Pollinator Species at Risk from the Expansion of Avocado Monoculture in Central Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Species Occurrence Data
2.2. Species Distribution Modeling
2.3. Effect of the Expansion of Avocado Monoculture on Pollinator Habitat
3. Results
3.1. Species Distribution Models
3.2. The Potential Distribution of Persea americana Mill
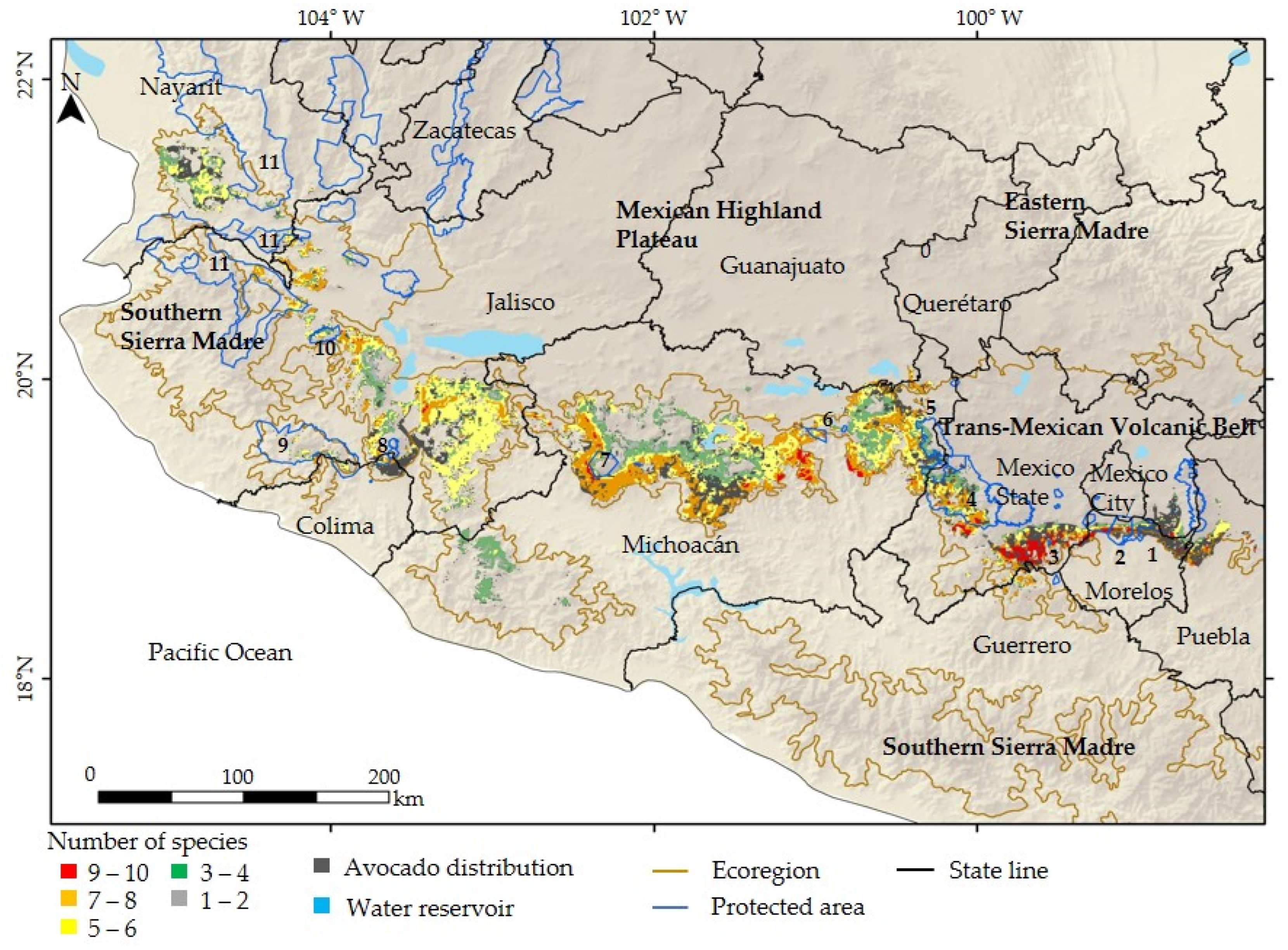
3.3. Pollinator Species Distribution and Habitat Loss
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffan-Dewenter, I.; Westphal, C. The interplay of pollinator diversity, pollination services and landscape change. J. Appl. Ecol. 2007, 45, 737–741. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Vanbergen, A.J.; Insect Pollinator Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualism: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Tylianakis, J.M.; Laliberté, E. The winners and losers of land use intensification: Pollinator community disassembly is non-random and alters functional diversity. Divers. Distrib. 2014, 20, 908–917. [Google Scholar] [CrossRef]
- Ganuza, C.; Redlich, S.; Uhler, J.; Tobisch, C.; Rojas-Botero, S.; Peters, M.K.; Zhang, J.; Benjamin, C.S.; Englmeier, J.; Ewald, J.; et al. Interactive effects of climate and land use on pollinator diversity differ among taxa and scales. Sci. Adv. 2022, 8, eabm9359. [Google Scholar] [CrossRef]
- Hansen, A.J.; Defries, R.S.; Turner, W. Land use change and biodiversity: A synthesis of rates and consequences during the period of satellite imagery. In Land Change Science; Gutman, G., Janetos, A.C., Justice, C.O., Moran, E.F., Mustard, J.F., Rindfuss, R.R., Skole, F., Lee, B., Cochrane, M.A., Eds.; Springer: New York, NY, USA, 2004; pp. 277–299. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Miljanic, A.S.; Loy, X.; Gruenewald, D.L.; Dobbs, E.K.; Gottlieb, I.G.W.; Fletcher, R.J.; Brosi, B.J. Bee communities in forestry production landscapes: Interactive effects of local-level management and landscape context. Landsc. Ecol. 2019, 34, 1015–1032. [Google Scholar] [CrossRef]
