Common Buckthorn Engineered Biochar (Rhamnus Cathartica), Calcined Quagga Mussel Shells (Dreissena Rostriformis), Pickled Steel, and Steel Slag as Filter Media for the Sorption of Phosphorus from Agricultural Runoff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Characterization of Materials
2.2.2. Batch Testing
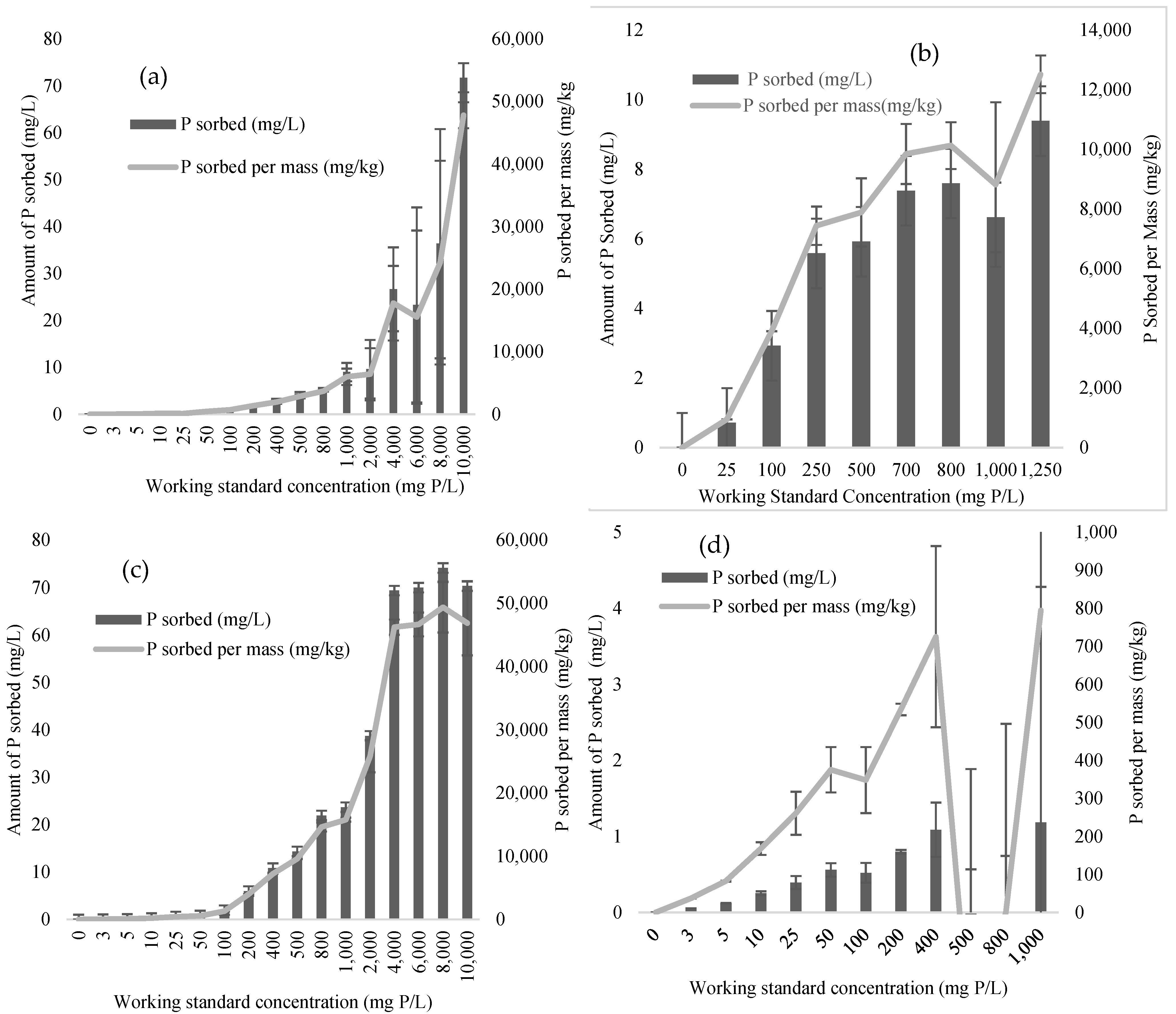
Isotherm Study
Kinetic Study
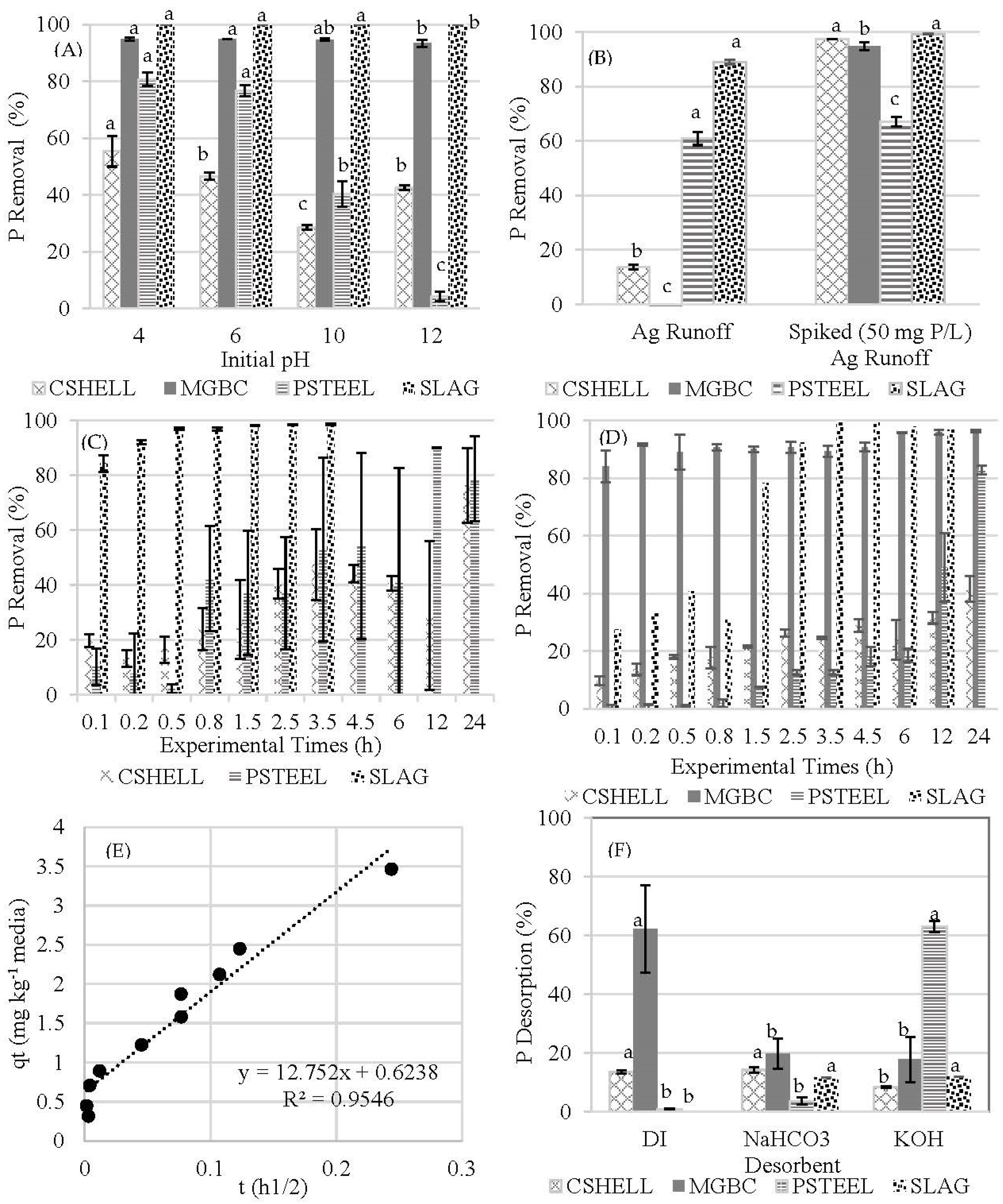
pH and Real Agricultural Runoff Performance Study
Desorption Study
2.3. Statistical Analysis and Evaluating Applicability of Filter Medias
3. Results
3.1. Characterization of Materials
3.2. Adsorption Isotherm Study
3.3. Impact of pH
3.4. Reaction Rate
3.5. Desorption
3.6. Efficacy of the Media in Agricultural Runoff
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, P.; Klump, J.V.; Guo, L. Variations in Chemical Speciation and Reactivity of Phosphorus between Suspended-Particles and Surface-Sediment in Seasonal Hypoxia-Influenced Green Bay. J. Great Lakes Res. 2018, 44, 864–874. [Google Scholar] [CrossRef]
- Robertson, D.M.; Saad, D.A. Nutrient Inputs to the Laurentian Great Lakes by Source and Watershed Estimated Using SPARROW Watershed Models. J. Am. Water Resour. Assoc. 2011, 47, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zhang, M.; Dahlgren, R.A.; Eitzel, M. A Review of Vegetated Buffers and a Meta-Analysis of Their Mitigation Efficacy in Reducing Nonpoint Source Pollution. J. Environ. Qual. 2010, 39, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Kalcic, M.; Crumpton, W.; Liu, X.; D’Ambrosio, J.; Ward, A.; Witter, J. Assessment of Beyond-the-Field Nutrient Management Practices for Agricultural Crop Systems with Subsurface Drainage. J. Soil Water Conserv. 