Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review
Abstract
:1. Introduction
1.1. Definition of Encapsulation
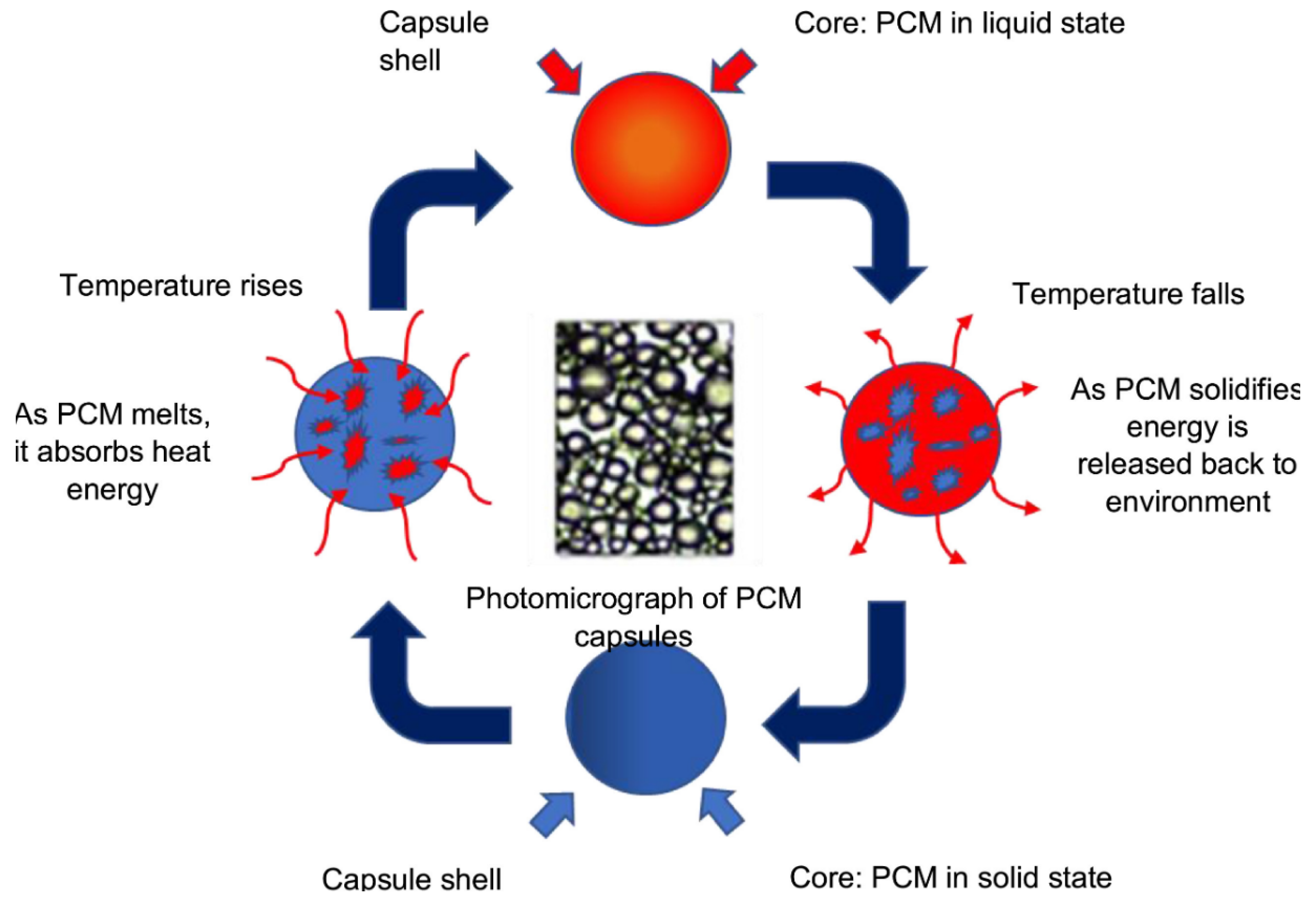
1.2. Working Principle of PCM Encapsulation
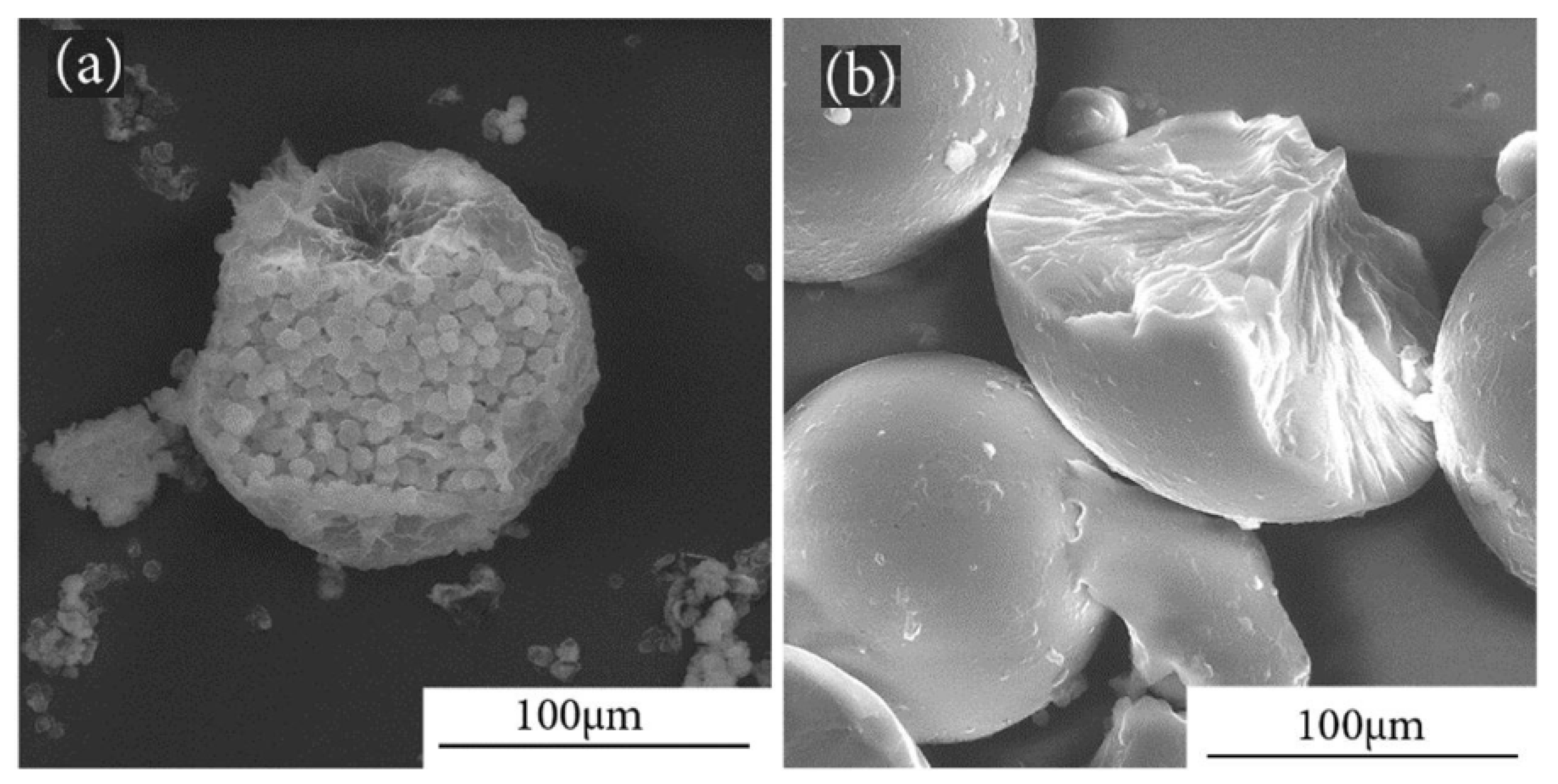
1.3. Morphology of PCM Microcapsules (mPCMs)
2. Manufacturing Methods of mPCMs
2.1. Chemical Methods
2.1.1. In Situ Polymerization
2.1.2. Interfacial Polymerization
2.1.3. Suspension Polymerization
Thermal Microencapsulation
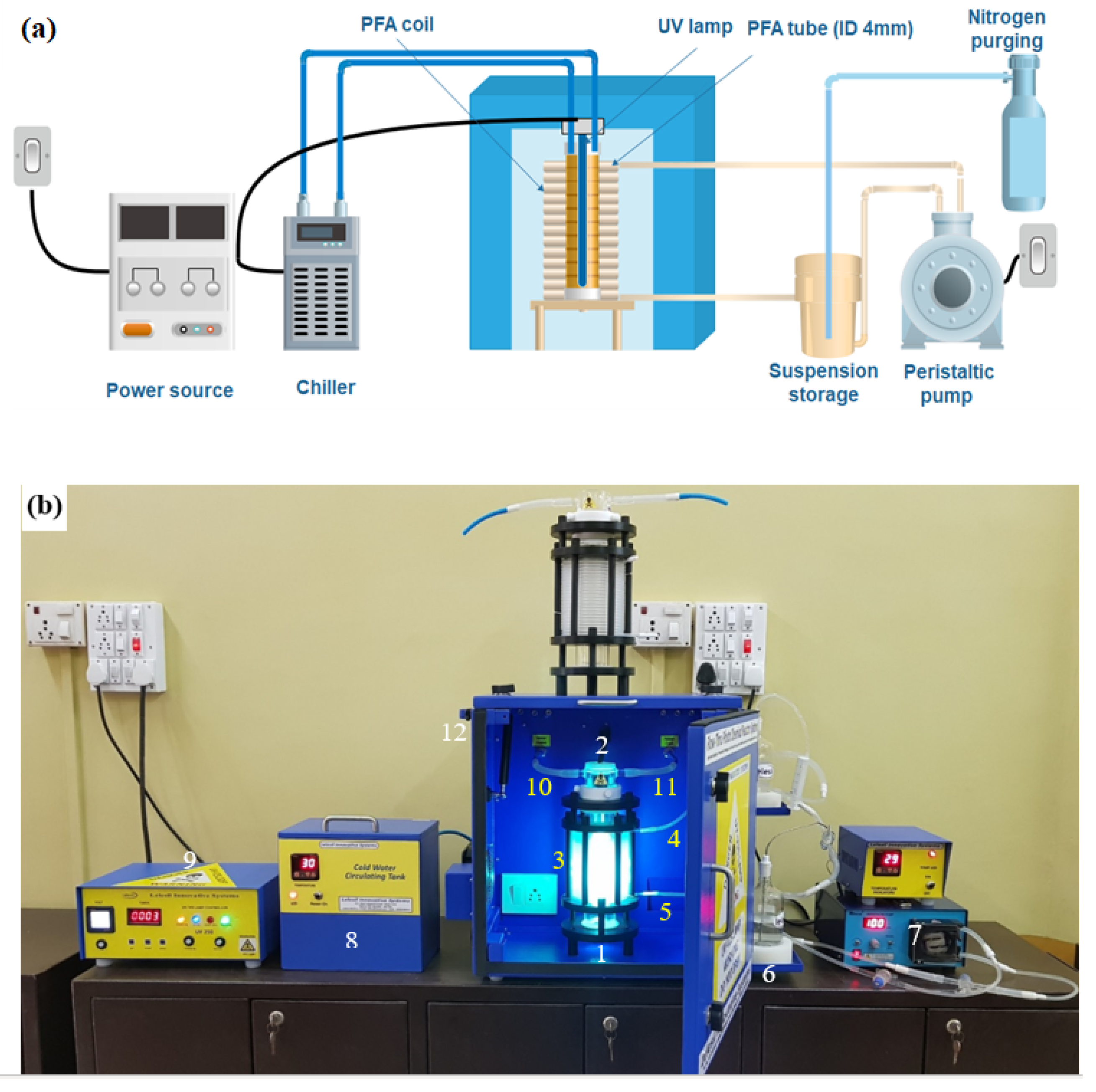
UV Microencapsulation
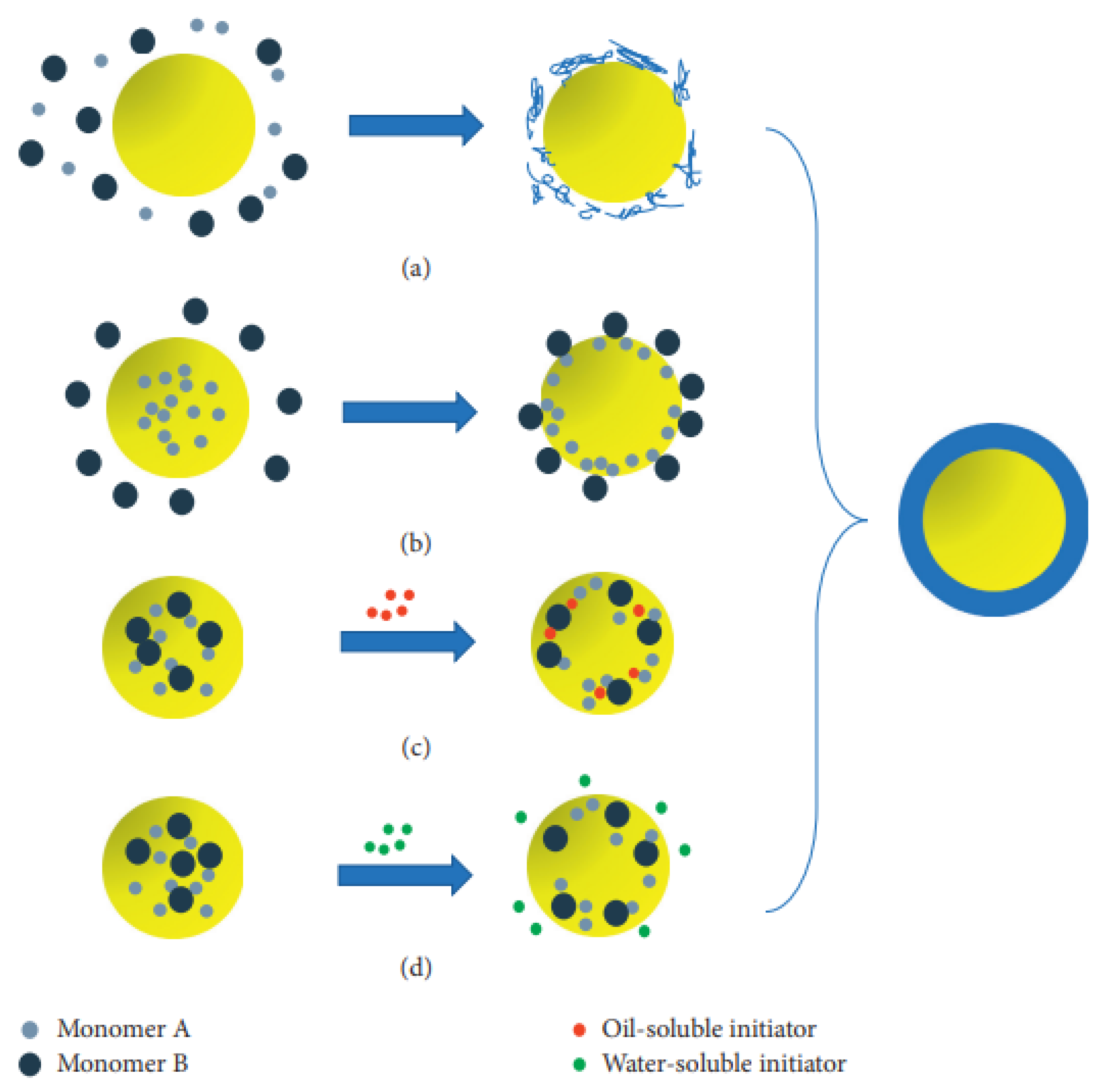
2.1.4. Emulsion Polymerization
2.2. Microencapsulation of PCMs with Other Methods and Shell Types
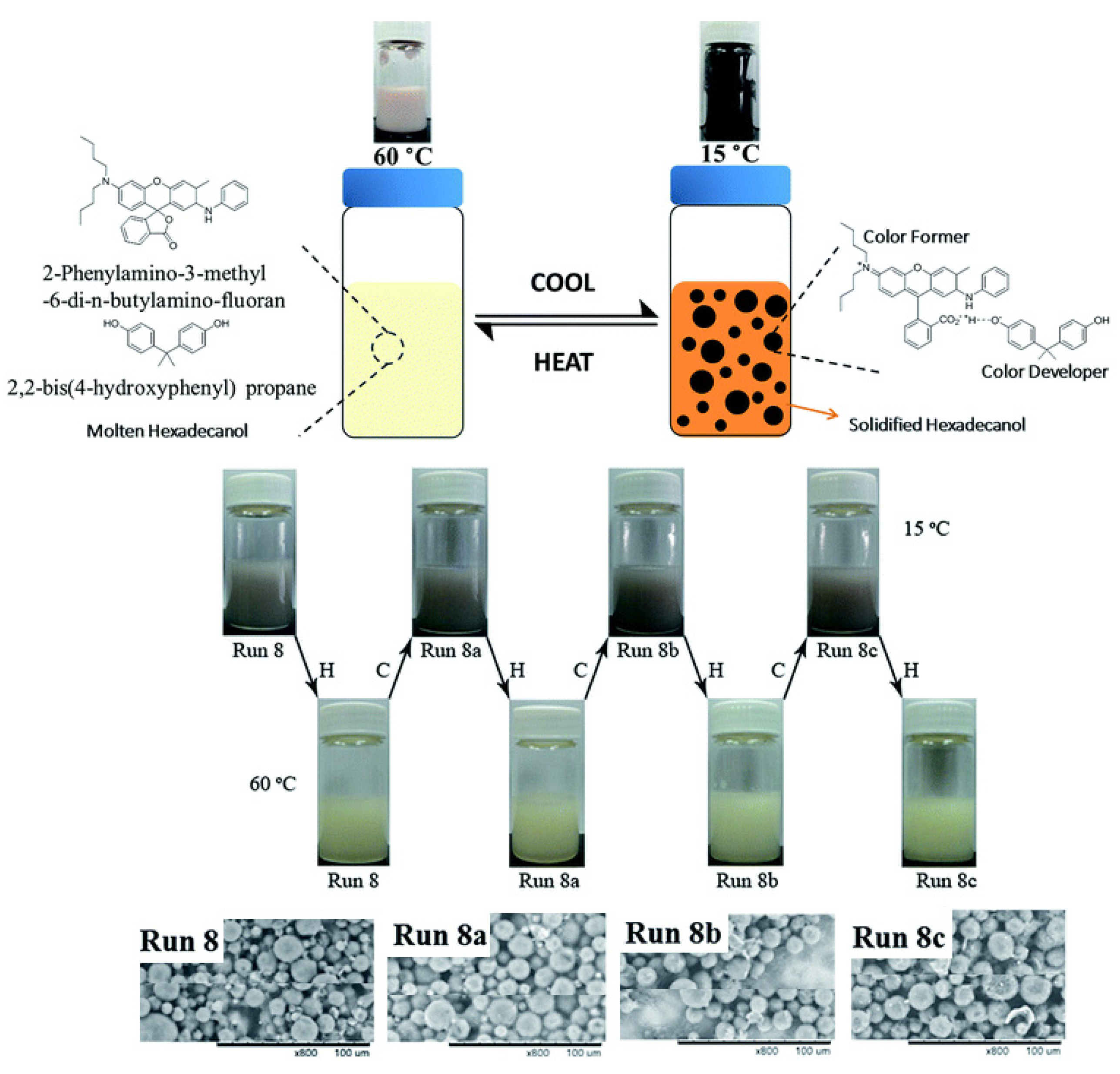
2.2.1. Coacervation-Phase Separation
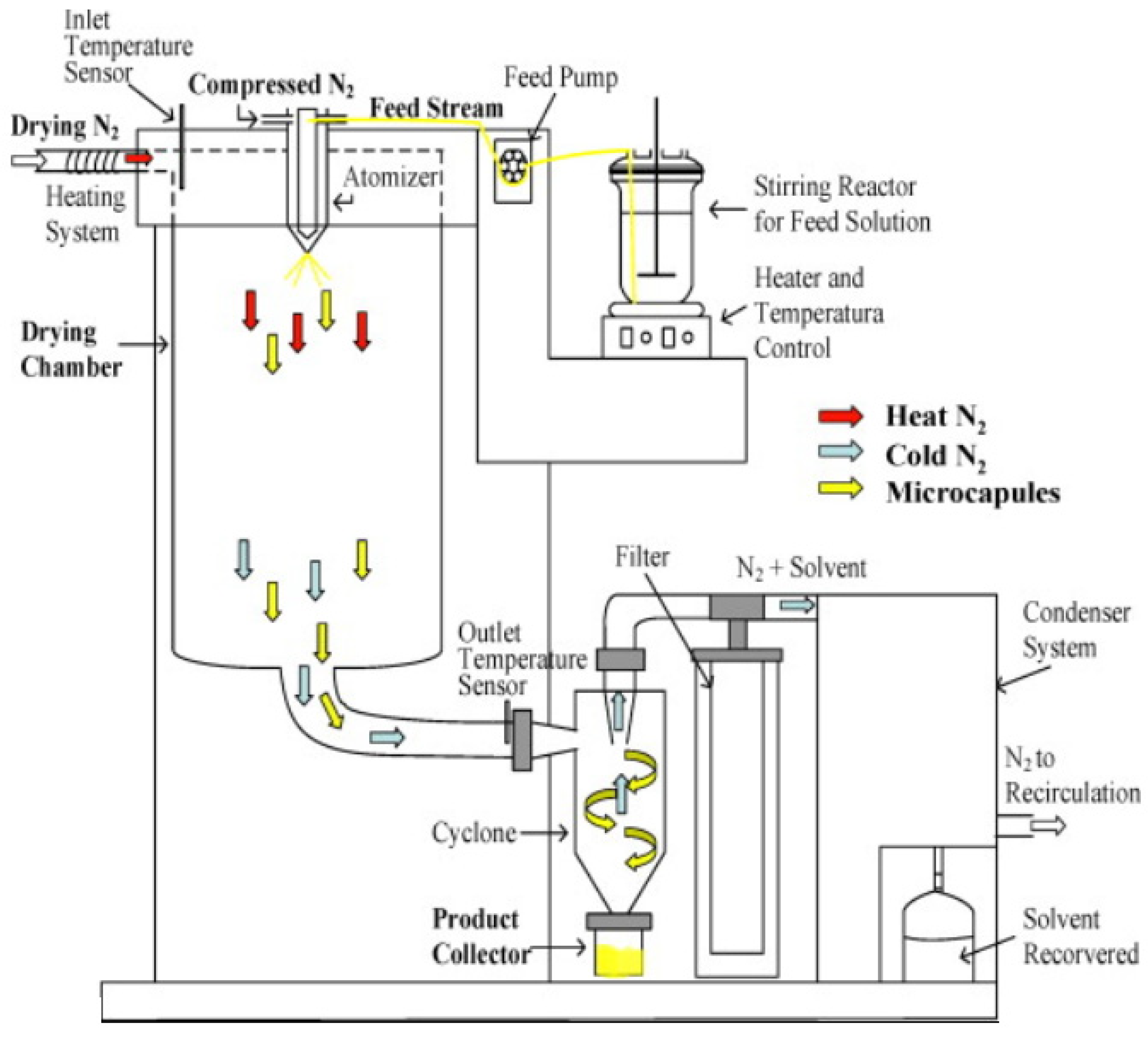
2.2.2. Spay Drying
- (1)
- Homogeneous liquid solution (feed stream) preparation—consists of phase change material and dis-solved polymer, which is achieved through the use of a proper solvent;
