Abstract
The population of the endangered southern greater glider (Petauroides volans) is rapidly declining across coastal lowlands in New South Wales, Australia. Here, we focus on a typical coastal lowland glider population in Seven Mile Beach National Park, New South Wales, that is declining primarily due to habitat fragmentation. This study aimed to assess the population’s status and viability in order to guide conservation efforts. Using the double-observer distance sampling method, we estimated the population size to be 347 gliders, with a density of 0.46 gliders/ha. Population viability analysis revealed a high extinction risk, with only a 1% chance of survival over the next 50 years. Fire was identified as the primary threat, followed by a low effective population size and inbreeding. The most effective conservation strategy involved genetic reinforcement through possible translocation and the installation of up to 50 nest boxes to improve habitat (hollow) availability.
1. Introduction
Over the past two decades, data collected at long-term monitoring sites have documented concerning reductions in southern greater glider, i.e., Petauroides volans, populations and instances of localised extinctions [,,]. Both central and southern greater gliders have only recently been uplisted in Australia to ‘endangered’ under the Environmental Protection and Biodiversity Conservation Act (1999). Southern greater gliders have physiological and ecological characteristics that increase their extinction potential, making them vulnerable to habitat loss and fragmentation [,,,], fire [,,], and climate change [,]. Also, as populations continue to decline and become isolated, they may also be threatened by genetic processes such as genetic drift and inbreeding depression [,]. It is important to investigate southern greater glider population viability across their range in order to appropriately manage the species and increase their chances of persistence into the future.
Recently, numerous southern greater glider populations living in forests of the south coast of New South Wales have been identified as having low effective population sizes, increasing their risk of extinction in the near future []. The southern greater glider population at Seven Mile Beach National Park is the most well studied of the southern greater glider populations in NSW and is listed as an endangered population []. In this location, the available habitat supports an estimated 335 gliders [] and the population has moderate genetic diversity, despite having a low effective population size []. However, Seven Mile Beach National Park is a closed population and thus especially vulnerable to stochastic events. Given that this population is similar in size and habitat characteristics to other populations nearby, it serves as a model for other populations on the NSW South Coast, and also acts as the ’early warning system’ in terms of extinction risk for other similar populations locally. Therefore, modelling the impact of threatening processes on this population and management strategies to mitigate their effects are urgently needed.
Population viability analysis (PVA) may be used to assist with the management of threatened populations and examines differing scenarios involving threatening processes []. PVA may be used for planning conservation efforts through the identification of parameters that population viability is particularly sensitive to [,]. It could also be used to assess the vulnerability of populations to extinction, the output of which may be used to set policy priorities and to allocate limited conservation resources []. Furthermore, PVA may be used to predict the likely response(s) of species to management options such as supplementation [,], prescribed burning [], facilitating dispersal [] and reducing predation risk by invasive species [].
PVA has been performed on greater glider populations in Australia [,,]. In two of these studies, the authors modelled a meta-population using previously collected data about initial population size and found some overlap with their empirical field data [,]. The most recent PVA on greater gliders found that road-crossing structures may make a positive contribution to greater glider conservation []. Importantly, all three published studies identified fragmentation effects as negatively impacting greater glider populations [,,].
To perform a PVA, accurate population estimates are vital []. The use of differing survey techniques can markedly change a population estimate [,,,], which then has a direct effect on the trajectory of the population modelled []. This is particularly true in cases where overestimates of initial population size lead to downstream mismanagement [,,]. For greater gliders, the double-observer distance sampling method has been proven to produce the most robust data []; thus, using this method is key to increasing the validity of conclusions drawn from PVA findings.
Further, there is a need to understand the threatening processes experienced by the species for which PVA is being undertaken [,,]. For southern greater gliders, heatwaves and fire are recognised as major threats across their distribution. Southern greater gliders are expected to respond negatively to heatwave events as their distribution is impacted by the number nights over 20 °C []. Recent studies have shown the southern greater glider to be contracting their distribution to higher elevation areas with cooler microclimates []. Additional threatening processes include increasing fire severity [,,]. The negative effects of fire on southern greater gliders can be observed up to 10 years after a wildfire []. Thus, PVA modelling under different climate regimes, both in the short and long term, will provide a better understanding of population persistence [].
In this study, we undertook a PVA of an endangered population of lowland coastal southern greater gliders; this population was used as a model population for similar populations found in the same geographic region. To increase the accuracy of the PVA modelling output, a current estimate of the population density was undertaken using double-observer distance sampling [], a survey method confirmed to provide accurate estimates of greater glider density. Once the population density was established, the identification of the locally relevant threatening processes was performed from previous studies undertaken on this population [,,,] and other southern greater glider populations in the broader geographic area [,]. Based on these studies, the key threatening processes affecting population viability locally and regionally were hollow limitation, wildfire, and heatwave scenarios.
2. Materials and Methods
2.1. Study Location
This study was conducted at Seven Mile Beach National Park (SMBNP), 110 km south of Sydney NSW (−34°49′ S, 150°45′ E). The dominant vegetation community has been previously described [,]. The National Park is isolated in the landscape, with the nearest contiguous forest 10 km to the northwest at the Barren Grounds Nature Reserve that forms part of the Illawarra Escarpment []. The need for a wildlife corridor, part of the Berry Bush Links programme, was recognised and established between SMBNP and Barren Grounds Nature Reserve to assist with southern greater glider dispersal and conservation management [].
2.2. Population Estimate
We estimated the population size and density of greater gliders at SMBNP using the double-observer distance sampling method []. Three rounds of surveys were completed to provide an accurate estimate of population size. A detailed description of the methods and results of the survey is provided (Supplementary File S1).
2.3. Population Viability Analysis
VORTEX v10 [] was used to model the SMBNP’s southern greater glider population. Using 1000 iterations of the model, the probability of persistence and the average population size were modelled over a 50-year period. A conservative base model was developed using parameter inputs determined from published studies and survey data (Table 1, Supplementary File S1). The fate of the population was also modelled in a further three main scenarios utilising the base model after adding different catastrophe regimes (or threatening processes) that SMBNP is likely to experience over the next 50 years (Table 2). Catastrophes were added into the system as three main categories: heatwaves, wildfires, and altered heatwave and fire regimes. Similar categorisations have also been utilised in previous PVAs for arboreal mammals [].