2018, 73, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T.A. How Effective Are Created or Restored Freshwater Wetlands for Nitrogen and Phosphorus Removal? A Systematic Review. Environ. Evid. 2016, 5, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kayhanian, M.; Mckenzie, E.R.; Leatherbarrow, J.E.; Young, T.M. Characteristics of Road Sediment Fractionated Particles Captured from Paved Surfaces, Surface Run-off and Detention Basins. Sci. Total Environ. 2012, 439, 172–186. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Zhao, X.; Jiang, C. Agricultural Runoff Pollution Control by a Grassed Swales Coupled with Wetland Detention Ponds System: A Case Study in Taihu Basin, China. Environ. Sci. Pollut. Res. 2016, 23, 9093–9104. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Kirkkala, T.; Ventelä, A.-M.; Tarvainen, M. Long-Term Field-Scale Experiment on Using Lime Filters in an Agricultural Catchment. J. Environ. Qual. 2012, 41, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter Materials for Phosphorus Removal from Wastewater in Treatment Wetlands—A Review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Nguyen, T.T.; Vu, N.D.; Soda, S.; Nguyen, T.H.H.; Nguyen, M.K.; Tran, T.V.H.; Dang, T.T.; et al. White Hard Clam (Meretrix Lyrata) Shells as Novel Filter Media to Augment the Phosphorus Removal from Wastewater. Sci. Total Environ. 