- (2)
- Atomization of the as-prepared solution by means of a carrier gas stream (such as compressed nitrogen);
- (3)
- Solvent evaporation, where the particles were dried by hot nitrogen stream (dried nitrogen) in the drying chamber, and then the final product was recovered in the collector, as shown in Figure 14.
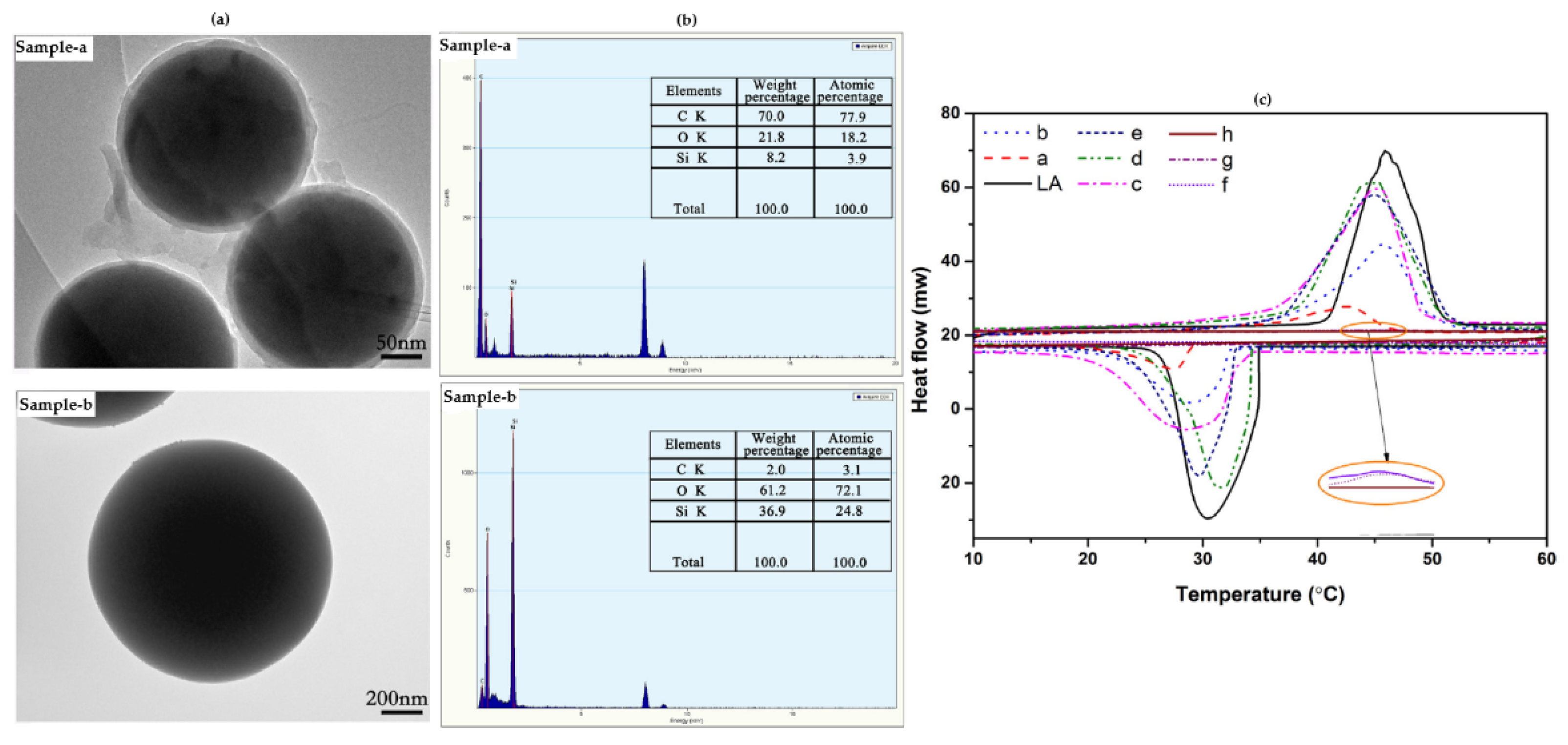
2.2.3. Sol–Gel Method
- (1)
- The formation of PCM O/W emulsion through the mixing of PCM with a surface-active solution containing surfactant (emulsifier);
- (2)
- The aqueous acidic phase (sol solution) prepared by dis-solving the precursor compound, e.g., tetraethyl orthosilicate (TEOS) or sodium silicate precursor in water;
- (3)
- Microcapsules formation via condensation polymerization by dropwise addition of the sol solution into the PCM O/W emulsion.
