Potential of Microwave Heating and Plasma for Biosecurity Applications
Abstract
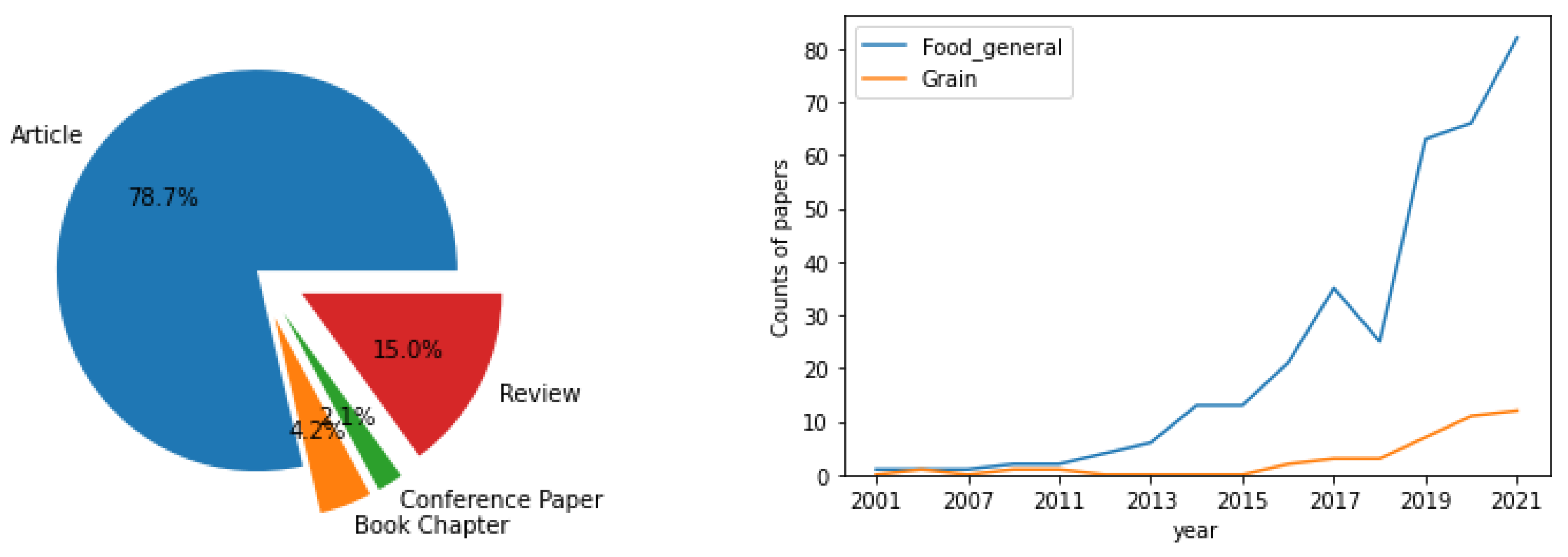
:1. Introduction
2. Thermal Disinfestation
3. Microwave Heat Sanitization
3.1. Effects on Soil Biota
3.2. Implications for Cropping Systems
| Microwave Frequency | Power Level | Irradiation Duration | Treatment Scenario | Target Weed Species | Percentage Weed/Seed Destruction | Reference |
|---|---|---|---|---|---|---|
| 39 MHz | – | 4–37 s | Pre-emergence | Hard Red Winter Wheat | 50% seed mortality | [27] |
| 2.45 GHz | 600 W | 60 s | Pre-emergence (dry, 4 h soaked and 46 h germinated seeds) | Zea mays, Arachis hypogaea, Prosopis juliflora, Cucumis sativus, Brassica spp., Rumex crispus, Echinochloa colonum, Amaranthus sp., Gossypium hirsutum, Glycine max, Sorghum vulgare and Triticum vulgare | 17% reduction in germination in dry seeds but 100% in case of moist seeds at 10 s of exposure | [15] |
| 2.45 GHz | 600 W | 8 s | Post-emergence (aquatic weed) | Duckweed (Wolffia punctat) | 50% | [28] |
| 2.45 GHz | 2–4 kW | Varying exposure time (not mention properly) | Pre- and post-emergences | Johonsongrass Moriningglory Redroot Pigweed Texas panicium Barnyardgrass Sunflower London rocket Rigseed euphrobia | For post-emergence MW treatment 309 J cm–2 energy was required for 100% control (field conditions) while for pre-emergence MW weed control 73 J cm−2 gave 85–100% control (Glass house conditions) | [29] |
| 2.45 GHz | 45–720 J cm−2 | No information | Pre-emergence | London rocket (13 cm deep in soil profile) and Sunflower (2.5 cm seeded depth) | 87% for London rocket and 93% for Sunflower | [30] |
| 2.45 GHz | 100–750 W | 120–1200 s | Pre-emergence | Clover and Turnip | 60–78% reduction in seeds germination | [31] |
| 2.45 GHz | 0.1–1.5 kW | Varying exposure time | Pre-emergence of seeds in soil | Black Medic, Barnyard grass, Foxtail purslane, Redroot pigweed, Large crabgrass | 50% | [32] |
| 2.45 GHz | – | 360 s | Pre-emergence | Brassica napus, Linum usitatissimum, Avena fatua | 85–95% | [33] |
| 9 GHz | 10–30 mW cm–2 | 22–24 h | Post emergence | Zea mays | 100% growth inhibitions | [34] |
| 2.45 GHz | 1.2 kW | 5–45 s | Pre-emergence | Trifolium and Medicago | 85% reduction in germination | [35] |
| 2.45 GHz | 500 W | 30 s | Pre-emergence | Avena fatua | 60% (based on seed moisture) | [36] |
| 2.45 GHz | 1.5 kW | 0, 10, 20, and 30 | Pre-emergence | Wild Oat and Wheat | 90–100% | [37] |
| 2.45 GHz | – | 120 s | Pre-emergence | Avena sativa and native weed seeds | Reduced weed seeds emergence | [38] |
| 2.45 GHz | 900 W | 4, 8, 16, 32, 64, 128, and 256 s | Post-emergence | Abutilon theophrasti, Pancium miliaceum, Lucerne and Rapeseed | Complete dehydrating of plants | [39] |
| 2.45 GHz | 800 W | 120, 240, 420 and 960 s | Pre-emergence | Rubber vine, Parthenium and Bellyache bush | 88% (Rubber vine), 67% (Parthenium) and 94% (Bellyache bush) mortality at 960 s irradiation | [40] |
| 2.45 GHz | 0.10–1.24 kWh m–2 | 30–300 s | Pre- and post-emergence | Malva parviflora and Triticum aestivum | 100% destruction of tested specie at 0.65 kWh m–2 | [41] |
| 2.45 GHz | 700 W | 120, 240, 320 and 720 s | Pre-emergence treatment of soil | Lolium perenne and Lolium rigidum | 100% seed mortality was achieved at 240 s of MW irradiation | [17] |
| 2.45 GHz | 750 W | 5, 15, 30 and 60 s | Pre- and post-emergence | Prickly Paddy melon | 100% debilitation of plants | [42] |
| 2.45 GHz | 2 kW | 5, 10, 15, 30, 60 s | Post-emergence | Ryegrass and Wild Radish | 100% mortality | [18] |
3.3. Microwave Treatment of Animal Fodder
3.4. Microwave Treatment of Timber for Pest Control
3.5. Scale-Up of Microwave Heating Systems
4. Grain Treatment Using Non-Thermal Plasma Technology for Pest and Pathogen Control
4.1. Grain Treatment with Cold Plasma
4.1.1. Disinfestation from Microorganisms and Pathogens
4.1.2. Disinfestation from Pests
4.1.3. Inactivation Mechanism
4.1.4. Effect of Process Parameters
4.1.5. Effect of Plasma Treatment on the Host Quality
| Material | Gas | Pressure | Temperature | Source | Pathogens | Treatment Time | Quality Change | Efficiency of Decontamination | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Wheat, bean, chickpea, soybean, barley, oat, rye, lentil, corn | Air or SF6 | Vacuum | Room | Aspergillus spp. and Penicillium spp. | 5–20 min | No change in germination and nutritive components (like gluten) | 3-log reduction after 15 min | [114] | |
| - | Air (3 m/s) | Atmospheric | <45 °C | DBD high-voltage pulse generator (13 kHz)-p = 560 W | Ascochyta pinodella, Fusarium culmorum | 60, 180, 360 s | - | 100% inhibition after 360 s; heating at 55 °C for 720 s did not remove the fungi | [80] |
| Blue lupine, catgut, honey clover, and soy | 5.28 MHz-RF-0.6 W/cm3 | Fusarium, black spot, Stemphiliosis, Anthracnose | 10–15 min | 10–20% increase in germination- more than 15 min decrease in G | 3–15% decrease in Fusarium, black spot, Stemphiliosis- no effect on Anthracnose | [115] | |||
| Wheat, spring barley, soy, field pea | Air | 40 pa (vacuum) | Max. 310 °C | RF-5.28 MHz-0.1–0.7 W/cm3 | Fungi | 2, 5, 7, 10, 15, 20 min | An increase of 4–7% in seed germination | 6–14% reduction | [116] |
