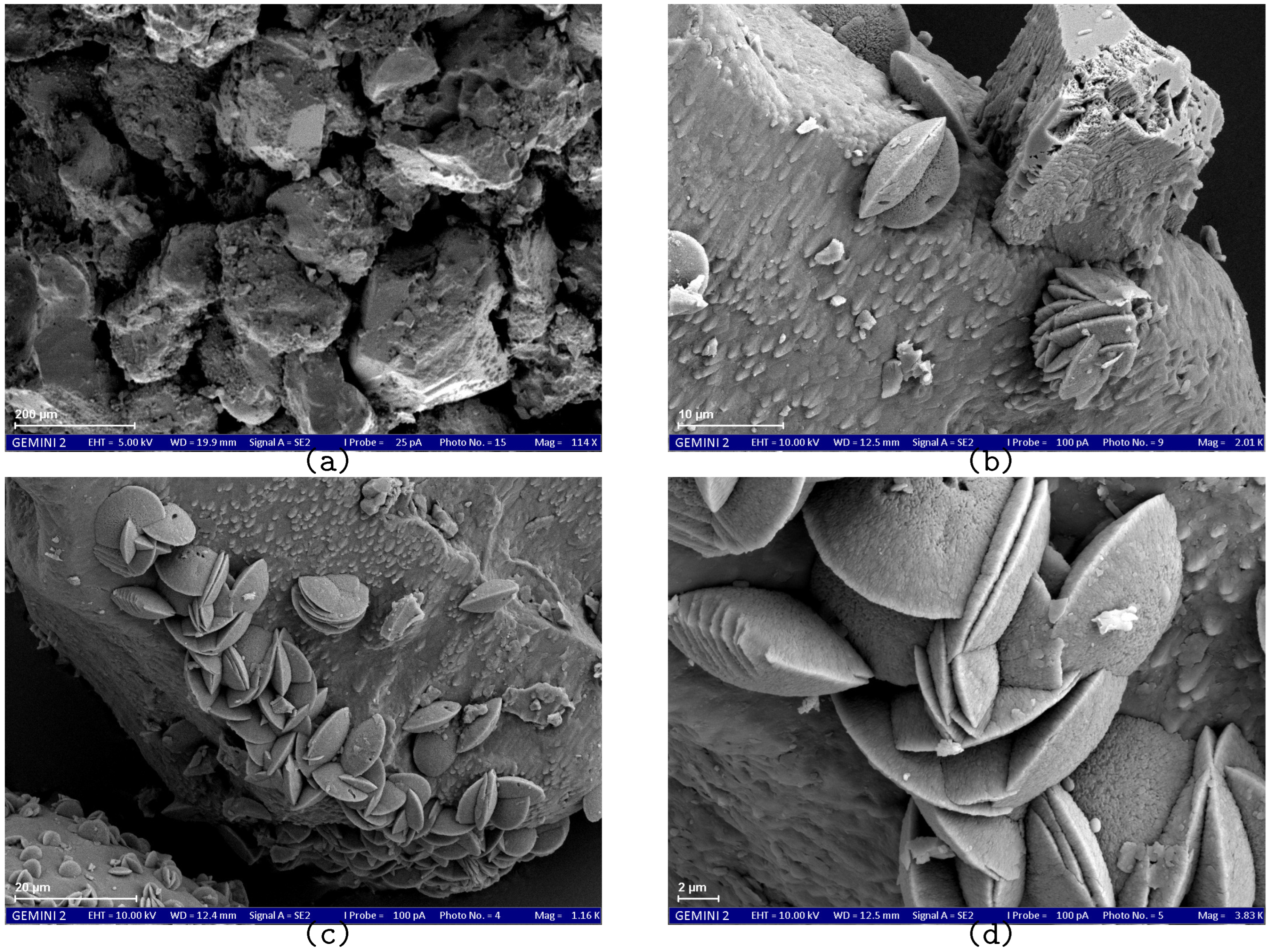
SEM results demonstrate the effect of the treatment method on the size and morphology of calcium carbonate crystals.
The results obtained for MICP-cemented samples were in line with the UCS values and calcium carbonate contents of naturally cemented sand.
The MICP method proposed in this study offers a possible time-efficient solution for enhancing the strength and the homogeneity of the precipitation distribution. The combined method may lay the groundwork for future research to reach higher strength by repeating the treatment with more cycles and to achieve an even higher homogeneity by flipping the sample inside the box containing the cementation solution upside down during the treatment to reverse the preferable flow induced by gravity. This study is limited to the laboratory scale with a few numbers of samples to mimic the behavior of naturally cemented soil. Further studies need to be carried out to examine the repeatability of the method, the compatibility with different grain size distributions of the soil, the cyclic behavior of the bio-cemented soil, the progressive bonding breakage, and the generalization for future engineering applications.