- Pfeiffer, V.; Silbernagel, J.; Guédot, C.; Zalapa, J. Woodland and floral richness boost bumble bee density in cranberry resource pulse landscapes. Landsc. Ecol. 2019, 34, 979–996. [Google Scholar] [CrossRef]
- Hellerstein, D.; Hitaj, C.; Smith, D.; Davis, A. Land Use, Land Cover, and Pollinator Health: A Review and Trend Analyses; USDA Economic Research Service: Washington, DC, USA, 2017; 41p. [Google Scholar]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Harrison, T.; Gibbs, J.; Winfree, R. Anthropogenic landscapes support fewer rare bee species. Landsc. Ecol. 2019, 34, 967–978. [Google Scholar] [CrossRef]
- Bennett, J.M.; Steets, J.A.; Burns, J.H.; Burkle, L.A.; Vamosi, J.C.; Wolowski, M.; Arceo-Gómez, G.; Burd, M.; Durka, W.; Ellis, A.G.; et al. Land use and pollinator dependency drives global patterns of pollen limitation in the Anthropocene. Nat. Commun. 2020, 11, 3999. [Google Scholar] [CrossRef]
- Cruz-López, D.F.; Caamal-Cauich, I.; Pat-Fernández, V.G.; Reza-Salgado, J. Competitiveness of Mexico´s Hass avocado exports in the world market. Rev. Mex. Cienc. Agric. 2022, 13, 355–362. [Google Scholar] [CrossRef]
- Barsimantov, J.; Navia-Antezana, J. Forest cover change and land tenure change in Mexico´s avocado region: Is community forestry related to reduced deforestation for high value crops? Appl. Geogr. 2012, 32, 844–853. [Google Scholar] [CrossRef]
- Mas, J.F.; Lemoine-Rodríguez, R.; González, R.; López-Sánchez, J.; Piña-Garduño, A.; Herrera-Flores, E. Assesment of deforestation rates in Michoacán at detailed scale through a hybrid classification method of SPOT images. Madera Bosques 2017, 23, 119–131. [Google Scholar] [CrossRef]
- Figueroa-Figueroa, D.K.; Ramírez-Dávila, J.F.; Antonio-Némiga, X.; González-Huerta, A. Mapping of avocado in the south of the state of Mexico by digital image processing sentinel-2. Rev. Mex. Cienc. Agric. 2020, 11, 865–879. [Google Scholar] [CrossRef]
- García-Jiménez, C.I.; Vargas-Rodríguez, Y.L. Passive government, organized crime, and massive deforestation: The case of western Mexico. Conserv. Sci. Pract. 2021, 3, e562. [Google Scholar] [CrossRef]
- SIAP. Estadística de Producción Agrícola. Available online: http://infosiap.siap.gob.mx/gobmx/datosAbiertos.php (accessed on 29 April 2022).