| Rice and lemon | Air | Atmospheric | Five discharge electrodes and a UV lamp, 7–10 kV | Mold and E. coli | 20 min for lemon and 90 min for rice seeds | No significant damage to the host | Complete eradication of the mold, combination of plasma and UV was more effective | [117] | |
| Rice seeds | Air | Atmospheric | <45 °C | DBD- 3 W, 30 kV | Seed-borne Gibberella fujikuroi | 120 s | No effect on the seedling emergence and height | More than 92% | [118] |
| Chickpea | Air | Max. 5 °C above ambient T | An electrode based on the surface micro-discharge (SMD) | Natural flora | 2–5 min | A strong improvement in the seed germination (89.2%), speed of germination (7.1 ± 0.1 seeds/day), and seed vigor, after 3 min (optimum 1 min) | 1–2 log reduction | [81] | |
| Barley and corn | Air | 15 Pa | Glow discharge, 100–200 W | Background fungi | 20 min | No change of germination and seedling length, an increase in corn fresh weight | 25% reduction in the number of fungi in barley seeds and 40% reduction in corn seeds | [119] | |
| Wheat | Argon (15 nL/min) with oxygen (5–10%) | 5 mbar | Thermal- max. surface T: 90 °C | RF-13.56 MHz-700–900 W | Bacillus amyloliquefaciens endospores | 30 s | No effect on flour and baking quality | 2 log reduction- spore inactivation is more with more O2 | [99] |
| Wheat | Air | Atmospheric | Low temperature | Cylindrical electrodes, 8 kV | Natural fungi | 3–30 s | No significant effect on seed germination and vigor | Significant reduction of all the present fungi after 10 s | [120] |
| Barley and wheat | 1: air-100 SCCM 2: air 8 bars | 1: low pressure-100 pa 2: atmospheric | 1: pulsed MW 2: gliding arc discharge-50 Hz | Mycotoxins, namely deoxynivalenol (DON), deoxynivalenol-3-glucoside (D3G), and trichothecene mycotoxins (T-2). | 1–5 min | 1: An increase in germination 2: A reduction in germination no nutritive change | 1 more effective than 2 | [121] | |
| Wheat | Pulsed argon (2.8 nL/min) | Atmospheric | Pulse frequency (5–15 kHz) and pulse voltage (6–10 kV) | Endospores of Geobacillus stearothermophilus (as a model) | 60 min | No change in gluten | 3 log reduction (1 log after 10 min) | [122] | |
| Maize | Air and N2 (3000 L/h) | Atmospheric | Non-thermal | 5–10 kV; 18–25 kHz (at a maximum power of 655 W)-nuzzle with 4 mm | Aspergillus flavus and Aspergillus parasiticus spores | 1–5 min | - | 5.48 and 5.20 log reduction | [123] |
| Rice | Air/ argon (2.5 L/min) | Atmospheric | <30 °C | Hybrid cold-discharge plasma with 10 × 10 twin-tip electrodes, 14 kV | Natural fungi | 1 min | An enhancement of seed wettability and germination | No fungi growth on the treated seedlings after 14 d while the untreated seedlings were invaded by fungi | [124] |
| Wheat | Air | Atmospheric | Cold | 100 W/cm3 (400 W) | Fusarium nivale, F. culmorum, Trichothecium Roseum, Aspergillus flavus, A. clavatus | 10–80 s for seeds and 30–300 s for microbes | 20–50 s: an increase in germination rate, dry weight, and vigor of seedlings | Fusarium spp. most sensitive; Fusarium nivale > F. culmorum > Trichothecium roseum > Aspergillus flavus > A. clavatus | [125] |
| Rice | L/min water) | Atmospheric | Surface discharge electrode, 20 kV, a 10 cm distance between the seeds and the end of the quartz tube | Fusarium fujikuroi, Burkholderia plantarii | 10 min (2 min interval shaking) | No adverse effect on the seed germination and seedling growth | A reduction of 18.1% (fungal blight) and 38.6% (bacterial blight) in the disease severity | [126] | |
| Rice culture | Air-130 sL/min-330 K | Atmospheric | Max. 60 °C | 4 W/cm2, 38 kV | Mycotoxins produced by Fusarium verticillioides, Fusarium avenaceum, Aspergillus nidulans, and Fusarium graminearum (FB1, EnnB, ST, and ZEN) | 60 s | - | Removed completely when pure, 2 log reduction after 30 s | [127] |
| Wheat and barley model media | Air | Atmospheric | DBD, 80 kV | Native microflora, pathogenic bacteria, and fungi: E. coli, Bacillus and Lactobacillus, B. atrophaeus endospores | 30 min | - | Maximum of 4.4 log reduction, more reduction on the hydrophobic surface than hydrophilic | [128] | |
| Wheat and barley | Air | Atmospheric | DBD, 80 kV | Native microflora, pathogenic bacteria | 5 and 20 min with 2 or 24 h retention time | No change in germination after 5 min, but a reduction after 20 min | Resistance to plasma treatment: E. coli > P. verrucosum (spores) > B. atrophaeus (vegetative cells) > B. atrophaeus (endospores) | [129] | |
| Soybean | O2/N2 (6 nL/min) | Atmospheric | Quasi-stationary DBD, 25 kV, 65 or 85 W | Seed-borne Diaporthe/Phomopsis complex | 1, 2, 3 min | 29% reduction in catalase activity, 30% increase in glutathione content | 49–81% reduction in the number of infected seed | [130] | |
| Pine seeds | Atmospheric | Diffuse coplanar surface barrier discharge (DCSBD) | Fusarium circinatum | 5–300 s | Dramatic reduction of germination at 60 s or more | 50% reduction after 10 s treatment | |||
| Maize and barley | Ar/N2-O2 (500 and 1000 SCCM) | 2–8 mbar | <40 °C | The afterglow of microwave discharge, 25 W | Fusarium graminearum and Fusarium verticillioides | 3 min (barley), 4 min (maize) | An improvement in the germination of contaminated seeds | With a mixture of gases, infected seeds reduced to below 10 or 40% and seed germination increased to 80% | [131] |
