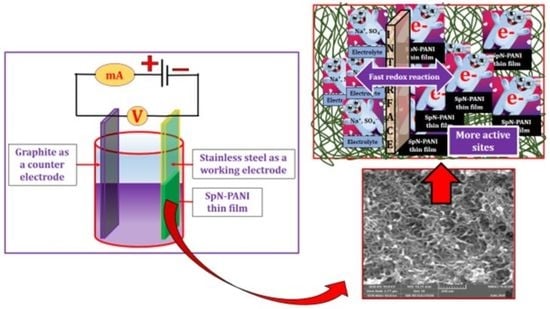
Spongy-Network-like Polyaniline Thin Films as Electrodes for a Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
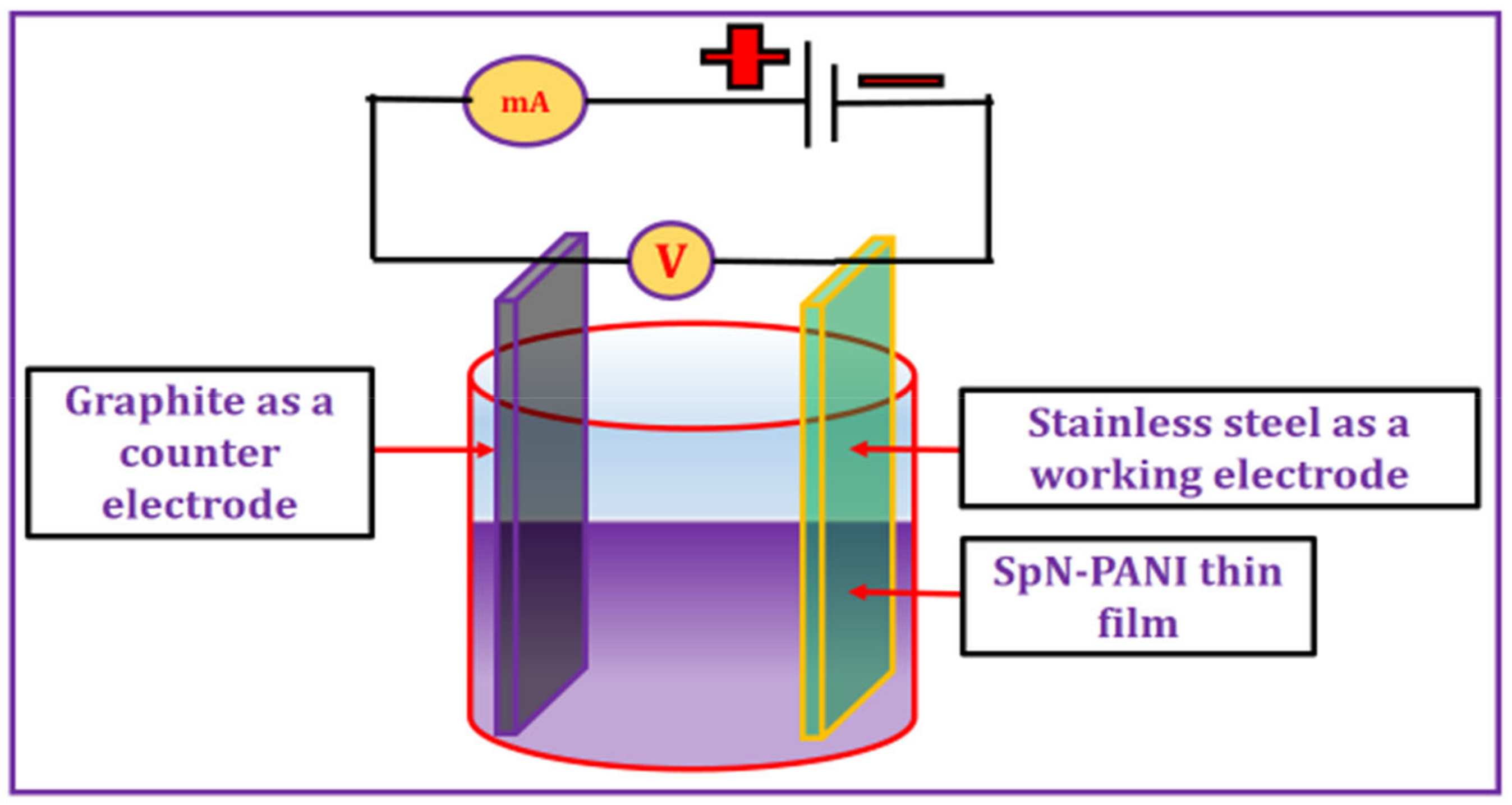
2.1. Synthesis of SpN-PANI Thin Film
2.2. Characterization Techniques
3. Results
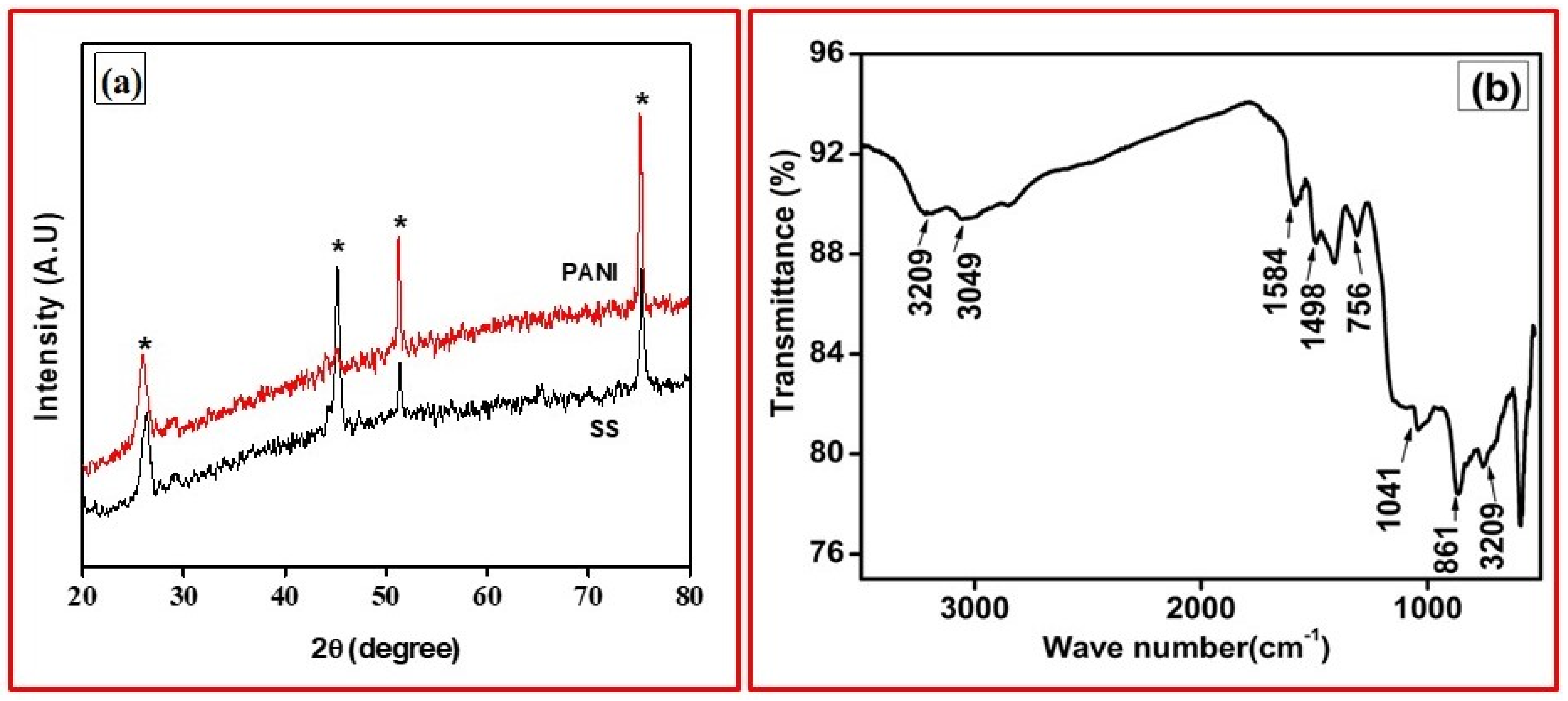
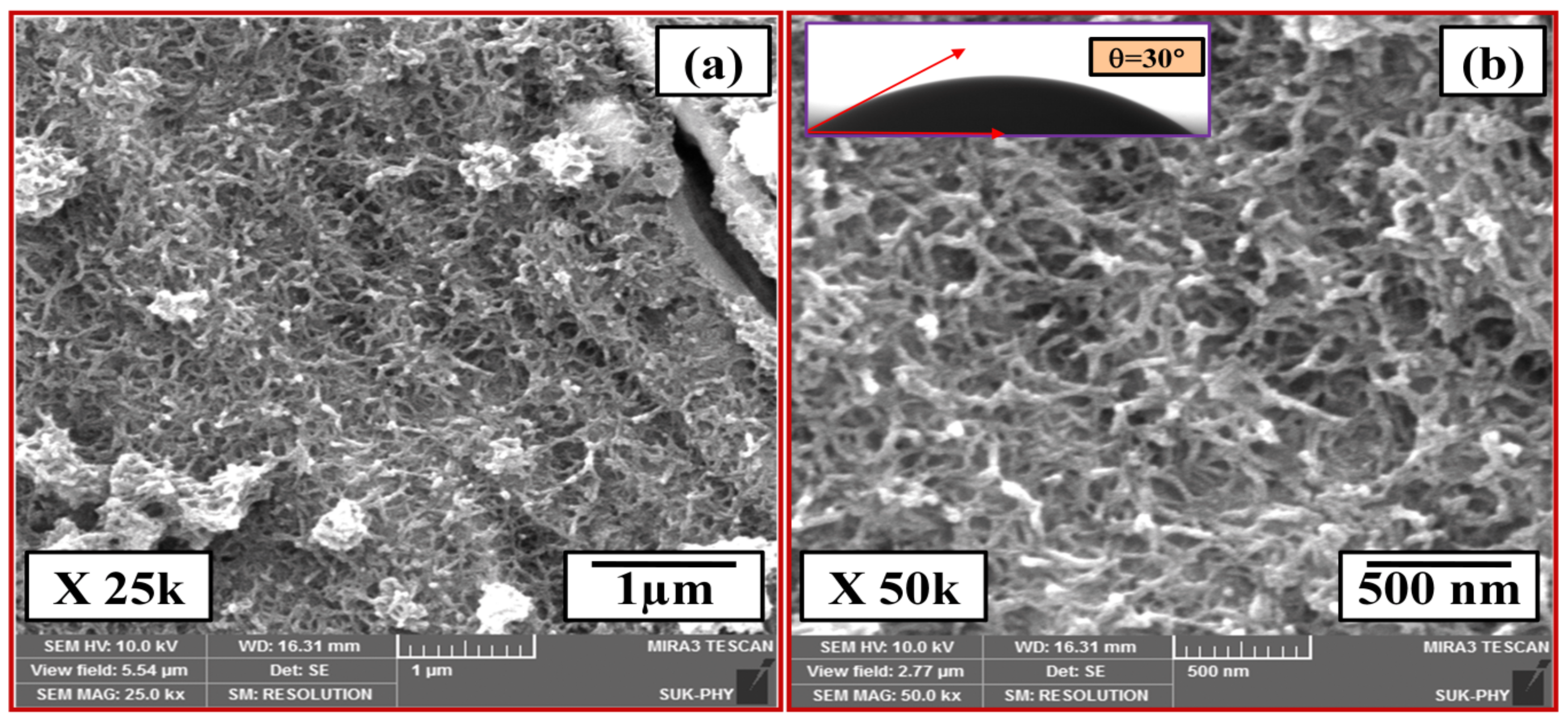
3.1. Structural and Surface Morphology Study
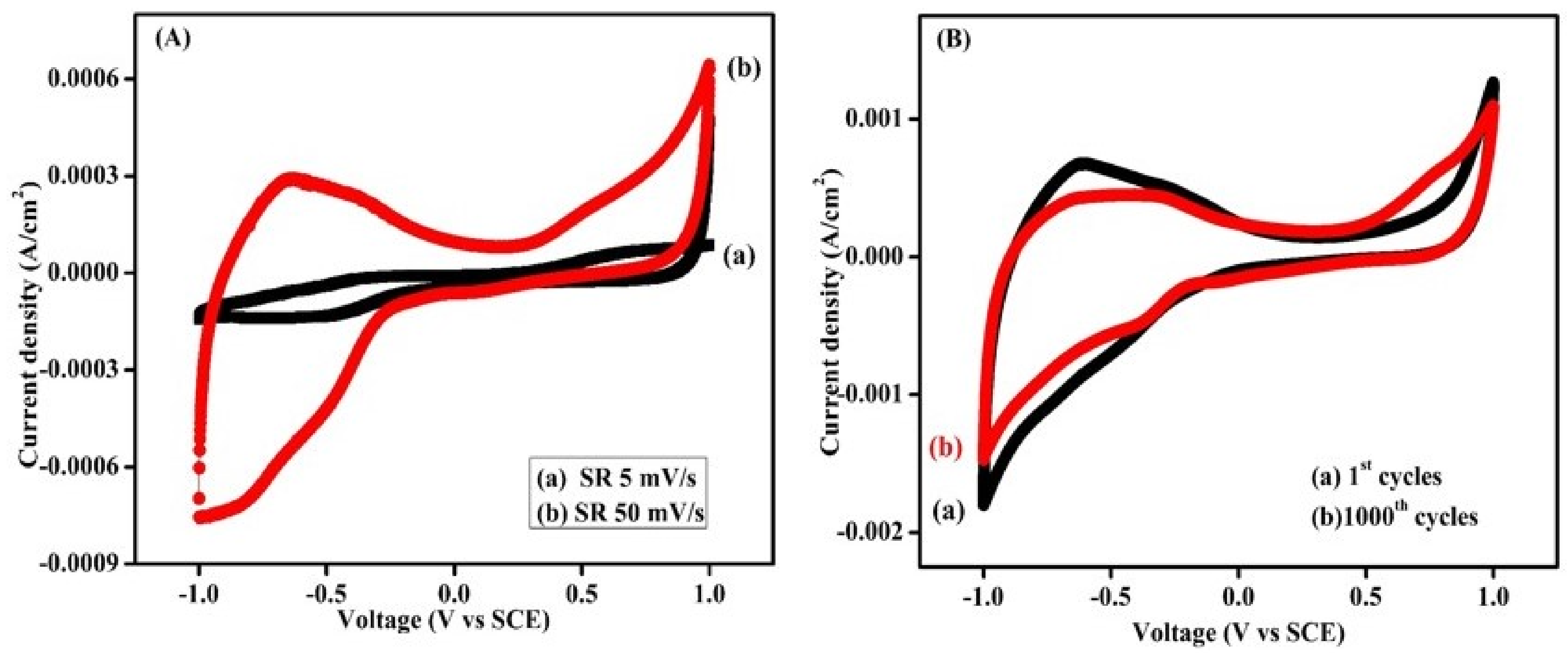
3.2. Cyclic Voltammetry (CV) Study
3.3. Stability Study
3.4. Galvanostatic Charge-Discharge (GCD) Study
3.5. Electrochemical Impedance Spectroscopy (EIS) Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing, and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline-based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Posdorfer, J.R.; Werner, B.; Wessling, B.; Heun, S.; Becker, H. Influence of Conductivity and Work Function of Polyaniline-Based HDL on PLED Device Performance. Proc. SPIE 2004, 5214, 188–196. [Google Scholar]
- Kharade, P.M.; Chavan, S.G.; Salunkhe, D.J.; Joshi, P.B.; Mane, S.M.; Kulkarni, S.B. Synthesis and characterization of PANI/MnO2 bi-layered electrode and its electrochemical supercapacitor properties. J. Mater. Sci. Mater. Electron. 