Table 1.
Assumptions used in VORTEX to develop a conservative base model for the probability of persistence and population size of greater gliders at Seven Miles Beach National Park (SMBNP) over a 50-year period.

Table 2.
Assumptions of three catastrophe models used in the PVA for southern greater gliders at Seven Mile Beach National Park (SMBNP): heatwave, fire, and altered regime.
2.4. Sensitivity Testing
The parameters relevant to threatening processes include carrying capacity, as this reflects habitat loss [,], inbreeding depression [] and catastrophes [,]. Catastrophes were chosen to reflect likely events that would cause significant impact on greater gliders: heatwaves [,,,], and fires [,,,,]. The percent of females breeding was low compared to other populations [], as this is usually 70% [,]. Therefore, the percentage of females breeding was also included as a parameter that may make the population vulnerable to extinction. Lastly, processes that directly affect mortality rates (i.e., disease and invasive species predation) are not recognised as major threatening processes acting on greater glider populations []. However, the investigation of how changes in stage-specific mortality rates can affect population viability is commonly incorporated into sensitivity analysis [,] and is seen to have an impact on the viability of other arboreal marsupial populations [,]. Thus, mortality rates were incorporated to investigate any vulnerability to changes in these parameters.
Input values were determined based on the best available data; however, there are some values with a higher degree of uncertainty. For example, the mating system can vary within and across different populations and varies from monogamy to polygamy depending on population density []. In previous PVA research undertaken for southern greater gliders, both monogamy and polygamy have been selected [,]. At SMBNP, some areas exhibit much higher densities of southern greater gliders than others [], and multiple observations of groups of greater gliders (2–4 individuals) are known to share a hollow (A. Gracanin, pers. obs.). Based on these data, polygamy was used in the model. However, the impact of selecting a monogamous system was nonetheless tested in the sensitivity analysis.
Mortality rates may differ across populations of southern greater gliders; however, in this study, we favoured the mortality rates used in a previous PVA for a species that occurs within a much closer geographic distance and latitude and is likely the same species [,] seen in other PVAs on the greater gliders (likely a different species) undertaken []. Using these mortality rates for SMBNP comes with a small degree of uncertainty. Density dependence was not incorporated into other southern greater glider PVA studies, including those that show congruency with field data [,]. However, density dependence may affect population viability [] and thus was incorporated into sensitivity analysis.
Where specific values were used, sensitivity testing was conducted through the systematic perturbation of base model assumptions [,,]. The parameters investigated included mortality [,,], carrying capacity [], and breeding rates []. As with PVAs performed on other species, assumptions were both increased and decreased in increments of 5% until a level of 20% was reached and a two tailed t-test was performed to investigate whether the deviation from base assumptions caused a significant difference to the population growth rate and mean time until extinction []. Furthermore, changes to ‘categorical’ parameters were investigated and these included the impact of density dependence and catastrophes. We also removed inbreeding depression and utilised a monogamous mating system that was tested against base model assumptions []. The influence of the frequency and severity of heatwaves and fires was determined via the same perturbation analysis detailed above; a t-test was performed for heatwave and fire scenario assumptions [].
2.5. Comparing Relative Effectiveness of Short-Term Management Scenarios Under Likely Scenarios
Possible management scenarios were modelled to investigate the relative impact of different strategies on three catastrophe regimes. While parts of a wildlife corridor have been planted, in its current state it will not be able to facilitate the movement of greater gliders for 50–70 years and will require significant further planting and hollow supplementation [].
Based on the population trajectory modelled, management options were selected based on prescribed greater glider conservation advice that can improve population numbers before a functional corridor is ready [,]. Consequently, the management scenario investigated was as follows:
- (a)
- Nest box placement: 50 boxes, 100 boxes, 200 boxes.
- (b)
- Genetic reinforcement: one, two and three translocations;
- (c)
- Combination approach: three translocation occasions over three levels of next box placement: 50, 100, 200.
Management scenarios were run for 100 years in order to be conservative about the functionality of the wildlife corridor. It is assumed that if a population can persist until a functional corridor exists, the population will be able to sustain itself via dispersal and gene flow in the landscape.
Nest box placement was included in the models as a potential way to increase carrying capacity. The use of nest boxes by southern greater gliders has rarely been recorded in the published literature; therefore, levels of effectiveness were drawn from other possum and glider species. The occupancy rates of nest boxes can vary greatly between studies and study species. For example, 10% of nest boxes were used by sugar and squirrel gilders [], and nest box occupancy in Leadbeater’s possum can range between 14% and 75% depending on the study site []. Improvements in the design of nest boxes can improve the uptake of the boxes []. As a result, using the most informed design [], it was assumed that a single nest box would contribute to a 0.25% increase in carrying capacity.
Reinforcement is the addition of translocated individuals into the population and has been recommended as a strategy to increase genetic diversity at SMBNP []. VORTEX assumes that the percent females breeding during supplementation remains the same. Consequently, reinforcement was modelled manually. Firstly, reinforcement was modelled by increasing the total number of individuals within the population. Each occasion of reinforcement assumed that 30 females were translocated. This model assumed a generous 80% success rate in establishing, leading to an increase in the initial population size by 24 females. Based upon reproductive rates in other greater glider populations, 70% of the 24 would reproduce each year [,]. Thus, 16.8 females were added into the breeding pool and the percentage of female breeding was adjusted. Each occasion was separated by five years. For subsequent occasions, average data from VORTEX output were utilised to generate the new input values. A combination approach was considered as the use of combined multiple management strategies may have greater success than the implementation of a single strategy by itself [].
3. Results
3.1. Population Trajectory
Under the base model scenario that assumes no catastrophes, 1000 iterations identified a zero chance of greater glider population persistence for another 50 years at SMBNP (Figure 1). The mean time until extinction was 26.93 years (Figure 1).