2020, 741, 140483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, R.; Zou, W.; Wei, W.; Li, J.; Wei, W.; Ni, B.; Chen, H. Integrating High-Efficiency Oxygen Evolution Catalysts Featuring Accelerated Surface Reconstruction from Waste Printed Circuit Boards via a Boriding Recycling Strategy. Appl. Catal. B Environ. 2021, 298, 120583. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, R.; Wei, W.; Wei, W.; Zou, W.; Li, J.; Ni, B.; Chen, H. Recycling Spent Water Treatment Adsorbents for Efficient Electrocatalytic Water Oxidation Reaction. Resour. Conserv. Recycl. 2022, 178, 106037. [Google Scholar] [CrossRef]
- Castro do Nascimento, A.; Figueiredo do Nascimento, B.; da Silva, M.P.; Silva Santos, R.; Pereira Neves, T.; de Araujo, C.M.B.; de Luna, F.E.T.; da Motta Sobrinho, M.A. Use of Charcoal from Gasification Residues in Adsorption Pilot Plant for the Practical Application of Circular Economy in Industrial Wastewater Treatment. Chem. Eng. Commun. 2022, 209, 1316–1333. [Google Scholar] [CrossRef]
- Nobaharan, K.; Novair, S.B.; Lajayer, B.A.; Van Hullebusch, E.D. Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Huong, P.T.; Jitae, K.; Giang, B.L.; Nguyen, T.D.; Thang, P.Q. Novel Lanthanum-Modified Activated Carbon Derived from Pine Cone Biomass as Ecofriendly Bio-Sorbent for Removal of Phosphate and Nitrate in Wastewater. Rend. Lincei. Sci. Fis. Nat. 2019, 30, 637–647. [Google Scholar] [CrossRef]