- Charre-Medellín, J.F.; Mas, J.; Chang-Martínez, L.A. Potential expansion of Hass avocado cultivation under climate change scenarios threatens Mexican mountain ecosystems. Crop Pasture Sci. 2021, 72, 291–301. [Google Scholar] [CrossRef]
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; López-Ortiz, E.I. Wild felids in temperate forest remnants in an avocado plantation landscape in Michoacán, México. Southwest. Nat. 2018, 63, 137–142. [Google Scholar] [CrossRef]
- Villamil, L.; Astier, M.; Merlín, Y.; Ayala-Barajas, R.; Ramírez-García, E.; Martínez-Cruz, J.; Devoto, M.; Gavito, M.E. Management practices and diversity of flower visitors and herbaceous plants in conventional and organic avocado orchards in Michoacán, Mexico. Agroecol. Sustain. Food Syst. 2018, 42, 530–551. [Google Scholar] [CrossRef]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Willcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef]
- SEMARNAT. Diagnóstico. Situación Actual de los Polinizadores en México; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2021; 152p. [Google Scholar]
- Pinkus-Rendon, M.; Parra-Tabla, V.; Meléndez-Ramírez, V. Floral resource use and interaction between Apis mellifera and native bees in cucurbit crops in Yucatán, Mexico. Can. Entomol. 2005, 137, 441–449. [Google Scholar] [CrossRef]
- Franco-Sánchez, M.A.; Leos-Rodríguez, J.A.; Salas-González, J.M.; Acosta-Ramos, M.; García-Munguía, A. Analysis of costs and competitiveness in avocado production in Michoacán, Mexico. Rev. Mex. Cienc. Agric. 2018, 9, 391–404. [Google Scholar] [CrossRef]
- De-la-Vega-Rivera, A.; Merino-Pérez, L. Socio-environmental impacts of the avocado boom in the Meseta Purépecha, Michoacán, Mexico. Sustainability 2021, 13, 7247. [Google Scholar] [CrossRef]
- SAGARPA. Planeación Agrícola Nacional 2017–2030: Aguacate Mexicano; Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: Mexico City, Mexico, 2017. Available online: https://www.gob.mx/cms/uploads/attachment/file/257067/Potencial-Aguacate.pdf (accessed on 29 April 2022).
- IUCN Red List of Threatened Species. Version 2021-3. Available online: https://www.iucnredlist.org (accessed on 29 April 2022).
- DOF. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019 (accessed on 22 April 2022).
- Sáenz-Ceja, J.E.; Pérez-Salicrup, D.R. Avocado cover expansion in the Monarch Butterfly Biosphere Reserve, central Mexico. Conservation 2021, 1, 299–310. [Google Scholar] [CrossRef]
- Burke, R.A.; Frey, J.K.; Gangulli, A.; Stoner, K.E. Species distribution modelling supports “nectar corridor” hypothesis for migratory nectarivorous bats and conservation of tropical dry forest. Divers. Distrib. 2019, 25, 1399–1415. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.; Nakamura, M.; Peterson, A.T. Predicting species’ distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility Occurrence Download. Available online: https://doi.org/10.15468/dl.cpqbbp (accessed on 24 March 2022).
- Urquhart, F.A.; Urquhart, N.R. Overwintering areas and migratory routes of the monarch butterfly (Danaus plexippus, Lepidoptera: Danaidae) in North America, with special reference to the western population. Can. Entomol. 1977, 109, 1583–1589. [Google Scholar] [CrossRef]
- Castañeda, S.; Botello, F.; Sánchez-Cordero, V.; Sarkar, S. Spatio-temporal distribution of monarch butterflies along their migratory route. Front. Ecol. Evol. 2019, 7, 400. [Google Scholar] [CrossRef]
- Google Earth Pro. Available online: https://www.google.com/intl/es/earth/download/gep/agree.html (accessed on 17 June 2022).
- Salazar-García, S.; Cossio-Vargas, L.E.; González-Durán, I.J.L. La fertilización de sitio específico mejoró la productividad del aguacate ´Hass´ en huertos sin riego. Agric. Tec. Mex. 2009, 35, 436–445. Available online: http://www.scielo.org.mx/pdf/agritm/v35n4/v35n4a9.pdf (accessed on 29 April 2022).