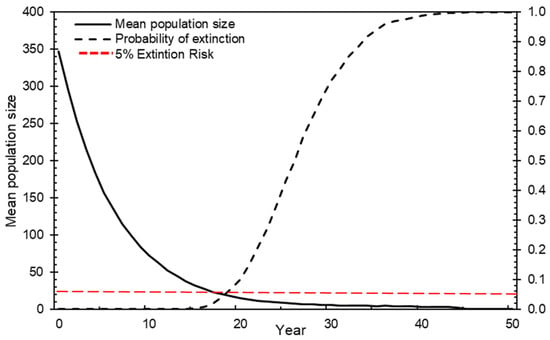
Figure 1.
Mean population size and probability of extinction of SMBNP southern greater gliders over 50-year period.
3.2. Sensitivity Testing
After testing 77 alternative models with altered input parameters, the southern greater glider population at SMBNP went extinct in 50 years (Supplementary File S2). No model had a probability of survival > 1%. The highest probability of survival was seen with no inbreeding effects, at 0.7%. Out of the 77 models, 69 of these had a significant impact of mean time until extinction. Thus, the mean time until extinction varied between 14.17 and 31.89 years.
The highest fire severity scenario produced the lowest amount of time until extinction and highest percent female breeding scenario provided the highest amount of time until extinction (Supplementary File S2). In all circumstances, the population growth rate was negative. Factors significantly influencing population growth rate included no inbreeding effects, 10% or more deviation in newborn mortality or percent females breeding, and 20% deviation in adult mortality. Furthermore, all catastrophic scenarios also had a negative impact on the outcome of the population. The order of lowest to greatest impact was heatwave, fire, and then altered regimes. Altering either frequency and severity of fire and increasing heatwave frequency had a significant impact on the population growth rate (Supplementary File S2). Perturbation of all fire parameters were significantly different from those of the fire base model. Throughout the analysis, the perturbation of carrying capacity values appeared to have no effect on the population trajectory.
3.3. Evaluating Short-Term Management Scenarios
This study investigated three different management strategies: (a) nest box placement, (b) reinforcement, and (c) a combination of three rounds of reinforcement and nest box placement. Each of these management strategies had three levels of effort. These were compared under three likely catastrophe regimes: (1) heatwave, (2) fire, and (3) altered regimes (Figure 2 and Figure 3). Out of the 27 scenarios modelled, only 4 scenarios had a chance of survival to 100 years > 1%. All four of these included three rounds of reinforcement and were performed under a heatwave regime. Within these models, the probability of extinction varied between 63 and 64%, and mean time until extinction was approximately 81–82 years. Across all management options, time until extinction had a negative relationship with the increasing level of catastrophe. As a result, the population trajectory decreased most rapidly under an altered regime. Furthermore, the relative impact of reinforcement (management strategy b) decreased with increasing catastrophe levels.
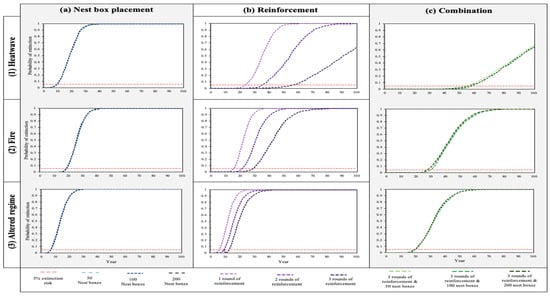
Figure 2.
The effect of management strategies on SMBNP greater glider probability of extinction over 1000 iterations using Vortex10. The probability of extinction, modelled over three levels of catastrophe—(1) heatwave, (2) fire, and (3) altered regime—and three management categories: (a) nest box placement, (b) reinforcement, or (c) a combination of both.
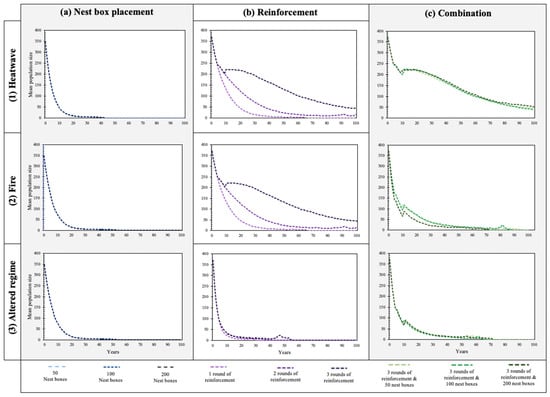
Figure 3.
The effect of management strategies on the mean population size of SMBNP greater gliders over 1000 iterations using Vortex10. The probability of extinction modelled over three levels of catastrophe: (1) heatwave, (2) fire, and (3) altered regime. Three management categories: (a) nest box placement, (b) reinforcement, (c) or a combination of both.
For both heatwave (1) and fire (2), nest box placement (management strategy a) did not change the population trajectory (Figure 3). The most influential factor with regard to mean time until extinction was reinforcement (management strategy b). This management category prolonged population existence between 10 and 56 and 5–26 years, respectively (Figure 2 and Figure 3). Population trajectory increased as levels of reinforcement were added to the model. Three rounds of reinforcement yielded the longest mean time until extinction. The use of a combined approach (management strategy c) had minimal effect on population trajectory, showing <1 year difference in mean time until extinction, than three rounds of reinforcement by itself. Within an altered regime (3), nest box placement (a) improved the mean time until extinction by 4 years (Figure 2 and Figure 3). Reinforcement prolonged the population’s existence by between 1 and 10 years. Three rounds of reinforcement yielded the best results.
A combined approach (3) had the best outcome for the population, prolonging population existence by between 23 and 24 years. For all catastrophe levels, the degree of nest box placement in management categories (a) and (b) showed <1 year change in the mean time until extinction. As a result, the most effective management strategy in terms of assisting the southern greater glider population across all catastrophe levels was the use of a combined approach of three rounds of reinforcement and the placement of 50 nest boxes in SMBNP.
4. Discussion
4.1. Population Estimate
Using the double-observer distance sampling method, southern greater gliders within SMBNP and adjacent sclerophyll forest had an estimated density of 0.46 individuals per ha (95% CI 0.21–0.84), resulting in an approximate population estimate of 347 individuals (95% CI 175–684). Additionally, there were new observations of greater gliders south of the 2013 tornado path, where gliders were absent in previous surveys (e.g., []). We found that the precision of estimates using the double-observer distance sampling method was greatly improved by increasing the number of repeat surveys for each transect. This was a factor that was not considered by Cripps et al. []. This is consistent with studies showing that repeat visitations to transects are required to increase the detectability of greater gliders [,,,]. This is because detectability is known to vary with prevailing environmental conditions []. If the survey is only undertaken on a single night, temporal fluctuation is unaccounted for. Even under ideal conditions, two repeat surveys are required to improve the accuracy of the estimates of glider surveys [].