- Hudson, R.M. Pickling and Descaling. Met. Finish. 1995, 93, 67–78. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of Nitrate and Phosphate Adsorption on Al-Modified Biochar: Influence of Al Content. Sci. Total Environ. 2018, 631–632, 859–903. [Google Scholar] [CrossRef]
- Bolster, C.H.; Hornberger, G.M. On the Use of Linearized Langmuir Equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Caio, B.; Fernandes, C.; Mendes, K.; Junior, A.; Da, V.; Caldeira, S.; Da Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; Souza, M.; et al. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- House, W.A. The Physico-Chemical Conditions for the Precipitation of Phosphate with Calcium. Environ. Technol. 1999, 20, 727–733. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.J. Phosphate Adsorption/Desorption Kinetics and P Bioavailability of Mg-Biochar from Ground Coffee Waste. J. Water Process Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Cho, S.; Ko, S.J.; Yoo, J.S.; Park, J.C.; Yoo, Y.H.; Kim, J.G. Optimization of Pickling Solution for Improving the Phosphatability of Advanced High-Strength Steels. Materials 2021, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Shilton, A.; Pratt, S.; Drizo, A.; Mahmood, B.; Banker, S.; Billings, L.; Glenny, S.; Luo, D. “Active” Filters for Upgrading Phosphorus Removal from Pond Systems. Water Sci. Technol. 2005, 51, 111–116. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Westholm, L.J.; Sillanpää, M. Steel Slag as a Low-Cost Sorbent for Metal Removal in the Presence of Chelating Agents. J. Ind. Eng. Chem. 2015, 27, 115–125. [Google Scholar] [CrossRef]