2014, 52, 37–41. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Salunkhe, R.R.; Jamadade, V.S.; Dubal, D.P.; Pawar, S.M.; Lokhande, C.D. Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Osaheni, J.A.; Jenekhe, S.A.; Vanherzeele, H.; Meth, J.S.; Sun, Y.; MacDiarmid, A.G. Nonlinear optical properties of polyanilines and derivatives. J. Phys. Chem. 1992, 96, 2830–2836. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Song, N.; Zhang, Y.; Yang, Y.; Wang, J. Synthesis of hollow polyaniline nano-capsules and their supercapacitor application. J. Power Sources 2014, 272, 915–921. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, F. Polyaniline nanofibers prepared by a facile electrochemical approach and their supercapacitor performance. J. Mater. Res. 2008, 23, 2326–2332. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yu, P.; Zhang, Q. Oriented Arrays of Polyaniline Nanorods Grown on Graphite Nanosheets for an Electrochemical Supercapacitor. Langmuir 2013, 29, 493–500. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. High performance electrochemical supercapacitor from electrochemically synthesized nanostructured polyaniline. Mater. Lett. 2006, 60, 1466–1469. [Google Scholar] [CrossRef]
- Amarnath, C.; Chang, J.; Kim, D.; Mane, R.; Han, S.H.; Sohn, D. Electrochemical supercapacitor application of electroless surface polymerization of polyaniline nanostructures. Mater. Chem. Phys. 2009, 113, 14–17. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Vinu, A.; Lokhande, C.D. Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim. Acta 2011, 56, 9482–9487. [Google Scholar] [CrossRef]
- Ghenaatian, H.; Mousavi, M.; Rahmanifar, M. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta 2012, 78, 212–222. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Jiu, J.; Liu, J.; Yan, H.; Suganuma, K. Polyaniline films with modified nanostructure for bifunctional flexible multicolor electrochromic and supercapacitor applications. Chem. Eng. J. 2018, 345, 290–299. [Google Scholar] [CrossRef]
- Jamadade, V.; Dhawale, D.; Lokhande, C. Studies on electrosynthesized leucoemeraldine, emeraldine and pernigraniline forms of polyaniline films and their supercapacitive behavior. Synth. Met. 2010, 160, 955–960. [Google Scholar] [CrossRef]