Even though we used a more accurate sampling methodology when compared to those of Vinson et al. [], our results showed that the population of gliders at SMBNP remains at a low-density; >10 greater gliders per 1 km (of transect) is considered a high-density population []. The result of the density estimation at SMBNP is within the range of other populations, e.g., 0.01 to 3.8 gliders/ha [,,,,]. Using the double-observer distance method, we found 0.08 fewer gliders/ha than Vinson et al. []. This may indicate a slight decline in the SMBNP population, but could also simply represent a discrepancy between density estimates that used two different survey methods.
4.2. Current Population Viability
In the base scenario and all sensitivity testing scenarios, there was <1% chance of population persistence in 50 years. The mean time until extinction, assuming no catastrophes, was 27 years. Furthermore, within the 77 alternate scenarios explored, including those with fire and heatwave catastrophes, the average time until extinction was between 14 and 32 years. As a result, the southern greater glider population at SMBNP is not viable in the medium to long term without focused interventions implementing conservation management strategies.
Populations of greater gliders using forested areas of 1000 ha or greater are deemed viable []. Given that the population modelled exists in 750 ha and is highly isolated, poor temporal population viability was expected. The predicted steep, negative population trajectory is consistent with the published literature, which indicates that greater gliders are facing population declines across their whole distribution [,,]. Notable declines (61%) were observed at the Blue Mountains World Heritage area between 2015 and 2020 []. Furthermore, greater gliders have been extirpated from Booderee National Park on the NSW South Coast for reasons that remain unclear []. There was likely a temporal decline occurring in the greater glider population at Booderee National Park, but it was undocumented as no population monitoring system was in place []. We suggest that the greater gliders of SMBNP have possibly undergone a decline in numbers since previous population estimates took place in 2019 []; therefore, the re-survey of the population undertaken here was warranted. Using Booderee National Park [] as a cautionary example has prompted the continual monitoring of SMBNP southern greater gliders [,,,], allowing the processes impacting their decline to be carefully documented. Lessons from both these southern greater glider populations can be used to assist with the management of other similar lowland coastal glider populations in the region.
4.3. Vulnerability of SMBNP to Threatening Processes
Through sensitivity testing, this study has been able to identify key processes that pose the largest threat to SMBNP population of greater gliders. The threatening parameters include fire, the percentage of females breeding, newborn mortality, and inbreeding effects. While fire had a greater effect on the population than heatwaves, we found heatwave frequency has a much greater impact than heatwave severity. Increasing the frequency by only 0.23% had a significant influence. Carrying capacity appeared to have no effect on the population trajectory. Thus, this study found fire to be the most important threatening process likely acting on the population because of projected increases in fire severity and frequency [].
Any changes to fire frequency and severity caused significant changes to the greater glider population growth rate and the mean time until extinction. Greater gliders can be directly killed on site by flames or in the aftermath due to a lack of food, the loss of hollow-bearing trees, and an increased risk of predation [,]. Furthermore, greater glider populations are slow to recover and recolonise burnt sites following fire and may take decades to return due to the low reproductive rate of the species and its limited dispersal capabilities [,]. Wildfire severity is correlated with greater glider density [,]. When the canopy is burnt (high-severity burn), greater glider densities can decline to 0.01/ha []. Furthermore, increases in the frequency of fire may compound these negative effects on glider population viability.
Mega fires that occurred over the summer of 2019–2020 significantly impacted the spread of greater gliders throughout their range [,]. Australia-wide, it was estimated that 29% of greater glider habitat was burned in the 2019–2020 mega-fire event []. Fire severity was severe or extreme (partial or full canopy consumption) in 37% of the greater glider habitat that burnt, suggesting that few gliders would persist in these areas [,,]. SMBNP escaped the direct impact of the mega fires of 2019–2020 and has not had a fire burn greater than 50% of park since 2002. However, our results suggest a high-severity fire will most likely cause population extinction when it occurs.
The effects of fire would be accelerated in this population due to the isolation of the SMBNP. There is only one main movement corridor along a thin strip of roadside habitat on Beach Road, Gerroa. Established through the habitat surveys conducted, the age and composition of habitat trees were deemed highly unlikely to support southern greater gliders []. Greater gliders are habitat specialists and have relatively small home ranges; they can be considered poor dispersers. At SMBNP, greater gliders took 10 years to recolonize the southern section of the park where they were no longer found after habitat trees were removed by a tornado []. Therefore, for this population, there is no chance of re-establishment after fire as SMBNP is completely isolated from other populations on the NSW South Coast []. As a result, land managers need to maintain appropriate fire regimes to decrease the risk of catastrophic and widescale fires within the park.
Altering the effective population size had a significant influence on the output of the PVA model; hence, this population is likely limited by their low effective population size. The effective population size refers to the number of individuals contributing to the production of the next generation. Within populations of greater gliders, it is assumed that 70% of females are reproductively breeding [,]. This is considerably higher than the level we used in this study, which was calculated from the effective population size []. When fire was excluded from the PVA, the low effective population size had the highest effect in terms of stymieing the population growth rate. When the effective population size is small (<50), it is likely the population has limited a ability for long-term persistence and may be at risk of increased genetic drift.
Another threatening process identified was inbreeding effects. This is a process that also stems from the isolation of SMBNP [] and the low effective population size []. The low effective population size causes higher genetic drift and therefore loss of genetic variation and increased inbreeding due to the restricted population size. Inbreeding reveals harmful alleles or lethal equivalents in the phenotype [,]. This decreases individual fitness, resistance to disease and parasites, and resilience in coping with environmental challenges []. Due to isolation and small effective population size, inbreeding effects may become more prominent []. Modelling subdivided populations of mountain brushtail possums caused a rapid decline in heterozygosity and allelic diversity []. However, mountain brushtail possums in the central highlands of Victoria showed some level of behavioural adaptability to avoid inbreeding, where non-pair bonded females were more likely to select genetically dissimilar mates []. For gliders at SMBNP, given both low effective population size and inbreeding effects are brought about and exacerbated by the isolation of the population, management may need to focus on assisting the immigration of new individuals into the population. This can be performed through implementing an effective wildlife corridor for greater glider movement [], or through the translocation of individuals to the population.