- Penn, C.; Livingston, S.; Shedekar, V.; King, K.; Williams, M. Performance of Field-Scale Phosphorus Removal Structures Utilizing Steel Slag for Treatment of Subsurface Drainage. Water 2020, 12, 443. [Google Scholar] [CrossRef]
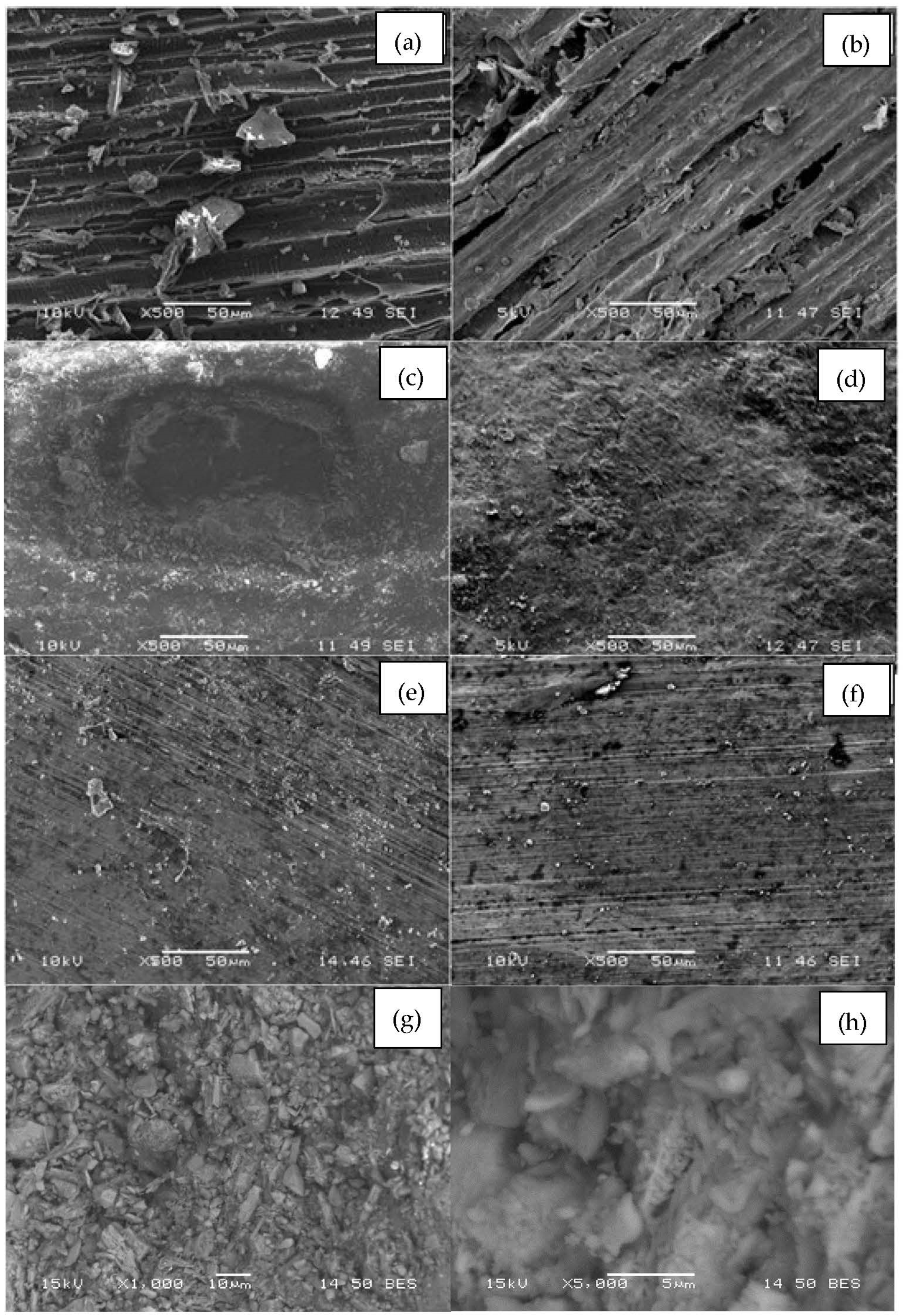
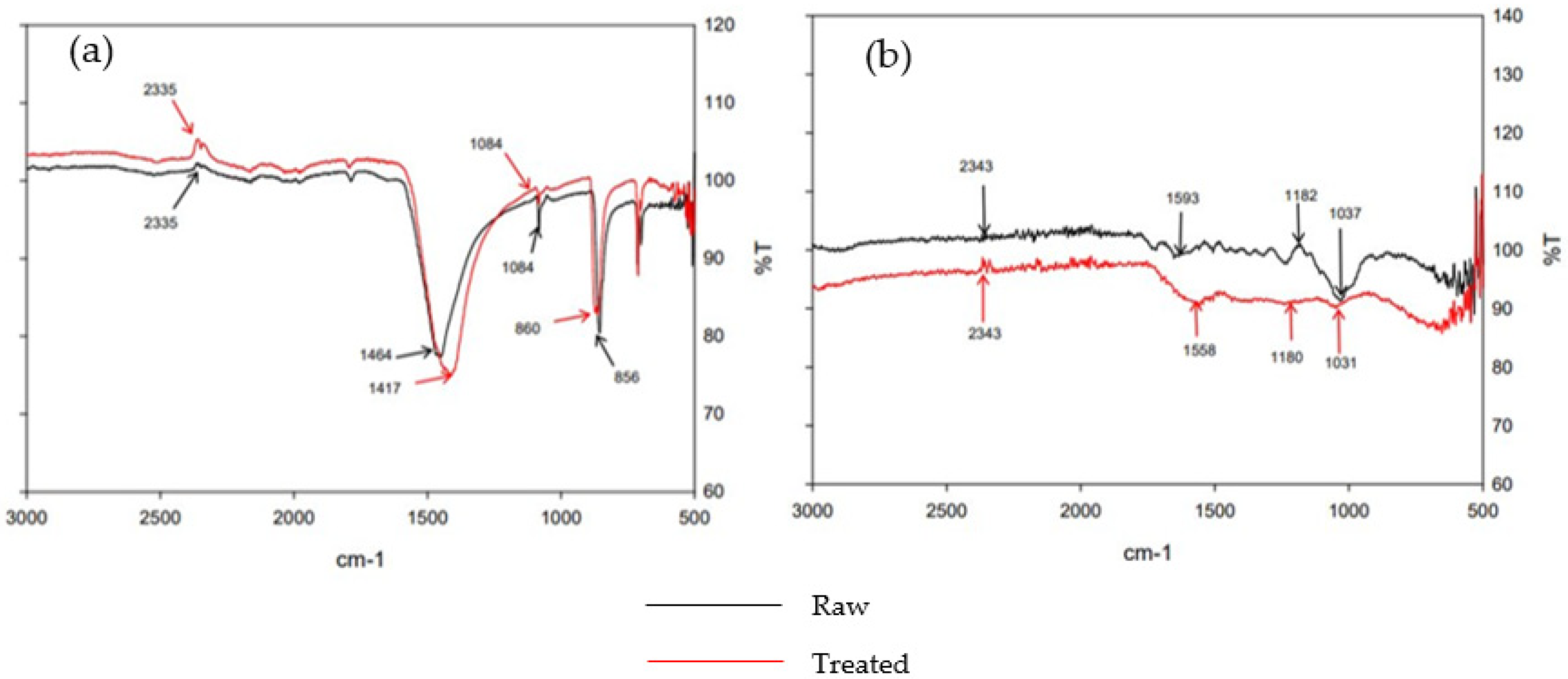
| Media | Pore Size (Radius Å) | Pore Volume (cc g−1) | Surface Area (m2 g−1) |
|---|---|---|---|
| Raw buckthorn | 15.6 | 0.0050 | 1.0 |
| MGBC | 17.5 | 0.0210 | 7.9 |
| Raw shell | 19.5 | 0.0110 | 6.0 |
| CSHELL | 19.7 | 0.0140 | 5.8 |
| Raw steel | 17.5 | 0.0000 | 0.1 |
| PSTEEL | 16.5 | 0.0000 | 0.3 |
| SLAG | 16.2 | 0.0150 | 3.2 |
| Isotherm Constants and Coefficients of Determination | ||||||||
|---|---|---|---|---|---|---|---|---|
| Isotherm model | Langmuir | Freundlich | ||||||
| Media | qm (mg kg−1) | KL (L mg−1) | R2 | KF (mg kg)(L kg−1)1/n | 1 n−1 | R2 | ||
| CSHELL | 64,419 a | 0.0005 | 0.84 | 91 | 1.18 | 0.92 | ||
| MGBC | 50,642 ab | 0.0019 | 0.92 | 2561 | 3.11 | 0.96 | ||
| SLAG | 14,541 bc | 0.003 | 0.86 | 602 | 2.36 | 0.87 | ||
| PSTEEL | 736 c | 0.0269 | 0.84 | 70 | 2.58 | 0.86 | ||
| Kinetic constants and coefficients of determination for the 50 mg P L−1 trials | ||||||||
| Kinetic model | Pseudo first-order model | Pseudo second-order model | ||||||
| Media | qe (mg kg−1) | K1 (1 h−1) | R2 | qe (mg kg−1) | k2 (g mg−1 h −1) | R2 | ||
| CSHELL | 0.15 | 0.67 | 0.67 | 0.16 | 24 | 0.99 | ||
| MGBC | 232 | 0.04 | 0.04 | 1250 | 0.01 | 0.99 | ||
| SLAG | 1.09 | 0.39 | 0.90 | 0.78 | 8.93 | 0.92 | ||