| Maize | Air | Atmospheric | DCSBD, 20 kV, 400 W | Natural microbiota and A. flavus, A. alternata, F. culmorum | 30–300 s | A decrease in water contact angle, removal of the lipid from the seed surface, no effect on seed germination up to 120 s | Complete elimination of natural bacteria and F. culmorum after 60 s and natural filamentous fungi after 180 s; A. flavus reduced to 10% and A. alternata reduced to 30% (from 100%) after 120 s | [83] | |
| Barley | Air | Atmospheric | DBD, 0–34 kV, 3500 Hz, 300 W, in an RH controlled chamber, electrode gap 5 mm, distance from barley 2 mm, | Deoxynivalenol (DON) | 6 and 10 min | No significant effect on germination, protein, beta-glucan, and moisture content | 48.9% and 54.4% reduction in DON | [132] | |
| Wheat | Air | Atmospheric | DBD, 80 kV, 50 Hz, diameter 15 mm, electrode distance 50 mm, sample holder height 20 mm, 2 g samples, direct and indirect exposure | Natural bacterial and fungal pathogens isolated from wheat | 0–20 min and 24 h of retention time | 20 min significantly reduced all pathogens, B. atrophaeus vegetative cells the highest reduction, B. atrophaeus spores the most resistant | [133] | ||
| Brown rice | Air | Atmospheric | Corona discharge plasma jet (CDPJ), 20 kV, | Aerobic bacteria, yeasts and molds | 10 min | No effect on germination, increased weight and length of the seedlings, improved phenolic content of seedling, no effect on sensory properties of seedlings | >1.5 log reduction in all contaminants | [134] | |
| canola grain, canola meal, and barley grains | Air | Atmospheric | DBD, 0–30 kV, 70% duty cycle, 10 μs pulse width, and 0–1 A current, the gap between sample and high voltage electrode 2 mm | Zearalenone (ZEA) | 3 min | 91.6, 83.2, and 64.8% reduction for canola grain, canola meal, and barley grains, respectively | [135] |
| Material | Gas | Pressure | Reactor/Plasma Generator | Pest | Treatment Time | Grain Quality Change | Efficiency of Decontamination | Reference |
|---|---|---|---|---|---|---|---|---|
| Air | Atmospheric | Plasma jet, 500 W, samples in plasma maintaining chamber, | Aphis gossypii, Bemisia tabaci, Helicoverpa armigera, Tetranychus kanzawai, and Thrips palmi | 3–20 min | LT50 of 6.3 and 9.6 min for B. tabaci and T. palmi | [136] | ||
| Rice | Air (70% RH), 10 L/h | Atmospheric | DBD, 200 V, 7.5–20 W, indirect exposure in fumigation chamber | Tribolium castaneum | 8, 16, 24 h | No change in cooking properties, texture, hydration, pasting profile, color, and moisture content | 100% mortality after 24 h compared to 86.67% mortality with 200 ppm Phosphine | [137] |
| Wheat (in package) | Atmospheric | DBD, 44–47 kV, | Rhyzopertha dominica | 4–7 min | Enhancement in milling yield, protein, and fiber content, reduced carbohydrate, increased lightness | 88.33% mortality at 47 kV-7 min treatment, after 24 h | [138] | |
| Argon, helium | 150 Kpa | RF (13.56 MHz), 100 W | Red flour beetle Tribolium castaneum | 0–90 s | He more efficient than Ar, adult more tolerant that larvae and pupae, 100% mortality with 90 s and He | [85] | ||
| Wheat | Air | Atmospheric | DBD, 15.9 W | Tribolium castaneum and Trogoderma granarium (adults and larvae) | Enhancement of seed germination, | Adult more tolerant than larvae, | [86] | |
| Chickpea | 0.5 mbar | RF (13.56 MHz), 40, 50 and 60 W | Callosobruchus chinensis | 10, 15, 20 min | 100% mortality of all life stages over 2 years of storage | [139] | ||
| Air | Atmospheric | DBD, 80 kV, 50 mm electrodes distance, direct and indirect exposure | Tribolium castaneum (egg, young and old larvae, pupae) | 0.5–5 min | 100% mortality after 24 h with 5 min direct exposure, adults most resistant stage, indirect exposure up to 20 min was less effective | [92] |
4.2. Plasma Apparatus for Seed Treatment
4.3. Indirect Treatment of the Seeds Using Plasma
4.3.1. Plasma Processed Air (PPA)
4.3.2. Plasma Activated Water (PAW)
4.4. Industrialization and Scale-Up
5. Brief Discussion
- Optimizing process parameters and developing a pilot-scale system for rice and grain treatment [155];
- Regulatory review, in-depth understanding of the effect of cold plasma on food functionality, combination with other conventional methods and development of prototypes [105];
- Shelf-life study based on quality parameters, analytical flavor analysis, and sensory analysis [112];
- Combination with other novel technologies such as nanotechnology for food surface decontamination [156];
- Development of large-scale systems and addressing the biofilm issue in the electrodes that could significantly affect laboratory results [77];
- Field studies with plasma treated seeds, unifying optimization of cold plasma parameters to be able to compare them for a specific grain and their pathogens [78];
- Optimization of the process parameters and appropriate equipment design with affordable prices for commercial-scale processing [157];
- Introducing plasma units or plasma dose standardization, mechanisms of action for microbial decontamination, and plasma “vaccination” of crops by activating their immune system [159];
- Scale-up of both microwave heating and microwave-generated plasma for continuous treatment must also be properly understood. This requires ongoing research and development.
6. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozone Secretariat: United Nations Environment Programme. The Montreal Protocol on Substances that Deplete the Ozone Layer; United Nations Environment Programme: Nairobi, Kenya, 2000. [Google Scholar]
- Sharp, J.L. Heat and Cold Treatments for Postharvest Quarantine Disinfestation of Fruit Flies (Diptera: Tephritidae) and Other Quarantine Pests. Fla. Entomol. 1993, 76, 212–218. [Google Scholar] [CrossRef]
- Metaxas, A.C.; Meredith, R.J. Industrial Microwave Heating; Peter Peregrinus: London, UK, 1983. [Google Scholar]
- Awida, M.H.; Shah, N.; Warren, B.; Ripley, E.; Fathy, A.E. Modeling of an industrial microwave furnace for metal casting applications. In Proceedings of the 2008 IEEE MTT-S International Microwave Symposium Digest, Atlanta, GA, USA, 15–20 June 2008; pp. 221–224. [Google Scholar]
- Horikoshi, S.; Brodie, G.; Takaki, K.; Serpone, N. Agritech: Innovative Agriculture Using Microwaves and Plasmas: Thermal and Non-Thermal Processing; Springer: Tokyo, Japan, 2022. [Google Scholar]
- Taheri, S.; Brodie, G.I.; Gupta, D.; Jacob, M.V. Microwave Atmospheric Plasma Processed Air and Argon for Disinfection of Lentil from BGM. In Proceedings of the 55th Annual Microwave Power Symposium (IMPI 55), Virtual, 28 June–1 July 2021; pp. 31–33. [Google Scholar]
- García, M.C.; Mora, M.; Esquivel, D.; Foster, J.E.; Rodero, A.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Microwave atmospheric pressure plasma jets for wastewater treatment: Degradation of methylene blue as a model dye. Chemosphere 2017, 180, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.O. A review and assessment of microwave energy for soil treatment to control pests. Trans. ASAE 1996, 39, 281–289. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Johnson, J.A.; Mitcham, E.; Hansen, J.D.; Hallman, G.; Drake, S.R.; Wang, Y. Dielectric Properties of Fruits and Insect Pests as related to Radio Frequency and Microwave Treatments. Biosyst. Eng. 2003, 85, 201–212. [Google Scholar] [CrossRef]
- Levitt, J. Response of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1980; Volume 1. [Google Scholar]
- Lepeschkin, W.W. Zur Kenntnis der Einwirkung supamaximaler Temperaturen auf die Pflanze. Ber. Dtsch. Bot. Ges. 1912, 30, 713–714. [Google Scholar]
- Shlevin, E.; Saguy, I.S.; Mahrer, Y.; Katan, J. Modeling the Survival of Two Soilborne Pathogens Under Dry Structural Solarization. Phytopathology 2003, 93, 1247–1257. [Google Scholar] [CrossRef]
- Trevisani, M.; Mancusi, R.; Valero, A. Thermal inactivation kinetics of shiga toxin-producing Escherichia coli in buffalo mozzarella curd. J. Dairy Sci. 2014, 97, 642–650. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.I.; Gupta, D.; Dadu, R.H.R. Effect of Microwave Radiation on Internal Inoculum of Ascochyta Blight in Lentil Seeds at Different Seed Moisture Contents. Trans. ASABE 2019, 62, 33–43. [Google Scholar] [CrossRef]
- Davis, F.S.; Wayland, J.R.; Merkle, M.G. Ultrahigh-Frequency Electromagnetic Fields for Weed Control: Phytotoxicity and Selectivity. Science 1971, 173, 535–537. [Google Scholar] [CrossRef]
- Davis, F. “Zapper” blasts weed seeds. New Zealand J. Agric. 1975, 53–54. [Google Scholar]
- Brodie, G.; Harris, G.; Pasma, L.; Travers, A.; Leyson, D.; Lancaster, C.; Woodworth, J. Microwave soil heating for controlling ryegrass seed germination. Trans. ASABE 2009, 52, 295–302. [Google Scholar] [CrossRef]
- Brodie, G.; Hollins, E. The Effect of Microwave Treatment on Ryegrass and Wild Radish Plants and Seeds. Glob. J. Agric. Innov. Res. Dev. 2015, 2, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Brodie, G.; Grixti, M.; Hollins, E.; Cole, M. The Effect of Microwave Soil Treatment on Key Soil Biota. In Proceedings of the 1st URSI Atlantic Radio Science Conference, Gran Canaria, Spain, 16–24 May 2015; p. 1189. [Google Scholar]
- Cooper, A.P.; Brodie, G. The effect of microwave radiation and soil depth on soil pH, N, P, K, SO4 and bacterial colonies. Plant Prot. Q. 2009, 24, 67–70. [Google Scholar]
- Vela, G.R.; Wu, J.F.; Smith, D. Effect of 2450 MHz microwave radiation on some soil microorganisms in situ. Soil Sci. 1976, 121, 44–51. [Google Scholar] [CrossRef]
- Khan, M.J.; Jurburg, S.D.; He, J.; Brodie, G.; Gupta, D. Impact of microwave disinfestation treatments on the bacterial communities of no-till agricultural soils. Eur. J. Soil Sci. 2019, 71, 1006–1017. [Google Scholar] [CrossRef]
- Hendricks, C.W.; Pascoe, N. Soil microbial biomass estimates using 2450 MHz microwave irradiation. Plant Soil 1988, 110, 39–47. [Google Scholar] [CrossRef]
- Brodie, G.; Khan, M.J.; Gupta, D. Microwave Soil Treatment and Plant Growth. In Sustainable Crop Production; Filho, M.C.M.T., Hasanuzzaman, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Khan, M.J.; Brodie, G.I. Microwave Weed and Soil Treatment in Rice Production. In Rice Crop—Current Development; Shah, F., Khan, Z.H., Iqbal, A., Eds.; IntechOpen: Vienna, Austria, 2018; pp. 99–127. [Google Scholar]
- Khan, M.J.; Brodie, G.I.; Gupta, D.; He, J. Microwave Soil Treatment Increases Soil Nitrogen Supply for Sustained Wheat Productivity. Trans. ASABE 2019, 62, 355–362. [Google Scholar] [CrossRef]
- Nelson, S.; Walker, E.R. Effects of radio-frequency electrical seed treatment. Agricltural Eng. 1961, 42, 688–691. [Google Scholar]
- Champ, M.; Davis, F.; Wayland, J. Ultrahigh-frequency electromagnetic radiation utilized for aquatic vegetation control. In Proceedings of the 26th Annual Conference of South Eastern Association Game and Fish Commissioners, Knoxville, TN, USA, 22–25 October 1972. [Google Scholar]
- Wayland, J.; Davis, F.; Merkle, M. Toxicity of an UHF device to plant seeds in soil. Weed Sci. 1973, 21, 161–162. [Google Scholar] [CrossRef]
- Menges, R.; Wayland, J. UHF Electromagnetic energy for weed control in vegetables. Weed Sci. 1974, 22, 584–590. [Google Scholar] [CrossRef]
- Hightower, N.C.; Burdette, E.; Burns, C. Investigation of the Use of Microwave Energy for Weed Seed and Wood Products Insect Control; Georgia Institute of Technology: Atlanta, GA, USA, 1974. [Google Scholar]
- Rice, R.P.; Putnam, A.R. Some factors which influence the toxicity of UHF energy to weed seeds. Weed Sci. 1977, 25, 179–183. [Google Scholar] [CrossRef]
- Bhartia, P.; Lebell, M.; Baron, J. Weed control with electromagnetic fields. J. Microw. Power 1977, 12, 52–54. [Google Scholar]
- Bigu-Del-Blanco, J.; Bristow, J.; Romero-Sierra, C. Effects of low-level microwave radiation on germination and growth rate in corn seeds. Proc. IEEE 1977, 65, 1086–1088. [Google Scholar] [CrossRef]