4.3.1. Carrying Capacity
A change in carrying capacity up to 20% appears to have no effect on the outcome of this population. The availability of hollows dictates the distribution of gliders found in SMBNP []; thus, carrying capacity is important for the population’s persistence. In this population model, deterministic factors and reproductive rates may be causing a decline that is below carrying capacity, thus showing no impact. Given this, the removal of crucial habitats is strongly advised against at SMBNP and the surrounding around (i.e., the Beach Road vegetation corridor). The preservation of habitat is a key requirement for the conservation of greater gliders and must be implemented in conjunction with other management options discussed above [].
4.3.2. Implications
At SMBNP, fire will cause considerable harm to the southern greater glider population, possibly causing relatively swift extinction. Thus, it is of the upmost importance to ensure appropriate fire regimes are maintained and, at a finer scale, that vegetative matter around and near den trees (known as ‘hoe raking’) is cleared before prescribed burns are undertaken. Furthermore, the past 200 years of fragmentation and isolation have already exacerbated the low effective population size []. This reinforces the published literature [,,,] on the negative impacts of fragmentation on arboreal mammals and further justifies the development of a wildlife corridor for greater gliders []. Short-term management strategies will need to be employed for greater gliders to survive when and if the corridor is functional, which may take 50–100 years [].
Under current heatwave and fire scenarios, reinforcement had the highest relative impact. The most effective course of action was three rounds of reinforcement, and this boosted the greater glider probability of survival to 100 years from 0% to 63%. The addition of nest boxes into the model was only beneficial at a level of 50 boxes and in an altered regime. Thus, the most versatile and effective model for the conservation of southern greater gliders at SMBNP is three rounds of reinforcement (each restocking 30 gliders) combined with the use of 50 nest boxes. Nest boxes have been recommended as a management strategy for greater gliders as they can provide additional sources of shelter for diurnal rest and breeding dens []. Species-specific nest box designs can improve habitat restoration for arboreal marsupials []. The design of nest boxes is currently being streamlined to improve successful greater glider colonisation through the inclusion of thermal insulation []. Greater gliders have been observed within these nest boxes []; however, the usage patterns at large landscape scales have not been studied, although some benefits have been identified in other arboreal mammal species [,,].
This study has provided evidence of the benefits of nest boxes in heightened fire regimes. This is consistent with the results of other studies that deploy nest boxes as a strategy to support populations after severe wildfires []. Severe fire can cause stag collapse, decreasing the number of available hollows across the landscape []. There is some evidence of arboreal marsupial nest box use post-fire within the first year after a fire, and up to 60 years after a severe wildfire [].
Under current heatwave and fire scenarios, reinforcement was the most effective management strategy for supporting the population of southern greater gliders at SMBNP. Out of three different levels of reinforcement, three rounds of reinforcement boosted the effective population size the most under all three catastrophe levels. This finding is consistent with the results from the PVA, which found that effective population size has a significant impact on greater glider population trajectory. Greater levels of reinforcement created a higher effective population size, thus having larger positive impacts on population trajectory. Reinforcement through translocation is likely to be the most effective option compared to nest box placement in terms of its ability to increase the probability of population persistence in the long-term.
Currently, there are no studies on the translocation of greater gliders. However, the investigation of their feasibility has been suggested [,]. Mammal translocation in Australia has a 62% success rate in studies with recorded outcomes []. However, translocations are expensive [] and could have unintended negative consequences, such as increasing the stress and mortality of relocated animals, exerting negative impacts on resident animals at the release sites, increasing conflicts with humans, and spreading of diseases otherwise not found in the new location []. More recently, a fine-scale understanding of the species microbiome has become warranted []. Thus, translocations need to be carefully considered. The chance of successful establishment used in this study was optimistic considering that rates may be much lower than 80% []. Nevertheless, this study has identified reinforcement to be an important management strategy that should be considered at SMBNP.
There are a variety of ecological and genetic factors that need to be met before translocation can occur (Supplementary File S3). Three out of six of these have been appropriately met at SMBNP. That is, there is good ecological understanding of the species, a clear identification of benefits a reintroduction programme would provide, and a release site that is known to sustain release of animals. However, whilst there is evidence for causes of decline, some of these cannot be eliminated. High heatwave frequency is of particular concern as that may impede the success of a translocation programme. Furthermore, research will need to include the a risk assessment and to carefully select the appropriate source population. A suitable course of action would be a trial reinforcement programme and consequent monitoring. This will help to identify whether translocation is feasible and has successful outcomes.
5. Conclusions
A combined approach would be the most beneficial management strategy pursue for southern greater gliders at SMBNP. Given the limited resources and pitfalls of translocation, the installation and uptake of nest boxes by greater gliders must be investigated. A nest box programme of habitat supplementation would be beneficial to understanding the effectiveness of habitat supplementation at SMBNP and for other southern greater glider populations in the region. The translocation of individuals should be considered only after nest box supplementation has occurred and its success has been evaluated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/conservation4040052/s1.
Author Contributions
Conceptualization, K.M.M. methodology, K.M.M., B.M. and A.G.; software, B.M.; validation, K.M.M., B.M. and A.G.; formal analysis, B.M.; investigation, K.M.M., B.M. and A.G.; resources, K.M.M.; data curation, K.M.M.; writing—original draft preparation, K.M.M., B.M. and A.G.; writing—review and editing, A.G. and K.M.M.; supervision, K.M.M. and A.G.; project administration, K.M.M.; funding acquisition, K.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research was approved under University of Wollongong Animal Ethics Committee protocol AE19/02.
Data Availability Statement
Data available on request to the corresponding author (K.M.M.).