- Tapia-Vargas, M.; Pedraza-Santos, M.E.; Larios-Guzmán, A.; Vidales-Fernández, I.; Guillén-Andrade, H.; Barradas-Vázquez, V.L. Variabilidad espacial de la lluvia por efecto de un sistema antigranizo en la franja aguacatera de Michoacán. Rev. Fitotec. Mex. 2012, 35, 91–96. Available online: http://www.scielo.org.mx/pdf/rfm/v35nspe5/v35nspe5a18.pdf (accessed on 29 April 2022). [CrossRef]
- Álvarez-Bravo, A.; Salazar-García, S.; Ruiz-Corral, J.A.; Medina-García, G. Escenarios de cómo el cambio climático modificará las zonas productoras de aguacate “hass” en Michoacán. Rev. Mex. Cienc. Agric. 2017, 19, 4035–4048. [Google Scholar] [CrossRef][Green Version]
- Cossio-Vargas, L.E.; Salazar-García, S.; González-Durán, I.J.L.; Medina-Torres, R. Fenología del aguacate ‘Hass’ en el clima semicálido de Nayarit, México. Rev. Chapingo Ser. Hortic. 2008, 14, 319–324. [Google Scholar] [CrossRef]
- Lara-Díaz, A.V.; Ramírez-Dávila, J.F.; Maldonado-Zamora, F.I.; Rivera-Martínez, R.; Acosta-Guadarrama, A.D.; Lára-Vázquez, F. Spatial modeling of the Oligonychus parseae (Tuttle, Baker and Abatiello, 1976) populations in the state of Mexico. Rev. Fitotec. Mex. 2020, 43, 411–419. Available online: https://revistafitotecniamexicana.org/documentos/43-4/6a.pdf (accessed on 29 April 2022).
- Reyes-Alemán, J.C.; Mejía-Caranza, J.; Monteagudo-Rodríguez, O.R.; Valdez-Pérez, M.E.; González-Díaz, J.G.; Espíndola-Barquera, M.C. Phenology of the ‘Hass’ avocado in the state of Mexico, Mexico. Rev. Chapingo Ser. Hortic. 2021, 27, 113–134. [Google Scholar] [CrossRef]
- Arenas-Navarro, A.; García-Oliva, F.; Torres-Miranda, A.; Téllez-Valdés, O.; Oyama, K. Environmental filters determine the distribution of tree species in a threatened biodiversity hotspot in western Mexico. Bot. Sci. 2020, 98, 219–237. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- INEGI. Conjunto de Datos Vectoriales Edafológico, Escala 1:250000 Serie II (Continuo Nacional). Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 29 April 2022).
- USGS. Global 30 Arc-Second Hydrological 1 Kilometer. Available online: https://lta.cr.usgs.gov/HYDRO1K (accessed on 29 April 2022).
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; Valdez-Leal, J.D.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruz, C.; Rubio-Rocha, Y. Distribución potencial histórica y contemporánea de la familia Psittacidae en México. Rev. Mex. Biodivers. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 April 2022).
- FAO. World Reference Base for Soil Resources 2006; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; 145p, Available online: https://www.fao.org/3/a0510e/a0510e.pdf (accessed on 22 June 2022).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10373. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Bastos-Araújo, M. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, UK, 2011; 316p. [Google Scholar]
- Peterson, T.; Papes, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Modell. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Escalante, T.; Rodríguez-Tapia, G.; Linaje, M.; Illoldi-Rangel, P.; González-López, R. Identification of areas of endemism from species distribution models: Thresholds selection and Nearctic mammals. TIP Rev. Espec. Cienc. Quim.-Biol. 2013, 16, 5–17. [Google Scholar] [CrossRef]
- INEGI. Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación, Escala 1:250,000 Serie VII (Capa Unión). Available online: https://www.inegi.org.mx/app/biblioteca/ficha.html?upc=889463842781 (accessed on 29 April 2022).
- EPA. Ecoregions of North America. Available online: https://www.epa.gov/eco-research/ecoregions-north-america (accessed on 29 April 2022).
- CONANP. Áreas Naturales Protegidas Federales de México. Available online: http://sig.conanp.gob.mx/website/pagsig/info_shape.htm (accessed on 29 April 2022).