- Crawford, A. Phytotoxicity threshold levels of microwave radiation for Trifolium and Medicago seeds. Seed Sci. Technol. 1977, 5, 671–676. [Google Scholar]
- Diprose, M.; Benson, F.; Willis, A. The effect of externally applied electrostatic fields, microwave radiation and electric currents on plants and other organisms, with special reference to weed control. Bot. Rev. 1984, 50, 171–223. [Google Scholar] [CrossRef]
- Lal, R.; Reed, W. The effect of microwave energy on germination and dormancy of wild oat seeds. Can. Agric. Eng. 1980, 22, 85–88. [Google Scholar]
- Barker, A.V.; Craker, L.E. Inhibition of Weed Seed Germination by Microwaves. Agron. J. 1991, 83, 302–305. [Google Scholar] [CrossRef]
- Sartorato, I.; Zanin, G.; Baldoin, C.; De Zanche, C. Observations on the potential of microwaves for weed control. Weed Res. 2006, 46, 1–9. [Google Scholar] [CrossRef]
- Bebawi, F.; Cooper, A.; Brodie, G.; Madigan, B.; Vitelli, J.; Worsley, K.; Davis, K. Effect of microwave radiation on seed mortality of rubber vine (Cryptostegia grandiflora R. Br.), parthenium (Parthenium hysterophorous L.) and bellyache bush (Jatropha gossypiifolia L.). Plant Prot. Q. 2007, 22, 136. [Google Scholar]
- Brodie, G.; Hamilton, S.; Woodworth, J. An assessment of microwave soil pasteurization for killing seeds and weeds. Plant Prot. Q. 2007, 22, 143. [Google Scholar]
- Brodie, G.; Ryan, C.; Lancaster, C. The Effect of Microwave Radiation on Prickly Paddy Melon (Cucumis myriocarpus). Int. J. Agron. 2012, 2012, 287608. [Google Scholar] [CrossRef]
- Almaiman, S.A.; Albadr, N.A.; Alsulaim, S.; Alhuthayli, H.F.; Osman, M.A.; Hassan, A.B. Effects of microwave heat treatment on fungal growth, functional properties, total phenolic content, and antioxidant activity of sorghum (Sorghum bicolor L.) grain. Food Chem. 2021, 348, 128979. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, W.M.; Al-Badri, K.S.L.; Alqaisi, M.R.M. Effect of Microwave Radiation on Bacteria, Fungi and Some Growth Characteristics of Cowpea Vigna unguiculata L. Gesunde Pflanz. 2020, 73, 161–167. [Google Scholar] [CrossRef]
- Tiwari, A.; Shanmugasundaram, S.; Jaganmohan, R.; Manickam, L. Dielectric heating-assisted disinfestation of black gram and its effect on protein profile: A comparative study on radio frequency and microwave heating. Legume Sci. 2021, 3, e83. [Google Scholar] [CrossRef]
- Speir, T.W.; Cowling, J.C.; Sparling, G.P.; West, A.W.; Corderoy, D.M. Effects of microwave radiation on the microbial biomass, phosphatase activity and levels of extractable N and P in a low fertility soil under pasture. Soil Biol. Biochem. 1986, 18, 377–382. [Google Scholar] [CrossRef]
- Deep, N.Y.; Tanupriya, A.; Monika, S.; Gupta, R.K. Microwave technology for disinfestation of cereals and pulses: An overview. J. Food Sci. Technol. 2014, 51, 3568–3576. [Google Scholar]
- Falciglia, P.P.; Vagliasindi, F.G.A. Techno-economic analysis of hydrocarbon-polluted soil treatment by using ex situ microwave heating: Influence of soil texture and soil moisture on electric field penetration, operating conditions and energy costs. J. Soils Sediments 2016, 16, 1330–1344. [Google Scholar] [CrossRef]
- Bhatta Kaudal, B.; Aponte, C.; Brodie, G. Biochar from biosolids microwaved-pyrolysis: Characteristics and potential for use as growing media amendment. J. Anal. Appl. Pyrolysis 2018, 130, 181–189. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. J. Environ. Chem. Eng. 2018, 6, 395–403. [Google Scholar] [CrossRef]
- Huss, A.R.; Schumacher, L.L.; Cochrane, R.A.; Poulsen, E.; Bai, J.; Woodworth, J.C.; Dritz, S.S.; Stark, C.R.; Jones, C.K. Elimination of Porcine Epidemic Diarrhea Virus in an Animal Feed Manufacturing Facility. PLoS ONE 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Tang, J.; Mitcham, E.J.; Wang, S.; Lurie, S. Heat Treatments for Postharvest Pest Control: Theory and Practice; CABI: Wallingford, UK, 2007. [Google Scholar]
- Mbata, G.N.; Phillips, T.W. Effects of temperature and exposure time on mortality of stored-product insects exposed to low pressure. J. Econ. Entomol. 2001, 94, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Long, R.; Zhang, D.; Hu, Z.; Pu, X. Effect of microwave treatment on chemical composition and in sacco digestibility of wheat straw in yak cow Asian-Australas. J. Anim. Sci. 2005, 18, 27–31. [Google Scholar]
- Sadeghi, A.A.; Shawrang, P. Effects of microwave irradiation on ruminal degradability and in vitro digestibility of canola meal. Anim. Feed Sci. Technol. 2006, 127, 45–54. [Google Scholar] [CrossRef]
- Sadeghi, A.A.; Shawrang, P. Effects of microwave irradiation on ruminal protein and starch degradation of corn grain. Anim. Feed Sci. Technol. 2006, 127, 113–123. [Google Scholar] [CrossRef]
- Brodie, G.; Rath, C.; Devanny, M.; Reeve, J.; Lancaster, C.; Harris, G.; Chaplin, S.; Laird, C. Effect of microwave treatment on lucerne fodder. Anim. Prod. Sci. 2010, 50, 124–129. [Google Scholar] [CrossRef]
- Lewis, V.R.; Haverty, M.I. Evaluation of six techniques for control of the western drywood termite (Isoptera: Kalotermitidae) in structures. J. Econ. Entomol. 1996, 89, 922–934. [Google Scholar] [CrossRef]
- Massa, R.; Panariello, G.; Pinchera, D.; Schettino, F.; Caprio, E.; Griffo, R.; Migliore, M.D. Experimental and numerical evaluations on palm microwave heating for Red Palm Weevil pest control. Sci. Rep. 2017, 7, 45299. [Google Scholar] [CrossRef]
- Lewis, V.R.; Power, A.B.; Haverty, M.I. Laboratory evaluation of microwaves for control of the western drywood termite. For. Prod. J. 2000, 50, 79–87. [Google Scholar]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Processing: Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Kabir, H.; Khan, M.J.; Brodie, G.; Gupta, D.; Pang, A.; Jacob, M.V.; Antunes, E. Measurement and modelling of soil dielectric properties as a function of soil class and moisture content. J. Microw. Power Electromagn. Energy 2020, 54, 3–18. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.; Jacob, M.V.; Antunes, E. Dielectric properties of chickpea, red and green lentil in the microwave frequency range as a function of temperature and moisture content. J. Microw. Power Electromagn. Energy 2018, 52, 198–214. [Google Scholar] [CrossRef]
- Vollmer, M. Physics of the microwave oven. Phys. Educ. 2004, 39, 74–81. [Google Scholar] [CrossRef]
- Al-Holy, M.; Wang, Y.; Tang, J.; Rasco, B. Dielectric properties of salmon (Oncorhynchus keta) and sturgeon (Acipenser transmontanus) caviar at radio frequency (RF) and microwave (MW) pasteurization frequencies. J. Food Eng. 2005, 70, 564–570. [Google Scholar] [CrossRef]