Acknowledgments
We acknowledge the Traditional Owners of the land on which this research was undertaken (Woddi Woddi). We pay our respects to Elders past and present. We also thank NSW National Parks and Wildlife Staff (Valda Corrigan), NSW National Parks Association (David Rush) and Berry LandCare group. Finally, we thank students from Team Quoll (University of Wollongong-Mikac Lab) for their assistance with fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wood, J.; McBurney, L.; MacGregor, C.; Youngentob, K.N.; Banks, S.; Lindenmayer, D.B. How to make a common species rare: A case against conservation complacency. Biol. Conserv. 2011, 144, 1663–1672. [Google Scholar]
- Smith, P.; Smith, J. Decline of the greater glider (Petauroides volans) in the lower Blue Mountains, New South Wales. Aust. J. Zool. 2018, 66, 103–114. [Google Scholar] [CrossRef]
- McLean, C.M.; Kavanagh, R.P.; Penman, T.; Bradstock, R. The threatened status of the hollow-dependent arboreal marsupial, the Greater Glider (Petauroides volans), can be explained by impacts from wildfire and selective logging. For. Ecol. Manag. 2018, 415–416, 19–25. [Google Scholar] [CrossRef]
- Kavanagh, R.P. Effects of variable-intensity logging and the influence of habitat. Pac. Conserv. Biol. 2000, 6, 18–30. [Google Scholar]
- Isaac, B.; White, J.; Ierodiaconou, D.; Cooke, R. Simplification of arboreal marsupial assemblages in response to increasing urbanization. PLoS ONE 2014, 9, e91049. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; Blair, D.; McBurney, L.; Taylor, C.; Scheele, B.C.; Westgate, M.J.; Robinson, N.; Foster, C. The response of arboreal marsupials to long-term changes in forest disturbance. Anim. Conserv. 2021, 24, 246–258. [Google Scholar] [CrossRef]
- Ashman, K.R.; Watchorn, D.J.; Lindenmayer, D.B.; Taylor, M.F. Is Australia’s environmental legislation protecting threatened species? A case study of the national listing of the greater glider. Pac. Conserv. Biol. 2022, 28, 277–289. [Google Scholar] [CrossRef]
- May-Stubbles, J.C.; Gracanin, A.; Mikac, K.M. Increasing fire severity negatively affects greater glider density. Wildl. Res. 2022, 49, 709–718. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J. Impact of the 2019–20 drought, heatwaves and mega- fires on Greater Gliders (Petauroides volans) in the Greater Blue Mountains World Heritage Area, New South Wales. Aust. Zool. 2022, 42, 164–181. [Google Scholar] [CrossRef]
- Wagner, B.; Baker, P.J.; Stewart, S.B.; Lumsden, L.F.; Nelson, J.L.; Cripps, J.K.; Durkin, L.K.; Scroggie, M.P.; Nitschke, C.R. Climate change drives habitat contraction of a nocturnal arboreal marsupial at its physiological limits. Ecosphere 2020, 11, e03262. [Google Scholar] [CrossRef]
- Lacy, R.C. Importance of genetic variation in mammalian populations. J. Mammal. 1997, 78, 320–335. [Google Scholar] [CrossRef]
- Knipler, M.L.; Gracanin, A.; Mikac, K.M. Conservation genomics of an endangered arboreal mammal following the 2019–2020 Australian megafire. Sci. Rep. 2023, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Greater Glider population at Seven Mile Beach National Park endangered population listing. In Proceedings of the Final Determination to List an Endangered Ecological Community under the Threatened Species Conservation Act 1995, Sydney, Australia, 16 December 2016. [Google Scholar]
- Vinson, S.G.; Johnson, A.P.; Mikac, K.M. Current estimates and vegetation preferences of an endangered population of the vulnerable greater glider at Seven Mile Beach National Park. Austral Ecol. 2020, 46, 303–314. [Google Scholar] [CrossRef]
- Akcakaya, H.R.; Sjögren-Gulve, P. Population viability analyses in conservation planning: An overview. Ecol. Bull. 2000, 48, 9–21. [Google Scholar]
- Cancino, J.; Rodrıguez-Estrella, R.; Miller, P. Using population viability analysis for management recommendations of the endangered endemic peninsular pronghorn. Acta Zool. Mex. 2010, 26, 173–189. [Google Scholar] [CrossRef][Green Version]
- Lacy, R.C. Lessons from 30 years of population viability analysis of wildlife populations. Zoo Biol. 2019, 38, 67–77. [Google Scholar] [CrossRef]
- Olsen, M.T.; Andersen, L.W.; Dietz, R.; Teilmann, J.; Härkönen, T.; Siegismund, H.R. Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Mol. Ecol. 2014, 23, 815–831. [Google Scholar] [CrossRef]
- Cremona, T.; Crowther, M.S.; Webb, J.K. High mortality and small population size prevent population recovery of a reintroduced mesopredator. Anim. Conserv. 2017, 20, 555–563. [Google Scholar] [CrossRef]
- Zilko, J.P.; Harley, D.; Pavlova, A.; Sunnucks, P. Applying population viability analysis to inform genetic rescue that preserves locally unique genetic variation in a critically endangered mammal. Diversity 2021, 13, 382. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Garnett, S.T.; Brook, B.W. Fire frequency matters more than fire size: Testing the pyrodiversity-biodiversity paradigm for at-risk small mammals in an Australian tropical savanna. Biol. Conserv. 2015, 186, 337–346. [Google Scholar] [CrossRef]
- Taylor, B.D.; Goldingay, R.L. Can road-crossing structures improve population viability of an urban gliding mammal? Ecol. Soc. 2009, 14, 13. [Google Scholar] [CrossRef]