| PSTEEL | 0.11 | 0.92 | 0.84 | 0.2 | 0.49 | 0.37 | ||
| Kinetic constants and coefficients of determination for the 2 mg P L−1 trials | ||||||||
| Kinetic model | Pseudo first-order model | Pseudo second-order model | ||||||
| Media | qe (mg kg−1) | K1 (1 h−1) | R2 | qe (mg kg−1) | k2 (g mg−1 h −1) | R2 | ||
| CSHELL | 0.05 | 0.00 | 0.00 | 0.11 | 160 | 0.98 | ||
| SLAG | 0.004 | −1.33 | 0.38 | 0.03 | 350 | 0.99 | ||
| PSTEEL | 0.03 | 0.01 | 0.25 | 0.27 | 7.14 | 0.39 | ||
| Evaluation Criteria | Weight | Ideal Media | CSHELL | EZEO | MGBC | PSTEEL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Score | Rank | Score | Rank | Score | Rank | Score | Rank | Score | ||||||
| Efficacy in ag runoff | 3 | High | 9 | Low | 3 | High | 9 | None | 0 | High | 6 | ||||
| P Sorption capacity | 2 | High | 6 | High | 6 | Low | 2 | High | 6 | Low | 2 | ||||
| Mechanical strength | 2 | High | 6 | Low | 2 | High | 6 | Low | 2 | High | 6 | ||||
| P removal kinetics | 1 | High | 3 | Medium | 2 | High | 3 | High | 3 | Low | 1 | ||||
| Reuse potential | 1 | High | 3 | Medium | 2 | Low | 1 | Low | 1 | High | 3 | ||||
| Availability in Midwest | 1 | High | 3 | Medium | 2 | Low | 1 | High | 3 | High | 3 | ||||
| Energy consumption | 1 | Low | 3 | Medium | 2 | High | 1 | High | 1 | Low | 3 | ||||
| Cost | 1 | Low | 3 | Low | 3 | High | 1 | Medium | 2 | High | 1 | ||||