- Sánchez-González, A.; López-Mata, L. Plant species richness and diversity along an altitudinal gradient in the Sierra Nevada, Mexico. Divers. Distrib. 2005, 11, 567–575. [Google Scholar] [CrossRef]
- Cortés-Flores, J.; Cornejo-Tenorio, G.; Ibarra-Manríquez, G. Flowering phenology and pollination syndromes in species with different growth forms in a Neotropical temperate forest of Mexico. Botany 2015, 93, 361–367. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E. Modelling the potential geographic distribution of an endangered pollination corridor in Mexico and the United States. Divers. Distrib. 2017, 23, 67–78. [Google Scholar] [CrossRef]
- Sáenz-Ceja, J.E.; Arenas-Navarro, M.; Torres-Miranda, A. Prioritization of conservation areas and vulnerability analyses of the genus Pinus L. (Pinaceae) in Mexico. J. Nat. Conserv. 2022, 67, 126171. [Google Scholar] [CrossRef]
- Dubrovina, I.A.; Bautista, F. Analysis of the suitability of various soil groups and types of climate for avocado growing in the state of Michoacán, Mexico. Eurasian Soil Sci. 2014, 47, 491–503. [Google Scholar] [CrossRef]
- Ramírez-Mejía, D.; Levers, C.; Mas, J.F. Spatial patterns and determinants of avocado frontier dynamics in Mexico. Reg. Environ. Chang. 2022, 22, 28. [Google Scholar] [CrossRef]
- Molina-Sánchez, A.; Delgado, P.; González-Rodríguez, A.; González, C.; Gómez-Tagle-Rojas, A.F.; Lopez-Toledo, L. Spatio-temporal approach for identification of critical conservation areas: A case study with two pine species from a threatened temperate forest in Mexico. Biodivers. Conserv. 2019, 28, 1863–1883. [Google Scholar] [CrossRef]
- Arima, E.Y.; Denvir, A.; Young, K.R.; González-Rodríguez, A.; García-Oliva, F. Modelling avocado-driven deforestation in Michoacán, Mexico. Environ. Res. Lett. 2022, 17, 034015. [Google Scholar] [CrossRef]
- Alves-Ferreira, P.; Boscolo, D.; Elsinor-Lopes, L.; Carvalheiro, L.G.; Biesmeijer, J.C.; Bernardo-da Rocha, P.L.; Felipe-Viana, B. Forest and connectivity loss simplify tropical pollination networks. Oecologia 2020, 192, 577–590. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Assessing role of major drivers in recent decline of monarch butterfly population in North America. Front. Environ. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Correa-Ayram, C.A.; Mendoza, M.E.; Etter, A.; Pérez-Salicrup, D.R. Effect of the landscape matrix condition for prioritizing multispecies connectivity conservation in a highly biodiverse landscape of central Mexico. Reg. Environ. Chang. 2019, 19, 149–163. [Google Scholar] [CrossRef]
- Rubí-Arriaga, M.; Franco-Malvaíz, A.L.; Rebollar-Rebollar, S.; Bobadilla-Soto, E.E.; Martínez-de la Cruz, I.; Siles-Hernández, Y. Situación actual del cultivo de aguacate (Persea americana Mill.) en el estado de México, México. Trop. Subtrop. Agroecosystems 2013, 16, 93–101. Available online: https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/1633 (accessed on 17 June 2022).