- Lunney, D.; Gresser, S.; O’neill, L.E.; Matthews, A.; Rhodes, J. The impact of fire and dogs on Koalas at Port Stephens, New South Wales, Using Population Viability Analysis. Pac. Conserv. Biol. 2007, 13, 189. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Lacy, R.C.; Pope, M.L. Testing a simulation model for population viability analysis. Ecol. Appl. 2000, 10, 580–597. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Ball, I.; Possingham, H.P.; McCarthy, M.A.; Pope, M.L. A landscape-scale test of the predictive ability of a spatially explicit model for population viability analysis. J. Appl. Ecol. 2001, 38, 36–48. [Google Scholar]
- Catling, P.C.; Burt, R.J.; Kooyman, R. A comparison of techniques used in a survey of the ground-dwelling and arboreal mammals in forests in north-eastern New South Wales. Wildl. Res. 1997, 24, 417–432. [Google Scholar] [CrossRef]
- Emerson, L.D.; Ballard, G.A.; Vernes, K. Conventional distance sampling versus strip transects and abundance indices for estimating abundance of greater gliders (Petauroides volans) and eastern ringtail possums (Pseudocheirus peregrinus). Wildl. Res. 2019, 46, 518–532. [Google Scholar] [CrossRef]
- Crowther, M.S.; Dargan, J.R.; Madani, G.; Rus, A.I.; Krockenberger, M.B.; McArthur, C.; Moore, B.D.; Lunney, D.; Mella, V.S. Comparison of three methods of estimating the population size of an arboreal mammal in a fragmented rural landscape. Wildl. Res. 2021, 48, 105–114. [Google Scholar] [CrossRef]
- Cripps, J.K.; Nelson, J.L.; Scroggie, M.P.; Durkin, L.K.; Ramsey, D.S.; Lumsden, L.F. Double-observer distance sampling improves the accuracy of density estimates for a threatened arboreal mammal. Wildl. Res. 2021, 48, 756–768. [Google Scholar] [CrossRef]
- McLoughlin, P.D.; Messier, F. Relative contributions of sampling error in initial population size and vital rates to outcomes of population viability analysis. Conserv. Biol. 2004, 18, 1665–1669. [Google Scholar] [CrossRef]
- Mason, F. The Newfoundland cod stock collapse: A review and analysis of social factors. Electron. Green J. 2002, 1. [Google Scholar] [CrossRef]
- Shelton, P.A. Did over-reliance on commercial catch rate data precipitate the collapse of northern cod? ICES J. Mar. Sci. 2005, 62, 1139–1149. [Google Scholar] [CrossRef]
- Ebenhard, T. Population viability analyses in endangered species management: The wolf, otter and peregrine falcon in Sweden. Ecol. Bull. 2000, 48, 143–163. [Google Scholar]
- Shaffer, M.; Watchman, L.H.; Snape, W.J., III; Latchis, I.K. Population viability analysis and conservation policy. In Population Viability Analysis; The University of Chicago Press: Chicago, IL, USA, 2002; pp. 123–142. [Google Scholar]
- Owens, G.; Heinsohn, R.; Crates, R.; Stojanovic, D. Long-term ecological data confirm and refine conservation assessment of critically endangered swift parrots. Anim. Conserv. 2023, 26, 450–463. [Google Scholar] [CrossRef]
- Legge, S.; Rumpff, L.; Woinarski, J.C.; Whiterod, N.S.; Ward, M.; Southwell, D.G.; Scheele, B.C.; Nimmo, D.G.; Lintermans, M.; Geyle, H.M.; et al. The conservation impacts of ecological disturbance: Time-bound estimates of population loss and recovery for fauna affected by the 2019–2020 Australian megafires. Glob. Ecol. Biogeogr. 2022, 31, 2085–2104. [Google Scholar] [CrossRef]
- Vinson, S.G.; Johnson, A.P.; Mikac, K.M. Thermal cameras as a survey method for Australian arboreal mammals: A focus on the greater glider. Aust. Mammal. 2020, 42, 367–374. [Google Scholar] [CrossRef]
- Hofman, M.; Gracanin, A.; Mikac, K.M. Greater glider (Petauroides volans) den tree and hollow characteristics. Aust. Mammal. 2022, 45, 127–137. [Google Scholar] [CrossRef]
- Gracanin, A.; Cappelletti, C.; Knipler, M.; Dallas, R.K.; Mikac, K.M. Exploring new grounds: Arboreal sugar gliders frequently observed spending time on the ground as seen on camera traps. Aust. Mammal. 2019, 42, 110–113. [Google Scholar] [CrossRef]
- Gracanin, A.; Mikac, K.M. Evaluating modelled wildlife corridors for the movement of multiple arboreal species in a fragmented landscape. Landsc. Ecol. 2023, 38, 1321–1337. [Google Scholar] [CrossRef]
- Lacy, R.C. Vortex: A computer simulation model for population viability analysis. Wildl. Res. 1993, 20, 45–65. [Google Scholar] [CrossRef]
- O’Grady, J.J.; Brook, B.W.; Reed, D.H.; Ballou, J.D.; Tonkyn, D.W.; Frankham, R. Realistic levels of in-breeding depression strongly affect extinction risk in wild populations. Biol. Conserv. 2006, 133, 42–51. [Google Scholar] [CrossRef]
- Harris, J.M.; Maloney, K.S. Petauroides volans (Diprotodontia: Pseudocheiridae). Mamm. Species 2010, 42, 207–219. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, C.H.; Smith, R.F.C. Studies on the marsupial glider, Schoinobates volans (Kerr): II. Population structure and regulatory mechanisms. J. Anim. Ecol. 1969, 38, 637–650. [Google Scholar] [CrossRef]
- Rübsamen, K.; Hume, I.D.; Foley, W.J.; Rübsamen, U. Implications of the large surface area to body mass ratio on the heat balance of the greater glider (Petauroides volans: Marsupialia). J. Comp. Physiol. B 1984, 154, 105–111. [Google Scholar] [CrossRef]
- Hofman, M.E. Hollow Use by Greater Gliders at Seven Mile Beach National Park. Bachelor’s Thesis, University of Wollongong, Wollongong, Australia, 2021. [Google Scholar]
- Anonymous. Illawarra: Climate Change Snapshot; Office of Environment and Heritage: Sydney, Australia, 2014. Available online: https://www.climatechange.environment.nsw.gov.au/sites/default/files/2021-06/Illawarra%20climate%20change%20snapshot.pdf (accessed on 21 November 2024).