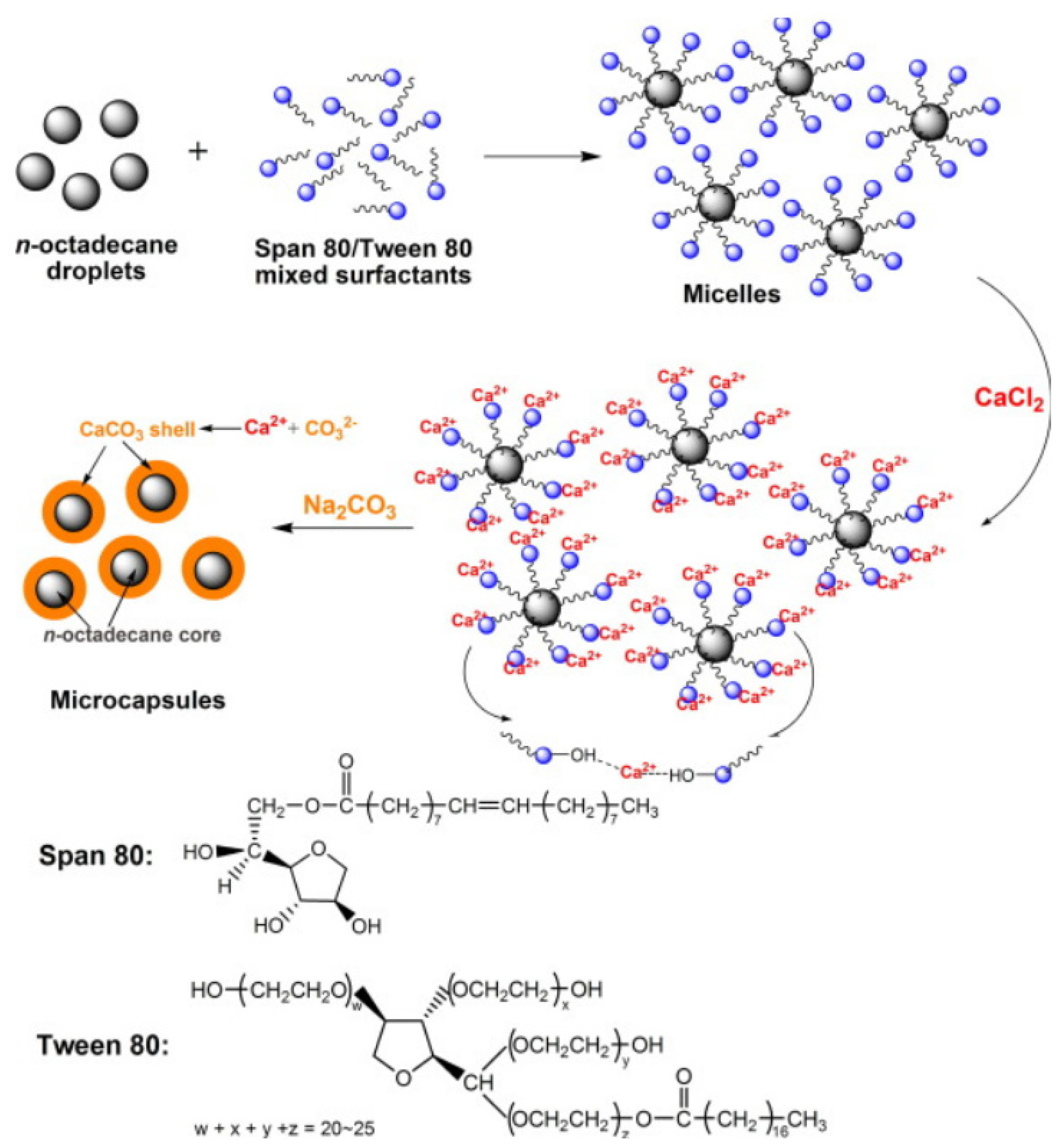
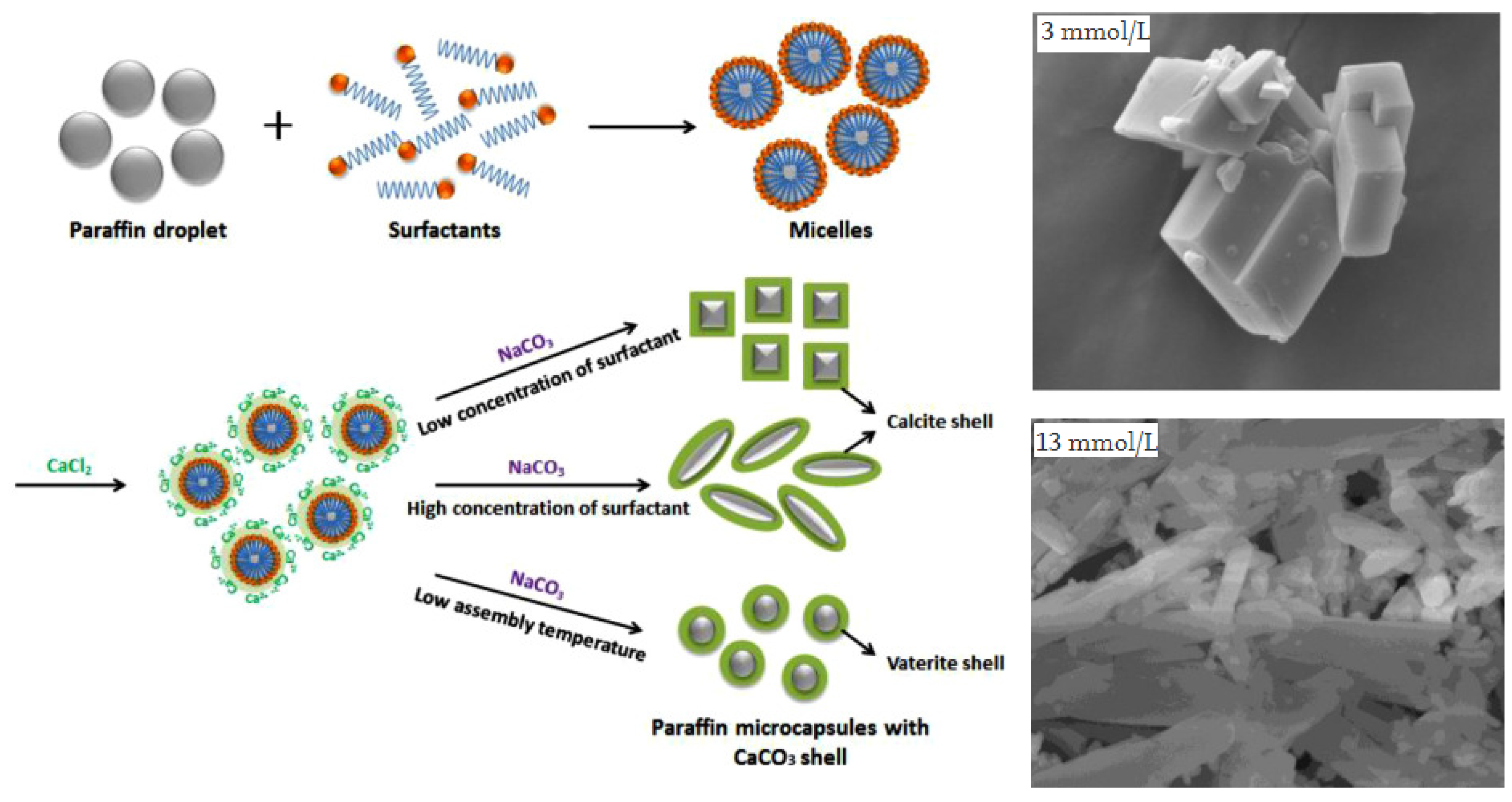
2.2.4. Self-Assembly Method
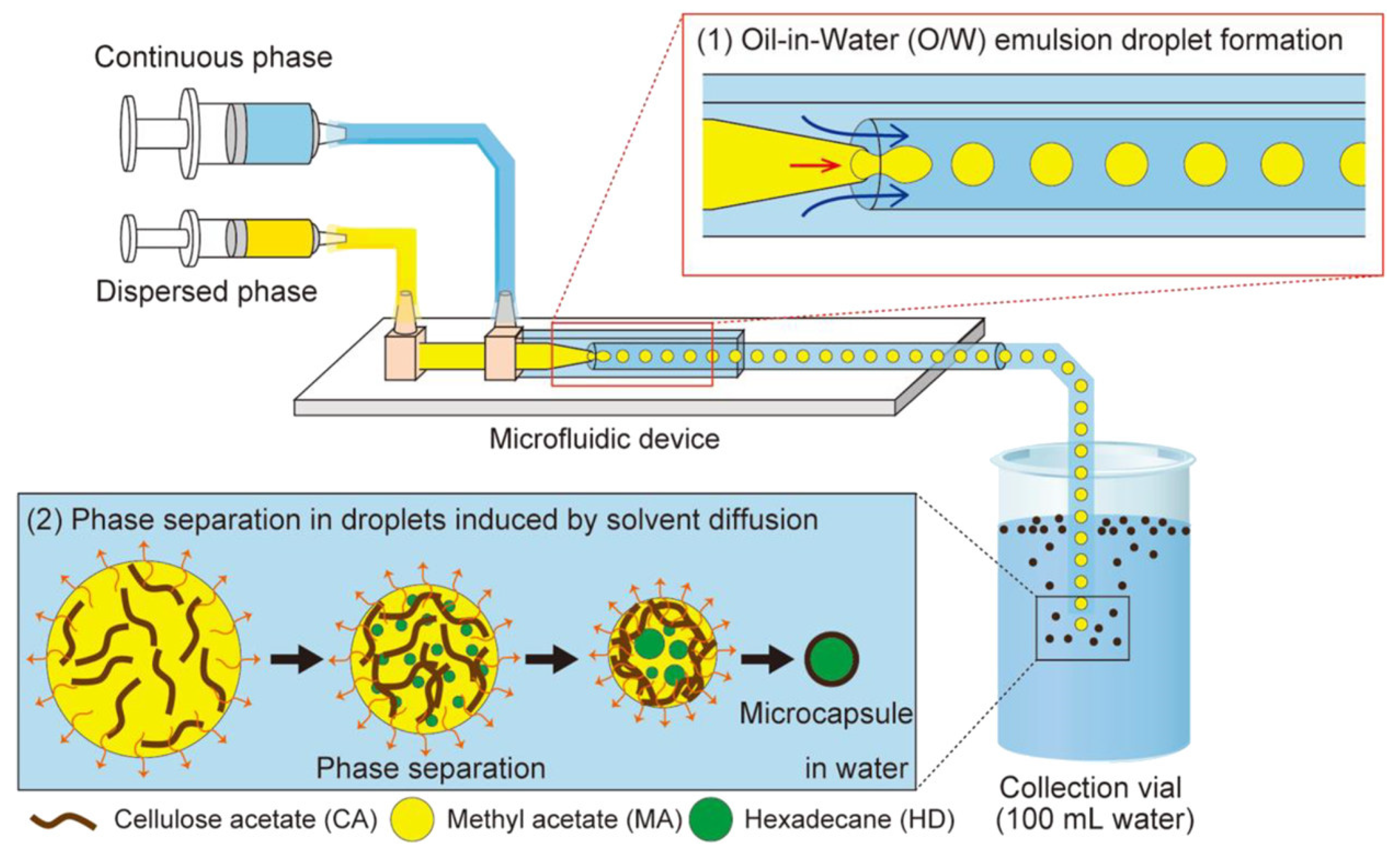
2.2.5. Microfluidic Method
- (1)
- Emulsification of n-octadecane, isophorone diisocyanate (IPDI), and dibutyltin dilaurate (DBTDL) in an aqueous mixture of tetraethylenepentamine (TEPA), poly (vinyl alcohol), and sodium dodecyl sulfate (SDS);
- (2)
- In situ polycondensation between TEPA and IPDI along and outside the tube length.
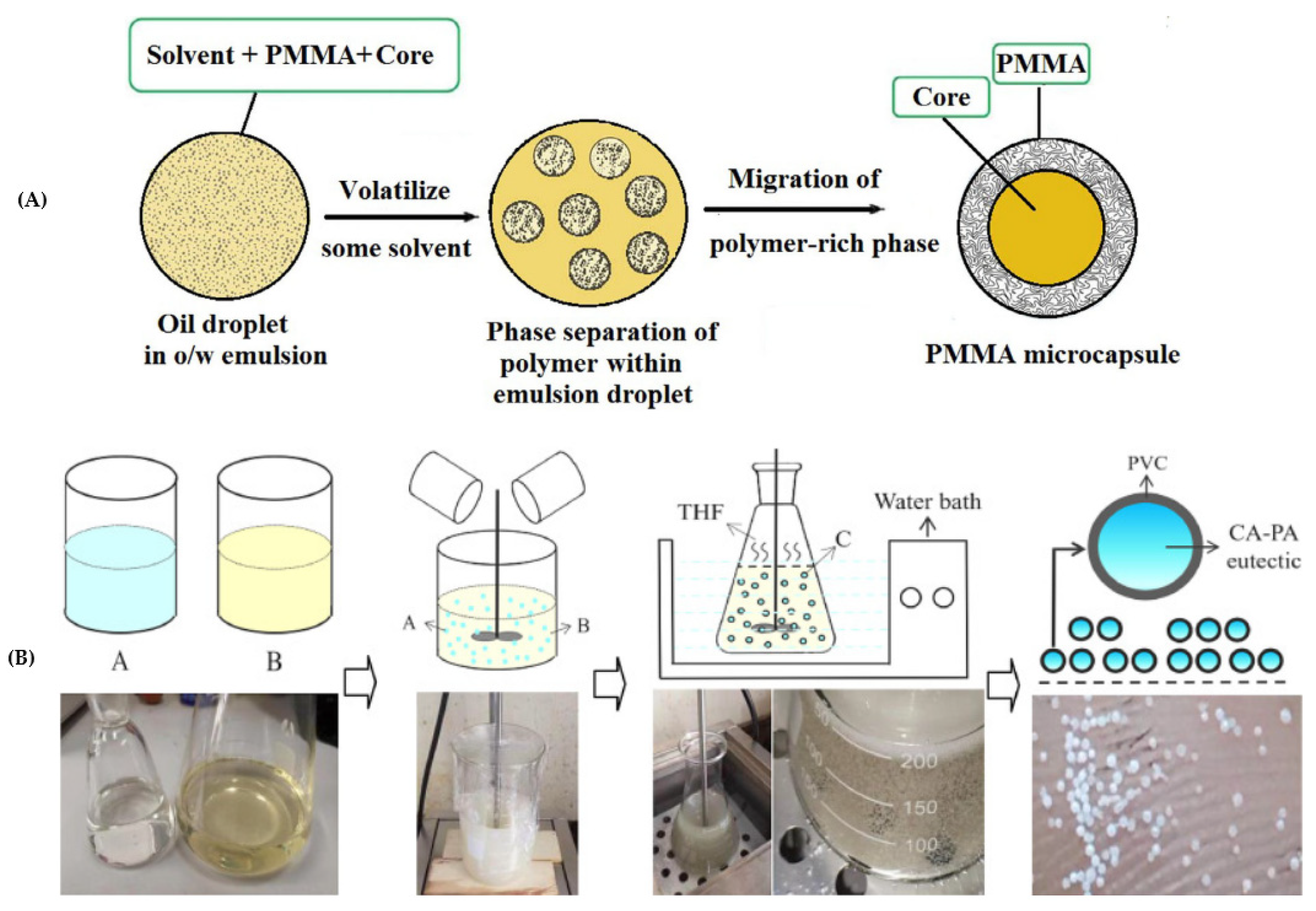
2.2.6. Solvent Evaporation/Phase Separation
2.2.7. Microencapsulation of Inorganic PCM
3. Thermophysical Enhancement of mPCMs
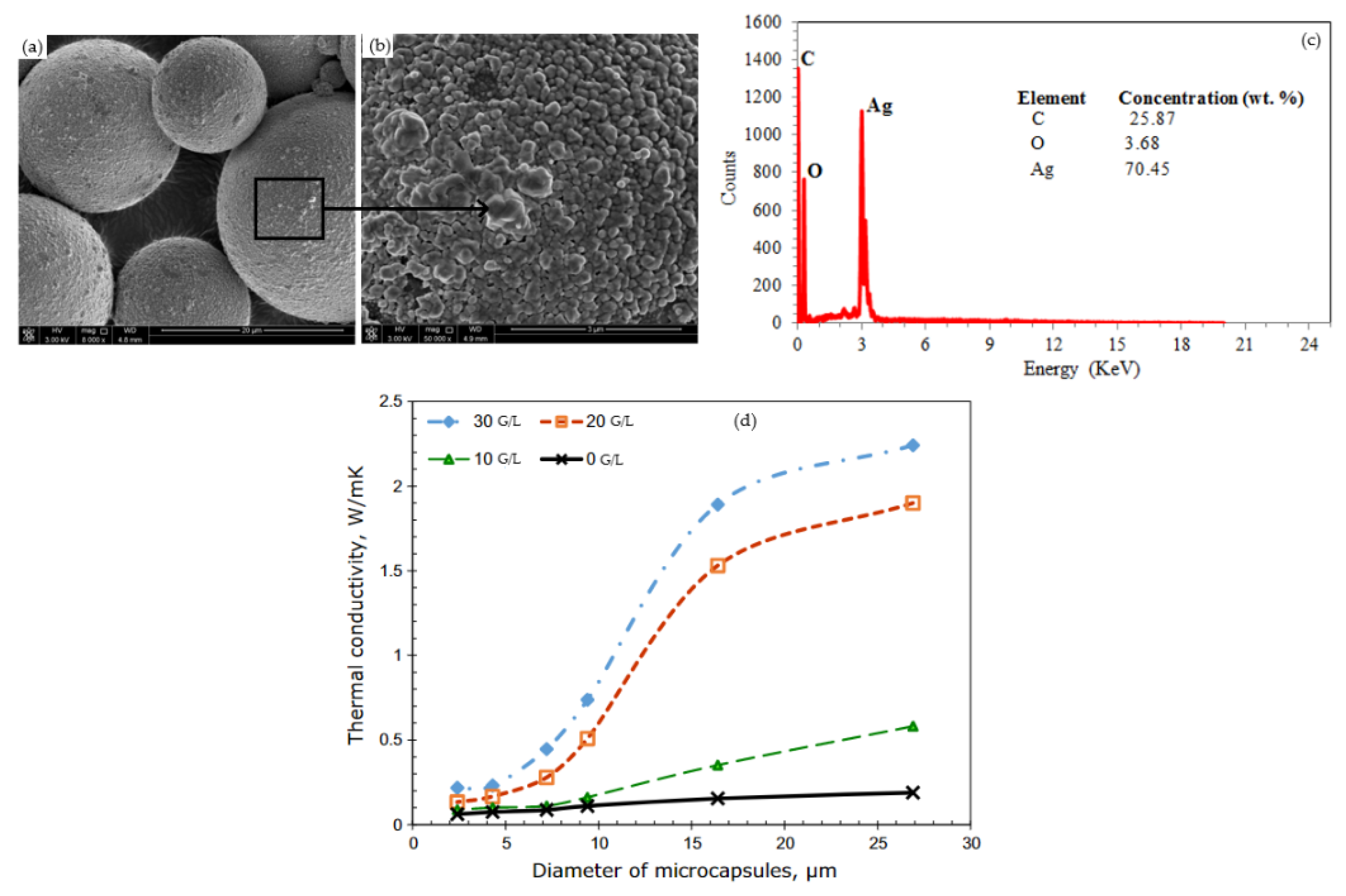
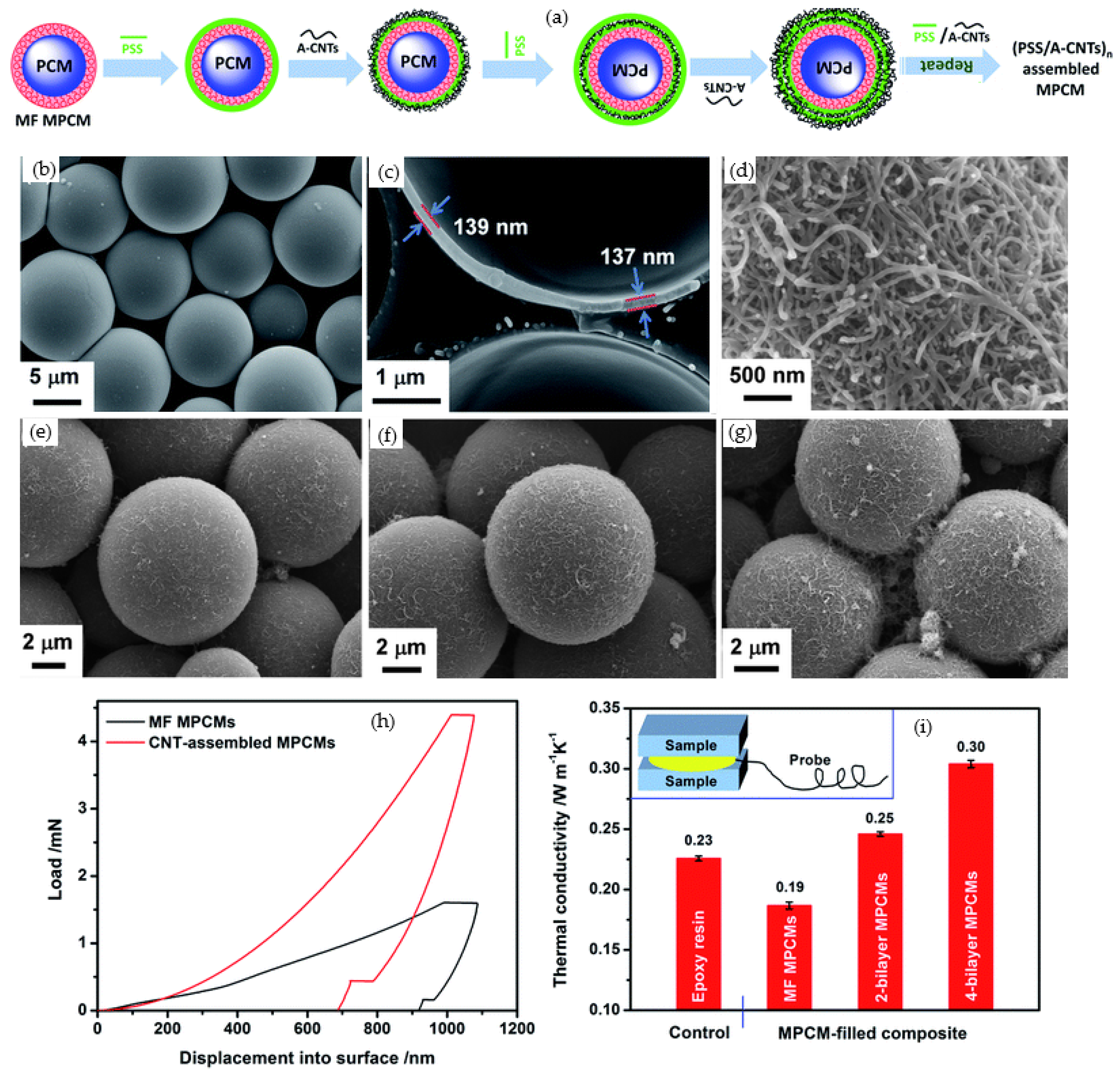
3.1. Heat Transfer Enhancement
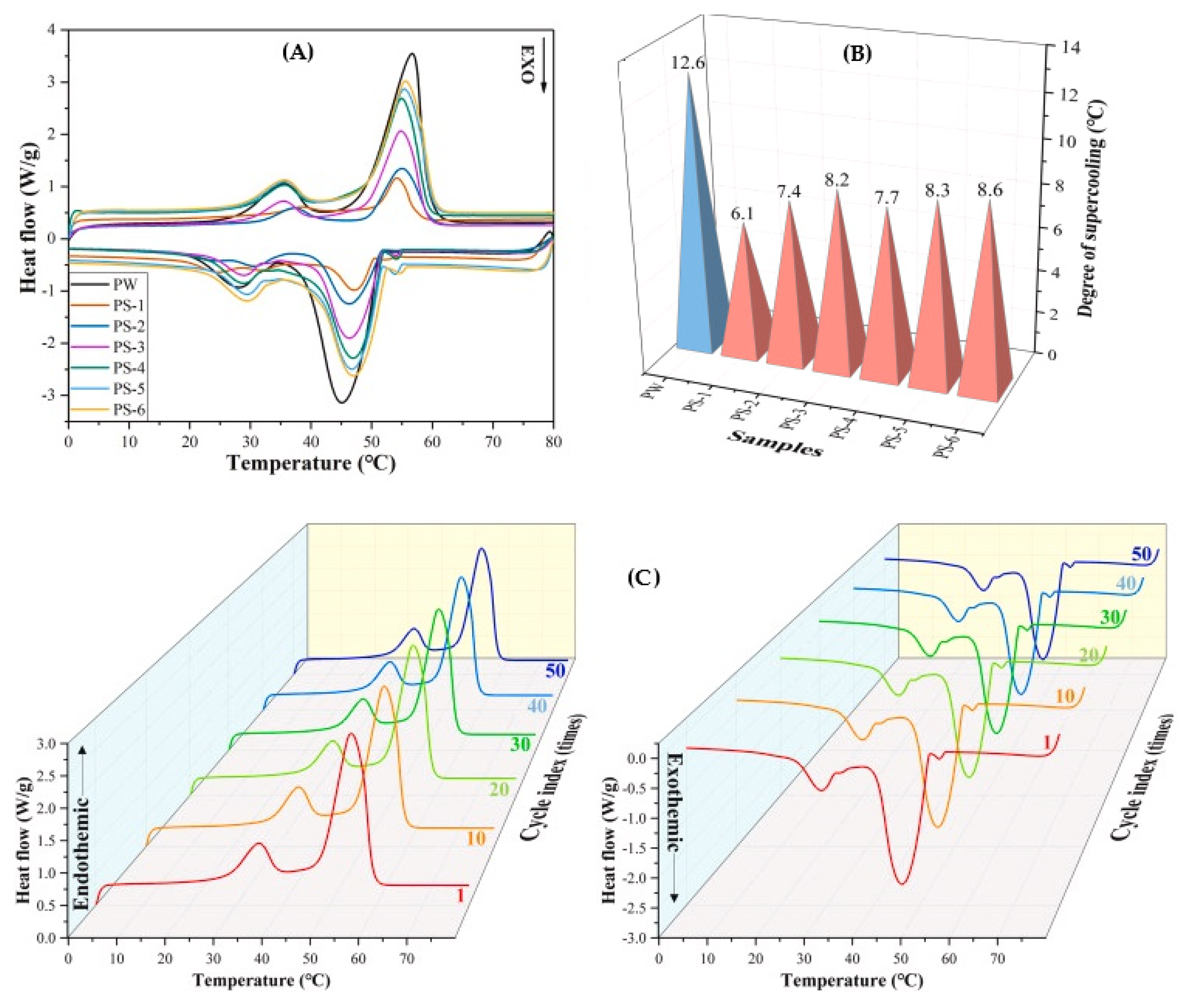
3.2. Supercooling Suppression
3.3. Mechanical Strength
4. Conclusions and Future Trends
Funding
Conflicts of Interest
Nomenclature
| PCMs | Phase change materials | PDVB | Poly(divinylbenzene) | GEL | Gelatine |
| mPCMs | Phase change materials microcapsules | LDPE | Low-density polyethylene | MC | Methylcellulose |
| TES | Thermal energy storage | EVA | Ethyl vinyl acetate | XG | Xanthan Gum |
| UV | Ultraviolet | PVC | Poly(vinyl chloride) | PAM | Poly(acrylamide) |
| MMA | Methyl methacrylate | HEMA | Hydroxyethyl methacrylate | PEI | Poly(ethylenimine) |
| BA | Butyl acrylate | PDMS | Poly(dimethyl siloxane) | SDS | Sodium dodecyl sulfate |
| MF | Melamine-formaldehyde | ABS | Acrylonitrile-butadiene-styrene | LED | light emitting diode |
| UF | Urea-formaldehyde | CNC | Cellulose nanocrystal | OA | Oxalic acid |
| UMF | Urea-melamine formaldehyde | SF | Silk fibroin | TNBT | Tetra-n-butyl titanate |
| PEG | Polyethylene glycol | CHI | Chitosan | TEOS | Tetraethyl orthosilicate |
| PU | Polyurethane | EC | Ethyl cellulose | TEM | Transmission electron microscopy |
| TDI | Toluene-2, 4-diisocyanate | GO | Graphene oxide | EDX | Energy-dispersive detector |
| DETA | Diethylenetriamine | SEM | Scanning electron microscope | DSC | Differential scanning calorimetry |
| PBMA | Poly(butyl methacrylate) | TGA | Thermogravimetric Analysis | PA | Palmitic acid |
| PS | Poly(styrene) | SMA | Styrene Maleic anhydride | PW | Paraffin wax |
| HD | Heptadecane | TSCD | Trisodium citrate dihydrate | CTAB | Cetyltrimethylammonium bromide |
| HD | n-hexadecane | IPDI | Isophorone diisocyanate | DBTDL | Dibutyltin dilaurate |
| TEPA | Tetraethylenepentamine | PVA | Poly (vinyl alcohol) | CA | Caprylic Acid |
| THF | Tetrahydrofuran | SiC | Silicon carbide | CNTs | Carbon nanotubes |
| PDA | polydopamine | CPCMs | composite phase change materials |
References
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Blakers, A.; Stocks, M.; Lu, B.; Cheng, C. A review of pumped hydro energy storage. Prog. Energy 2021, 3, 022003. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Chavan, S.; Rudrapati, R.; Manickam, S. A comprehensive review on current advances of thermal energy storage and its applications. Alex. Eng. J. 2022, 61, 5455–5463. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Ruggles, T.H.; Rinaldi, K.; Dowling, J.A.; Duan, L.; Caldeira, K.; Lewis, N.S. The role of concentrated solar power with thermal energy storage in least-cost highly reliable electricity systems fully powered by variable renewable energy. Adv. Appl. Energy 2022, 6, 100091. [Google Scholar] [CrossRef]
- Nuytten, T.; Claessens, B.; Paredis, K.; Van Bael, J.; Six, D. Flexibility of a combined heat and power system with thermal energy storage for district heating. Appl. Energy 2013, 104, 583–591. [Google Scholar] [CrossRef]
- Sadeghi, G. Energy storage on demand: Thermal energy storage development, materials, design, and integration challenges. Energy Storage Mater. 2022, 46, 192–222. [Google Scholar] [CrossRef]
- Enescu, D.; Chicco, G.; Porumb, R.; Seritan, G. Thermal Energy Storage for Grid Applications: Current Status and Emerging Trends. Energies 2020, 13, 340. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Martorell, I.; Miró, L.; Fernández, A.I.; Barreneche, C.; Cabeza, L.F.; Fernández, A.I.; Barreneche, C. 1—Introduction to thermal energy storage systems. In Advances in Thermal Energy Storage Systems, 2nd ed.; Cabeza, L.F., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 1–33. [Google Scholar]
- Mahon, H.; O’Connor, D.; Friedrich, D.; Hughes, B. A review of thermal energy storage technologies for seasonal loops. Energy 2022, 239, 122207. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Esen, M.; Ayhan, T. Development of a model compatible with solar assisted cylindrical energy storage tank and variation of stored energy with time for different phase change materials. Energy Convers. Manag. 1996, 37, 1775–1785. [Google Scholar] [CrossRef]
- Esen, M.; Durmuş, A.; Durmuş, A. Geometric design of solar-aided latent heat store depending on various parameters and phase change materials. Sol. Energy 1998, 62, 19–28. [Google Scholar] [CrossRef]
- Esen, M. Thermal performance of a solar-aided latent heat store used for space heating by heat pump. Sol. Energy 2000, 69, 15–25. [Google Scholar] [CrossRef]
- Rajabifar, B. Enhancement of the performance of a double layered microchannel heatsink using PCM slurry and nanofluid coolants. Int. J. Heat Mass Transf. 2015, 88, 627–635. [Google Scholar] [CrossRef]
- Wu, W.; Bostanci, H.; Chow, L.C.; Hong, Y.; Wang, C.M.; Su, M.; Kizito, J.P. Heat transfer enhancement of PAO in microchannel heat exchanger using nano-encapsulated phase change indium particles. Int. J. Heat Mass Transf. 2013, 58, 348–355. [Google Scholar] [CrossRef]
- Roberts, N.S.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Farid, M.M. Efficacy of using slurry of metal-coated microencapsulated PCM for cooling in a micro-channel heat exchanger. Appl. Therm. Eng. 2017, 122, 11–18. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, J.-H.; Seo, J.; Jeong, S.-G.; Kim, S. Application of PCM thermal energy storage system to reduce building energy consumption. J. Therm. Anal. Calorim. 2013, 111, 279–288. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Auckaili, A.; Farid, M. Chapter11—Cooling of milk on dairy farms: An application of a novel ice encapsulated storage system in New Zealand. In Food Engineering Innovations Across the Food Supply Chain; Juliano, P., Knoerzer, K., Sellahewa, J., Nguyen, M.H., Buckow, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 207–228. [Google Scholar]
- Al-Shannaq, R.; Farid, M.M. 10—Microencapsulation of phase change materials for thermal energy storage systems. In Advances in Thermal Energy Storage Systems, 2nd ed.; Cabeza, L.F., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 269–329. [Google Scholar]
- Benita, S. Microencapsulation: Methods and Industrial Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Cao, R.; Zhang, Z.; Du, J.; Peng, X. Encapsulated Dye/Polymer Nanoparticles Prepared via Miniemulsion Polymerization for Inkjet Printing. ACS Omega 2018, 3, 7380–7387. [Google Scholar] [CrossRef] [Green Version]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Boh Podgornik, B.; Šandrić, S.; Kert, M. Microencapsulation for Functional Textile Coatings with Emphasis on Biodegradability—A Systematic Review. Coatings 2021, 11, 1371. [Google Scholar] [CrossRef]
- Al Shannaq, R.; Farid, M.M. 10—Microencapsulation of phase change materials (PCMs) for thermal energy storage systems. In Advances in Thermal Energy Storage Systems; Cabeza, L.F., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 247–284. [Google Scholar]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M.; et al. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2, 20170020. [Google Scholar]
- Sánchez-Silva, L.; Tsavalas, J.; Sundberg, D.; Sánchez, P.; Rodriguez, J.F. Synthesis and Characterization of Paraffin Wax Microcapsules with Acrylic-Based Polymer Shells. Ind. Eng. Chem. Res. 2010, 49, 12204–12211. [Google Scholar] [CrossRef]
- Lashgari, S.; Arabi, H.; Mahdavian, A.R.; Ambrogi, V. Thermal and morphological studies on novel PCM microcapsules containing n-hexadecane as the core in a flexible shell. Appl. Energy 2017, 190, 612–622. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Valdes, A.; Ramos, M.; Beltran, A.; Garrigos, M.C. Recent Trends in Microencapsulation for Smart and Active Innovative Textile Products. Curr. Org. Chem. 2018, 22, 1237–1248. [Google Scholar] [CrossRef]
- Yan, W.-M.; Ho, C.J.; Tseng, Y.-T.; Qin, C.; Rashidi, S. Numerical study on convective heat transfer of nanofluid in a minichannel heat sink with micro-encapsulated PCM-cooled ceiling. Int. J. Heat Mass Transf. 2020, 153, 119589. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.P.; Tyagi, I.; Rawat, A.; Sinha, S. Microencapsulation of a eutectic PCM using in situ polymerization technique for thermal energy storage. Int. J. Energy Res. 2020, 44, 3854–3864. [Google Scholar]