- Figueroa, F.; Sánchez-Cordero, V. Effectiveness of natural protected areas to prevent land use and land cover change in Mexico. Biodivers. Conserv. 2008, 17, 3223–3240. [Google Scholar] [CrossRef]
- Villers-Ruiz, L.; Trejo-Vázquez, I. Climate change in Mexican forests and natural protected areas. Glob. Environ. Chang. 1998, 8, 141–157. [Google Scholar] [CrossRef]
- Chávez-González, H.; González-Guillén, M.J.; Hernández-de la Rosa, P. Methodologies to find priority areas for the conservation of natural ecosystems. Rev. Mex. Cienc. For. 2015, 6, 8–23. [Google Scholar] [CrossRef][Green Version]
- Brandon, K.; Gorenflo, L.J.; Rodrigues, A.S.L.; Waller, R.W. Reconciling biodiversity conservation, people, protected areas, and agricultural suitability in Mexico. World Dev. 2005, 33, 1403–1418. [Google Scholar] [CrossRef]
- Arizmendi, M.C.; Berlanga, H.; Rodríguez-Flores, C.; Vargas-Canales, V.; Montes-Leyva, L.; Lira, R. Hummingbird conservation in Mexico: The natural protected areas system. Nat. Areas J. 2016, 36, 366–376. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E., Jr.; Moreno-Talamantes, A.; Flores-Maldonado, J.J. Impacts of land cover change on the plant resources of an endangered pollinator. PeerJ 2021, 9, e11990. [Google Scholar] [CrossRef]
- Martínez-Méndez, N.; Aguirre-Planter, E.; Eguiarte, L.E.; Jaramillo-Correa, J.P. Modelado del nicho ecológico de las especies del género Abies (Pinaceae) en México: Algunas implicaciones taxonómicas y para la conservación. Bot. Sci. 2016, 94, 5–24. [Google Scholar] [CrossRef]
- Olaya-Arenas, P.; Kaplan, I. Quantifying pesticide exposure risk for monarch caterpillars on milkweeds bordering agricultural land. Front. Ecol. Evol. 2019, 7, 223. [Google Scholar] [CrossRef]
- Castañeda-Vildózola, A.; Equihua-Martínez, A.; Valdés-Carrasco, J.; Barrientos-Priego, A.F.; Ish-Am, G.; Gazit, S. Insectos polinizadores del aguacatero en los estados de México y Michoacán. Rev. Chapingo Ser. Hortic. 1999, 5, 129–136. [Google Scholar]
- Lara-Pulido, J.A.; Guevara-Sanginés, A.; Torres-Rojo, J.M.; Núñez-Hernández, J.M.; Riojas, J.; Pérez-Cirera, V.; Breceda, K.; Barragán, M.J.; Ezzine-de-Blas, D.; Jiménez-Quiroga, C. Honey-guacamole: Assessment of pollination environmental service in avocado production in Michoacan, Mexico. Acta Univ. 2021, 31, e3083. [Google Scholar] [CrossRef]
- Martínez-López, O.; Koch, J.B.; Martínez-Morales, M.A.; Navarrete-Gutiérrez, D.; Enríquez, E.; Vandame, R. Reduction in the potential distribution of bumble bees (Apidae: Bombus) in Mesoamerica under different climate change scenarios: Conservation implications. Glob. Chang. Biol. 2021, 27, 1772–1787. [Google Scholar] [CrossRef]
- Prieto-Torres, D.A.; Nuñez-Rosas, L.E.; Remolina-Figueroa, D.; Arizmendi, M.C. Most hummingbirds lose under climate change and land-use change: Long-term conservation implications. Perspect. Ecol. Conserv. 2021, 19, 487–499. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E., Jr. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 9, 14048. [Google Scholar] [CrossRef]