- Goldingay, R.L.; McHugh, D.; Parkyn, J.L. Multiyear monitoring of threatened iconic arboreal mammals in a mid-elevation conservation reserve in eastern Australia. Ecol. Evol. 2022, 12, e8935. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.C.; Driscoll, D.; Smith, A.L.; Gill, A.M. Fire severity and landscape context effects on arboreal marsupials. Biol. Conserv. 2013, 167, 137–148. [Google Scholar] [CrossRef]
- Anonymous; Department of Climate Change, Energy, the Environment and Water. Conservation Advice for Petauroides volans (Greater glider (Southern and Central)); Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2022. Available online: http://www.environment.gov.au/biodiversity/threatened/species/pubs/254-conservation-advice-05072022.pdf (accessed on 22 November 2024).
- Pacioni, C.; Williams, M.R.; Lacy, R.C.; Spencer, P.B.; Wayne, A.F. Predators and genetic fitness: Key threatening factors for the conservation of a bettong species. Pac. Conserv. Biol. 2017, 23, 200–212. [Google Scholar] [CrossRef]
- Whitehead, T.; Vernes, K.; Goosem, M.; Abell, S.E. Invasive predators represent the greatest extinction threat to the endangered northern bettong (Bettongia tropica). Wildl. Res. 2018, 45, 208–219. [Google Scholar] [CrossRef]
- Moro, D.; Dunlop, J.; Williams, M.R. Northern quoll persistence is most sensitive to survivorship of juveniles. Wildl. Res. 2019, 46, 165–175. [Google Scholar] [CrossRef]
- Chaudhary, V.; Oli, M.K. A critical appraisal of population viability analysis. Conserv. Biol. 2020, 34, 26–40. [Google Scholar] [CrossRef]
- Lunney, D. Effects of logging, fire, and drought on possums and gliders in the coastal forests near Bega, NSW. Wildl. Res. 1987, 14, 263–274. [Google Scholar] [CrossRef]
- Durant, R.; Luck, G.W.; Matthews, A. Nest-box use by arboreal mammals in aperi-urban landscape. Wildl. Res. 2009, 36, 565–573. [Google Scholar] [CrossRef]
- Harley, D.K.P. A role for nest boxes in the conservation of Leadbeater’s possum (Gymnobelideus leadbeateri). Wildl. Res. 2006, 33, 385–395. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Rueegger, N.N.; Grimson, M.J.; Taylor, B.D. Specific nest box designs can improve habitat restoration for cavity-dependent arboreal mammals. Restor. Ecol. 2015, 23, 482–490. [Google Scholar] [CrossRef]
- Howard, I.; Ridley, J.C.; Blanchard, W.; Ashman, K.R.; Lindenmayer, D.B.; Head, M.L.; Youngentob, K.N. Helping wildlife beat the heat: Testing strategies to improve the thermal performance of nest boxes. Aust. Zool. 2022, 42, 534–560. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kavanagh, R.P.; McCarthy, M.A.; Burgman, M.A. Estimating and dealing with detectability in occupancy surveys for forest owls and arboreal marsupials. J. Wildl. Manag. 2005, 69, 905–917. [Google Scholar] [CrossRef]
- MacHunter, J.; Brown, G.; Loyn, R.; Lumsden, L. Survey Standards: Greater Glider, Petauroides Volans; The Department of Sustainability and Environment: Melbourne, Australia, 2011. Available online: https://www.vic.gov.au/sites/default/files/2020-12/8-Greater-Glider-Survey-Standards-FINALv1.0_2MAY11.doc (accessed on 22 November 2024).
- Possingham, H.P.; Lindenmayer, D.B.; Norton, T.W.; Davies, I. Metapopulation viability analysis of greater glider Petauroides volans in a wood production area. Biol. Conserv. 1994, 70, 227–236. [Google Scholar] [CrossRef]
- Blyton, M.D.; Shaw, R.E.; Peakall, R.; Lindenmayer, D.B.; Banks, S.C. The role of relatedness in mate choice by an arboreal marsupial in the presence of fine-scale genetic structure. Behav. Ecol. Sociobiol. 2016, 70, 313–321. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Wood, J.; MacGregor, C.; Foster, C.; Scheele, B.; Tulloch, A.; Barton, P.; Banks, S.; Robinson, N.; Dexter, N.; et al. Conservation conundrums and the challenges of managing unexplained declines of multiple species. Biol. Conserv. 2018, 221, 279–292. [Google Scholar] [CrossRef]
- Knipler, M.L.; Dowton, M.; Clulow, J. Genome-wide SNPs detect fine-scale genetic structure in threatened populations of squirrel glider Petaurus norfolcensis. Conserv. Genet. 2022, 23, 541–558. [Google Scholar] [CrossRef]
- Knipler, M.L.; Downton, M.; Mikac, K.M. Limited genetic structure detected in sugar gliders (Petaurus breviceps) using genome-wide SNPs. Aust. Mammal. 2022, 45, 41–52. [Google Scholar] [CrossRef]
- Murphy, J.K.; Dunn, S.C. An assessment of nest box occupancy and program effectiveness in a bushfire recovery program in Warrumbungle National Park, northern inland New South Wales. Victorian Nat. 2021, 138, 36–44. [Google Scholar]
- Short, J. The Characteristics and Success of Vertebrate Translocations Within Australia; Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2010; Available online: https://researchportal.murdoch.edu.au/esploro/outputs/book/The-characteristics-and-success-of-vertebrate/991005542576007891 (accessed on 18 May 2024).
- Weise, F.L.; Stratford, K.J.; Rudolf, J.V.V. Financial Costs of Large Carnivore Translocations—Accounting for Conservation. PLoS ONE 2014, 9, e105042. [Google Scholar] [CrossRef] [PubMed]
- Chipman, R.; Slate, D.; Rupprecht, C.; Mendoza, M. Downside Risk of Wildlife Translocation; USDA National Wildlife Research Center—Staff Publications: 1896; U.S. Department of Agriculture: Animal and Plant Health Inspection Service: Lincoln, NE, USA, 2008; Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/1896/?utm_source=digitalcommons.unl.edu%2Ficwdm_usdanwrc%2F1896&utm_medium=PDF&utm_campaign=PDFCoverPages (accessed on 18 May 2024).
- Clough, J.; Schwab, S.; Mikac, K. Gut Microbiome Profiling of the Endangered Southern Greater Glider (Petauroides volans) after the 2019–2020 Australian Megafire. Animals 2023, 13, 583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).