- Han, S.; Chen, Y.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. Effects of processing conditions on the properties of paraffin/melamine-urea-formaldehyde microcapsules prepared by in situ polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124046. [Google Scholar] [CrossRef]
- Kumar, G.N.; Al-Aifan, B.; Parameshwaran, R.; Ram, V.V. Facile synthesis of microencapsulated 1-dodecanol/melamine-formaldehyde phase change material using in-situ polymerization for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125698. [Google Scholar] [CrossRef]
- Dhivya, S.; Hussain, S.I.; Jeya Sheela, S.; Kalaiselvam, S. Experimental study on microcapsules of Ag doped ZnO nanomaterials enhanced Oleic-Myristic acid eutectic PCM for thermal energy storage. Thermochim. Acta 2019, 671, 70–82. [Google Scholar] [CrossRef]
- Singh, J.; Parvate, S.; Vennapusa, J.R.; Maiti, T.K.; Dixit, P.; Chattopadhyay, S. Facile method to prepare 1-dodecanol@poly(melamine-paraformaldehyde) phase change energy storage microcapsules via surfactant-free method. J. Energy Storage 2022, 49, 104089. [Google Scholar] [CrossRef]
- Cai, C.; Ouyang, X.; Zhou, L.; Liu, G.; Wang, Y.; Zhu, G.; Yao, J.; Militky, J.; Venkataraman, M.; Zhang, G. Co-solvent free interfacial polycondensation and properties of polyurea PCM microcapsules with dodecanol dodecanoate as core material. Sol. Energy 2020, 199, 721–730. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernández, A.I.; Farid, M.M. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Ansari, J.A.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Ikutegbe, C.A.; Farid, M.M. A Rapid Method for Low Temperature Microencapsulation of Phase Change Materials (PCMs) Using a Coiled Tube Ultraviolet Reactor. Energies 2021, 14, 7867. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Dickinson, M.; Farid, M. Supercooling elimination of phase change materials (PCMs) microcapsules. Energy 2015, 87, 654–662. [Google Scholar] [CrossRef]
- Chaiyasat, P.; Islam, M.Z.; Chaiyasat, A. Preparation of poly(divinylbenzene) microencapsulated octadecane by microsuspension polymerization: Oil droplets generated by phase inversion emulsification. RSC Adv. 2013, 3, 10202–10207. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Altıntaş, A. Preparation, characterization and latent heat thermal energy storage properties of micro-nanoencapsulated fatty acids by polystyrene shell. Appl. Therm. Eng. 2014, 73, 1160–1168. [Google Scholar] [CrossRef]
- Karaipekli, A.; Erdoğan, T.; Barlak, S. The stability and thermophysical properties of a thermal fluid containing surface-functionalized nanoencapsulated PCM. Thermochim. Acta 2019, 682, 178406. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Wang, Y.; Han, N.; Wang, J.; Wang, N.; Zhang, X. Chitosan composite microencapsulated comb-like polymeric phase change material via coacervation microencapsulation. Carbohydr. Polym. 2018, 200, 602–610. [Google Scholar] [CrossRef]
- Deveci, S.S.; Basal, G. Preparation of PCM microcapsules by complex coacervation of silk fibroin and chitosan. Colloid Polym. Sci. 2009, 287, 1455. [Google Scholar] [CrossRef]
- Demirbağ, S.; Aksoy, S.A. Encapsulation of phase change materials by complex coacervation to improve thermal performances and flame retardant properties of the cotton fabrics. Fibers Polym. 2016, 17, 408–417. [Google Scholar] [CrossRef]
- Zhang, H.; Balram, A.; Tiznobaik, H.; Shin, D.; Santhanagopalan, S. Microencapsulation of molten salt in stable silica shell via a water-limited sol-gel process for high temperature thermal energy storage. Appl. Therm. Eng. 2018, 136, 268–274. [Google Scholar] [CrossRef]
- Chang, C.C.; Tsai, Y.L.; Chiu, J.J.; Chen, H. Preparation of phase change materials microcapsules by using PMMA network-silica hybrid shell via sol-gel process. J. Appl. Polym. Sci. 2009, 112, 1850–1857. [Google Scholar] [CrossRef]
- Subramanian, A.; Appukuttan, S. Microencapsulation of Stearic Acid into Strontium Titanate Shell by Sol-Gel Approach for Thermal Energy Storage. ChemistrySelect 2019, 4, 7818–7823. [Google Scholar] [CrossRef]
- Qian, T.; Dang, B.; Chen, Y.; Jin, C.; Qian, J.; Sun, Q. Fabrication of magnetic phase change n-eicosane@ Fe3O4/SiO2 microcapsules on wood surface via sol-gel method. J. Alloys Compd. 2019, 772, 871–876. [Google Scholar] [CrossRef]
- Zou, L.; Li, S.; Li, L.; Ji, W.; Li, Y.; Cheng, X. Synthesis of TiO2 shell microcapsule-based phase change film with thermal energy storage and buffering capabilities. Mater. Today Sustain. 2022, 18, 100119. [Google Scholar] [CrossRef]
- Ji, W.; Cheng, X.; Chen, S.; Wang, X.; Li, Y. Self-assembly fabrication of GO/TiO2@paraffin microcapsules for enhancement of thermal energy storage. Powder Technol. 2021, 385, 546–556. [Google Scholar] [CrossRef]
- Yuan, K.; Liu, J.; Fang, X.; Zhang, Z. Novel facile self-assembly approach to construct graphene oxide-decorated phase-change microcapsules with enhanced photo-to-thermal conversion performance. J. Mater. Chem. A 2018, 6, 4535–4543. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Electrostatic interaction-based self-assembly of paraffin@ graphene microcapsules with remarkable thermal conductivity for thermal energy storage. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 120–127. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Methaapanon, R.; Kornbongkotmas, S.; Ataboonwongse, C.; Soottitantawat, A. Microencapsulation of n-octadecane and methyl palmitate phase change materials in silica by spray drying process. Powder Technol. 2020, 361, 910–916. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Valverde, J.L.; Rodríguez, J.F.; Barber, A.H.; Cubillo, J.J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm®RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Microencapsulation and thermal properties of myristic acid with ethyl cellulose shell for thermal energy storage. Appl. Energy 2018, 231, 494–501. [Google Scholar] [CrossRef]
- Xing, J.; Zhou, Y.; Yang, K.; Chang, J.; Yu, Y.; Cai, L.; Shi, S.Q.; Huang, Z. Microencapsulation of fatty acid eutectic with polyvinyl chloride shell used for thermal energy storage. J. Energy Storage 2021, 34, 101998. [Google Scholar] [CrossRef]
- Khan, A.; Saikia, P.; Saxena, R.; Rakshit, D.; Saha, S. Microencapsulation of phase change material in water dispersible polymeric particles for thermoregulating rubber composites—A holistic approach. Int. J. Energy Res. 2020, 44, 1567–1579. [Google Scholar] [CrossRef]
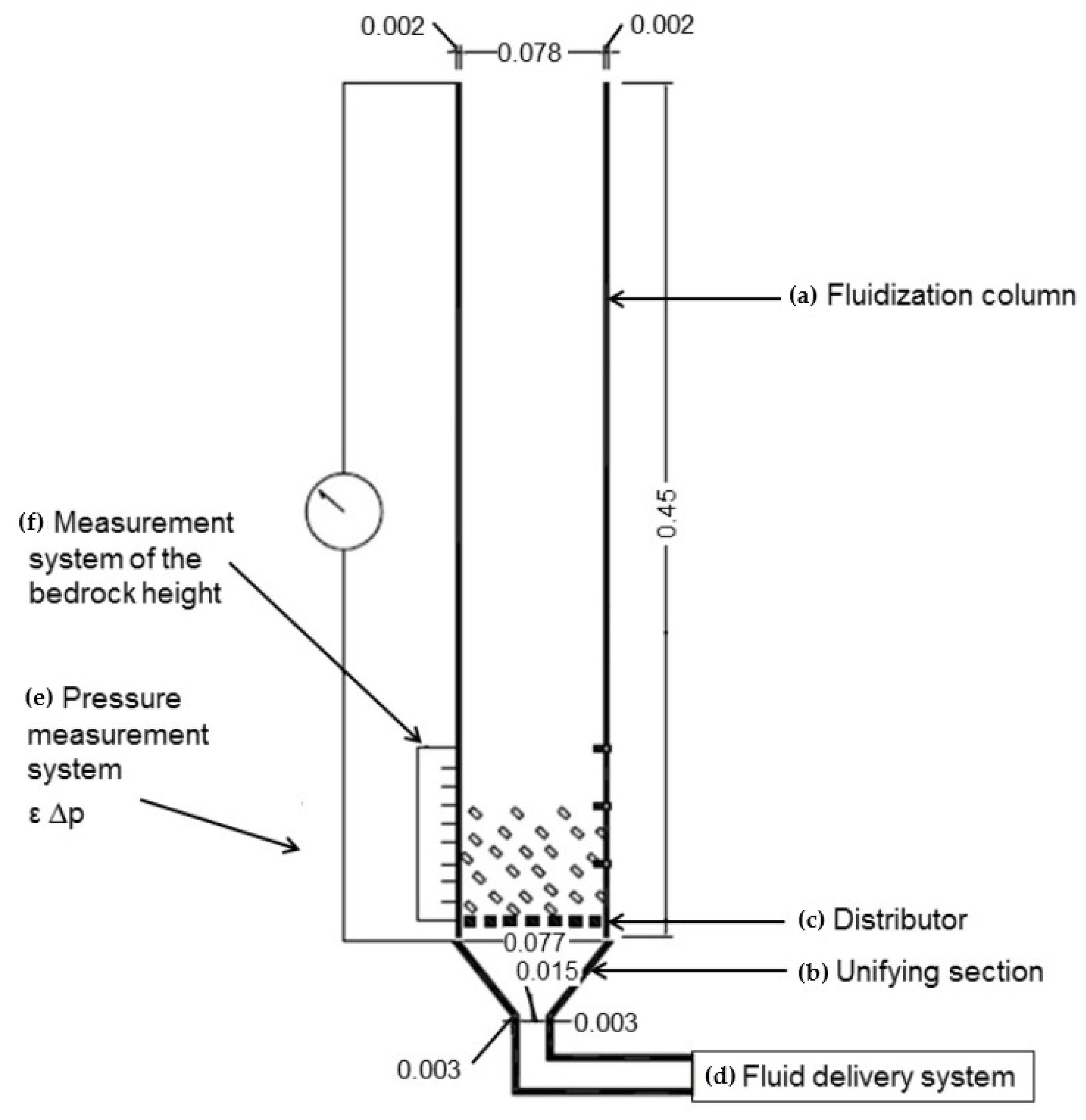
- Ushak, S.; Cruz, M.J.; Cabeza, L.F.; Grágeda, M. Preparation and Characterization of Inorganic PCM Microcapsules by Fluidized Bed Method. Materials 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lone, S.; Lee, H.M.; Kim, G.M.; Koh, W.-G.; Cheong, I.W. Facile and highly efficient microencapsulation of a phase change material using tubular microfluidics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 61–67. [Google Scholar] [CrossRef]
- Fu, Z.; Su, L.; Li, J.; Yang, R.; Zhang, Z.; Liu, M.; Li, J.; Li, B. Elastic silicone encapsulation of n-hexadecyl bromide by microfluidic approach as novel microencapsulated phase change materials. Thermochim. Acta 2014, 590, 24–29. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ogawa, M.; Katayama, R.; Yonemura, K.; Nakao, S.-I. A facile microencapsulation of phase change materials within silicone-based shells by using glass capillary devices. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 297–303. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Mortazavi, S.M.; Khayamian, T. Preparation of calcium alginate microcapsules containing n-nonadecane by a melt coaxial electrospray method. J. Electrost. 2015, 73, 56–64. [Google Scholar] [CrossRef]
- Peng, G.; Dou, G.; Hu, Y.; Sun, Y.; Chen, Z. Phase Change Material (PCM) Microcapsules for Thermal Energy Storage. Adv. Polym. Technol. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tao, X.-M.; Yick, K.-L.; Wang, X.-C. Structure and thermal stability of microencapsulated phase-change materials. Colloid Polym. Sci. 2004, 282, 330–336. [Google Scholar] [CrossRef]
- Choi, J.-K.; Lee, J.G.; Kim, J.H.; Yang, H.-S. Preparation of microcapsules containing phase change materials as heat transfer media by in-situ polymerization. J. Ind. Eng. Chem. 2001, 7, 358–362. [Google Scholar]