| CUMULATIVE SCORE | 36 | 22 | 24 | 18 | 25 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holly, M.A.; Sanford, J.R.; Forsythe, P.S.; Silva, M.R.; Lakich, D.D.; Swan, C.K.; Leonard, K.A. Common Buckthorn Engineered Biochar (Rhamnus Cathartica), Calcined Quagga Mussel Shells (Dreissena Rostriformis), Pickled Steel, and Steel Slag as Filter Media for the Sorption of Phosphorus from Agricultural Runoff. Conservation 2022, 2, 726-738. https://doi.org/10.3390/conservation2040047
Holly MA, Sanford JR, Forsythe PS, Silva MR, Lakich DD, Swan CK, Leonard KA. Common Buckthorn Engineered Biochar (Rhamnus Cathartica), Calcined Quagga Mussel Shells (Dreissena Rostriformis), Pickled Steel, and Steel Slag as Filter Media for the Sorption of Phosphorus from Agricultural Runoff. Conservation. 2022; 2(4):726-738. https://doi.org/10.3390/conservation2040047
Chicago/Turabian StyleHolly, Michael A., Joseph R. Sanford, Patrick S. Forsythe, Marcia R. Silva, Daniel D. Lakich, Camryn K. Swan, and Keenan A. Leonard. 2022. "Common Buckthorn Engineered Biochar (Rhamnus Cathartica), Calcined Quagga Mussel Shells (Dreissena Rostriformis), Pickled Steel, and Steel Slag as Filter Media for the Sorption of Phosphorus from Agricultural Runoff" Conservation 2, no. 4: 726-738. https://doi.org/10.3390/conservation2040047
APA StyleHolly, M. A., Sanford, J. R., Forsythe, P. S., Silva, M. R., Lakich, D. D., Swan, C. K., & Leonard, K. A. (2022). Common Buckthorn Engineered Biochar (Rhamnus Cathartica), Calcined Quagga Mussel Shells (Dreissena Rostriformis), Pickled Steel, and Steel Slag as Filter Media for the Sorption of Phosphorus from Agricultural Runoff. Conservation, 2(4), 726-738. https://doi.org/10.3390/conservation2040047