- Inamdar, A.; Kim, Y.; Sohn, J.; Im, H. Supercapacitive Characteristics of Electrodeposited Polyaniline Thin Films Grown on Indium-doped Tin-oxide Substrates. J. Korean Phys. Soc. 2011, 59, 145–149. [Google Scholar] [CrossRef]
- Li, N.; Xiao, Y.; Xu, C.; Li, H.; Yang, X. Facile Preparation of Polyaniline Nanoparticles via Electrodeposition for Supercapacitors. Int. J. Electrochem. Sci. 2013, 8, 1181–1188. [Google Scholar]
- Zhang, H.; Zhao, Q.; Zhou, S.; Liu, N.; Wang, X.; Li, J.; Wang, F. Aqueous dispersed conducting polyaniline nanofibers: Promising high specific capacity electrode materials for supercapacitor. J. Power Sources 2011, 196, 10484–10489. [Google Scholar] [CrossRef]
- Kharade, P.M.; Thombare, J.V.; Babar, A.R.; Bulakhe, R.N.; Kulkarni, S.B.; Salunkhe, D.J. Electrodeposited nanoflakes like hydrophilic Co3O4 as a supercapacitor electrode. J. Phys. Chem. Solids 2018, 120, 207–210. [Google Scholar] [CrossRef]
- Navale, Y.H.; Ingole, S.M.; Navale, S.T.; Stadler, F.J.; Mane, R.S.; Naushad Mu Patil, V.B. Electro-synthesized fibrous polyaniline electrode as an active electrochemical supercapacitor material. J. Colloid Interface Sci. 2017, 487, 458–464. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Kharade, P.M.; Thombare, J.V.; Kadam, S.L.; Kulkarni, S.B.; Salunkhe, D.J. Layered PPy/Cr2O3 as a supercapacitor electrode with improved electrochemical performance. J. Mater. Sci. Mater. Electron. 2017, 28, 17908–17916. [Google Scholar] [CrossRef]
- Luo, Y.S.; Jiang, J.; Zhou, W.W.; Yang, H.P.; Luo, J.S.; Qi, X.Y.; Zhang, H.; Yu, D.Y.W.; Li, C.M.; Yu, T. Self-assembly of well-ordered whisker-like manganese oxide arrays on carbon fiber paper and its application as electrode material for supercapacitors. J. Mater. Chem. 2012, 22, 8634–8640. [Google Scholar] [CrossRef]

| Material | Conc. | Specific Capacitance (F.g−1) | Electrolyte | Method of Synthesis | Reference |
|---|---|---|---|---|---|
| Polyaniline | 0.2 M Aniline + 0.2 M H2SO4 | 473 | 0.5 M H2SO4 | Electrodeposition | [16] |
| Polyaniline nanograins | 0.2 M Aniline + 0.2 M APS + 0.2 M H2SO4 | 503 | 1.0 M H2SO4 | Template-free and seedless method | [12] |
| Polyaniline nanofibers | 0.1 M Aniline + 0.1 M H2SO4 | 508.7 | 1.0 M H2SO4 | Electrodeposition | [20] |
| Polyaniline nanotube | 0.2 mL Aniline + 1 M HCl + 0.1 g of K2Cr2O7 | 510 | 1.0 M H2SO4 | Chemical Polymerization | [21] |
| Polyaniline nanocapsules | 0.96 g Aniline + 0.57 APS | 502 | 1.0 M H2SO4 | Interfacial Polymerization | [7] |