- Konuklu, Y.; Erzin, F. Preparation of pentadecane/poly(melamine-urea-formaldehyde) microcapsules for thermal energy storage applications. Int. J. Energy Res. 2019, 43, 6322–6326. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Lopez, V.; Cuenca, N.; Valverde, J.L. Poly(urea-formaldehyde) microcapsules containing commercial paraffin: In situ polymerization study. Colloid Polym. Sci. 2018, 296, 1449–1457. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Huang, R.; Wang, N.; Wang, J.; Zhang, X. Microstructure regulation of microencapsulated bio-based n-dodecanol as phase change materials via in situ polymerization. New J. Chem. 2017, 41, 14696–14707. [Google Scholar] [CrossRef]
- Konuklu, Y.; Unal, M.; Paksoy, H.O. Microencapsulation of caprylic acid with different wall materials as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 120, 536–542. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chang, T.; Wang, J.; Wang, X.; Zhou, G. Phase change material microcapsules with melamine resin shell via cellulose nanocrystal stabilized Pickering emulsion in-situ polymerization. Chem. Eng. J. 2022, 428, 131164. [Google Scholar] [CrossRef]
- Mustapha, A.N.; Zhang, Y.; Zhang, Z.; Ding, Y.; Li, Y. A systematic study on the reaction mechanisms for the microencapsulation of a volatile phase change material (PCM) via one-step in situ polymerisation. Chem. Eng. Sci. 2022, 252, 117497. [Google Scholar] [CrossRef]
- Lawson, C.M.; Virgallito, D.R.; Lawson, J. Methods for Making Low Remnant Free Formaldehyde Microcapsules and Microcapsules Made by Same. EP3337604B1, 24 March 2021. [Google Scholar]
- Li, W.; Zhang, X.-X.; Wang, X.-C.; Niu, J.-J. Preparation and characterization of microencapsulated phase change material with low remnant formaldehyde content. Mater. Chem. Phys. 2007, 106, 437–442. [Google Scholar] [CrossRef]
- Šumiga, B.; Knez, E.; Vrtačnik, M.; Savec, V.F.; Starešinic, M.; Boh, B. Production of Melamine-Formaldehyde PCM Microcapsules with Ammonia Scavenger used for Residual Formaldehyde Reduction. Acta Chim. Slov. 2011, 58, 14–25. [Google Scholar]
- Su, J.-F.; Wang, S.-B.; Zhang, Y.-Y.; Huang, Z. Physicochemical properties and mechanical characters of methanol-modified melamine-formaldehyde (MMF) shell microPCMs containing paraffin. Colloid Polym. Sci. 2011, 289, 111–119. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, S.-B.; Zhou, J.-W.; Huang, Z.; Zhao, Y.-H.; Yuan, X.-Y.; Zhang, Y.-Y.; Kou, J.-B. Fabrication and interfacial morphologies of methanol–melamine–formaldehyde (MMF) shell microPCMs/epoxy composites. Colloid Polym. Sci. 2011, 289, 169–177. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, X.-Y.; Wang, S.-B.; Zhao, Y.-H.; Zhu, K.-Y.; Yuan, X.-Y. Interface stability behaviors of methanol-melamine-formaldehyde shell microPCMs/epoxy matrix composites. Polym. Compos. 2011, 32, 810–820. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Lu, S.; Shen, T.; Xing, J.; Song, Q.; Shao, J.; Zhang, J.; Xin, C. Preparation and characterization of cross-linked polyurethane shell microencapsulated phase change materials by interfacial polymerization. Mater. Lett. 2018, 211, 36–39. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.-C.; Yan, W.-M.; Amani, M. Microencapsulated n-eicosane PCM suspensions: Thermophysical properties measurement and modeling. Int. J. Heat Mass Transf. 2018, 125, 792–800. [Google Scholar] [CrossRef]
- Nikpourian, H.; Bahramian, A.R.; Abdollahi, M. On the thermal performance of a novel PCM nanocapsule: The effect of core/shell. Renew. Energy 2020, 151, 322–331. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.; Youngblood, J.P. Synthesis and Characterization of Microencapsulated Phase Change Materials with Poly(urea−urethane) Shells Containing Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Gao, Y.; Geng, X.; Wang, X.; Han, N.; Zhang, X.; Li, W. Synthesis and characterization of microencapsulated phase change materials with chitosan-based polyurethane shell. Carbohydr. Polym. 2021, 273, 118629. [Google Scholar] [CrossRef]
- Dumistracel, I.; Ponchel, G.; Danila, G.; Duchene, D.; Carpov, A. Poly(vinylbenzyl chloride) microsphere synthesis and their chemical modifications. J. Microencapsul. 2000, 17, 45–55. [Google Scholar] [CrossRef]
- Abd El-Mageed, A.I.A.; Dyab, A.K.F.; Mohamed, L.A.; Taha, F.; Essawy, H.A. Suspension polymerization for fabrication of magnetic polystyrene microspheres stabilized with Hitenol BC-20. Polym. Bull. 2022, 79, 3379–3393. [Google Scholar] [CrossRef]
- Bux, J.; Manga, M.S.; Hunter, T.N.; Biggs, S. Manufacture of poly(methyl methacrylate) microspheres using membrane emulsification. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150134. [Google Scholar] [CrossRef]
- Cai, S.; Weng, Z.; Zheng, Y.; Zhao, B.; Gao, Z.; Gao, C. High porosity microspheres with functional groups synthesized by thiol–yne click suspension polymerization11Electronic supplementary information (ESI) available. Polym. Chem. 2016, 7, 7400–7407. [Google Scholar] [CrossRef]
- Chaiyasat, P.; Chaiyasat, A.; Boontung, W.; Promdsorn, S.; Thipsit, S. Preparation and Characterization of Poly(divinylbenzene) Microcapsules Containing Octadecane. Mater. Sci. Appl. 2011, 2, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Supatimusro, D.; Promdsorn, S.; Thipsit, S.; Boontung, W.; Chaiyasat, P.; Chaiyasat, A. Poly(divinylbenzene) Microencapsulated Octadecane for Use as a Heat Storage Material: Influences of Microcapsule Size and Monomer/Octadecane Ratio. Polym.-Plast. Technol. Eng. 2012, 51, 1167–1172. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Sánchez, P. Influence of different suspension stabilizers on the preparation of Rubitherm RT31 microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 62–66. [Google Scholar] [CrossRef]
- Qiu, X.; Song, G.; Chu, X.; Li, X.; Tang, G. Preparation, thermal properties and thermal reliabilities of microencapsulated n-octadecane with acrylic-based polymer shells for thermal energy storage. Thermochim. Acta 2013, 551, 136–144. [Google Scholar] [CrossRef]
- Qiu, X.; Song, G.; Chu, X.; Li, X.; Tang, G. Microencapsulated n-alkane with p(n-butyl methacrylate-co-methacrylic acid) shell as phase change materials for thermal energy storage. Sol. Energy 2013, 91, 212–220. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 157, 216–222. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material encapsulation in poly (methyl methacrylate) shell: A review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef] [Green Version]
- Sarı, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Microencapsulated n-octacosane as phase change material for thermal energy storage. Sol. Energy 2009, 83, 1757–1763. [Google Scholar] [CrossRef]
- Ma, S.; Song, G.; Li, W.; Fan, P.; Tang, G. UV irradiation-initiated MMA polymerization to prepare microcapsules containing phase change paraffin. Sol. Energy Mater. Sol. Cells 2010, 94, 1643–1647. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Xia, T.D.; Zhang, T.; Feng, H.X. Fabrication and performances of microencapsulated paraffin composites with polymethylmethacrylate shell based on ultraviolet irradiation-initiated. Mater. Chem. Phys. 2012, 135, 181–187. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Xia, T.; Zhao, W.; Yang, W. Effects of fabricated technology on particle size distribution and thermal properties of stearic–eicosanoic acid/polymethylmethacrylate nanocapsules. Sol. Energy Mater. Sol. Cells 2014, 120, 481–490. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Zhang, Y.; Wang, Y. Microencapsulation of stearic acid with polymethylmethacrylate using iron (III) chloride as photo-initiator for thermal energy storage. Chin. J. Chem. Eng. 2017, 25, 1524–1532. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Chen, L.; Song, G.; Tang, G. Facile and low energy consumption synthesis of microencapsulated phase change materials with hybrid shell for thermal energy storage. J. Phys. Chem. Solids 2017, 111, 207–213. [Google Scholar] [CrossRef]
- Farid, M.; Al-Shannaq, R.; Al-Muhtaseb, S.; Kurdi, J. Method for Low Temperature Microencapsulation of Phase Change Materials. U.S. Patent 10,913,882, 9 February 2021. [Google Scholar]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. Polymerization. In Encyclopedia Britannica; Encyclopaedia Britannica: Chicago, IL, USA, 2020; Available online: https://www.britannica.com/science/polymerization (accessed on 7 July 2022).