- Guan, D.; Younce, F.; Tang, J.; Pathak, S.; Komarov, V. Heating Characteristics of Mashed Potato in a 915 MHz Microwave-Circulated Water Combination Heating System. In Proceedings of the 2003 ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 1. [Google Scholar]
- Harris, G.A.; Brodie, G.I.; Ozarska, B.; Taube, A. Design of a Microwave Chamber for the Purpose of Drying of Wood Components for Furniture. Trans. Am. Soc. Agric. Biol. Eng. 2011, 54, 363–368. [Google Scholar]
- Chow Ting Chan, T.V.; Reader, H.C. Computational design of a multiple magnetron cavity. In Proceedings of the 4th IEEE Afrocon Conference, Stellenbosch, South Africa, 24–27 September 1996; pp. 523–527. [Google Scholar]
- Benford, J. History and Future of the Relativistic Magnetron. In Proceedings of the International Conference on the Origins and Evolution of the Cavity Magnetron 2010, Bournemouth, UK, 19–20 April 2010; pp. 40–45. [Google Scholar]
- Meredith, R. Engineers’ Handbook of Industrial Microwave Heating; The Institute of Electrical Engineers: Stevenage, UK, 1998. [Google Scholar]
- Meredith, R.J. A three axis model of the mode structure of multimode cavities. J. Microw. Power Electromagn. Energy 1994, 29, 31–44. [Google Scholar] [CrossRef]
- Pollak, J.; Moisan, M.; Kéroack, D.; Séguin, J.; Barbeau, J. Plasma Sterilisation within Long and Narrow Bore Dielectric Tubes Contaminated with Stacked Bacterial Spores. Plasma Processes Polym. 2008, 5, 14–25. [Google Scholar] [CrossRef]
- Moreau, S.; Moisan, M.; Tabrizian, M.; Barbeau, J.; Pelletier, J.; Ricard, A.; Yahia, L.H. Using the flowing afterglow of a plasma to inactivate Bacillus subtilis spores: Influence of the operating conditions. J. Appl. Phys. 2000, 88, 1166. [Google Scholar] [CrossRef]
- Petitpas, G.; Rollier, J.D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrog. Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- Ohta, T. Chapter 8—Plasma in Agriculture. In Cold Plasma in Food and Agriculture; Academic Press: San Diego, CA, USA, 2016; pp. 205–221. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.E.; Chung, M.-S.; Min, S.C. Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiol. 2015, 51, 74–80. [Google Scholar] [CrossRef]
- Scholtz, V.; Jirešová, J.; Šerá, B.; Julák, J. A Review of Microbial Decontamination of Cereals by Non-Thermal Plasma. Foods 2021, 10, 2927. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Vogel-Mikuš, K. Development of Cold Plasma Technologies for Surface Decontamination of Seed Fungal Pathogens: Present Status and Perspectives. J. Fungi 2021, 7, 650. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.-H.; Lee, M.-H.; Han, D.-W.; Chung, K.-H.; Hyun, S.O.; Park, J.-C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Avramidis, G.; Stüwe, B.; Wascher, R.; Bellmann, M.; Wieneke, S.; von Tiedemann, A.; Viöl, W. Fungicidal effects of an atmospheric pressure gas discharge and degradation mechanisms. Surf. Coat. Technol. 2010, 205, S405–S408. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.-F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Processing 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Sayed, W.A.A.; Hassan, R.S.; Sileem, T.M.; Rumpold, B.A. Impact of plasma irradiation on Tribolium castaneum. J. Pest Sci. 2021, 94, 1405–1414. [Google Scholar] [CrossRef]
- Abotaleb, A.O.; Badr, N.F.; Rashed, U.M. Assessment of the potential of non-thermal atmospheric pressure plasma discharge and microwave energy against Tribolium castaneum and Trogoderma granarium. Bull. Entomol. Res. 2021, 111, 528–543. [Google Scholar] [CrossRef]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef]
- Guo, J.; Huang, K.; Wang, J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control 2015, 50, 482–490. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Radhakrishnan, M.; Anandakumar, S.; Shanmugasundaram, S.; Anandharamakrishnan, C. Disinfestation techniques for major cereals: A status report. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1125–1155. [Google Scholar] [CrossRef] [PubMed]
- Sutar, S.A.; Thirumdas, R.; Chaudhari, B.B.; Deshmukh, R.R.; Annapure, U.S. Effect of cold plasma on insect infestation and keeping quality of stored wheat flour. J. Stored Prod. Res. 2021, 92, 101774. [Google Scholar] [CrossRef]
- Ziuzina, D.; Van Cleynenbreugel, R.; Tersaruolo, C.; Bourke, P. Cold plasma for insect pest control: Tribolium castaneum mortality and defense mechanisms in response to treatment. Plasma Processes Polym. 2021, 18, 2000178. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Processes Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control 2015, 55, 221–229. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric. Sci. 2011, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Butscher, D.; Schlup, T.; Roth, C.; Müller-Fischer, N.; Gantenbein-Demarchi, C.; Rudolf von Rohr, P. Inactivation of microorganisms on granular materials: Reduction of Bacillus amyloliquefaciens endospores on wheat grains in a low pressure plasma circulating fluidized bed reactor. J. Food Eng. 2015, 159, 48–56. [Google Scholar] [CrossRef]
- Mošovská, S.; Medvecká, V.; Gregová, M.; Tomeková, J.; Valík, Ľ.; Mikulajová, A.; Zahoranová, A. Plasma inactivation of Aspergillus flavus on hazelnut surface in a diffuse barrier discharge using different working gases. Food Control 2019, 104, 256–261. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.M.; Bourke, P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Moiseev, T.; Misra, N.; Cullen, P.; Mosnier, J.; Keener, K.; Bourke, P. Influence of high voltage atmospheric cold plasma process parameters and role of relative humidity on inactivation of Bacillus atrophaeus spores inside a sealed package. J. Hosp. Infect. 2014, 88, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Surowsky, B.; Bußler, S.; Schlüter, O.K. Chapter 7—Cold Plasma Interactions with Food Constituents in Liquid and Solid Food Matrices. In Cold Plasma in Food and Agriculture; Academic Press: San Diego, CA, USA, 2016; pp. 179–203. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef]
- Li, J.; Xiang, Q.; Liu, X.; Ding, T.; Zhang, X.; Zhai, Y.; Bai, Y. Inactivation of soybean trypsin inhibitor by dielectric-barrier discharge (DBD) plasma. Food Chem. 2017, 232, 515–522. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiate). LWT—Food Sci. Technol. 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Niu, D.; Zhou, Y.-X.; Cheng, J.-H.; Bekhit, A.E.-D.; Aadil, R.M. Oxidation induced by dielectric-barrier discharge (DBD) plasma treatment reduces soybean agglutinin activity. Food Chem. 2021, 340, 128198. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Recent trends in the application of cold plasma for the modification of plant proteins—A review. Future Foods 2022, 5, 100119. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Yousefi, M.; Nematollahi, A.; Khorshidian, N. Effect of atmospheric cold plasma treatment on technological and nutrition functionality of protein in foods. Eur. Food Res. Technol. 2021, 247, 1579–1594. [Google Scholar] [CrossRef]