- Brittain, C.A.; Vighi, M.; Bommarco, R.; Settele, J.; Potts, S.G. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 2010, 11, 106–115. [Google Scholar] [CrossRef]
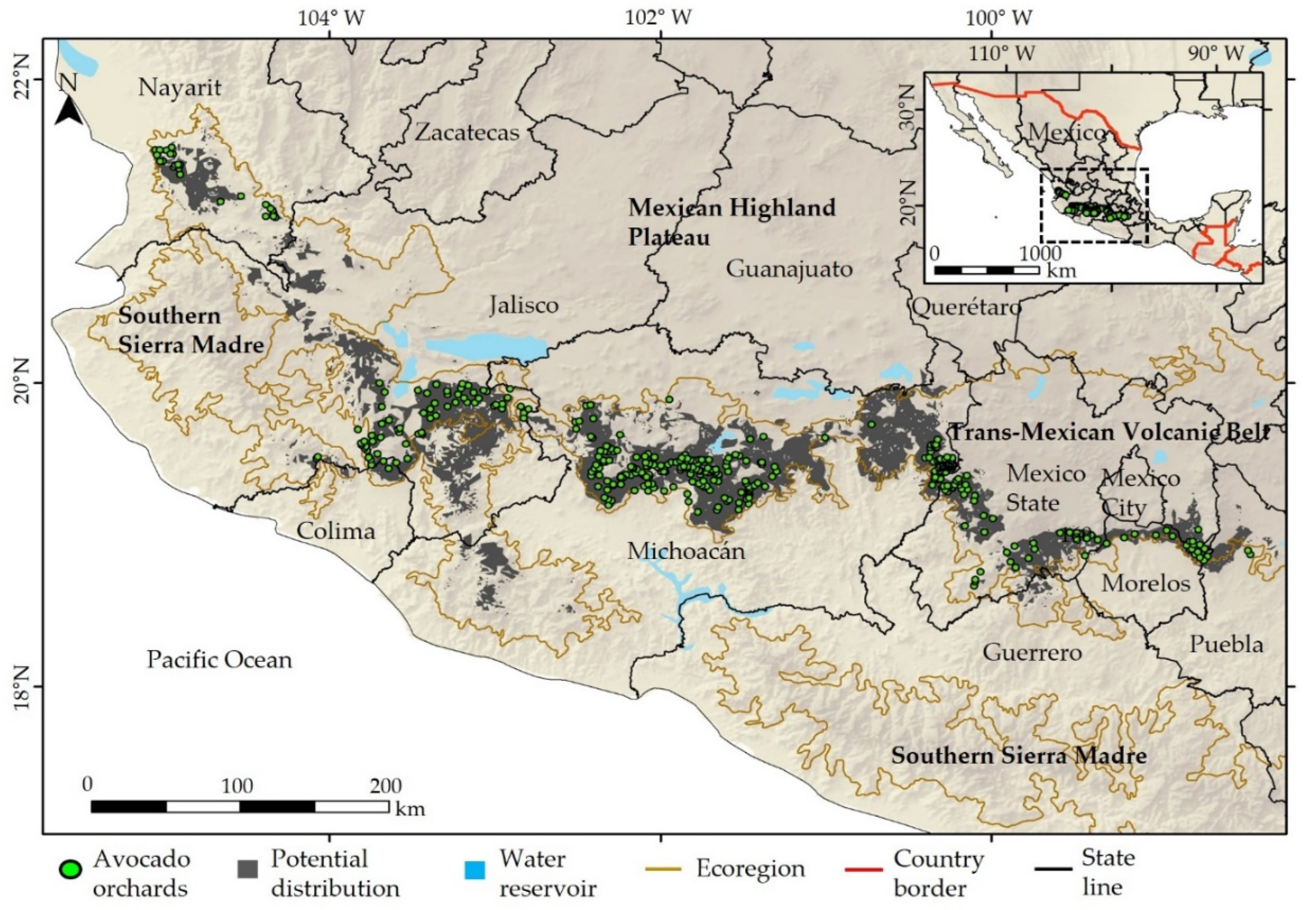


| Scientific Name | Common Name | Family | Habitat | Distribution | Risk Category | Number of Occurrences | |
|---|---|---|---|---|---|---|---|
| IUCN Red List | Mexican Endangered Species Act | ||||||
| Class Insecta | |||||||
| Bombus brachycephalus Handlirsch | Short-headed bumble bee | Apidae | POF, TDF | MX, CA | EN | - | 73 |
| Bombus diligens Smith | Abejón bumble bee | Apidae | POF, TDF | MX | NT | - | 162 |
| Bombus steindachneri Handlirsch | Steindachner’s bumble bee | Apidae | POF, TDF | MX | EN | - | 194 |
| Danaus plexippus Linnaeus | Monarch butterfly | Nymphalidae | FF, PF, POF | CN, US, MX | EN | PR | 668 |
| Class Mammalia | |||||||
| Choeronycteris mexicana Tschudi | Mexican long-tongued bat | Phyllostomidae | TEF, TDF, POF | US, MX, CA | - | A | 536 |
| Corynorhinus mexicanus Allen | Mexican big-eared bat | Vespertilionidae | TDF, POF, PF | MX | NT | - | 175 |
| Leptonycteris nivalis Saussure | Mexican long-nosed bat | Phyllostomidae | DS, POF, PF | US, MX | - | A | 239 |
| Leptonycteris yerbabuenae Martínez & Villa | Lesser long-nosed bat | Phyllostomidae | DS, TDF, POF | US, MX | - | PR | 790 |
| Class Aves | |||||||
| Selasphorus rufus Gmelin | Rufous hummingbird | Trochilidae | DS, POF, PF | CN, US, MX | - | A | 1976 |
| Tilmatura dupontii Lesson | Sparkling-tailed woodstar | Trochilidae | TDF, POF | MX, CA | - | A | 567 |
| Key | Bioclimatic Variable |
|---|---|
| Bio01 | Annual mean temperature |
| Bio02 | Mean diurnal range |
| Bio03 | Isothermality |
| Bio04 | Temperature seasonality |
| Bio05 | Maximal temperature of the warmest month |
| Bio06 | Minimum temperature of the coldest month |
| Bio07 | Temperature annual range |
| Bio08 | Mean temperature of the wettest quarter |
| Bio09 | Mean temperature of the driest quarter |
| Bio10 | Mean temperature of the warmest quarter |
| Bio11 | Mean temperature of the coldest quarter |
| Bio12 | Annual precipitation |
| Bio13 | Precipitation of the wettest month |
| Bio14 | Precipitation of the driest month |
| Bio15 | Precipitation seasonality |
| Bio16 | Precipitation of the wettest quarter |
| Bio17 | Precipitation of the driest quarter |
| Bio18 | Precipitation of the warmest quarter |
| Bio19 | Precipitation of the coldest quarter |
| Elev | Elevation |
| Soil | Soil type * |
| Species | AUC | Partial-ROC | Z-Value | p-Value | Variable and Contribution (%) | ||
|---|---|---|---|---|---|---|---|
| Persea americana | 0.982 | 1.962 | 8434.6 | <0.001 | Soil (25.4) | Elev (17.7) | Bio16 (15.9) |
| Bombus brachycephalus | 0.963 | 1.856 | 1071.7 | <0.001 | Elev (26.9) | Bio16 (23.1) | Bio12 (12.3) |
| Bombus diligens | 0.953 | 1.827 | 2401.7 | <0.001 | Elev (32.3) | Bio04 (22.4) | Bio13 (16.8) |
| Bombus steindachneri | 0.928 | 1.685 | 821.8 | <0.001 | Bio15 (31.2) | Bio06 (15.2) | Bio04 (13.3) |