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Biçer, A.; Altuntaş, A.; Bilgin, C. Micro/nanoencapsulated n-nonadecane with poly(methyl methacrylate) shell for thermal energy storage. Energy Convers. Manag. 2014, 86, 614–621. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Kapar, S.; Ataeian, P.; da Silva Bernardes, J.; Berry, R.; Zhao, W.; Zhou, G.; Tam, K.C. Microencapsulation of Phase Change Materials with Polystyrene/Cellulose Nanocrystal Hybrid Shell via Pickering Emulsion Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 17756–17767. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.O.; Unal, M. Nanoencapsulation of n-alkanes with poly(styrene-co-ethylacrylate) shells for thermal energy storage. Appl. Energy 2015, 150, 335–340. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.; Zainal, Z.; Rusli, R. Nano-Encapsulated n-Nonadecane Using Vinyl Copolymer Shell for Thermal Energy Storage Medium. Macromol. Res. 2015, 23, 658–669. [Google Scholar] [CrossRef]
- Alay Aksoy, S.; Alkan, C.; Tözüm, M.S.; Demirbağ, S.; Altun Anayurt, R.; Ulcay, Y. Preparation and textile application of poly(methyl methacrylate-co-methacrylic acid)/n-octadecane and n-eicosane microcapsules. J. Text. Inst. 2017, 108, 30–41. [Google Scholar] [CrossRef]
- Şahan, N.; Nigon, D.; Mantell, S.C.; Davidson, J.H.; Paksoy, H. Encapsulation of stearic acid with different PMMA-hybrid shell materials for thermotropic materials. Sol. Energy 2019, 184, 466–476. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.O.; Unal, M.; Konuklu, S. Microencapsulation of a fatty acid with Poly(melamine–urea–formaldehyde). Energy Convers. Manag. 2014, 80, 382–390. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Samui, A.B.; Mahanwar, P.A. Melamine-formaldehyde microencapsulated n-Tetracosane phase change material for solar thermal energy storage in coating. Sol. Energy Mater. Sol. Cells 2020, 215, 110676. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S.; Fei, X.; Zhao, H.; Lou, X. Enhanced mechanical properties and thermal conductivity of paraffin microcapsules shelled by hydrophobic-silicon carbide modified melamine-formaldehyde resin. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125219. [Google Scholar] [CrossRef]
- Hussain, S.I.; Kalaiselvam, S. Nanoencapsulation of oleic acid phase change material with Ag2O nanoparticles-based urea formaldehyde shell for building thermal energy storage. J. Therm. Anal. Calorim. 2020, 140, 133–147. [Google Scholar] [CrossRef]
- Saraç, E.G.; Öner, E.; Kahraman, M.V. Microencapsulated organic coconut oil as a natural phase change material for thermo-regulating cellulosic fabrics. Cellulose 2019, 26, 8939–8950. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wang, K.; Huang, Y.; Chen, Z. Highly efficient photothermal conversion capric acid phase change microcapsule: Silicon carbide modified melamine urea formaldehyde. J. Colloid Interface Sci. 2021, 582, 30–40. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S.; Pethurajan, V. Heat transfer performance of graphene nano-platelets laden micro-encapsulated PCM with polymer shell for thermal energy storage based heat sink. Appl. Therm. Eng. 2019, 156, 237–249. [Google Scholar] [CrossRef]
- Siddhan, P.; Jassal, M.; Agrawal, A.K. Core content and stability of n-octadecane-containing polyurea microencapsules produced by interfacial polymerization. J. Appl. Polym. Sci. 2007, 106, 786–792. [Google Scholar] [CrossRef]
- Lu, S.; Xing, J.; Zhang, Z.; Jia, G. Preparation and characterization of polyurea/polyurethane double-shell microcapsules containing butyl stearate through interfacial polymerization. J. Appl. Polym. Sci. 2011, 121, 3377–3383. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Hu, D.; Wu, L. Synthesis and characterization of microencapsulated methyl laurate with polyurethane shell materials via interfacial polymerization in Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124958. [Google Scholar] [CrossRef]
- Lashgari, S.; Mahdavian, A.R.; Arabi, H.; Ambrogi, V.; Marturano, V. Preparation of acrylic PCM microcapsules with dual responsivity to temperature and magnetic field changes. Eur. Polym. J. 2018, 101, 18–28. [Google Scholar] [CrossRef]
- Pradhan, R.; Ramaswamy, A.P. Encapsulation of paraffin wax by rigid cross-linked poly (styrene divinylbenzene-acrylic acid) and its thermal characterization. SN Appl. Sci. 2019, 1, 859. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yang, R. Efficient preparation and characterization of paraffin-based microcapsules by emulsion polymerization. J. Appl. Polym. Sci. 2019, 136, 47552. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Kahraman Döğüşcü, D.; Biçer, A. Micro/nano-encapsulated n-heptadecane with polystyrene shell for latent heat thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 126, 42–50. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-encapsulated organic phase change material based on copolymer nanocomposites for thermal energy storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, J.; Li, H.; Li, X. Cuprous oxide modified nanoencapsulated phase change materials fabricated by RAFT miniemulsion polymerization for thermal energy storage and photothermal conversion. Powder Technol. 2022, 399, 117189. [Google Scholar] [CrossRef]
- Wu, B.; Shi, L.; Zhang, Q.; Wang, W.-J. Microencapsulation of 1-hexadecanol as a phase change material with reversible thermochromic properties. RSC Adv. 2017, 7, 42129–42137. [Google Scholar] [CrossRef] [Green Version]
- Hawlader, M.N.A.; Uddin, M.S.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.S.; Zhu, H.J. Preparation and evaluation of a novel solar storage material: Microencapsulated paraffin. Int. J. Sol. Energy 2000, 20, 227–238. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, Q.; Lv, W.; Song, Z. Novel phase separation method for the microencapsulation of oxalic acid dihydrate/boric acid eutectic system in a hybrid polymer shell for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127369. [Google Scholar] [CrossRef]
- Machado, M.H.; Almeida, A.d.R.; Maciel, M.V.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the appropriate wall materials for spray-drying microencapsulation of natural bioactive ingredients: Taking phenolic compounds as examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Arpagaus, C. Chapter Four—Production of food bioactive-loaded nanoparticles by nano spray drying. In Nanoencapsulation of Food Ingredients by Specialized Equipment; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 151–211. [Google Scholar]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Lu, X.; Zhao, X.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Effect of alkaline pH on formation of lauric acid/SiO2 nanocapsules via sol-gel process for solar energy storage. Sol. Energy 2019, 185, 374–386. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Singh, J.K.; Lee, D.-E.; Park, T. Synthesis and application of paraffin/silica phase change nanocapsules: Experimental and numerical approach. J. Energy Storage 2022, 51, 104407. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Morphology-controlled synthesis of microencapsulated phase change materials with TiO2 shell for thermal energy harvesting and temperature regulation. Energy 2019, 172, 599–617. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Qu, Z.; Ren, J.; Xiong, C. Microencapsulated capric–stearic acid with silica shell as a novel phase change material for thermal energy storage. Appl. Therm. Eng. 2014, 70, 546–551. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Ghulam, M.; Zhang, J.; Wei, Q. Synthesis of a Novel Nanoencapsulated n-Eicosane Phase Change Materialwith Inorganic Silica Shell Material for Enhanced Thermal Properties throughSol-Gel Route. J. Text. Sci. Eng. 2017, 7, 292. [Google Scholar]
- Li, C.; He, G.; Yan, H.; Yu, H.; Song, Y. Synthesis of microencapsulated stearic acid with amorphous TiO2 as shape-stabilized PCMs for thermal energy storage. Energy Procedia 2018, 152, 390–394. [Google Scholar] [CrossRef]
- Pourmohamadian, H.; Sheikhzadeh, G.A.; Rahimi-Nasrabadi, M.; Tabrizi, H.B. Fabrication and characterization of microencapsulated PA with SiO2 shell through sol–gel synthesis via sodium silicate precursor. J. Mater. Sci. Mater. Electron. 2017, 28, 9990–9997. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Sun, L.; Xie, T.; Yang, K.; Li, Z. Preparation and characterization of high efficiency microencapsulated phase change material based on paraffin wax core and SiO2 shell derived from sodium silicate precursor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126905. [Google Scholar] [CrossRef]
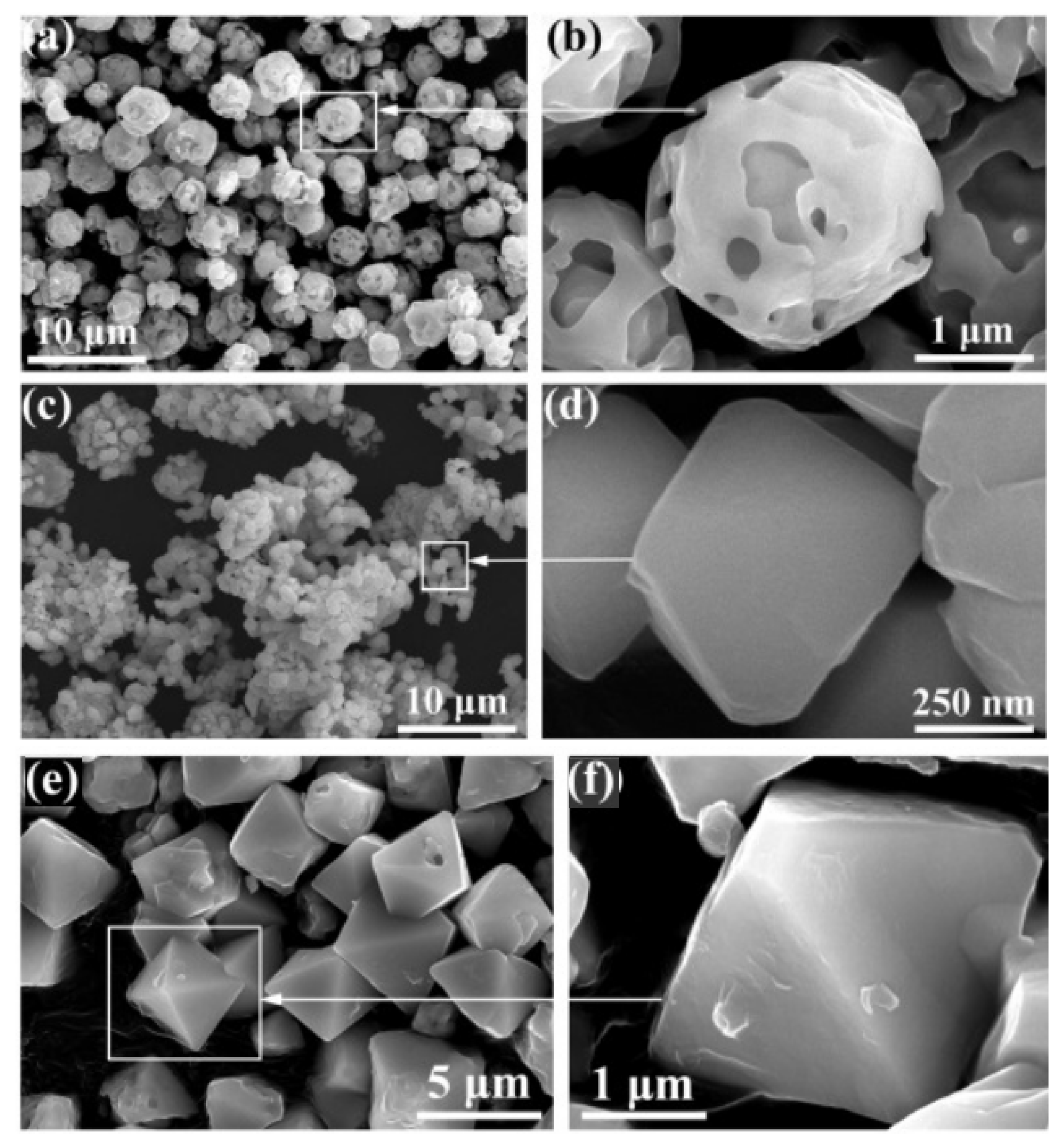
- Yu, S.; Wang, X.; Wu, D. Self-Assembly Synthesis of Microencapsulated n-Eicosane Phase-Change Materials with Crystalline-Phase-Controllable Calcium Carbonate Shell. Energy Fuels 2014, 28, 3519–3529. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, T.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Self-Assembly Synthesis and Properties of Microencapsulated n-Tetradecane Phase Change Materials with a Calcium Carbonate Shell for Cold Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 3074–3080. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Microencapsulated Paraffin Phase-Change Material with Calcium Carbonate Shell for Thermal Energy Storage and Solar-Thermal Conversion. Langmuir 2018, 34, 14254–14264. [Google Scholar] [CrossRef]