| SpN-polyaniline | 0.1 M Aniline + 0.1 M H2SO4 | 580 | 0.5 M Na2SO4 | Galvanostatic Electrodeposition | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharade, P.M.; Thombare, J.V.; Dhasade, S.S.; Deokar, S.S.; Salunkhe, D.J.; Tamboli, M.S.; Patil, S.S. Spongy-Network-like Polyaniline Thin Films as Electrodes for a Supercapacitor. Micro 2022, 2, 541-548. https://doi.org/10.3390/micro2030035
Kharade PM, Thombare JV, Dhasade SS, Deokar SS, Salunkhe DJ, Tamboli MS, Patil SS. Spongy-Network-like Polyaniline Thin Films as Electrodes for a Supercapacitor. Micro. 2022; 2(3):541-548. https://doi.org/10.3390/micro2030035
Chicago/Turabian StyleKharade, P. M., J. V. Thombare, S. S. Dhasade, S. S. Deokar, D. J. Salunkhe, Mohaseen S. Tamboli, and Santosh S. Patil. 2022. "Spongy-Network-like Polyaniline Thin Films as Electrodes for a Supercapacitor" Micro 2, no. 3: 541-548. https://doi.org/10.3390/micro2030035
APA StyleKharade, P. M., Thombare, J. V., Dhasade, S. S., Deokar, S. S., Salunkhe, D. J., Tamboli, M. S., & Patil, S. S. (2022). Spongy-Network-like Polyaniline Thin Films as Electrodes for a Supercapacitor. Micro, 2(3), 541-548. https://doi.org/10.3390/micro2030035