| Danaus plexippus | 0.944 | 1.732 | 1934.4 | <0.001 | Bio07 (23.5) | Elev (18.9) | Bio09 (11.4) |
| Choeronycteris mexicana | 0.817 | 1.313 | 867.8 | <0.001 | Bio06 (28.5) | Elev (15.8) | Bio07 (13.2) |
| Corynorhinus mexicanus | 0.957 | 1.693 | 1250.3 | <0.001 | Elev (59.7) | Bio05 (7.7) | Bio04 (5.0) |
| Leptonycteris nivalis | 0.897 | 1.555 | 1142.6 | <0.001 | Bio07 (27.1) | Bio19 (16.3) | Elev (14.1) |
| Leptonycteris yerbabuenae | 0.828 | 1.373 | 884.3 | <0.001 | Bio06 (37.8) | Bio15 (29.3) | Bio12 (5.4) |
| Selasphorus rufus | 0.835 | 1.502 | 2448.7 | <0.001 | Bio15 (19.4) | Elev (11.7) | Bio10 (10.7) |
| Tilmatura dupontii | 0.936 | 1.759 | 3134 | <0.001 | Bio16 (30.6) | Bio04 (15) | Bio15 (8.7) |
| Species | Ecoregions | Potential Distribution Mexico (km2) | Habitat Loss Mexico (%) | Potential Distribution TMVB (km2) | Habitat Loss TMBV (%) |
|---|---|---|---|---|---|
| Bombus brachycephalus | CASM, ESM, SSM, TMVB | 34,702 | 26.5 | 14,840 | 57.8 |
| Bombus diligens | ESM, MHP, SSM, TMVB, WSM | 51,933 | 23.1 | 18,347 | 56.2 |
| Bombus steindachneri | SSM, TMVB, WSM | 104,355 | 5.4 | 14,279 | 36.1 |
| Danaus plexippus | ESM, MHP, TMVB | 31,286 | 16.7 | 9777 | 48.9 |
| Choeronycteris mexicana | CASM, ESM, MC, MHP, SSM, TMVB, TTSP, WD, WPCP, WSM, WSMP | 353,704 | 0.8 | 27,601 | 30.3 |
| Corynorhinus mexicanus | ESM, MHP, TMVB, WSM, WSMP | 84,720 | 9.1 | 29,477 | 25.1 |
| Leptonycteris nivalis | ESM, ID, MHP, TMVB, SSM, WD, WSM | 162,902 | 6.6 | 24,122 | 38.6 |
| Leptonycteris yerbabuenae | ESM, ID, MHP, SSM, SPCP, TMVB, WD, WPCP | 256,467 | 3.1 | 16,994 | 34.8 |
| Selasphorus rufus | MHP, SSM, TMVB, WSM | 181,356 | 9.4 | 42,626 | 33.9 |
| Tilmatura dupontii | CASM, SSM, TMVB | 56,240 | 15.5 | 12,408 | 54.4 |
| Number of Species | Potential Extent (km2) | Protected Extent (km2) | Protected Areas |
|---|---|---|---|
| 9–10 | 653 | 56 | 1, 2, 4 |
| 7–8 | 4674 | 443 | 1, 2, 3, 4, 6, 8, 11 |
| 5–6 | 6549 | 500 | 1, 2, 3, 4, 6, 7, 8, 9, 10, 11 |
| 3–4 | 4303 | 530 | 1, 2, 4, 5, 7, 9, 10, 11 |
| 1–2 | 481 | 60 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáenz-Ceja, J.E.; Sáenz-Reyes, J.T.; Castillo-Quiroz, D. Pollinator Species at Risk from the Expansion of Avocado Monoculture in Central Mexico. Conservation 2022, 2, 457-472. https://doi.org/10.3390/conservation2030031
Sáenz-Ceja JE, Sáenz-Reyes JT, Castillo-Quiroz D. Pollinator Species at Risk from the Expansion of Avocado Monoculture in Central Mexico. Conservation. 2022; 2(3):457-472. https://doi.org/10.3390/conservation2030031
Chicago/Turabian StyleSáenz-Ceja, Jesús E., J. Trinidad Sáenz-Reyes, and David Castillo-Quiroz. 2022. "Pollinator Species at Risk from the Expansion of Avocado Monoculture in Central Mexico" Conservation 2, no. 3: 457-472. https://doi.org/10.3390/conservation2030031
APA StyleSáenz-Ceja, J. E., Sáenz-Reyes, J. T., & Castillo-Quiroz, D. (2022). Pollinator Species at Risk from the Expansion of Avocado Monoculture in Central Mexico. Conservation, 2(3), 457-472. https://doi.org/10.3390/conservation2030031