- Sarı, A.; Saleh, T.A.; Hekimoğlu, G.; Tyagi, V.V.; Sharma, R.K. Microencapsulated heptadecane with calcium carbonate as thermal conductivity-enhanced phase change material for thermal energy storage. J. Mol. Liq. 2021, 328, 115508. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Modified Phase Change Microcapsules with Calcium Carbonate and Graphene Oxide Shells for Enhanced Energy Storage and Leakage Prevention. ACS Sustain. Chem. Eng. 2018, 6, 5182–5191. [Google Scholar] [CrossRef]
- Wang, T.; Tong, J.; Li, X.; Wang, S.; Deng, J. Research on morphological control and temperature regulation of phase change microcapsules with binary cores for electronics thermal management. Thermochim. Acta 2021, 706, 179079. [Google Scholar] [CrossRef]
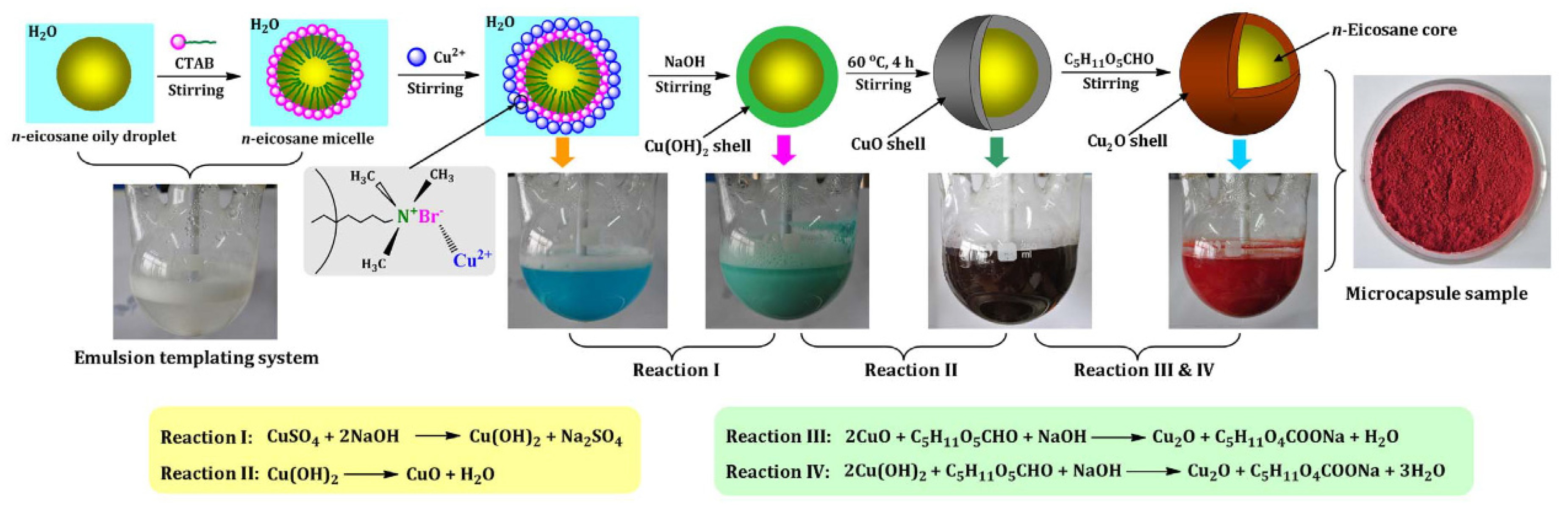
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Cai, T.; Yang, W.; Chen, Z.; Yang, A.; Jiang, J.; Ding, B.; Zhu, L.; He, C.; Zhou, Y.; Zhang, K. Morphology-controllable paraffin@lead tungstate microcapsules for gamma radiation shielding and thermal energy storage. J. Energy Storage 2022, 50, 104245. [Google Scholar] [CrossRef]
- Du, J.; Ibaseta, N.; Guichardon, P. Characterization of polyurea microcapsules synthesized with an isocyanate of low toxicity and eco-friendly esters via microfluidics: Shape, shell thickness, morphology and encapsulation efficiency. Chem. Eng. Res. Des. 2022, 182, 256–272. [Google Scholar] [CrossRef]
- Shi, T.; Hu, P.; Wang, J. Preparation of Polyurea Microcapsules Containing Phase Change Materials Using Microfluidics. ChemistrySelect 2020, 5, 2342–2347. [Google Scholar] [CrossRef]
- Rahman, A.; Dickinson, M.E.; Farid, M.M. Microencapsulation of a PCM through membrane emulsification and nanocompression-based determination of microcapsule strength. Mater. Renew. Sustain. Energy 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Sakai, Y.; Sugimori, N.; Ikeda, T.; Monzen, M.; Ono, T. Microfluidic Production of Monodisperse Biopolymer Microcapsules for Latent Heat Storage. ACS Mater. Au 2022, 2, 250–259. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hayaty, M.; Navarchian, A.H. Morphological study of polymethyl methacrylate microcapsules filled with self-healing agents. Appl. Surf. Sci. 2017, 399, 721–731. [Google Scholar] [CrossRef]
- Salaün, F.; Devaux, E.; Bourbigot, S.; Rumeau, P. Influence of the solvent on the microencapsulation of an hydrated salt. Carbohydr. Polym. 2010, 79, 964–974. [Google Scholar] [CrossRef]
- Huang, J.; Wang, T.; Zhu, P.; Xiao, J. Preparation, characterization, and thermal properties of the microencapsulation of a hydrated salt as phase change energy storage materials. Thermochim. Acta 2013, 557, 1–6. [Google Scholar] [CrossRef]
- Tangsiriratana, E.; Skolpap, W.; Patterson, R.J.; Sriprapha, K. Thermal properties and behavior of microencapsulated sugarcane wax phase change material. Heliyon 2019, 5, e02184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Campagne, C.; Salaün, F. Preparation of n-Alkane/Polycaprolactone Phase-Change Microcapsules via Single Nozzle Electro-Spraying: Characterization on Their Formation, Structures and Properties. Appl. Sci. 2020, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, S.; Zhao, Y.; Ling, Z.; Zhang, Z.; Fang, X. Compatible paraffin@SiO2 microcapsules/polydimethylsiloxane composites with heat storage capacity and enhanced thermal conductivity for thermal management. Compos. Sci. Technol. 2022, 218, 109192. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Lv, Z.; Hua, Q.; Liu, L.; Wang, B.; Tang, J. Synthesis of high latent heat lauric acid/silica microcapsules by interfacial polymerization method for thermal energy storage. J. Energy Storage 2021, 33, 102059. [Google Scholar] [CrossRef]
- Ke, W.-D.; Wu, X.-W.; Zhang, J.-L. In situ polymerization of organic and inorganic phase change microcapsule and enhancement of infrared stealth via nano iron. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127124. [Google Scholar] [CrossRef]
- Chen, M.; Qian, Z.; Liu, H.; Wang, X. Size-tunable CaCO3@n-eicosane phase-change microcapsules for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128470. [Google Scholar] [CrossRef]
- Yamada, Y.; Horibe, A. Thermal properties and related core/shell structure of n-tetracosane microencapsulated by calcium carbonate. Appl. Therm. Eng. 2020, 178, 115512. [Google Scholar] [CrossRef]
- Pourmohamadian, H.; Rahimi-Nasrabadi, M.; sobhani-nasab, A.; Sheikhzadeh, G.A.; Basirat Tabrizi, H. Experimental Study of the Thermal Properties of Microencapsulated Palmitic Acid Composites with CuCO3 Shell as Thermal Energy Storage Materials. ChemistrySelect 2019, 4, 6501–6505. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Hassabo, A.; Mohamed, A.; Wang, H.; Popescu, C.; Moller, M. Metal salts rented in silica microcapsules as inorganic phase change materials for textile usage. Inorg. Chem. Indian J. 2015, 10, 59–65. [Google Scholar]
- Chang, Z.; Wang, K.; Wu, X.; Lei, G.; Wang, Q.; Liu, H.; Wang, Y.; Zhang, Q. Review on the preparation and performance of paraffin-based phase change microcapsules for heat storage. J. Energy Storage 2022, 46, 103840. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Fang, G. Encapsulation of inorganic phase change thermal storage materials and its effect on thermophysical properties: A review. Sol. Energy Mater. Sol. Cells 2022, 241, 111747. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhao, T. Fabrication and characterization of poly(melamine-formaldehyde)/silicon carbide hybrid microencapsulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol. Energy Mater. Sol. Cells 2018, 183, 82–91. [Google Scholar] [CrossRef]
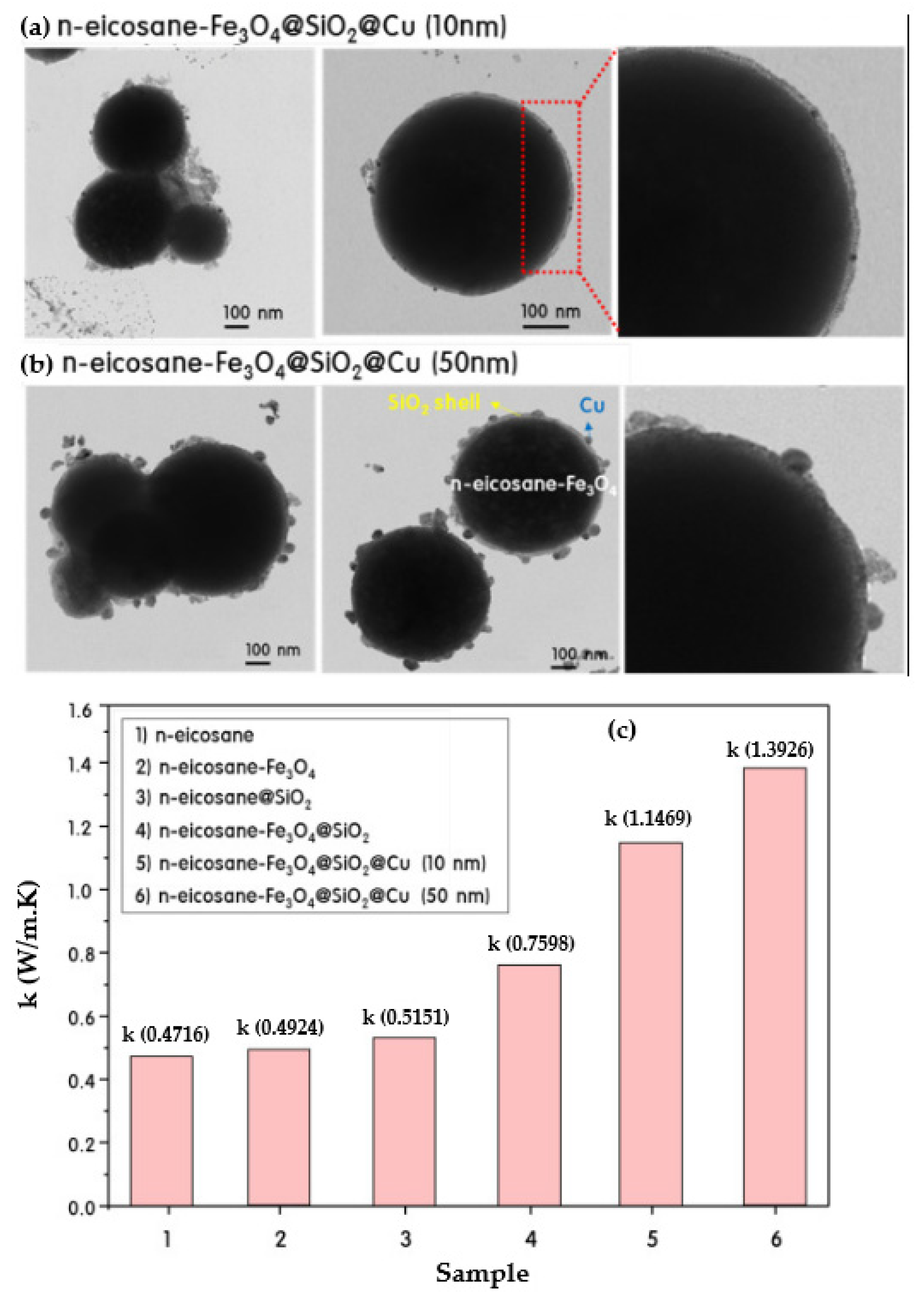
- Do, J.Y.; Son, N.; Shin, J.; Chava, R.K.; Joo, S.W.; Kang, M. n-Eicosane-Fe3O4@SiO2@Cu microcapsule phase change material and its improved thermal conductivity and heat transfer performance. Mater. Des. 2021, 198, 109357. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Liang, H.; Yan, X. Preparation and characterization of PMMA/TiO2 hybrid shell microencapsulated PCMs for thermal energy storage. Energy 2019, 167, 1031–1039. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, S.; Kang, M.; Liu, Y.; Zhang, F.; Qu, W.; Guan, Y.; Li, S. The thermal behavior and flame retardant performance of phase change material microcapsules with modified carbon nanotubes. Energy 2022, 240, 122821. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Farid, M. Innovative method of metal coating of microcapsules containing phase change materials. Sol. Energy 2016, 129, 54–64. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Grishanov, D.A.; Sladkevich, S.; Xu, Z.J.; Sakharov, K.A.; Prikhodchenko, P.V.; Lev, O. Doubly Coated, Organic–Inorganic Paraffin Phase Change Materials: Zinc Oxide Coating of Hermetically Encapsulated Paraffins. Adv. Mater. Interfaces 2019, 6, 1900368. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Wang, T.; Zhu, P. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Thermochim. Acta 2019, 675, 1–8. [Google Scholar] [CrossRef]
- Kang, T.; You, Y.; Jun, S. Supercooling preservation technology in food and biological samples: A review focused on electric and magnetic field applications. Food Sci. Biotechnol. 2020, 29, 303–321. [Google Scholar] [CrossRef]
- Lei, K.; Bao, J.; Zhao, X.; Wang, H.; Zou, D. Supercooling suppression of metal-based microencapsulated phase change material (MEPCM) for thermal energy storage. Chem. Eng. J. 2022, 446, 137020. [Google Scholar] [CrossRef]
- Cao, F.; Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 2014, 113, 1512–1518. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Fan, Y.-F.; Tao, X.-M.; Yick, K.-L. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Colloid Interface Sci. 2005, 281, 299–306. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, D.; Jiao, X.; Zhang, Y.; Shea, K.J.; Lu, X.; Qiu, G. Preparation, Properties, and Supercooling Prevention of Phase Change Material n-Octadecane Microcapsules with Peppermint Fragrance Scent. Ind. Eng. Chem. Res. 2015, 54, 8130–8136. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Design and synthesis of microcapsules with cross-linking network supporting core for supercooling degree regulation. Energy Build. 2021, 253, 111437. [Google Scholar] [CrossRef]