- Warne, G.R.; Williams, P.M.; Pho, H.Q.; Tran, N.N.; Hessel, V.; Fisk, I.D. Impact of cold plasma on the biomolecules and organoleptic properties of foods: A review. J. Food Sci. 2021, 86, 3762–3777. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.I.; Gupta, D.; Jacob, M.V. Afterglow of atmospheric non-thermal plasma for disinfection of lentil seeds from Botrytis Grey Mould. Innov. Food Sci. Emerg. Technol. 2020, 66, 102488. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Kadyrov, M.; Beljavsky, V.; Gvozdov, A.; Shik, A.; Antonuk, A. The effect of plasma treatment of seeds of some grains and legumes on their sowing quality and productivity. Rom. J. Phys 2011, 56, 139–143. [Google Scholar]
- Filatova, I.; Azharonok, V.; Shik, A.; Antoniuk, A.; Terletskaya, N. Fungicidal effects of plasma and radio-wave pre-treatments on seeds of grain crops and legumes. In Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Berlin/Heidelberg, Germany, 2012; pp. 469–479. [Google Scholar]
- Hayashi, N.; Yagyu, Y.; Yonesu, A.; Shiratani, M. Sterilization characteristics of the surfaces of agricultural products using active oxygen species generated by atmospheric plasma and UV light. Jpn. J. Appl. Phys. 2014, 53, 05FR03. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Cho, J.; Tsai, T.-C.; Staack, D.; Kang, M.-H.; Roh, J.-H.; Shin, D.-B.; Cromwell, W.; Gross, D. A Non-thermal plasma seed treatment method for management of a seedborne fungal pathogen on rice seed. Crop Sci. 2014, 54, 796–803. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.; Surdu-Bob, C.; Karaca, G.; Erper, I. Effect of glow discharge plasma on germination and fungal load of some cereal seeds. Rom. Rep. Phys. 2015, 67, 617–624. [Google Scholar]
- Kordas, L.; Pusz, W.; Czapka, T.; Kacprzyk, R. The Effect of Low-Temperature Plasma on Fungus Colonization of Winter Wheat Grain and Seed Quality. Pol. J. Environ. Stud. 2015, 24, 433–438. [Google Scholar]
- Kriz, P.; Petr, B.; Zbynek, H.; Jaromir, K.; Pavel, O.; Petr, S.; Miroslav, D. Influence of plasma treatment in open air on mycotoxin content and grain nutriments. Plasma Med. 2015, 5, 145–158. [Google Scholar] [CrossRef]
- Butscher, D.; Zimmermann, D.; Schuppler, M.; von Rohr, P.R. Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge. Food Control 2016, 60, 636–645. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control 2016, 70, 1–8. [Google Scholar] [CrossRef]
- Khamsen; Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) Seed Sterilization and Germination Enhancement via Atmospheric Hybrid Nonthermal Discharge Plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef] [PubMed]
- Zahoranová, A.; Henselova, M.; Hudecova, D.; Kaliňáková, B.; Kováčik, D.; Medvecka, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Processing 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. The potential of atmospheric air cold plasma for control of bacterial contaminants relevant to cereal grain production. Innov. Food Sci. Emerg. Technol. 2017, 44, 36–45. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef]
- Pérez Pizá, M.C.; Prevosto, L.; Zilli, C.; Cejas, E.; Kelly, H.; Balestrasse, K. Effects of non–thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 2018, 49, 82–91. [Google Scholar] [CrossRef]
- Szőke, C.; Nagy, Z.; Gierczik, K.; Székely, A.; Spitkól, T.; Zsuboril, Z.T.; Galiba, G.; Marton, C.L.; Kutasi, K. Effect of the afterglows of low pressure Ar/N2-O2 surface-wave microwave discharges on barley and maize seeds. Plasma Processes Polym. 2018, 15, 1700138. [Google Scholar] [CrossRef]
- Feizollahi, E.; Iqdiam, B.; Vasanthan, T.; Thilakarathna, M.S.; Roopesh, M.S. Effects of Atmospheric-Pressure Cold Plasma Treatment on Deoxynivalenol Degradation, Quality Parameters, and Germination of Barley Grains. Appl. Sci. 2020, 10, 3530. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Bourke, P. Effects of cold plasma on wheat grain microbiome and antimicrobial efficacy against challenge pathogens and their resistance. Int. J. Food Microbiol. 2020, 335, 108889. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Puligundla, P.; Mok, C. Cold plasma decontamination of brown rice grains: Impact on biochemical and sensory qualities of their corresponding seedlings and aqueous tea infusions. LWT 2020, 131, 109508. [Google Scholar] [CrossRef]
- Feizollahi, E.; Roopesh, M. Degradation of Zearalenone by Atmospheric Cold Plasma: Effect of Selected Process and Product Factors. Food Bioprocess Technol. 2021, 14, 2107–2119. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kim, H.-S.; Park, M.-R. Plasma-based organism evaluation equipment using atmospheric-pressure plasma jets: Efficacy for controlling insect pests. J. Asia-Pac. Entomol. 2019, 22, 868–873. [Google Scholar] [CrossRef]
- Paul, A.; Mahendran, R. Mortality of Tribolium castaneum and quality changes in Oryza sativa by indirect exposure to Non-Thermal Plasma. Front. Adv. Mater. Res. 2020, 2, 26–40. [Google Scholar] [CrossRef]
- Madathil, R.V.; Thirugnanasambandan Kalaivendan, R.G.; Paul, A.; Radhakrishnan, M. In package control of Rhyzopertha dominica in wheat using a continuous atmospheric jet cold plasma system. Front. Adv. Mater. Res. 2021, 3, 10–25. [Google Scholar] [CrossRef]
- Pathan, F.; Deshmukh, R.; Annapure, U. Potential of cold plasma to control Callosobruchus chinensis (Chrysomelidae: Bruchinae) in chickpea cultivars during four year storage. Sci. Rep. 2021, 11, 13425. [Google Scholar] [CrossRef]
- Lebedev, Y.A. Microwave discharges at low pressures and peculiarities of the processes in strongly non-uniform plasma. Plasma Sources Sci. Technol. 2015, 24, 053001. [Google Scholar] [CrossRef]
- Lebedev, Y.A. Microwave discharges: Generation and diagnostics. J. Phys. Conf. Ser. 2010, 257, 012016. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Schlüter, O.; Gniffke, H.; Ehlbeck, J. Decontamination and sensory properties of microbiologically contaminated fresh fruits and vegetables by microwave plasma processed air (PPA). J. Food Processing Preserv. 2015, 39, 653–662. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Krohmann, U.; Winter, J.; Schlüter, O.; Weltmann, K.D.; Ehlbeck, J. Decontamination of microbiologically contaminated specimen by direct and indirect plasma treatment. Plasma Processes Polym. 2012, 9, 569–575. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. A Inactivation Efficacy and Mechanisms of Gas Plasma and Plasma Activated Water Against Aspergillus flavus Spores and Biofilms: A Comparative Study. Appl. Environ. Microbiol. 2020, 86, 9. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K.K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Risa Vaka, M.; Sone, I.; García Álvarez, R.; Walsh, J.L.; Prabhu, L.; Sivertsvik, M.; Noriega Fernández, E. Towards the Next-Generation Disinfectant: Composition, Storability and Preservation Potential of Plasma Activated Water on Baby Spinach Leaves. Foods 2019, 8, 692. [Google Scholar] [CrossRef]