- Meng, X.; Qin, S.; Fan, H.; Huang, Z.; Hong, J.; Xu, X.; Ouyang, X.; Chen, D.-Z. Long alkyl chain-grafted carbon nanotube-decorated binary-core phase-change microcapsules for heat energy storage: Synthesis and thermal properties. Sol. Energy Mater. Sol. Cells 2020, 212, 110589. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Li, M.; Yang, H.; Li, B. Improvements in the thermal conductivity and mechanical properties of phase-change microcapsules with oxygen-plasma-modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2017, 134, 45269. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Wu, Z. Enhancement in thermal property and mechanical property of phase change microcapsule with modified carbon nanotube. Appl. Energy 2014, 127, 166–171. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Zhang, H.; Wan, X.-J.; Chen, D.-Z.; Chen, X.-F.; Ye, X.; Ouyang, X.; Qin, S.-Y.; Wen, H.-X.; Tang, J.-N. Carbon nanotube-enhanced double-walled phase-change microcapsules for thermal energy storage. J. Mater. Chem. A 2017, 5, 7482–7493. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, L.-X.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, L.-X.; Ren, L.; Huang, Z. Mechanical properties and thermal stability of double-shell thermal-energy-storage microcapsules. J. Appl. Polym. Sci. 2007, 103, 1295–1302. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Microencapsulated n-octadecane with different methylmethacrylate-based copolymer shells as phase change materials for thermal energy storage. Energy 2012, 46, 188–199. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Song, G.; Chu, X.; Tang, G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol. Energy Mater. Sol. Cells 2012, 98, 283–293. [Google Scholar] [CrossRef]












| Category | Methods | Common Shell | PCM | Ref. |
|---|---|---|---|---|
| Chemical | In situ polymerization | MF, UF, PEG modified MF | Organic/ inorganic | [37,38,39,40,41] |
| Interfacial polymerization | PU, TDI, DETA, Polyamide | Organic/ inorganic | [42] | |
| Suspension polymerization | PMMA, PBMA, PS, PDVB | Organic | [43,44,45,46,47] | |
| Emulsion/mini emulsion polymerization | PMMA, PS, SBA, PS-MMA | Organic/ inorganic | [48,49] | |
| Physical–chemical | Coacervation | Gum Arabic/gelatine, agar agar/gum Arabic, UF, MF, UMF, Chitosan/gum Arabic | Organic | [50,51,52] |
| Sol–gel method | SiO2, TiO2, Fe3O4/SiO2, PMMA/SiO2, SrTiO3 | Organic/ inorganic | [53,54,55,56,57] | |
| Self-assembly | CaCO3, Cu2O, TiO2 | Organic | [58,59,60,61] | |
| Ionic gelation | Organic | |||
| Physical–mechanical | Spray drying | Gelatine acacia, LDPE, EVA | Organic | [62,63] |
| Solvent evaporation | PVC, ethyl cellulose, MMA-co-HEMA | Organic | [64,65,66] | |
| Vacuum impregnation | n/a | Organic | n/a | |
| Fluidized bed | Acrylic | Organic/ inorganic | [67] | |
| Pan coating | n/a | Organic | n/a | |
| Air-suspension coating | n/a | Organic | n/a | |
| Vibration nozzle | n/a | Organic | n/a | |
| Centrifugal extrusion | n/a | Organic | n/a | |
| Chemical–mechanical | Microfluidic method | Silicone, PDMS | Organic | [68,69,70] |
| Melt coaxial electrospray method | Sodium alginate | Organic | [71] |
| Materials | Examples | Consideration and Comments |
|---|---|---|
| Monomers |
|
|
| Initiators |
|
|
| Surfactants |
|
|
| Other recipes |
|
|
| Core | Shell | Tmp (°C) | PS (µm or/nm) | LHm (J/g) | CC (wt.%) | Ref. | |
|---|---|---|---|---|---|---|---|
| In situ polymerization | Paraffin | MUF | 32.4 | 3.02 ± 0.42 μm | 134.3 | 77.1 | [38] |
| n-octadecane | MF | 25.17 | 3 μm | 185.1 | 84.3 | [80] | |
| n-octadecane | UMF | 25.2 | 0.2–5.6 μm | 172.7–190.6 | 71.4–78.8 | [73] | |
| pentadecane | MUF | 12.5 | n/a | 84.5−88.2 | 48.5–50.60 | [75] | |
| capric acid | UF | 33.9 | 0.28 μm | 88 | 78.6 | [121] | |
| n-Tetracosane | MF | 54.2 | n/a | 134.7 | 72.4 | [122] | |
| n-dodecanol | GO-modified MF | 26.3 | n/a | 125.2 | 64.8 | [123] | |
| paraffin | H-SiC-modified MF | 52.3 | n/a | 93.2 | 65.1 | [124] | |
| 1-Dodecanol | MPF | 22.9 | n/a | 169.5 | 88.6 | [41] | |
| oleic acid | Ag2O-UF | 5.4 | 71.7 | 54.8 | [125] | ||
| coconut oil | MF | 21.1 | n/a | 81.9 | 76.2 | [126] | |
| capric acid | nano-SiC-modified MUF | 32.9 | n/a | 97.8 | 65.7 | [127] | |
| 1-dodecanol | MF | 22.1 | 490.2 nm | 79.5 | 40.9 | [39] | |
| Caprylic acid | UF | 19.3 | 200 nm–1.5 μm | 93.9 | 59 | [79] | |
| Paraffin | nanoplatelets laden/UF | 63.2 | 60–65 µm | 110.7–116.7 | 52.2–55.1 | [128] | |
| Interfacial polymerization | methyl laurate | CNCs- urea–urethane | 5.48 ± 0.05 | n/a | 148.4 | 66.1 | [92] |
| n-octadecane | PU | 25.2 | 10–14 μm | 220.1 | 71 | [129] | |
| butyl stearate | PU | 23.2 | 1–5 μm | 85 | 69.7 | [130] | |
| dodecanol dodecanoate | PU | 31.2 | 10 to 40 μm | 103.4–140.3 | 54–74.5 | [42] | |
| butyl stearate | PU | 23.2 | - | 80.6 | 74.3 | [89] | |
| Methyl laurate | nano-TiO2-PU | 7.4 | 150–350 μm | 147.71 | 83.3 | [131] | |
| paraffin wax | PU | 25–185 nm | 153.9 | 80.2 | [91] | ||
| n–octadecane | Fe3O4-PU | 29.34 | 35–500 μm | 165.7 | 83.6 | [68] | |
| Suspension polymerization | n-hexadecane | Fe3O4-modified PMMA | 18 | 180 µm | 53.1 | 24.4 | [132] |
| Paraffin@RT21 | Cross-linked PMMA | 21 | 5–10 µm | 113.4 | 85.6 | [43] | |
| n-hexadecane | PMMA or/ poly(BA-co-MMA | 18.3 | n/a | 63.1 | 28.9 | [33] | |
| Paraffin@RT21 | Cross-linked PMMA | 21 | n/a | 93.1 | 66.5 | [45] | |
| Paraffin wax | Poly (DVB/St/AA) | 56.1 | 200–500 µm | 62.4 | 41.1 | [133] | |
| n-octadecane | PDVB | 30 | n/a | 184.0 | 76.3 | [98] | |
| Emulsion/miniemulsion polymerization | Paraffin | PMMA | 29.8 | n/a | 75.6 | 72.5 | [134] |
| heptadecane | PMMA | 20.3 | 0.14–0.40 μm | 81.5 | 38 | [114] | |
| n-heptadecane | PSt | 21.4 | 1–15 µm | 136.9 | 63.3 | [135] | |
| n-octadecane | Poly(St-MMA) | 30.9 | 102 ± 11nm | 117.3 | 49.0 | [136] | |
| n-octadecane | Cu2O/PMMA | 27.2 | n/a | 102.66 | 70.0 | [137] |
| Method | Core | Shell | Tmp (°C) | PS (µm or/nm) | LHm (J/g) | CC (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|
| Complex coacervation | oxalic acid dihydrate | EC-ABS | 91.3 | 57.7–95.3 µm | 178.4 | 61.9 | [141] |
| Complex coacervation | sugarcane wax−Al2O3 composite | gelatine−gum Arabic | 70.7 | n/a | 59.7 | 87.6 | [173] |
| Complex coacervation | n-eicosane | SF-CHI | 38.0 | 23 µm | 149.5 | 61.8 | [51] |
| Spray drying | Rubitherm®RT27 | LDPE-EVA copolymer | 29.4 | n/a | 98.1 | 49.3 | [63] |
| Electro-Spraying | n-hexadecane | Polycaprolactone | 18.0 | 21.5 ± 3.1 µm | 113.2 ± 7.2 | 81.1 ± 5.2 | [174] |
| Sol–gel method | Paraffin | SiO2 | 48–50 | n/a | 161.4 | 80.0 | [175] |
| Sol–gel method | lauric acid | SiO2 | 44.2 | n/a | 186.6 | 78.6 | [176] |
| Sol–gel method | Paraffin | GO/TiO2 | 51.53 | n/a | 160.8 | 81.9 | [58] |
| Self-assembly | Stearic acid | CaCO3 | 56.6 | n/a | 161 | 91.2 | [177] |
| Self-assembly | n-eicosane | CaCO3 | 37.2 | 740 nm–1.54 µm | 100–131.5 | 44.8–58.9 | [178] |
| Self-assembly | n-tetracosane | CaCO3 | 51.0 | n/a | 134.0 | 53 | [179] |
| Self-assembly | n-eicosane | Cu2O | 38.70 | n/a | 165.3 | 61.6 | [164] |
| Self-assembly | palmitic acid | CuCO3 | 66.9 | n/a | 89.3 | 43.9 | [180] |
| Microfluidic method | n-hexadecane | cellulose acetate | 18.0 | 88.3 µm | 176.0 | 66.0 | [169] |
| Microfluidic method | n-octadecane | Fe3O4-polyurea | 29.3 | 35–500 μm | 165.7 | 83.6 | [68] |
| Solvent evaporation | myristic acid | ethyl cellulose | 54.7 | n/a | 122.6 | 60.0 | [64] |
| Solvent evaporation | CA-PA eutectic | polyvinyl chloride | 17.1 | n/a | 92.1 | 57.7 | [65] |
| Material | MgCl2·6H2O | Bischofite |
|---|---|---|
| PCM |  |  |
| PCM Microcapsule |  |  |
| Microscopic view X10 |  |  |
| Method | Advantages | Disadvantages |
|---|---|---|
| In situ polymerization |
|
|
| Interfacial polymerization |
|
|
| Suspension polymerization |
|
|
| Emulsion/mini emulsion polymerization |
|
|
| Coacervation |
|
|
| Sol–gel method |
|
|
| Spray drying |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shannaq, R.; Farid, M.M.; Ikutegbe, C.A. Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review. Micro 2022, 2, 426-474. https://doi.org/10.3390/micro2030028
Al-Shannaq R, Farid MM, Ikutegbe CA. Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review. Micro. 2022; 2(3):426-474. https://doi.org/10.3390/micro2030028
Chicago/Turabian StyleAl-Shannaq, Refat, Mohammed M. Farid, and Charles A. Ikutegbe. 2022. "Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review" Micro 2, no. 3: 426-474. https://doi.org/10.3390/micro2030028
APA StyleAl-Shannaq, R., Farid, M. M., & Ikutegbe, C. A. (2022). Methods for the Synthesis of Phase Change Material Microcapsules with Enhanced Thermophysical Properties—A State-of-the-Art Review. Micro, 2(3), 426-474. https://doi.org/10.3390/micro2030028