- Darmanin, M.; Kozak, D.; de Oliveira Mallia, J.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Generation of plasma functionalized water: Antimicrobial assessment and impact on seed germination. Food Control 2020, 113, 107168. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef]
- Kang, M.H.; Jeon, S.S.; Shin, S.M.; Veerana, M.; Ji, S.-H.; Uhm, H.-S.; Choi, E.-H.; Shin, J.H.; Park, G. Dynamics of nitric oxide level in liquids treated with microwave plasma-generated gas and their effects on spinach development. Sci. Rep. 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhao, C.; Mo, Q.; Zhou, Y.; Huang, K. An electrodeless atmospheric microwave plasma jet for efficient degradation of antibiotic norfloxacin. J. Environ. Manag. 2021, 291, 112729. [Google Scholar] [CrossRef]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of plasma technology from the lab to the food industry. Plasma Processes Polym. 2018, 15, 1700085. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, W.K.; Lee, J.W.; Chung, M.-S.; Oh, S.-W.; Shin, J.-K.; Min, S.C. Microbial Decontamination of Rice Germ Using a Large-Scale Plasma Jet-Pulsed Light-Ultraviolet-C Integrated Treatment System. Food Bioprocess Technol. 2021, 14, 542–553. [Google Scholar] [CrossRef]
- Misnal, M.F.I.; Redzuan, N.; Zainal, M.N.F.; Ahmad, N.; Raja Ibrahim, R.K.; Agun, L. Cold Plasma: A Potential Alternative for Rice Grain Postharvest Treatment Management in Malaysia. Rice Sci. 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Xiang, Q.; Huangfu, L.; Dong, S.; Ma, Y.; Li, K.; Niu, L.; Bai, Y. Feasibility of atmospheric cold plasma for the elimination of food hazards: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2021, 54, 1539–1547. [Google Scholar] [CrossRef]
- Kaur, M.; Hüberli, D.; Bayliss, K. Cold plasma: Exploring a new option for management of postharvest fungal pathogens, mycotoxins and insect pests in Australian stored cereal grain. Crop Pasture Sci. 2020, 71, 715–724. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
| Author | Title | Main Finding | Reference |
|---|---|---|---|
| Almaiman et al. | Effects of microwave heat treatment on fungal growth, functional properties, total phenolic content, and antioxidant activity of sorghum (Sorghum bicolor L.) grain | Microwave heating significantly reduced fungal incidence in the sorghum grain. No significant changes were found in the crude protein and digestibility of protein, water holding capacity, and oil holding capacity of sorghum; however, application of higher microwave caused a sharp reduction in the protein solubility, foaming capacity, emulsion capacity, and the emulsion stability. Conversely, a significant increase in total phenolic content and antioxidant activity was observed after microwave heat treatment. | [43] |
| Mahdi et al. | Effect of Microwave Radiation on Bacteria, Fungi and Some Growth Characteristics of Cowpea Vigna unguiculata L. | Microwave soil treatment significantly reduced fungi and bacteria that could be cultured from the soil. At the highest energy level (15 kJ kg−1 soil) both fungi and bacteria were eliminated. Cowpea above ground biomass and root mass significantly increased (103% increase above the control) for moderate doses of microwave energy (10 kJ kg−1 soil), but almost returned to the same level as the untreated control for very high doses of microwave energy (15 kJ kg−1 soil). | [44] |
| Tiwari et al. | Dielectric heating-assisted disinfestation of black gram and its effect on protein profile: A comparative study on radio frequency and microwave heating | Pulse beetle (Callosobruchus maculatus) in blackgram (Vigna mungo (L.) Hepper) kernels, were subjected to radio frequency and microwave heating. The pupa stage of the insect’s life cycle was found to be more resilient to heat treatment than eggs, larvae, and adults. There were also measurable changes in the amino acid profile of the blackgram. | [45] |
| Speir et al. | Effects of microwave radiation on the microbial biomass, phosphatase activity and levels of extractable N and P in a low fertility soil under pasture | Microwave irradiation was investigated as a controlled soil biocidal treatment which could selectively kill microbial biomass. Under the experimental conditions chosen, irradiation of the soil sample for 90 s gave a kill of microbial biomass equal to that achieved by CHCl3 fumigation. Extractable mineral N was increased after incubation of irradiated soil, and after 90 s irradiation was only slightly lower than that of fumigated soil. | [46] |
| Yadav et al. | Microwave technology for disinfestation of cereals and pulses: An overview | Microwaves may be an alternate to chemical methods of killing insects in grain as their application do not leave any undesirable residues and thus might be very effective for controlling insect infestation compared to other available methods. Microwave disinfestation can provide a continuous process to allow large quantities of products to pass in a shorter period. Microwave disinfestation is considered safe and competitive alternative method to fumigation as it avoids environmental pollution. | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, S.; McFarlane, D.J.; Mattner, S.W.; Brodie, G.I. Potential of Microwave Heating and Plasma for Biosecurity Applications. Thermo 2022, 2, 312-333. https://doi.org/10.3390/thermo2030022
Taheri S, McFarlane DJ, Mattner SW, Brodie GI. Potential of Microwave Heating and Plasma for Biosecurity Applications. Thermo. 2022; 2(3):312-333. https://doi.org/10.3390/thermo2030022
Chicago/Turabian StyleTaheri, Saeedeh, Dylan John McFarlane, Scott William Mattner, and Graham Ian Brodie. 2022. "Potential of Microwave Heating and Plasma for Biosecurity Applications" Thermo 2, no. 3: 312-333. https://doi.org/10.3390/thermo2030022
APA StyleTaheri, S., McFarlane, D. J., Mattner, S. W., & Brodie, G. I. (2022). Potential of Microwave Heating and Plasma for Biosecurity Applications. Thermo, 2(3), 312-333. https://doi.org/10.3390/thermo2030022






